Highlight
Multiple abiotic factors can combine to alter crop quality and rates of herbivore attack. Aphids benefit from elevated CO2 and root damage, but these effects are neutralized by increased temperatures.
Key words: Aboveground–belowground interactions, aphid, climate change, legume, root herbivore, simulated herbivory.
Abstract
Changes in host plant quality, including foliar amino acid concentrations, resulting from global climate change and attack from multiple herbivores, have the potential to modify the pest status of insect herbivores. This study investigated how mechanically simulated root herbivory of lucerne (Medicago sativa) before and after aphid infestation affected the pea aphid (Acyrthosiphon pisum) under elevated temperature (eT) and carbon dioxide concentrations (eCO2). eT increased plant height and biomass, and eCO2 decreased root C:N. Foliar amino acid concentrations and aphid numbers increased in response to eCO2, but only at ambient temperatures, demonstrating the ability of eT to negate the effects of eCO2. Root damage reduced aboveground biomass, height, and root %N, and increased root %C and C:N, most probably via decreased biological nitrogen fixation. Total foliar amino acid concentrations and aphid colonization success were higher in plants with roots cut early (before aphid arrival) than those with roots cut late (after aphid arrival); however, this effect was counteracted by eT. These results demonstrate the importance of amino acid concentrations for aphids and identify individual amino acids as being potential factors underpinning aphid responses to eT, eCO2, and root damage in lucerne. Incorporating trophic complexity and multiple climatic factors into plant–herbivore studies enables greater insight into how plants and insects will interact in the future, with implications for sustainable pest control and future crop security.
Introduction
Host plant quality and suitability for herbivorous insects shapes the extent to which plants are attacked (Bernays and Chapman, 1994), and sudden improvements in host plant quality can lead to pest outbreaks (Barbosa et al., 2012). Biotic and abiotic factors often induce changes in secondary metabolites that might deleteriously affect herbivores (Iason et al., 2012), but these can also change the nutritional quality of plants, leading to increased susceptibility of plants to herbivores (White, 1984; Johnson et al., 2009). This is particularly true for mobile herbivores that have short generation times and respond quickly to changes in host plant nutrition, such as aphids (Dixon, 1998; Douglas, 2003). In particular, aphids respond to changes in the amino acid quality of phloem sap, which has been shown to be affected by both global climate change (Harrington et al., 1995; Newman, 2003; Pritchard et al., 2007; Sun and Ge, 2011) and root herbivory (Johnson et al., 2012 2013). To the authors’ knowledge, how these factors operate and interact remains largely unknown. In this study, experiments were carried out to examine how elevated temperature (eT) and atmospheric carbon dioxide concentrations (eCO2), as well as simulated root herbivory, acted alone and together to affect foliar amino acids and plant susceptibility to aphids.
Future CO2 concentrations (from current levels of 400 μmol mol–1 to >550 μmol mol–1 by 2050) are likely to be accompanied by increased temperatures (1–4 °C within this century) and, while a number of studies have observed effects of eCO2 on aphid populations, the combination of eT and eCO2 has received little attention (Newman, 2003; Himanen et al., 2008; Murray et al., 2013). In general, eCO2 is expected to increase plant growth by accelerating rates of photosynthesis, which reduces tissue quality and increases the carbon to nitrogen (C:N) ratio (Robinson et al., 2012). This can be exacerbated when increased plant growth leads to N limitation (Rogers et al., 2009). Legumes, such as lucerne (Medicago sativa L.), can, however, avoid N limitation and maintain tissue N concentrations under eCO2 by enhancing biological N fixation (Soussana and Hartwig, 1995; Johnson and McNicol, 2010). This can alter the dynamics of foliar-feeding aphids (Douglas, 1993), which tend to perform better on plants with higher N (Nowak and Komor, 2010; Sun and Ge, 2011) and amino acid concentrations (Ponder et al., 2000; Karley et al., 2002; Guo et al., 2014). In contrast, eT may combat eCO2 effects on plant nutrient quality by decreasing biological N fixation, often associated with a low tolerance of N-fixing rhizobial bacteria to increased temperatures (Zahran, 1999; Whittington et al., 2013), which ultimately decreases amino acid concentrations and aphid abundance (Ryalls et al., 2013a ).
Plants, in addition to being attacked by aboveground herbivores, often have to contend with root damage by herbivores, which may increase amino acid concentrations in the foliage and, in turn, make the plant more susceptible to aphid attack aboveground (Masters et al., 1993; Johnson et al., 2009). This might be different for legumes, however, since the roots are the source of N acquisition through biological N fixation. The general prediction that root herbivory usually has beneficial effects on aboveground aphids may also be altered by changes in herbivore synchronization (Erb et al., 2011). In a meta-analysis, Johnson et al. (2012) identified the sequence of herbivore arrival (e.g. whether aphids arrive before or after roots are damaged) as the most influential factor affecting aboveground–belowground linkages. Root damage before aphids arrive may impair water uptake and lead to a stress-related accumulation of amino acids in the phloem, but crucially gives the plant a chance to recover and regain hydraulic properties (e.g. phloem turgor) which might allow aphids to capitalize on this. Root recovery is often rapid and can be overcompensatory (i.e. more root nodules are produced) in legumes (Quinn and Hall, 1992; Ryalls et al., 2013b ). Conversely, if aphids are already feeding on a plant when root damage occurs, root recovery and stress-related increases in foliar nutrients may be less likely because the plant remains in stress. This mirrors the pulsed stress hypothesis (Huberty and Denno, 2004), which posits that phloem-feeding insects exhibit poor performance on continuously stressed plants, yet respond positively when plant recovery is possible.
Using the model legume, lucerne, this study combines aboveground sap feeding by the pea aphid, Acyrthosiphon pisum (Harris), and simulated root herbivory in the form of root cutting, to characterize the effects of herbivore arrival sequence on aboveground–belowground interactions. While artificial herbivory may not mimic natural damage exactly (Blossey and Hunt-Joshi, 2003), it enables control of the type, timing, and intensity of damage, especially in complex systems such as this (Hjältén, 2004; Rogers and Siemann, 2004; Borowicz, 2010). Lucerne itself is the most important and widely grown forage legume worldwide (Small, 2011). The late 1970s saw the invasion of Australia by three aphid pests, including the pea aphid, which devastated lucerne stands in eastern Australia (Bouton, 2012). The development of resistant cultivars has helped to abate the aphid problem, but outbreaks driven by environmental factors and changes in the nature and intensity of trophic interactions (e.g. release from natural enemies) still occur (Zarrabi et al., 1995; Reddy and Hodges, 2000; Humphries et al., 2012; Ryalls et al., 2013a ).
