Abstract
Objective
Children with congenital heart disease (CHD) have loss of intestinal epithelial barrier function (EBF), which increases their risk for post-operative sepsis and organ dysfunction. We do not understand how post-operative cardiopulmonary support or the inflammatory response to cardiopulmonary bypass (CPB) might alter intestinal EBF. We examined variation in a panel of plasma biomarkers to reflect intestinal EBF (cellular and paracellular structure and function) after CPB and in response to routine ICU care.
Design
Prospective cohort
Setting
University medical center cardiac intensive care unit
Patients
Twenty children aged newborn to 18 years undergoing repair or palliation of CHD with CPB.
Interventions
We measured baseline and repeated plasma FABP2, citrulline, claudin 3, and dual sugar permeability test (DSPT) to reflect intestinal epithelial integrity, epithelial function, paracellular integrity, and paracellular function, respectively. We measured baseline and repeated plasma pro-inflammatory (IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL4, IL10) cytokines, known to modulate intestinal EBF in murine models of CPB.
Measurements and Main Results
All patients had abnormal baseline FABP2 concentrations (mean 3815.5 pg/mL), (normal 41–336 pg/mL). Cytokine response to CPB was associated with early, but not late changes in plasma concentrations of FABP2 and citrulline. Variation in biomarker concentrations over time were associated with aspects of ICU care indicating greater severity of illness: claudin 3, FABP2, and DSPT ratio were associated with symptoms of feeding intolerance (p<0.05) while FABP2 was positively associated with vasoactive-inotrope score (VIS) (p=0.04). Citrulline was associated with larger arteriovenous O2 saturation difference (p=0.04) and had a complex relationship with VIS.
Conclusions
Children undergoing CPB for repair or palliation of CHD are at risk for intestinal injury and often present with evidence for loss of intestinal epithelial integrity pre-operatively. Greater severity of illness requiring increased cardiopulmonary support rather than the inflammatory response to CPB seems to mediate late post-operative intestinal EBF.
Keywords: Cardiac intensive care, Intestinal epithelial barrier Function, Vasopressin, Epinephrine, Congenital heart disease, Post-operative
Introduction
Children undergoing surgical repair or palliation of congenital heart disease (CHD) are at high risk of post-operative organ dysfunction, sepsis, and gastrointestinal complications1,2. One proposed mechanism for post-operative sepsis and organ dysfunction after cardiopulmonary bypass (CPB) is the development of intestinal epithelial barrier dysfunction1. Intestinal ischemia-reperfusion injury occurring after cardiopulmonary bypass (CPB) worsens intestinal epithelial barrier function, permitting bacteria or bacterial products to translocate across the intestinal epithelial barrier and into the bloodstream1. In murine models, intestinal epithelial barrier dysfunction is also associated with loss of local and remote organ immune function3,4. The gastrointestinal tract, through intestinal epithelial barrier functions, may therefore impact post-operative outcomes and complication rates after CPB. Previously thought to be static, intestinal epithelial barrier function is adaptable throughout the gastrointestinal tract and is regulated by diverse extracellular stimuli, including nutrients, medications, commensal and pathogenic organisms, and cytokines5–9. We do not have a clear understanding of how routine ICU care or the inflammatory response to CPB impact intestinal epithelial barrier function.
Minimally invasive assessment of intestinal epithelial barrier function is possible using plasma biomarkers, which reflect downstream structural and functional changes to the intestinal epithelial barrier, and through functional sugar permeability testing10,11. Intestinal Fatty Acid Binding Protein (FABP2) is a cytosolic protein found primarily in mature enterocytes in the small intestine has been used as a biomarker of early intestinal ischemia and injury in children and adults after cardiopulmonary bypass1,12. FABP2 plasma concentration correlates with intestinal epithelial injury and re-epithelialization in animal models of ischemia-reperfusion injury10,13. Functional enterocyte mass in turn, can be measured by the concentrations of circulating citrulline, a non-essential amino acid in humans14. Plasma concentrations of citrulline reflect enterocyte citrulline synthesis, and correlate with functional enterocyte mass in stem cell transplant patients, and in pediatric short bowel syndrome15,16. Transmembrane tight junction proteins such as claudins are the primary determinants of gastrointestinal paracellular barrier integrity8,17. Plasma claudin 3 is a non-invasive marker for early intestinal tight junction loss, and is localized to the epithelial tight junctions8. Recent studies have shown a strong relationship between intestinal tight junction loss and claudin 3 plasma concentrations in a rat hemorrhagic shock model, during developmental maturation of the gastrointestinal tract, and in children undergoing surgery8,18,19. Dual sugar permeability testing (DSPT) relies on the differential intestinal paracellular and cellular permeability of larger (lactulose) and smaller (mannitol) molecules20. Simultaneous co-ingestion of lactulose and mannitol are used as controls for gastric emptying, intestinal fluid volume, gastrointestinal transit time, and renal excretion which are thought to affect each molecule equally20. The ratio of urinary excretion reflects small intestinal permeability.
Intestinal epithelial barrier function after repair or palliation of CHD offers a novel target for the prevention of post-operative sepsis and organ dysfunction; sources of ongoing morbidity in this patient population1,2. In this study, we aimed at understanding how cytokine response to CPB and characteristics of routine post-operative management affected immediate and late post-operative intestinal epithelial barrier function. We sought to identify risk factors for worsened intestinal epithelial barrier function in children with CHD. We investigated a panel of plasma biomarkers to reflect intestinal epithelial cellular and paracellular structure (FABP2 and claudin 3), as well as cellular and paracellular function (citrulline and DSPT) with simultaneous assessment of pro- and anti-inflammatory response to CPB.