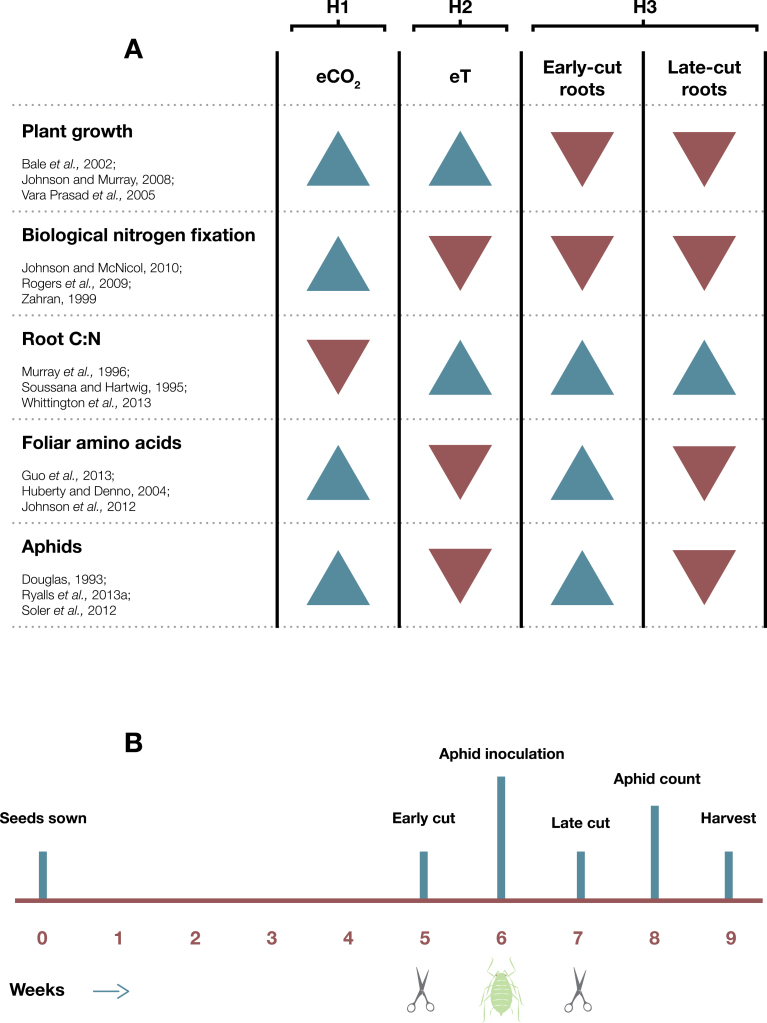
This study specifically aimed to determine the effects of: eT and eCO2, individually and in combination, on lucerne growth (height and biomass), chemistry (root C:N and foliar amino acid concentrations and composition), and pea aphid abundance; and simulated root herbivory (root cutting before and after aphid arrival) on lucerne growth, chemistry, and pea aphids. It was hypothesized that: (i) eCO2 would increase lucerne growth and promote biological N fixation (i.e. decrease root C:N), which would increase foliar amino acid concentrations and ultimately aphid abundance. In contrast, (ii) eT would increase lucerne growth but reduce biological N fixation (i.e. increase root C:N), which would reduce foliar amino acid concentrations and aphid abundance; and (iii) root cutting would reduce lucerne growth and biological N fixation (i.e. increase root C:N) generally. Early root cutting would benefit aphids via increased foliar amino acid concentrations because stress and recovery of lucerne would be possible. In contrast, root cutting during aphid feeding would present above- and belowground stress, making recovery less likely, and would negatively impact aphids via decreased foliar amino acid concentrations. Hypothesized treatment effects of early and late root cutting, elevated temperature, and CO2 on aphids and plant characteristics are summarized in Fig. 1A.
Fig. 1.
Hypothesized treatment effects of root cutting (before and after aphid infestation), elevated temperature, and CO2 on aphids and plant characteristics, with example references given as a basis for the hypotheses. H1, H2, and H3 refer to hypotheses (i), (ii), and (iii), respectively (A). Methodological timeline of experimental events (B).
Materials and methods
Glasshouse conditions
Experiments were conducted in four glasshouse chambers at the University of Western Sydney Hawkesbury campus, NSW (latitude –33.611141, longitude 150.745315) in which CO2 was maintained at either ambient CO2 concentrations, aCO2 (400 μmol mol–1), or at eCO2 (640 μmol mol–1), and temperature was maintained at either ambient temperature, aT (26/18 °C day/night on a 15:9 light:dark cycle), or eT (30/22 °C day/night on a 15:9 light:dark cycle). aT (26 °C) represents the average daily maximum temperature for Richmond, NSW over the last 30 years, and eT was consistent with the predicted maximum temperature increase of 4 °C for this region within this century (CSIRO, 2007–2014). Humidity was controlled at 55%, and no significant differences in light conditions were observed between chambers (Ryalls et al., 2013b ). Temperature, CO2, and humidity within glasshouse chambers were monitored continuously throughout the experiment, and plants were randomly reallocated among positions within chambers twice weekly to minimize potential within-chamber effects. The experiment itself was repeated three times, which was incorporated into statistical models to reduce the influence of pseudo-replication of CO2 and temperature treatments (Newman et al., 2011).
Aphid cultures
Acyrthosiphon pisum adults used in the experiment were taken from four established cultures, all originating from an individual parthenogenetic adult female collected from a local lucerne field in Richmond, NSW (latitude –33.609189, longitude 150.746953) in January 2013. Cultures were maintained on propagated lucerne plants in each of the four experimental chambers for at least six generations prior to the inoculation period.
Experimental procedure
Lucerne plants (54 plants of cultivar ‘Sequel’) in each of the four chambers (aCO2, aT; eCO2, aT; aCO2, eT; and eCO2, eT) were distributed randomly among six treatments: aphids only (A), early cut no aphids (EC), early cut and aphids (ECA), late cut no aphids (LC), late cut and aphids (LCA), and control plants with no cutting or aphids (C), giving nine replicates of each treatment in each chamber. Five weeks after sowing, treatments EC and ECA were subjected to early root damage by severing the entire root system 35mm below the soil surface using a sharp steel blade inserted in a narrow opening cut into the plastic pot. One week later, treatments A, ECA, and LCA were inoculated with two teneral adult pea aphids. Organza bags (125×170mm) were secured around each plant to confine individual aphids to the plant. One week after aphid inoculation, treatments LC and LCA were subjected to simulated late root herbivore damage using the same technique as for those cut earlier. Aphids were counted and removed a week later (8 weeks after sowing). One week after aphids were removed, roots were separated from soil and shoots and all plant material was snap-frozen in liquid N and stored at –20 °C. Material was then freeze-dried and weighed to determine dry mass before chemical analysis. Plant heights (from ground level to the base of the highest leaf) were measured 5 (early cutting period), 6 (aphid inoculation period), and 9 (harvest period) weeks after planting (Fig. 1B).