Materials and Methods
Patients
We obtained institutional review board approval for this research from the University of Arizona, Human Subjects Protection Program. We approached parents for signed written consent for all eligible children scheduled for CHD surgery in the pre-anesthesia clinic at Diamond Children’s Medical Center in Tucson, AZ. Inclusion criteria were age greater than 37 weeks corrected gestational age and a diagnosis of CHD requiring surgical repair or palliation with the use of CPB. Exclusion criteria identified patients with baseline gastrointestinal epithelial barrier dysfunction, patients likely to have an abnormal inflammatory response to CPB, or who had contraindications to DSPT: pre-existing gastrointestinal diagnosis (such as history of necrotizing enterocolitis, short bowel syndrome, protein losing enteropathy, cow milk protein allergy, or inflammatory bowel disease), immune disorder diagnosis, active intracranial bleeding, and anuric renal failure.
Operative and Post-operative Management
All operations were performed by two surgeons. CPB and anesthetic regimens were per usual care. Mannitol was a routine component of CPB prime in our study subjects, but as our DSPT testing occurred either prior to CPB or at 48 and 72 hours, mannitol given with CPB should not impact our results. None of our study patients received pre-operative steroids. Perioperative antibiotic [second generation Cephalosporin (Cefuroxime)] was administered in all patients except in cases of documented penicillin allergy, or history of methicillin resistant S. aureus infection. Use of caudal or spinal morphine was monitored as it may impact splanchnic perfusion. Patients were prescribed intermittent intravenous or oral furosemide post-operatively as per usual care.
Blood samples
Blood samples for measurement of plasma FABP2, claudin 3, and citrulline were collected from indwelling intravascular catheters pre-operatively after induction of general anesthesia, but prior to CPB, and at 6, 12, 24, 48, and ≥120 hours post-operatively. Final samples at ≥120 hours were collected between 120 and 168 hours post-operatively to evaluate the return to baseline values. Blood collection occurred during steady state time periods for vasoactive infusions. Blood samples were collected from arterial catheters, in place for clinical monitoring, immediately placed into K+ EDTA (BD Vacutainer, Franklin Lakes, NJ) collection tubes. To identify any effect from CPB blood on serial biomarker concentrations, we collected CPB bypass circuit blood samples intra-operatively from the self-circulating CPB circuits prior to connection to the patient. Blood and urine samples (below) were immediately stored at 4°C, spun at 3400rpm for 15 minutes within 4 hours of collection, and plasma stored at −80°C until analysis.
Clinical data included candidate factors likely to alter intestinal epithelial barrier function. Candidate patient and treatment factors were chosen based on literature review. We collected multiple parameters including; patient demographics and vital statistics, cardiac diagnoses, type of surgical repair, CPB characteristics, anesthetic regimen, hemodynamic variables, laboratory values related to organ function and adequacy of circulation, in addition to fluid and nutritional management characteristics. Vasoactive-inotrope score (VIS) was determined at time of sample collection and was previously validated21. As no validated post-operative feeding intolerance score exists for children, we scored feeding intolerance as a cumulative count variable for symptoms occurring over the previous 24 hours (use of antiemetic, abdominal distention, vomiting, diarrhea, and GI bleed). Each variable was scored as present or absent over the previous 24 hours, as such the minimum score was 0 and maximum score was 5. Patients were all scored by a single investigator (KT).
Analysis of Plasma FABP2 and Claudin 3 Concentrations
Plasma concentrations of human FABP2 were evaluated by ELISA according to the manufacturer’s protocol (Standards range: 10 000pg/ml to 156.25 pg/ml) (R&D Systems, Minneapolis, MN). Claudin 3 concentration was evaluated by ELISA in plasma from patients according to manufacturer’s instruction (standard range from 20ng/ml to 0.312ng/ml) (Uscn life science Inc, Wuhan, China).
Analysis of Citrulline Concentration
Plasma was deproteinized via acetone precipitation. Samples were separated on Phenomenex Luna HILIC 200A SB-C18, 3 µm, 150 × 2.00 mm column (Torrance, CA) using Paradigm MS4B - multi-dimensional separations module (Michrom BioResources, Inc., Auburn, CA) with an internal standard of L-Citrulline (5–13C, 99%; 4,4,5,5 – D4, 95%- Cambridge Isotope Laboratories, Inc., Andover, MA). AB SCIEX API 3000 triple-quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) controlled by Analyst 1.5.1 in-line with the HPLC was used for citrulline detection and quantitative analysis. Mass spectrometric analysis was performed using Multiple Reaction Monitoring (MRM-MS) scan type in positive mode with a TurboIonSpray source.
Analysis of Plasma Cytokines
We analyzed baseline and serial pro-inflammatory (TNF-α, IFN-γ, IL-6) and anti-inflamatory (IL-4, and IL-10) cytokines known to impact intestinal epithelial tight junction and enterocyte integrity in animal models of intestinal ischemia-reperfusion injury. Cytokine concentrations were evaluated using an xMAP assay (Millipore, Billerica, MA) and Luminex 100 platform (Liquichip; Qiagen, Valencia, CA.)
Dual Sugar Permeability Testing
Subjects were given 2ml/kg of lactulose/mannitol (lactulose 5gm/100ml + mannitol 2gm/100ml) solution via nasogastric tube (NGT) in the operating room after induction of general anesthesia. This test was repeated at 48 and ≥120 hours either orally of via NGT, if in place. Urine was collected for 6 hours, placed in a urine collection tube with preservative (boric acid), and further processed similar to blood samples. Not all patients completed the DSPT at 48 and 120 hours due to refusal to drink sugar solution, or if they were discharged to home before the study day. Lactulose and mannitol in urine were determined by HPLC at Baylor College of Medicine, as previously described22.