Chemical analyses
Four samples, selected at random, from each treatment combination per run were chosen for chemical analysis (288 samples overall). Freeze-dried roots and shoots were ball-milled for 90 s to a fine powder. Soluble amino acids were extracted from milled shoot samples (10mg) by shaking in 1.5ml of 80% ethanol for 20min at 50 °C. After centrifugation at 12 000 relative centrifugal force (rcf) for 5min to remove solids, 1ml of supernatant was removed and evaporated to dryness at 30 °C in a vacuum concentrator (Eppendorf). Amino acids were analysed by reverse-phase high-performance liquid chromatography (HPLC) in an Agilent 1260 Infinity HPLC system after pre-column derivatization using phenylisothiocyanate (PITC) (Crafts-Brandner, 2002; Reason, 2003). Derivatization steps were as follows: dried extracts were redissolved in 100 μl of coupling solution (acetonitrile:pyridine:triethylamine:water 10:5:2:3), vortexed, and redried under vacuum at 30 °C. They were then redissolved in 100 μl of coupling solution, followed by 5 μl of PITC, vortexed, and allowed to react for 5min at room temperature before redrying at 30 °C. A 100 μl aliquot of Milli-Q water was then added, vortexed, and redried at 45 °C. Samples were redissolved in 250 μl of analysis solvent (water:acetonitrile 7:2) and filtered through a 0.45 μm 4mm PVDF filter prior to HPLC analysis. HPLC was performed using an Agilent Poroshell 120 EC-C18 column (4.6×75mm, 2.7 μm) at a column temperature of 40 °C. The gradient, composed of eluent A (1 litre of Milli-Q water, 19g of sodium acetate trihydrate, 0.5ml of triethylamine, pH 5.7, 63.8ml of acetonitrile, 1.07ml of EDTA dipotassium salt 1g l–1 solution) and eluent B (acetonitrile:water 6:4), was as follows: 0–3.75min, 0–40% B; 3.75–4.5min, 40–80% B; 4.5–5.75min, 80–100% B; 5.75–7min, 100% B; 7–9min, 100–0% B. The flow rate was 1.4ml min–1. Amino acid derivatives were detected by ultraviolet absorbance at 254nm. Amino acid standards (5, 10, 15, 20 and 25 nmol) containing 17 amino acids (see below) and an internal standard (12.5 nmol l–1 norleucine) were used to calibrate the analysis. Nine essential amino acids (i.e. those that cannot be synthesized by insects de novo), namely arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, and valine (Morris, 1991), and eight non-essential amino acids (alanine, aspartic acid, cysteine, glutamic acid, glycine, proline, serine, and tyrosine) were detected using this method. Foliar amino acid composition was measured as it has been demonstrated to be a reliable indicator of phloem amino acid composition (Winter et al., 1992; Johnson et al., 2014). Root C and N concentrations of 4–6mg of milled root samples were determined using a Carlo Erba CE1110 elemental analyser with thermal conductivity and mass spectrometric detection (of N2 and CO2). The percentage of N and C in the sample was calculated by comparison with known standards.
Statistical analyses
Linear mixed effect models were produced using the nlme (v3.1–109) and lme4 (v0.999999-2) statistical packages for aphid abundance and aphid colonization, respectively, in the R statistical interface v3.0.1. Models described the effects of simulated root herbivory, eT, and eCO2 on aphids. The fixed terms included were plant-damage treatment (C, A, EC, ECA, LC, and LCA), temperature (26 °C and 30 °C), and CO2 (ambient and elevated), as well as the interactions between these terms. The random terms included were experimental run, with chamber as a nested factor, to account for pseudoreplication and chamber effects that could confound temperature and CO2 effects. The dependent variable ‘aphid abundance’ was log+1 transformed to normalize the model-standardized residuals. Aphid colonization (i.e. the proportion of plants hosting aphids at harvest) was analysed using a binomial error structure. Models were reduced by deleting non-significant fixed terms in a stepwise manner using Akaike information criterion (AIC) values and associated P-values taken from analysis of variance (ANOVA) model comparisons. Post-hoc Tukey’s tests and the R package LMERConvenienceFunctions were used for pairwise comparisons of means for treatment and interaction effects. The effects of simulated root herbivory, aphid presence, eT, and eCO2 on lucerne shoot biomass, height, and root biomass were also analysed with mixed models using nlme in R. Dependent variables were log-transformed to normalize model residuals. The random terms included were run/chamber and fixed terms were reduced in a stepwise manner, as above. Temperature, CO2, and plant-damage treatment effects on total, essential, and individual amino acid concentrations were also analysed using linear mixed models with log-transformed dependent variables to normalize residuals. Similar models with log-transformed dependent variables were used to determine the effects of temperature, CO2, and plant-damage treatment on root %N, C, and C:N. Principal components analysis (PCA) and permutational multivariate analysis of variance (PERMANOVA) were used to explore the impacts of eT, eCO2, and plant-damage treatment on amino acid composition. Groupings of individual amino acids were determined using a correlation matrix, and PERMANOVA results were compared with those obtained from individual mixed models.
Results
Impacts of eT and eCO2
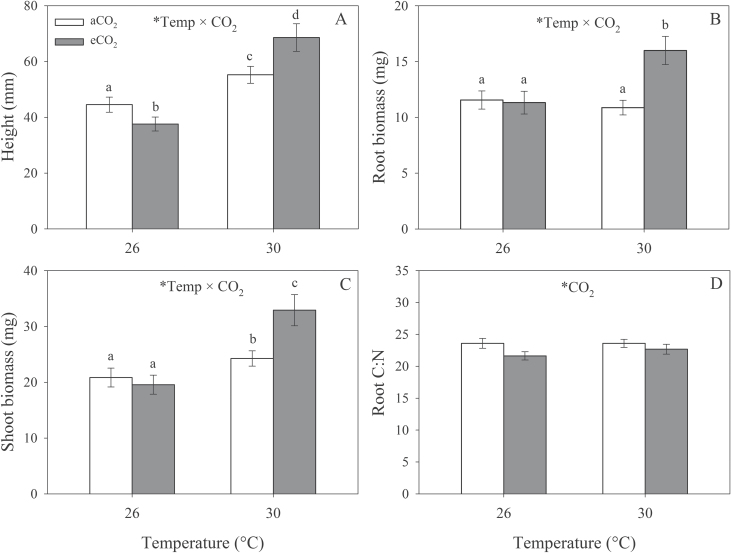
Temperature in interaction with CO2 had a significant effect on lucerne height (F 1,6=7.80; P=0.032), root biomass (F 1,6=21.76; P=0.003), and shoot biomass (F 1,6=6.45; P=0.044). At 30 °C, plants were taller under eCO2, whereas, at 26 °C, plants were shorter under eCO2 (Fig. 2A). Lucerne root and shoot mass increased under eCO2 at 30 °C, but no effects of eCO2 were observed at 26 °C (Fig. 2B, C). eCO2 decreased root C:N significantly (F 1,8=7.90; P=0.023) (Fig. 2D) but did not significantly increase root %N (F 1,8=4.55; P=0.065) or decrease root %C (F 1,8=2.59; P=0.146) individually.
Fig. 2.
Effects of elevated temperature and CO2 on plant height (A), root biomass (B), shoot biomass (C), and root C:N (D). Mean values (± SE) are shown. Statistically significant effects are indicated by * (P<0.05). Bars with the same letters were not significantly different (P<0.05).