Data Analysis
Data were analyzed using STATA SE/11 software. We report raw numbers for type of cardiac diagnosis and surgical procedure performed. For continuous variables with normal or skewed distributions we report means with standard errors, or medians with interquartile ranges, respectively. As citrulline values were normally distributed, the effect of binary variables on citrulline were evaluated with a Student’s t-test. When comparing the relationship of two continuous variables we report the results of pairwise correlations, with missing values handled by pairwise deletion. The effect of continuous and categorical variables over time on repeated log-transformed plasma FABP2, log-transformed claudin 3, and citrulline was evaluated with multi-level mixed-effects linear regression. The effect of categorical variables on plasma FABP2, claudin 3, and citrulline values were evaluated with Kruskal Wallis. The effect of binary variables on plasma values for FABP2 and claudin 3 was tested with Mann-Whitney ranksum, and with the Student’s t-test for citrulline. A p value below 0.05 was considered significant for all tests.
Results
We measured baseline and serial post-operative plasma FABP2, citrulline, claudin 3, and pro- and anti-inflammatory cytokines in 20 children undergoing repair or palliation of CHD with the use of CPB. Table 1 lists the cardiac diagnoses and operations performed. Patient demographics, cardiac bypass duration, and hospital length of stay are listed in Table 2. Creatinine clearance remained normal for all study subjects. Patients maintained >1ml/kg/hour of urine output. Median cumulative percent fluid overload [(Fluid in-Fluid out/admit weight (kg)) × 100] at post-operative day 5 was +0.8% (IQR -6.6–+10.1%). At the time of blood sample collection, all subjects were within one standard deviation of age-appropriate mean arterial pressures. Caudal morphine or bupivicaine were not associated with changes in post-operative FABP2, claudin 3, or citrulline (p>0.05).
Table 1.
Patient Demographic Information
| Patient characteristic | N=20 |
|---|---|
| Median age, months (IQR) | 17 (3.1–76.9) |
| Median weight, kg (IQR) | 9.3 (6.6–22.5) |
| Male gender, N (%) | 14 (70) |
| Median CPB time, min (IQR) | 102 (78–126) |
| Mortality, N (%) | 0(0) |
| Median hospital length of stay, days (IQR) | 15 (7–31) |
IQR, interquartile range; kg, kilogram; CPB, cardiopulmonary bypass.
Table 2.
Surgical Diagnoses and Operation Performed
| Diagnoses | N | Operation | N |
|---|---|---|---|
| TOF | 2 | TOF repair | 2 |
| Septal defects | 8 | Tricuspid valve repair | 1 |
| AV canal defects | 3 | Arterial switch | 1 |
| Aortic/subaortic stenosis | 4 | Fontan revision | 1 |
| Coarctation of the aorta/hypoplastic arch | 1 | RVOT repair | 2 |
| Mitral valve defects | 2 | Septal defect repair | 8 |
| TAPVR | 1 | RV-PA conduit | 5 |
| Pulmonary stenosis/atresia | 3 | Subaortic/aortic stenosis repair | 3 |
| Pulmonary insufficiency | 3 | Coarctation/hypoplastic arch repair | 1 |
| DORV | 2 | Mitral valve repair | 2 |
| Ebstein’s anomaly | 1 | Ross procedure | 1 |
| AV canal repair | 1 | ||
| TAPVR repair | 1 |
AV, atrioventricular; TAPVR, total anomalous pulmonary venous return; DORV, double outlet right ventricle; RVOT, right ventricular outflow tract; RV-PA, right ventricle to pulmonary artery.
Total number of diagnoses and operations performed exceeds 20 as some patients had multiple defects which were repaired.
Baseline Plasma Biomarkers
At baseline, children with CHD had > 10-fold higher mean (SEM) plasma FABP2 concentrations, 3815 (976) pg/ml compared to healthy children (normal range 41–336 pg/ml)23 (Figure 1). Subjects had normal baseline mean (SEM) plasma citrulline, 28.9 (2.2) µmol/L [normal range (14–39µmol/L)24], and claudin 3 mean (SEM), was 3.7 (2.0) ng/ml (Figure 1). We did not find differences between type of cardiac defect and any plasma biomarkers either at baseline or post-operatively (p>0.05). We did not find significant differences in the plasma FABP2, claudin 3, or citrulline with surgical transition from hypoxemic to normoxemic circulation, with aortic cross clamp time, or with correction of shunt (p>0.05).
Figure 1.
Change in Minimally Invasive Plasma Intestinal Epithelial Barrier Function Biomarkers and Regulatory Cytokines after Cardiopulmonary Bypass.
FABP2, Intestinal Fatty Acid Binding Protein; IL-10, Interleukin 10; IL-4, Interleukin 4; IL-6, Interleukin 6; TNF-α, Tumor Necrosis Factor α; INF-γ, Interferon γ.
a. Baseline and post-CPB pro-inflammatory response over time; b. Baseline and post-CPB anti-inflammatory response over time; c–f. Change over time of plasma FABP2, claudin 3, citrulline, and dual sugar permeability test (DSPT- Urinary Lactulose/Mannitol Ratio), respectively. Early (<48 hours) but not late changes in plasma biomarkers of enterocyte integrity (FABP2) were associated with post-bypass inflammatory cascade. Plasma FABP2 levels rise in response to CPB (p=0.01). Mean citrulline levels are normal pre-operatively and fall post-operatively, but remained within the age-appropriate normal range (14–39µmol/L)24. Repeated claudin 3 and Lactulose/Mannitol ratios were significantly associated (p<0.01) and rise remote from cardiopulmonary bypass (CPB). *Claudin 3, FABP2, Citrulline, and Lactulose/Mannitol ratios had statistically significant change over time, p<0.05, mixed effects linear regression with time zero as the referent group.
Serial Plasma FABP2
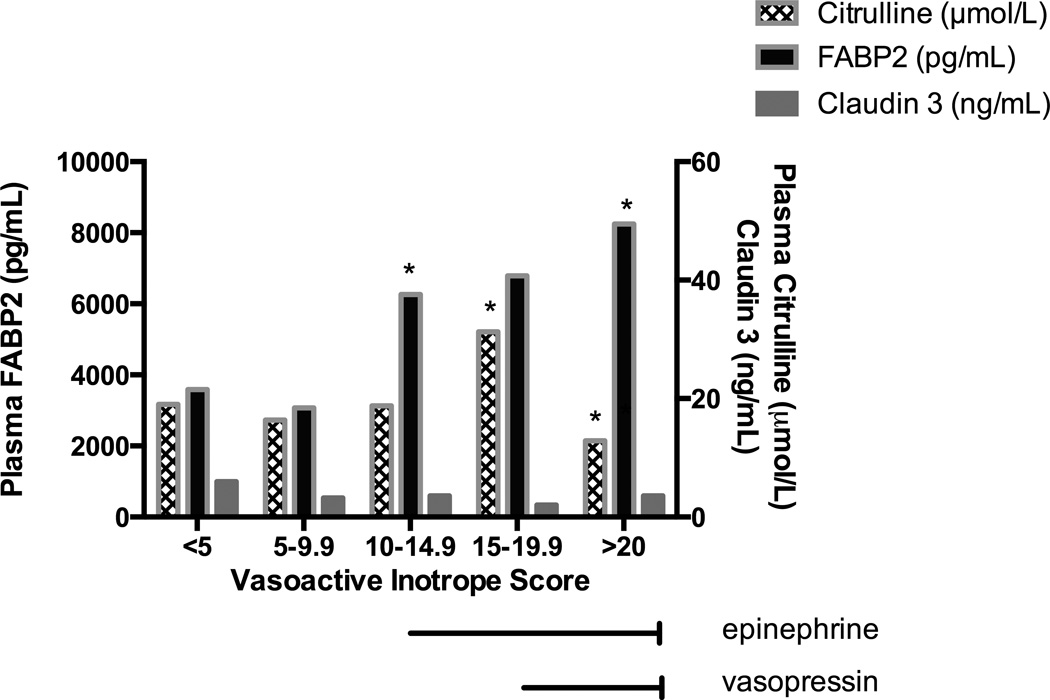
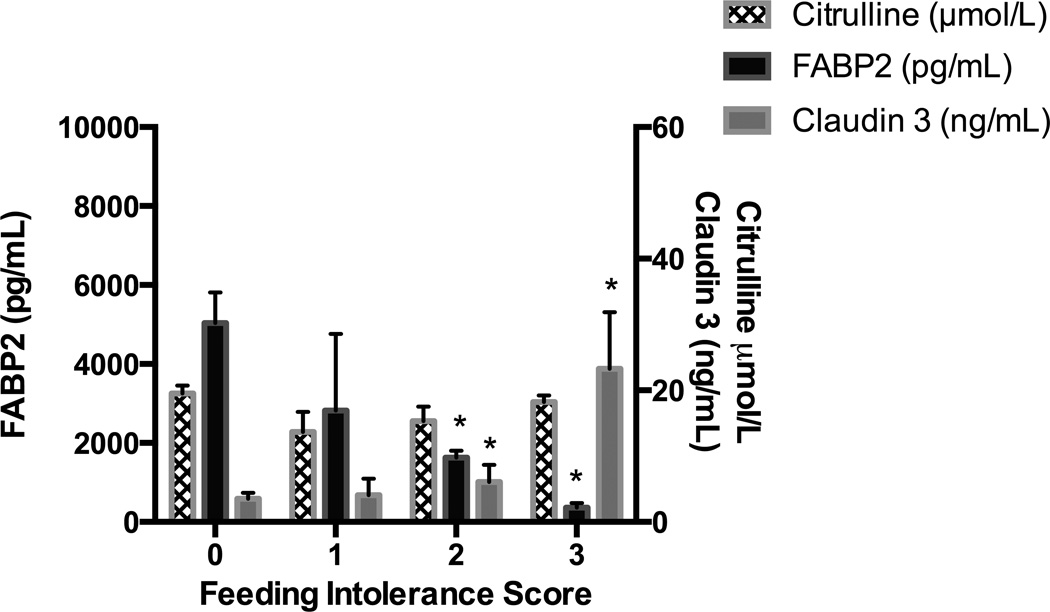
Both peak and change over time of plasma FABP2 were associated with clinical variables indicating greater severity of illness. Peak post-operative plasma FABP2 was associated with CPB duration (p=0.01). Higher plasma FABP2 over time was associated with use of epinephrine and vasopressin infusions, but not with dopamine or milrinone at any dose (p<0.03) (Figure 2). In our study population, vasopressin was used only after initial epinephrine titration for subjects with continued post-operative hypotension. Subjects with VIS >20, who received combined vasopressin and epinephrine had the highest plasma FABP2 concentrations (p=<0.01) (Figure 2). FABP2 was associated with symptoms of feeding intolerance (p=0.02) (Figure 3). Any enteral nutrition (EN) by post-operative day 2 was associated with improved (decreased) plasma FABP2 concentrations (p=0.02).
Figure 2.
Plasma FABP2 and Citrulline Change with Vasoactive-Inotrope Score.