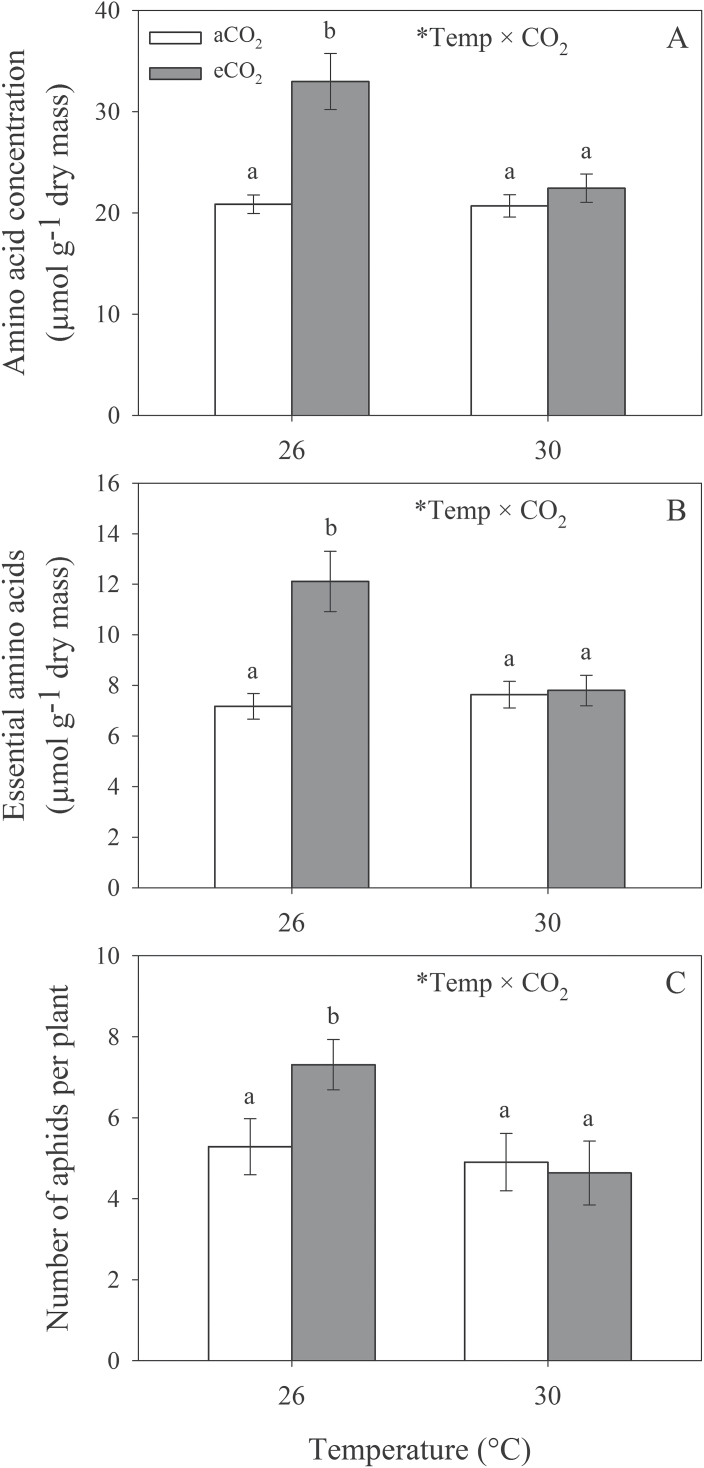
Significant interactions of temperature and CO2 were also observed for total (F 1,287=7.63; P=0.006) and essential amino acid concentrations (F 1,292=10.072; P=0.002), as well as aphid abundance (F 1,6=6.45; P=0.044), whereby eCO2 significantly increased total amino acids (Fig. 3A), essential amino acids (Fig. 3B), and pea aphid numbers (Fig. 3C) at 26 °C but not at 30 °C. Results from individual amino acid analyses (Table 1; Supplementary Table S1 available at JXB online) showed that eT decreased concentrations of arginine, aspartic acid, glutamic acid, and histidine, and eCO2 increased concentrations of alanine, glutamic acid, leucine, lysine, phenylalanine, proline, and serine. The interaction of eT and eCO2 affected glycine and glutamic acid, whereby concentrations of both amino acids increased under eCO2, but only at 26 °C.
Fig. 3.
Effects of elevated temperature and CO2 on total amino acids (A), essential amino acids (B), and aphid abundance (C). Mean values (± SE) are shown. Statistically significant effects are indicated by * (P<0.05). Bars with the same letters were not significantly different (P<0.05).
Table 1.
Summary of final model statistical analyses of individual amino acid responses to plant damage (Treatment), temperature, and CO2 treatments
| Amino acid | CO2 | Temperature | Treatment | Temperature×CO2 | Treatment× Temperature | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F 1,6 | P | F 1,6 | P | F 5,264 | P | F 1,6 | P | F 5,264 | P | |
| Alanine (Ala)1 | 28.26 | 0.002 | – | – | 3.48 | 0.005 | – | – | – | – |
| Argininea (Arg)2 | – | – | 14.41 | 0.009 | 2.41 | 0.037 | – | – | – | – |
| Aspartic acid (Asp)2 | – | – | 22.9 | 0.003 | 3.62 | 0.004 | – | – | – | – |
| Cysteine (Cys)4 | – | – | – | – | – | – | – | – | – | – |
| Glutamic acid (Glu)3 | 10.33 | 0.02 | 17.23 | 0.006 | 5.78 | <0.001 | – | – | – | – |
| Glycine (Gly)1 | – | – | – | – | – | – | 8.99 | 0.024 | – | – |
| Histidinea (His)2 | – | – | 11.66 | 0.014 | 4.79 | <0.001 | – | – | – | – |
| Isoleucinea (Ile)1 | – | – | – | – | 2.77 | 0.019 | – | – | – | – |
| Leucinea (Leu)1 | 9.22 | 0.023 | – | – | – | – | – | – | – | – |
| Lysinea (Lys)1,2,3,5 | 23.91 | 0.003 | – | – | – | – | – | – | 10.88 | <0.001 |
| Methioninea (Met)4 | – | – | – | – | 3.83 | 0.002 | 15.49 | 0.008 | – | – |
| Phenylalaninea (Phe)1 | 10.64 | 0.017 | – | – | – | – | – | – | 3.74 | 0.003 |
| Proline (Pro)1 | 7.43 | 0.034 | – | – | 2.36 | 0.04 | – | – | – | – |
| Serine (Ser)1 | 9.25 | 0.023 | – | – | 2.31 | 0.045 | – | – | – | – |
| Threoninea (Thr)1 | – | – | – | – | – | – | – | – | – | – |
| Tyrosine (Tyr)5 | – | – | – | – | – | – | – | – | 6.99 | <0.001 |
| Valinea (Val)1 | – | – | – | – | – | – | – | – | – | – |
Treatments that were not significant (–) and interactive effects that are not displayed were absent from the final models. All dependent variables were log transformed to standardize residuals. Superscript numbers indicate groupings used for PERMANOVA based on correlations (>0.5).
a Essential amino acids as defined by Morris (1991).
PCA revealed no clear separation of treatments, although greater variation was seen in plants grown in eCO2 at 26 °C. The first two principal components (PC 1 and PC 2) accounted for 62% of the variation in the data set (Supplementary Fig. S1 at JXB online). PERMANOVA on correlated amino acid groups, consisting of group 1 (alanine, glycine, isoleucine, leucine, lysine, phenylalanine, proline, serine, threonine, and valine), group 2 (arginine, aspartic acid, histidine, and lysine) and four individual amino acids (glutamic acid, methionine, cysteine, and tyrosine), confirmed patterns observed in individual models (see Supplementary Table S2 for full results), whereby group 1 was significantly affected by CO2 and group 2 was significantly affected by temperature (Supplementary Fig, S2A and C, respectively). Temperature and CO2 interactively affected all ungrouped individuals.