FABP2, Intestinal Fatty Acid Binding Protein; VIS, vasoactive infusion score.
FABP2, Intestinal Fatty Acid Binding Protein
*p<0.05
In mixed effects linear regression model with the lowest VIS as the referent group, we evaluate repeated measures of plasma FABP2 and citrulline over time. All patients with vasoactive infusion score (VIS) > 10 were on epinephrine infusions. All patients with VIS > 20 were also receiving vasopressin infusions.
Figure 3.
Plasma Claudin 3 and FABP2 are Associated with Symptoms of Post-Operative Feeding Intolerance
FABP2, Intestinal Fatty Acid Binding Protein
*p<0.05
Feeding Intolerance Score is a count variable of the number of feeding intolerance symptoms experienced by a patient in the preceeding 24 hours. Claudin 3 (p=0.02) concentrations and FABP2 (p=0.02) were significantly associated with symptoms of feeding intolerance, mixed effects linear regression.
Serial Plasma Citrulline
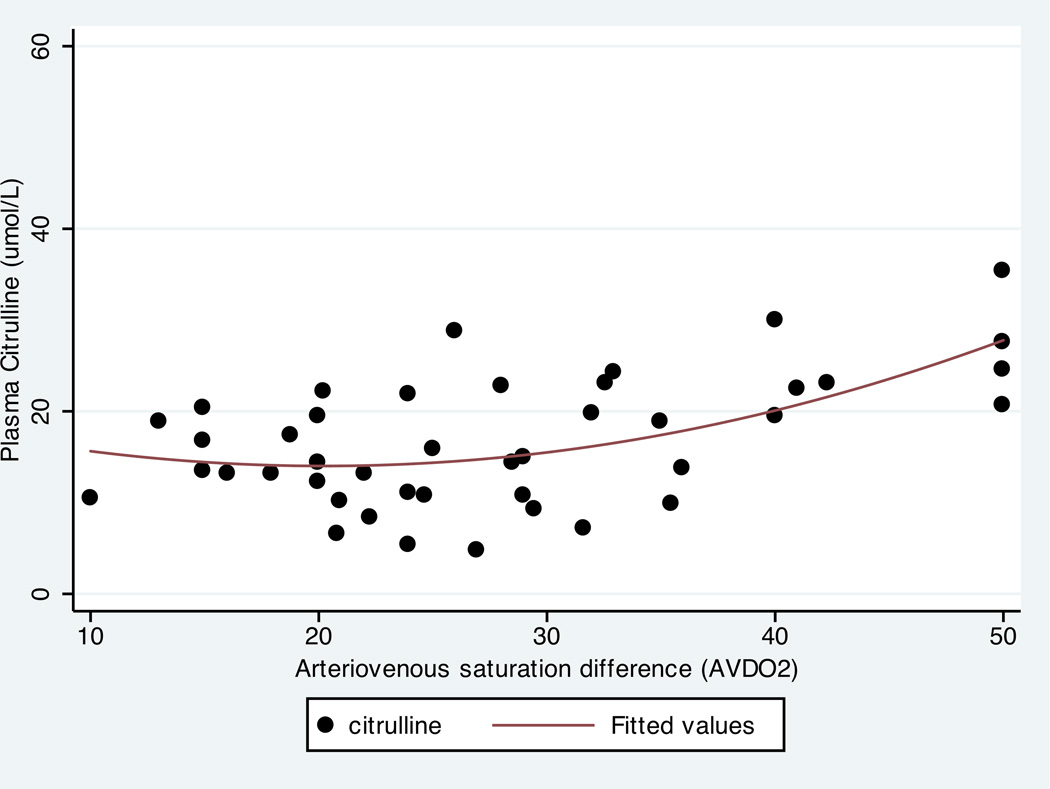
Citrulline concentrations were also associated with clinical factors indicating more severe acute illness. Rise in plasma citrulline concentrations over time was positively associated with VIS in all but the highest VIS group, with low citrulline concentrations associated with VIS > 20 (p=0.02), indicating lower functional enterocyte mass for patients on high dose epinephrine and vasopressin infusions (Figure 2). Consistent with chronic mitochrondrial adaptations to poor intestinal perfusion, citrulline correlated with arterio-venous oxygenation difference (AVDO2) difference, with larger AVDO2 associated with higher citrulline concentration (r=0.51,p=0.02). VIS and AVDO2 were poorly correlated, (r=0.1, p=0.6).
Serial Plasma Claudin 3
Plasma claudin 3 concentrations did not rise after CPB, but were elevated remote from CPB at 120 hours (p<0.001) (Figure 1). Several clinical factors indicating greater severity of illness remote from CPB were also associated with plasma claudin 3 concentrations; positive cumulative percent fluid overload (p=0.03), symptoms of feeding intolerance (emesis, use of promotility agents, abdominal distention, diarrhea, gastrointestinal hemorrhage) (p=0.01) (Figure 3) and duration of antibiotic treatment (p=0.02). Unlike FABP2, Claudin 3 concentrations were not associated with CPB time or VIS (p>0.05).
Dual Sugar Permeability Testing
On a subset of patients we performed dual sugar permeability testing (n=12), and evaluated small intestinal permeability at baseline, then at 48 and ≥120 hours (Figure 1). Consistent with tight junctions as the primary determinants of intestinal epithelial barrier permeability, claudin 3 values correlated with repeated lactulose/mannitol ratios over time, (r=0.45, p<0.001) (Figure 1), confirming claudin 3 as a test of intestinal epithelial tight junction function in our patient population. Lactulose/mannitol ratios rose remote from CPB (p<0.001).