Impacts of root damage
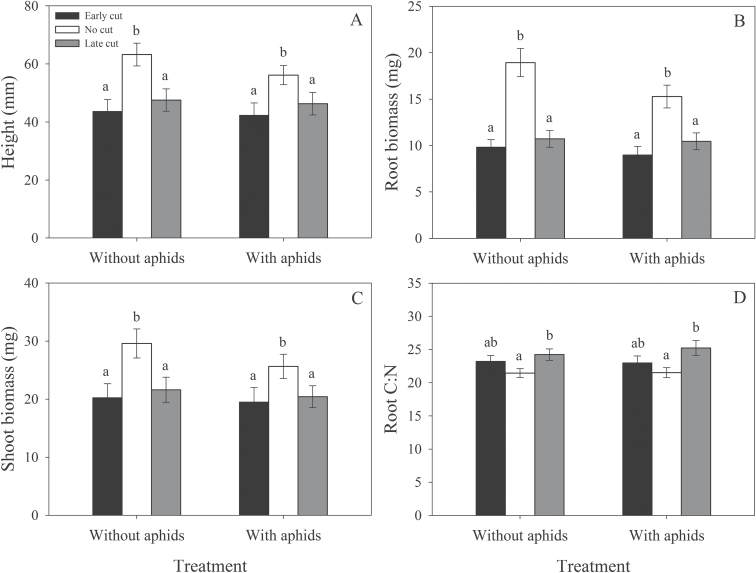
Plant-damage treatment had a significant effect on lucerne height (F 5,504=10.73; P<0.001), whereby control (uncut) plants were significantly taller than those with damaged roots (Fig. 4A). Similar results were found for both root (F 5,496=22.76; P<0.001) and shoot biomass (F 5,495=7.44; P<0.001). Overall, early cutting and late cutting reduced root mass by 46% and 37%, respectively (Fig. 4B). Similarly, shoot mass was reduced by 26% and 22% in early and late-cut plants, respectively (Fig. 4C). Significant effects of plant-damage treatment were also seen for root %N (F 5,207=2.58; P=0.028), %C (F 5,207=5.05; P<0.001), and C:N (F 5,207=3.33; P=0.006), whereby control plants had a significantly higher root %N and lower root %C and C:N than late-cut plants. Consistent general increases in C:N were seen across cutting treatments depending on the time at which the plants were cut (Fig. 4D).
Fig. 4.
Effects of plant-damage treatment on plant height (A), root biomass (B), shoot biomass (C), and root C:N (D). Mean values (± SE) are shown. Bars with the same letters were not significantly different (P<0.05).
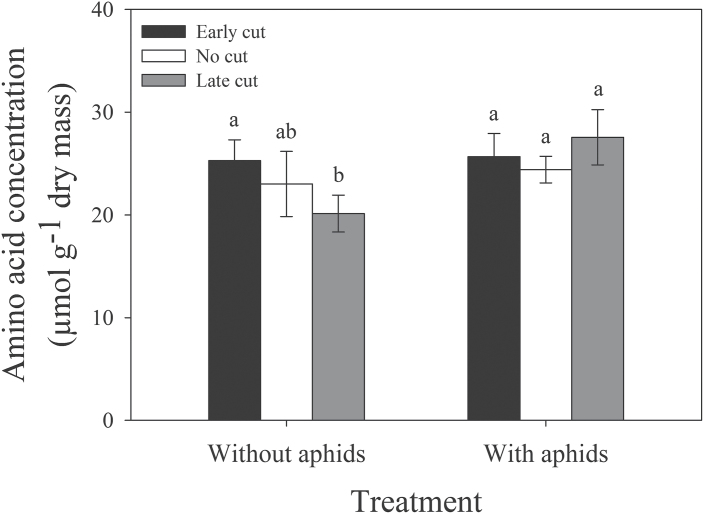
Plant-damage treatment also significantly affected total amino acids (F 1,287=3.042; P=0.011). but had no effect on essential amino acids. On plants without aphids present, total amino acid concentrations were greater in roots that were cut early compared with those cut late, although neither early- or late-cut plants were significantly different from uncut controls, which contained intermediate concentrations. When aphids were present, amino acid concentrations were generally higher, but no significant root-damage effects were detected. However, late-cut plants with aphids contained significantly higher concentrations of amino acids than late-cut plants without aphids (Fig. 5). Similar to individual models (Table 1), glutamic acid and group 2 amino acids were significantly affected by plant-damage treatment (Supplementary Table S2 at JXB online). Group 2 amino acids showed dramatic increases in late-cut plants when aphids were present (Supplementary Fig. S2D).
Fig. 5.
Effects of plant-damage treatment on total foliar amino acid concentrations. Mean values (± SEs) are shown. Bars with the same letters were not significantly different (P<0.05).
Interaction effects of environment and root damage
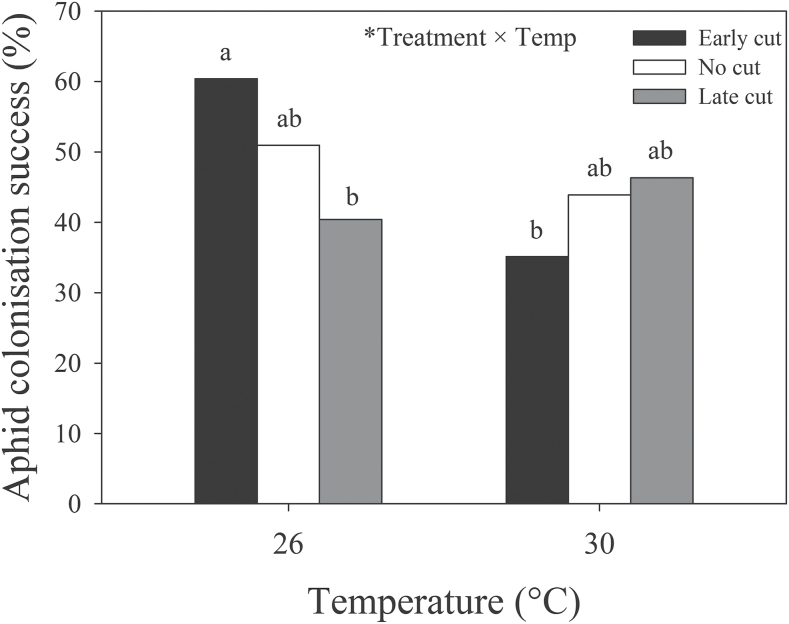
Plant-damage treatment and temperature interactively affected lysine, phenylalanine, and tyrosine (Table 1). Specifically, at 26 °C, concentrations of all three amino acids were consistently higher when plants were cut early compared with control plants or plants cut late. Additionally, at 30 °C, concentrations of all three amino acids were significantly higher when late-cut plants were colonized by aphids compared with other treatments. Lysine was strongly correlated with group 1, group 2, glutamic acid, and tyrosine (Supplementary Table S2 at JXB online). Plant-damage treatment in interaction with temperature also had a significant effect on aphid colonization success, whereby, at 26 °C, pea aphid colonization success was significantly higher when lucerne roots were cut before aphid inoculation compared with those cut after aphid inoculation, although neither was significantly different from uncut control plants (Fig. 6). Aphids were significantly more successful at colonizing plants with roots that were cut early at 26 °C compared with 30 °C. No significant differences between treatments were seen at 30 °C. Significant effects of different factors and their associated figures are summarized in Fig. 7.
Fig. 6.
Effects of root cutting on aphid colonization success (i.e. percentage of plants with aphids present upon harvest) under ambient and elevated temperatures. A statistically significant effect is indicated by * (P<0.05). Bars with the same letters were not significantly different (P<0.05).
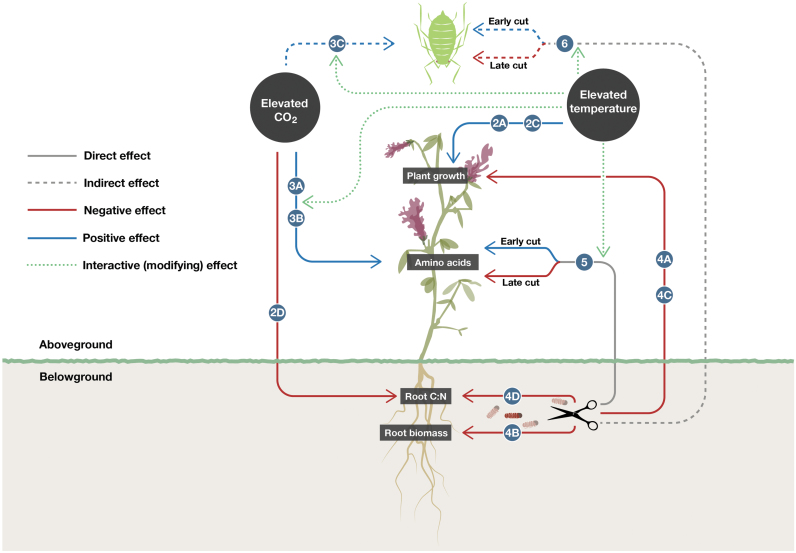
Fig. 7.
Schematic diagram of the significant positive and negative effects of temperature, CO2, and root cutting treatments on plant characteristics and aphids. Numbers and letters correspond to the figures associated with those effects.
Discussion
This study is the first to incorporate the effects of both eT and eCO2 on the sequence of herbivore arrival within an aboveground–belowground framework. These combined approaches incorporating multiple factors enable greater understanding of how plants and insects will interact in the future (Ryalls et al., 2013a ). The results demonstrate that eT can counter the positive effects of eCO2 and root damage on amino acid concentrations and aphid populations, with important implications for future aphid outbreaks and crop security.
Impacts of eT and eCO2
Expected increases in aboveground biomass and height in response to eCO2 were observed at 30 °C, although, at 26 °C, eCO2 decreased height and had no effect on biomass. This is probably due to the high numbers of aphids present on the plants under these conditions since data were analysed across all treatments. The decrease in root C:N at eCO2 suggests that lucerne increases biological N fixation to compensate for N limitation under these conditions. The hypothesis that elevated CO2 stimulates N fixation in legumes is broadly supported (Rogers et al., 2009).
Concentrations of amino acids in group 2 significantly decreased under eT, suggesting that these amino acids were the main temperature response drivers, although no overall decrease in total amino acid concentrations was observed under eT. Total concentrations did, however, increase in response to eCO2 at 26 °C, and the amino acids in group 1 were the major drivers of this CO2 response. Studies have reported both positive and negative effects of eCO2 on amino acid concentrations, although plant type plays an important role in determining the direction of the effect (Docherty et al., 1997; Himanen et al., 2008; Rogers et al., 2009). While tissue dilution effects of eCO2 often lead to decreases in amino acid concentrations in plants (Sun and Ge, 2011), legumes often show increases in amino acid concentrations under eCO2 due to increases in biological N fixation (Guo et al., 2013). Responses of different cultivars, varieties, or genotypes of the same species to eCO2, however, can also vary. For example, Johnson et al. (2014) found 86% and 56% increases in essential amino acid concentrations and pea aphid colonization success, respectively, on the cultivar ‘Sequel’ under eCO2, whereas essential amino acid concentrations and aphid colonization success decreased by 53% and 33%, respectively, on the cultivar ‘Genesis’ under the same conditions. In other words, some cultivars may become more or less susceptible to aphid attack under climate change conditions, an important consideration for determining future outcomes. Further study combining both eCO2 and eT would help to clarify the effects of climate change on aphid responses to different cultivars.
This study used the widely grown public variety ‘Sequel’, with similar results to those found by Johnson et al. (2014) using the same cultivar. As with that study, aphid numbers were lower than the reproductive potential of A. pisum when feeding on susceptible plants and cultivars (e.g. the lucerne cultivar, ‘Hunter River’). This most probably reflects aphid populations in the field since only cultivars with some aphid resistance are now grown; ‘Hunter River’ was phased out >30 years ago (Ryalls et al., 2013a ). The exact mechanisms underpinning lucerne resistance to aphids are poorly characterized (Ryalls et al., 2013a ), but probably include plant traits conferring both antibiosis (e.g. low fecundity) and antixenosis (e.g. inability to colonize) resistance. Evidence was seen for both in the present study. In addition to primary chemistry, secondary chemistry may play a role in lucerne resistance to aphids. In particular, saponins have also been identified as potentially important factors associated with aphid resistance (Goławska et al., 2014). Moreover, the extent to which endosymbionts (obligate and facultative) might help aphids cope with such induced changes in primary and secondary chemistry are unknown in this system, but could also play a role. Simultaneously quantifying both saponins and amino acids, while considering the role of endosymbionts, might therefore provide an important insight into lucerne susceptibility to aphids.
Effects of eT and eCO2 on aphids mirrored their effects on amino acid concentrations, suggesting that aphids rely on amino acid concentrations to reproduce and colonize effectively on lucerne. In general, when aphids were present, amino acid concentrations were higher, suggesting that pea aphids promote amino acid metabolism in lucerne to favour population growth. Similar results were found for pea aphid populations fed on N-fixing-deficient M. truncatula plants, which had decreased activities of N assimilation-related enzymes and amino acid concentrations under eCO2, compared with increases seen in control plants (Guo et al., 2013). Pea aphids performed better on plants grown under eCO2 conditions at 26 °C but not at 30 °C. Such negation of eCO2 effects by eT has also been shown in other systems (e.g. Murray et al., 2013), demonstrating the importance of considering multiple climate change factors. Both plant amino acid and aphid responses to eCO2 were either reduced or negated at 30 °C, suggesting that future temperature increases may have beneficial effects on lucerne by reducing aphid numbers, although this will also depend on how aphid natural enemies are affected by such changes (Ryalls et al., 2013a ).
Impacts of root damage and interactions with climate
Aboveground biomass and height of control plants were expectedly higher than those with severed roots. Overall and relative losses in root and shoot mass due to root cutting were similar to the overall reductions in root biomass (36.3%) and aboveground growth (16.3%) that were averaged over 85 experimental studies by Zvereva and Kozlov (2012). Root C:N was higher in plants with severed roots, which is likely to be due to the removal of nodules containing N-fixing rhizobial bacteria and the subsequent impairment of biological N fixation. Root cutting may also have caused carbohydrates to be diverted away from the foliage towards the roots (Blossey and Hunt-Joshi, 2003; Johnson et al., 2011). Pea aphids responded positively to early-cut plants, associated with an increase in amino acid concentrations, but this effect was reversed by eT. One essential amino acid that may be important to consider is lysine, which was strongly correlated with other amino acid groups and closely matched amino acid and aphid responses to different treatments. For example, concentrations of lysine were higher at eCO2 (also shown by Johnson et al., 2014) and in early-cut plants maintained at 26 °C. In these conditions, aphids may respond to specific amino acids, including lysine, and even influence their composition in the phloem (e.g. Guo et al., 2013), as suggested by the increase in total amino acid concentration in late-cut plants with aphids present compared with those without aphids present. If aphids are already present on the plant when roots are severed, stress-related increases in foliar N and amino acid concentrations are less likely (Huberty and Denno, 2004) and may be the reason why aphid colonization decreased on late-cut plants at 26 °C. Realistically, lucerne is likely to go through periods of discontinuous stress resulting from variable root damage that would allow aphids to take advantage of periodic increases in foliar N or amino acid concentrations (Huberty and Denno, 2004; Johnson et al., 2012). In this case, changes in temporal patterns of herbivore arrival under future conditions would be important to consider.