Pro-and Anti-Inflammatory Response to CPB
As expected, CPB time was associated with the magnitude of the measured pro-and anti-inflammatory response. CPB time had a strong positive correlation with peak IL-6 concentrations), r=0.63 and a moderate negative correlation with IL-4 and IFN-γ concentrations, r=−0.35 and −0.42, respectively. We identified significant associations between the pro- and anti-inflammatory response to CPB and early changes in plasma biomarkers of intestinal epithelial and tight junction integrity. In linear mixed effects models examining the interaction between repeated pro-and anti-inflammatory cytokines and intestinal epithelial integrity (FABP2 concentrations) over time, FABP2 was associated with both pro-inflammatory cytokines (IL-6 and TNF-α) and anti-inflammatory cytokines (IL-10), (p<0.05) during the initial 48 hours after surgery, but not later after CPB. Changes in intestinal epithelial tight junction integrity (plasma claudin 3 concentrations) over time were associated with IL-6 (p=0.02), but surprisingly, not with TNF-α (p=0.48), TFNα as we would expect from murine models of intestinal tight junction regulation. We found no association between plasma citrulline and inflammatory response to CPB.
Discussion
We found that children with CHD have evidence for baseline abnormalities in intestinal enterocyte integrity, made transiently worse after surgical correction with CPB. Impaired baseline enterocyte integrity may be in response to altered circulation in the setting of CHD. While pro and anti-inflammatory cytokine responses to CPB were associated with changes in plasma biomarkers of intestinal epithelial barrier function over the initial 48 hours, characteristics of routine ICU care indicating greater severity of illness were associated with changes in plasma biomarker concentrations indicative of intestinal epithelial barrier dysfunction throughout the 5-day study period. We found that peak plasma FABP2 concentrations which occurred within 48 hours of surgery, correlated with CPB duration, as has been previously demonstrated. We found an association with VIS and FABP2 concentrations over time. The highest post-operative plasma FABP2 concentrations were seen for children on vasopressin and epinephrine infusions. Vasopressin is used as a vasopressor in children with CHD, but may have negative effects on the intestinal epithelial barrier through restriction in splanchnic blood flow25. In this single-center pilot investigation it is not possible to determine if type or dose of vasoactive infusion alters intestinal barrier function, or if intestinal barrier dysfunction is indicative of severity of illness. However, we have demonstrated that intestinal epithelial barrier dysfunction is common in post-operative children with CHD and is worse for the patients with more severe acute illness. Whether we can improve intestinal epithelial barrier function by altering post-operative care practices requires further investigations.
Consistent with observations that children on vasopressor infusions have intolerance of enteral feeding, we found an association between intestinal epithelial barrier injury and high dose vasopressor infusions. Importantly, we also found that claudin 3 and FABP2 concentrations were associated with symptoms of feeding intolerance. While our finding of improved enterocyte integrity with any early EN supports the role of early EN in gut protection, it may be that children who cannot be fed have a priori worse intestinal epithelial barrier function. Previous studies have shown that for infants with necrotizing enterocolitis (NEC), elevated plasma FABP2 concentrations after resumption of EN is a marker of poor outcome26, suggesting that biomarkers of intestinal epithelial barrier function could reflect readiness for EN. These biomarkers require further study as possible targets for EN readiness and tolerance. Large practice variation exists with regard to delivery of EN by type and dose of vasoactive infusion27. A multi-centered study would be necessary to determine if biomarkers of intestinal epithelial barrier function could predict safe enteral feeding for children with hemodynamic instability.
Chronic hypoxia may promote an adaptive response in mitochondrial function in intestinal epithelial cells28. Mitochondrial nitric oxide synthase is functionally upregulated in hypoxia and is necessary for the conversion of citrulline from arginine29. At some threshold these adaptive responses may fail and result in decreased citrulline concentrations. Mitochondrial adaptation to hypoxia may explain our findings of higher citrulline with larger AVDO2 difference and the complex relationship with VIS group. Further investigations are needed to identify how citrulline concentrations vary in response to cardiac output in the settings of chronic and acute low cardiac output syndromes and hypoxia. It is possible that mitochondrial adaptations to chronic hypoxia and impaired perfusion are overcome only with high dose vasopressors or with more severe impairments in oxygen delivery.
Intestinal epithelial barrier dysfunction can result in translocation of bacterial products into the bloodstream, decreased gastrointestinal intraepithelial lymphocyte populations, and loss of IgA mediated mucosal immunity in the gastrointestinal and respiratory tract, negatively affecting host immune response while also increasing exposure to pathogens10,30,31. Our observed changes in claudin 3 concentrations and DSPT ratios over time are consistent with endotoxin activity assays after CPB1. Restoration of intestinal epithelial barrier function is associated with restored local and remote organ mucosal immunity in animal models of critical illness and offers a potential target to reduce post-operative hospital-acquired infection risk and organ dysfunction32.
Plasma claudin 3 concentrations were associated with antibiotic duration. Two of our 20 patients had documented post-operative infections, other patients continued on antibiotics for suspected post-operative infections, later discontinued with negative culture results. Prolonged antibiotic use for suspected infections may have negative effects on intestinal epithelial barrier function. Mechanisms for associations between antibiotic duration and claudin 3 concentrations could reflect changes in the gastrointestinal microbiome, known to regulate murine paracellular tight junction permeability8,33. Systematic evaluation of change in gut microbiome is necessary to determine if this is a mechanism for increased intestinal epithelial barrier permeability after CPB.
We observed expected changes in the pro and anti-inflammatory response after CPB34. We did not identify expected changes in claudin 3 concentrations in response to TNF-α and IFN-γ in our pilot population. In murine models of ischemia-reperfusion injury, TNF-α and IFN-γ are primary determinants of intestinal tight junction regulation. It is possible that early loss of claudin 3 expression was prevented by a protective effect of post-CPB anti-inflammatory responses. Pro and anti-inflammatory cytokines may have opposing effects on the intestinal epithelial barrier and the balance of the response determines maintenance or loss of function34,35. For example, IL-6 in murine models promotes intestinal epithelial proliferation and healing and could be protective if elevated in the setting of ischemia-reperfusion injury, while IFN-γ is cytotoxic6,35. IL-10 causes upregulation of tight junctions, while TNF-α causes fulminant apoptosis and tight junction reorganization9,36. When the cytokine response to CPB abated and resulted in reduced IL-10 concentrations, we found increased paracellular permeability by both lactulose/mannitol ratios and rise in plasma claudin 3 concentrations. Unlike previous reports in children with trauma and sepsis, we did not find an association between the magnitude of the pro-inflammatory response and plasma citrulline concentrations37. This may reflect differences in magnitude and type of inflammatory response and vasoactive infusion use in our patients after CPB.
Limitations
Our study was a single center, observation pilot investigation, not powered to detect differences in many clinical outcome variables, including sepsis rates and organ dysfunction. We may not correctly identify clinical factors that, in a larger patient cohort, would be significantly associated with changes in intestinal epithelial barrier function. In this manner, some of our negative findings could represent type II errors. We may also find false positive associations or type I errors. Given our small sample size, and inadequate risk adjustment strategies in the post-operative CHD population we were not able to adequately adjust for illness severity. A larger sample size, across several centers would be needed to control for illness severity. While citrulline is a product of glutamine metabolism and could be altered in the setting of sepsis38, none of our patients were septic, so it is unlikely that this is impacting our results. Claudin 3 is expressed in the lung and lung injury could confound our results39. Our one subject with acute lung injury did not have elevated claudin 3 concentrations. It is unlikely that the claudin 3 concentrations in the plasma reflect a pulmonary source. Dual sugar permeability testing can be affected in the setting of fluid overload, altered gastrointestinal perfusion, renal failure and may be unreliable in the setting of critical illness40. In our study, lactulose/mannitol ratio testing was used in combination with a panel of plasma biomarkers to facilitate detection of outliers.
Conclusions
Children undergoing CPB during repair or palliation of CHD are at risk for intestinal injury and often present with evidence for loss of intestinal epithelial integrity pre-operatively. Characteristics of cardiopulmonary and nutritional support, indicating greater severity of illness, may impact intestinal epithelial barrier function throughout the post-operative course.
Figure 4.
Plasma Citrulline Concentration Correlates with AVDO2
AVDO2, Arteriovenous oxygen saturation difference
Higher citrulline concentration correlated with larger AVDO2 which may suggest adaptive responses to hypoxia and poor cardiac output in children with CHD (r=0.51, p=0.02).
Acknowledgements
The authors would like to thank Yelena Feinstein and George Tsaprailis for citrulline analysis, Bonnie LaFleur for statistical planning, Katie Kowalek for data collection, and the department of Pediatrics at the University of Arizona for funds for this research. This research is supported by grant funding from NIH/NICHD PCCTSDP (5K12HD047349-09). Mass spectrometry and proteomics data were acquired by the Arizona Proteomics Consortium supported by NIEHS grant ES06694 to the SWEHSC, NIH/NCI grant CA023074 to the AZCC and by the BIO5 Institute of the University of Arizona.
References Cited
- 1.Pathan N, Burmester M, Adamovic T, et al. Intestinal injury and endotoxemia in children undergoing surgery for congenital heart disease. American journal of respiratory and critical care medicine. 2011;184:1261–1269. doi: 10.1164/rccm.201104-0715OC. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler DS, Jeffries HE, Zimmerman JJ, Wong HR, Carcillo JA. Sepsis in the Pediatric Cardiac Intensive Care Unit. World JPediatrCongenitHeart Surg. 2011;2:393–399. doi: 10.1177/2150135111403781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wildhaber BE, Yang H, Spencer AU, Drongowski RA, Teitelbaum DH. Lack of enteral nutrition--effects on the intestinal immune system. JSurgRes. 2005;123:8–16. doi: 10.1016/j.jss.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Sacks GS, Kudsk KA. Maintaining mucosal immunity during parenteral feeding with surrogates to enteral nutrition. NutrClinPract. 2003;18:483–488. doi: 10.1177/0115426503018006483. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Ralls MW, Xiao W, Miyasaka E, Herman RS, Teitelbaum DH. Loss of enteral nutrition in a mouse model results in intestinal epithelial barrier dysfunction. Annals of the New York Academy of Sciences. 2012;1258:71–77. doi: 10.1111/j.1749-6632.2012.06572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin X, Zimmers TA, Zhang Z, Pierce RH, Koniaris LG. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut. 2010;59:186–196. doi: 10.1136/gut.2008.151175. [DOI] [PubMed] [Google Scholar]
- 7.Ohta K, Omura K, Hirano K, et al. The effects of an additive small amount of a low residual diet against total parenteral nutrition-induced gut mucosal barrier. American journal of surgery. 2003;185:79–85. doi: 10.1016/s0002-9610(02)01108-x. [DOI] [PubMed] [Google Scholar]