Understanding how multiple climate change factors shape crop susceptibility to insect pests is clearly a priority for achieving food security (Gregory et al., 2009), with the amount damaged by insects sufficient to feed 1 billion people (Birch et al., 2011). The present study demonstrates how some aspects of climate change can promote aphid pests, with simulated root herbivory having similar positive effects on the same pests. This could clearly be problematic for plant production. Both these effects, mediated by increases in foliar amino acids, were, however, negated by predicted increases in temperature. Research into future patterns of crop susceptibility to insect pests is at a crossroads (Gregory et al., 2009). Logistic constraints often limit the ability to test multiple climatic factors and multitrophic factors, but, nonetheless, a realistic insight into plant–insect interactions will only be obtained if this challenge is met.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Principal component analysis of foliar amino acid data with attribute loadings on the first two components PC 1 and PC 2.
Figure S2. Temperature, CO2, and plant-damage treatment effects on average amino acid concentrations (mean ±SE) of group 1 and group 2 amino acids.
Table S1. Single-factor treatment effects of CO2, temperature, and herbivore damage on individual amino acids (μmol g–1 dry mass).
Table S2. Results from multivariate permutational analysis (PERMANOVA) of temperature, CO2, and plant-damage treatment effects on different groups of amino acids.
Acknowledgements
This work was undertaken as part of a PhD research project funded by the Hawkesbury Institute for the Environment (University of Western Sydney). We would like to thank Lisa Bromfield and Aidan Hall for glasshouse assistance, Christopher Mitchell for HPLC assistance, Hilary Stuart-Williams for analysing carbon and nitrogen concentrations, and Benjamin Punzalan for providing the schematic diagrams.
References
- Bale JS, Masters GJ, Hodkinson ID, et al. 2002. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biology 8, 1–16. [Google Scholar]
- Barbosa P, Letourneau DK, Agrawal AA. 2012. Insect outbreaks revisited. West Sussex, UK: John Wiley & Sons. [Google Scholar]
- Bernays EA, Chapman RF. 1994. Host-plant selection by phytophagous insects. New York: Springer. [Google Scholar]
- Birch ANE, Begg GS, Squire GR. 2011. How agro-ecological research helps to address food security issues under new IPM and pesticide reduction policies for global crop production systems. Journal of Experimental Botany 62, 3251–3261. [DOI] [PubMed] [Google Scholar]
- Blossey B, Hunt-Joshi TR. 2003. Belowground herbivory by insects: influence on plants and aboveground herbivores. Annual Review of Entomology 48, 521–547. [DOI] [PubMed] [Google Scholar]
- Borowicz VA. 2010. The impact of arbuscular mycorrhizal fungi on strawberry tolerance to root damage and drought stress. Pedobiologia 53, 265–270. [Google Scholar]
- Bouton JH. 2012. Breeding lucerne for persistence. Crop and Pasture Science 63, 95–106. [Google Scholar]
- Crafts-Brandner SJ. 2002. Plant nitrogen status rapidly alters amino acid metabolism and excretion in Bemisia tabaci . Journal of Insect Physiology 48, 33–41. [DOI] [PubMed] [Google Scholar]
- CSIRO. 2007–2014. Climate change in Australia: technical report. Melbourne: CSIRO. [Google Scholar]
- Dixon AFG. 1998. Aphid ecology. London: Chapman & Hall. [Google Scholar]
- Docherty M, Wade F, Hurst D, Whittaker J, Lea P. 1997. Responses of tree sap-feeding herbivores to elevated CO2 . Global Change Biology 3, 51–59. [Google Scholar]
- Douglas AE. 1993. The nutritional quality of phloem sap utilized by natural aphid populations. Ecological Entomology 18, 31–38. [Google Scholar]
- Douglas AE. 2003. The nutritional physiology of aphids. Advances in Insect Physiology 31, 73–140. [Google Scholar]
- Erb M, Robert CAM, Hibbard BE, Turlings TCJ. 2011. Sequence of arrival determines plant-mediated interactions between herbivores. Journal of Ecology 99, 7–15. [Google Scholar]
- Goławska S, Sprawka I, Łukasik I. 2014. Effect of saponins and apigenin mixtures on feeding behavior of the pea aphid, Acyrthosiphon pisum Harris. Biochemical Systematics and Ecology 55, 137–144. [Google Scholar]
- Gregory PJ, Johnson SN, Newton AC, Ingram JSI. 2009. Integrating pests and pathogens into the climate change/food security debate. Journal of Experimental Botany 60, 2827–2838. [DOI] [PubMed] [Google Scholar]
- Guo H, Sun Y, Li Y, Liu X, Zhang W, Ge F. 2014. Elevated CO2 decreases the response of the ethylene signaling pathway in Medicago truncatula and increases the abundance of the pea aphid. New Phytologist 201, 279–291. [DOI] [PubMed] [Google Scholar]
- Guo H, Sun Y, Li Y, Tong B, Harris M, Zhu-Salzman K, Ge F. 2013. Pea aphid promotes amino acid metabolism both in Medicago truncatula and bacteriocytes to favor aphid population growth under elevated CO2 . Global Change Biology 19, 3210–3223. [DOI] [PubMed] [Google Scholar]
- Harrington R, Bale JS, Tatchell GM. 1995. Aphids in a changing climate. In: Harrington R, Stork NE, eds. Insects in a changing environment. London: Academic Press, 126–155. [Google Scholar]
- Himanen SJ, Nissinen A, Dong W-X, Nerg A-M, Stewart CN, Poppy GM, Holopainen JK. 2008. Interactions of elevated carbon dioxide and temperature with aphid feeding on transgenic oilseed rape: are Bacillus thuringiensis (Bt) plants more susceptible to nontarget herbivores in future climate? Global Change Biology 14, 1437–1454. [Google Scholar]
- Hjältén J. 2004. Simulating herbivory: problems and possibilities. In: Weisser W, Siemann E, eds. Insects and ecosystem function. Ecological Studies, Vol. 173 Berlin: Springer, 243–255. [Google Scholar]
- Huberty AF, Denno RF. 2004. Plant water stress and its consequences for herbivorous insects: a new synthesis. Ecology 85, 1383–1398. [Google Scholar]
- Humphries AW, Peck DM, Robinson SS, Rowe T, Oldach K. 2012. A new biotype of bluegreen aphid (Acyrthosiphon kondoi Shinji) found in south-eastern Australia overcomes resistance in a broad range of pasture legumes. Crop and Pasture Science 63, 893–901. [Google Scholar]
- Iason GR, Dicke M, Hartley SE. 2012. The ecology of plant secondary metabolites: from genes to global processes. New York: Cambridge University Press. [Google Scholar]
- Johnson SN, Barton AT, Clark KE, Gregory PJ, McMenemy LS, Hancock RD. 2011. Elevated atmospheric carbon dioxide impairs the performance of root-feeding vine weevils by modifying root growth and secondary metabolites. Global Change Biology 17, 688–695. [Google Scholar]
- Johnson SN, Clark KE, Hartley SE, Jones TH, McKenzie SW, Koricheva J. 2012. Aboveground–belowground herbivore interactions: a meta-analysis. Ecology 93, 2208–2215. [DOI] [PubMed] [Google Scholar]
- Johnson SN, Hawes C, Karley AJ. 2009. Reappraising the role of plant nutrients as mediators of interactions between root- and foliar-feeding insects. Functional Ecology 23, 699–706. [Google Scholar]
- Johnson SN, McNicol JW. 2010. Elevated CO2 and aboveground–belowground herbivory by the clover root weevil. Oecologia 162, 209–216. [DOI] [PubMed] [Google Scholar]
- Johnson SN, Mitchell C, McNicol JW, Thompson J, Karley AJ. 2013. Downstairs drivers— root herbivores shape communities of above-ground herbivores and natural enemies via changes in plant nutrients. Journal of Animal Ecology 82, 1021–1030. [DOI] [PubMed] [Google Scholar]
- Johnson SN, Murray PJ. 2008. Root feeders: an ecosystem perspective. Wallingford, UK: CABI. [Google Scholar]
- Johnson SN, Ryalls JMW, Karley AJ. 2014. Global climate change and crop resistance to aphids: contrasting responses of lucerne genotypes to elevated atmospheric carbon dioxide. Annals of Applied Biology 165, 62–72. [Google Scholar]
- Karley AJ, Douglas AE, Parker WE. 2002. Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. Journal of Experimental Biology 205, 3009–3018. [DOI] [PubMed] [Google Scholar]
- Masters G, Brown V, Gange A. 1993. Plant mediated interactions between above- and below-ground insect herbivores. Oikos 66, 148–151. [Google Scholar]
- Morris JG. 1991. Nutrition. In: Prosser CL, ed. Environmental and metabolic animal physiology. New York: John Wiley & Sons, 231–276. [Google Scholar]
- Murray TJ, Ellsworth DS, Tissue DT, Riegler M. 2013. Interactive direct and plant-mediated effects of elevated atmospheric [CO2] and temperature on a eucalypt-feeding insect herbivore. Global Change Biology 19, 1407–1416. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Hatch DJ, Cliquet JB. 1996. Impact of insect root herbivory on the growth and nitrogen and carbon contents of white clover (Trifolium repens) seedlings. Canadian Journal of Botany 74, 1591–1595. [Google Scholar]
- Newman JA. 2003. Climate change and cereal aphids: the relative effects of increasing CO2 and temperature on aphid population dynamics. Global Change Biology 10, 5–15. [Google Scholar]
- Newman JA, Anand M, Henry HAL, Hunt S, Gedalof Z. 2011. Methods for studying the impacts of climatic change. In: Climate change biology. Wallingford, UK: CABI, 50 ––70. [Google Scholar]
- Nowak H, Komor E. 2010. How aphids decide what is good for them: experiments to test aphid feeding behaviour on Tanacetum vulgare (L.) using different nitrogen regimes. Oecologia 163, 973–984. [DOI] [PubMed] [Google Scholar]
- Ponder KL, Pritchard J, Harrington R, Bale JS. 2000. Difficulties in location and acceptance of phloem sap combined with reduced concentration of phloem amino acids explain lowered performance of the aphid Rhopalosiphum padi on nitrogen deficient barley (Hordeum vulgare) seedlings. Entomologia Experimentalis et Applicata 97, 203–210. [Google Scholar]
- Pritchard J, Griffiths B, Hunt EJ. 2007. Can the plant-mediated impacts on aphids of elevated CO2 and drought be predicted? Global Change Biology 13, 1616–1629. [Google Scholar]
- Quinn MA, Hall MH. 1992. Compensatory response of a legume root-nodule system to nodule herbivory by Sitona hispidulus . Entomologia Experimentalis et Applicata 64, 167–176. [Google Scholar]
- Reason AJ. 2003. Validation of amino acid analysis methods. In: Smith BJ, ed. Protein sequencing protocols. 2nd Ed., Totowa: Humana Press. [DOI] [PubMed] [Google Scholar]
- Reddy KR, Hodges H. 2000. Climate change and global crop productivity. Wallingford, UK: CABI. [Google Scholar]
- Robinson EA, Ryan GD, Newman JA. 2012. A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytologist 194, 321–336. [DOI] [PubMed] [Google Scholar]
- Rogers A, Ainsworth EA, Leakey ADB. 2009. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiology 151, 1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers WE, Siemann E. 2004. Invasive ecotypes tolerate herbivory more effectively than native ecotypes of the Chinese tallow tree Sapium sebiferum. Journal of Applied Ecology 41, 561–570. [Google Scholar]
- Ryalls JMW, Riegler M, Moore BD, Johnson SN. 2013a. Biology and trophic interactions of lucerne aphids. Agricultural and Forest Entomology 15, 335–350. [Google Scholar]
- Ryalls JMW, Riegler M, Moore BD, Lopaticki G, Johnson SN. 2013b. Effects of elevated temperature and CO2 on aboveground–belowground systems: a case study with plants, their mutualistic bacteria and root/shoot herbivores. Frontiers in Plant Science 4, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E. 2011. Alfalfa and relatives: evolution and classification of Medicago. Ottawa, Canada: NRC Research Press. [Google Scholar]
- Soler R, Van der Putten WH, Harvey JA, et al. 2012. Root herbivore effects on aboveground multitrophic interactions: patterns, processes and mechanisms. Journal of Chemical Ecology 38, 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussana JF, Hartwig UA. 1995. The effects of elevated CO2 on symbiotic N2 fixation: a link between the carbon and nitrogen cycles in grassland ecosystems. Plant and Soil 187, 321–332. [Google Scholar]
- Sun Y, Ge F. 2011. How do aphids respond to elevated CO2? Journal of Asia-Pacific Entomology 14, 217–220. [Google Scholar]
- Vara Prasad PV, Allen LH, Boote KJ. 2005. Crop responses to elevated carbon dioxide and interaction with temperature. Journal of Crop Improvement 13, 113–155. [Google Scholar]
- White T. 1984. The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 63, 90–105. [DOI] [PubMed] [Google Scholar]
- Whittington HR, Tilman D, Powers JS. 2013. Consequences of elevated temperatures on legume biomass and nitrogen cycling in a field warming and biodiversity experiment in a North American prairie. Functional Plant Biology 40, 1147–1158. [DOI] [PubMed] [Google Scholar]
- Winter H, Lohaus G, Heldt HW. 1992. Phloem transport of amino acids in relation to their cytosolic levels in barley leaves. Plant Physiology 99, 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahran HH. 1999. Rhizobium–legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiology and Molecular Biology Reviews 63, 968–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrabi AA, Berberet RC, Caddel JL. 1995. New biotype of Acyrthosiphon kondoi (Homoptera: Aphididae) on alfalfa in Oklahoma. Journal of Economic Entomology 88, 1461–1465. [DOI] [PubMed] [Google Scholar]
- Zvereva EL, Kozlov MV. 2012. Sources of variation in plant responses to belowground insect herbivory: a meta-analysis. Oecologia 169, 441–452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.