- 8.Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. AmJPathol. 2012;180:626–635. doi: 10.1016/j.ajpath.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song HL, Lu S, Liu P. Tumor necrosis factor-alpha induces apoptosis of enterocytes in mice with fulminant hepatic failure. World journal of gastroenterology : WJG. 2005;11:3701–3709. doi: 10.3748/wjg.v11.i24.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grootjans J, Lenaerts K, Derikx JP, et al. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. AmJPathol. 2010;176:2283–2291. doi: 10.2353/ajpath.2010.091069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grootjans J, Thuijls G, Verdam F, Derikx JP, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World JGastrointestSurg. 2010;2:61–69. doi: 10.4240/wjgs.v2.i3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes JHt, Lieberman JM, Probert CB, et al. Elevated intestinal fatty acid binding protein and gastrointestinal complications following cardiopulmonary bypass: a preliminary analysis. The Journal of surgical research. 2001;100:192–196. doi: 10.1006/jsre.2001.6237. [DOI] [PubMed] [Google Scholar]
- 13.Thuijls G, van WK, Grootjans J, et al. Early diagnosis of intestinal ischemia using urinary and plasma fatty acid binding proteins. AnnSurg. 2011;253:303–308. doi: 10.1097/SLA.0b013e318207a767. [DOI] [PubMed] [Google Scholar]
- 14.Bailly-Botuha C, Colomb V, Thioulouse E, et al. Plasma citrulline concentration reflects enterocyte mass in children with short bowel syndrome. Pediatric research. 2009;65:559–563. doi: 10.1203/PDR.0b013e31819986da. [DOI] [PubMed] [Google Scholar]
- 15.Rhoads JM, Plunkett E, Galanko J, et al. Serum citrulline levels correlate with enteral tolerance and bowel length in infants with short bowel syndrome. The Journal of pediatrics. 2005;146:542–547. doi: 10.1016/j.jpeds.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Merlin E, Minet-Quinard R, Pereira B, et al. Non-invasive biological quantification of acute gastrointestinal graft-versus-host disease in children by plasma citrulline. Pediatric transplantation. 2013;17:683–687. doi: 10.1111/petr.12128. [DOI] [PubMed] [Google Scholar]
- 17.Turner JR. Intestinal mucosal barrier function in health and disease. NatRevImmunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 18.Thuijls G, Derikx JP, de Haan JJ, et al. Urine-based detection of intestinal tight junction loss. Journal of clinical gastroenterology. 2010;44:e14–e19. doi: 10.1097/MCG.0b013e31819f5652. [DOI] [PubMed] [Google Scholar]
- 19.Thuijls G, Derikx JP, van Wijck K, et al. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Annals of surgery. 2010;251:1174–1180. doi: 10.1097/SLA.0b013e3181d778c4. [DOI] [PubMed] [Google Scholar]
- 20.Rao AS, Camilleri M, Eckert DJ, et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. AmJPhysiol GastrointestLiver Physiol. 2011;301:G919–G928. doi: 10.1152/ajpgi.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2010;11:234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 22.Shulman RJ, Schanler RJ, Lau C, Heitkemper M, Ou CN, Smith EO. Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. PediatrRes. 1998;44:519–523. doi: 10.1203/00006450-199810000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Derikx JP, van Waardenburg DA, Thuijls G, et al. New Insight in Loss of Gut Barrier during Major Non-Abdominal Surgery. PloS one. 2008;3:e3954. doi: 10.1371/journal.pone.0003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepage N, McDonald N, Dallaire L, Lambert M. Age-specific distribution of plasma amino acid concentrations in a healthy pediatric population. Clinical chemistry. 1997;43:2397–2402. [PubMed] [Google Scholar]
- 25.Alten JA, Borasino S, Toms R, Law MA, Moellinger A, Dabal RJ. Early initiation of arginine vasopressin infusion in neonates after complex cardiac surgery. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2012;13:300–304. doi: 10.1097/PCC.0b013e31822f1753. [DOI] [PubMed] [Google Scholar]
- 26.Reisinger KW, Derikx JP, Thuijls G, et al. Noninvasive measurement of intestinal epithelial damage at time of refeeding can predict clinical outcome after necrotizing enterocolitis. Pediatric research. 2013;73:209–213. doi: 10.1038/pr.2012.160. [DOI] [PubMed] [Google Scholar]
- 27.Mehta NM, McAleer D, Hamilton S, et al. Challenges to optimal enteral nutrition in a multidisciplinary pediatric intensive care unit. JPEN JParenterEnteral Nutr. 2010;34:38–45. doi: 10.1177/0148607109348065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell metabolism. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Lacza Z, Puskar M, Figueroa JP, Zhang J, Rajapakse N, Busija DW. Mitochondrial nitric oxide synthase is constitutively active and is functionally upregulated in hypoxia. Free radical biology & medicine. 2001;31:1609–1615. doi: 10.1016/s0891-5849(01)00754-7. [DOI] [PubMed] [Google Scholar]
- 30.Nose K, Yang H, Sun X, et al. Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: a potential mechanism for the preservation of epithelial barrier function. JInterferon Cytokine Res. 2010;30:67–80. doi: 10.1089/jir.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson CD, Kudsk KA, Fukatsu K, Renegar KB, Zarzaur BL. Route of nutrition influences generation of antibody-forming cells and initial defense to an active viral infection in the upper respiratory tract. AnnSurg. 2003;237:565–573. doi: 10.1097/01.SLA.0000059991.89316.B8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kudsk KA, Wu Y, Fukatsu K, et al. Glutamine-enriched total parenteral nutrition maintains intestinal interleukin-4 and mucosal immunoglobulin A levels. JPEN JParenterEnteral Nutr. 2000;24:270–274. doi: 10.1177/0148607100024005270. [DOI] [PubMed] [Google Scholar]
- 33.Larmonier CB, Laubitz D, Hill FM, et al. Reduced colonic microbial diversity is associated with colitis in NHE3-deficient mice. American journal of physiology Gastrointestinal and liver physiology. 2013;305:G667–G677. doi: 10.1152/ajpgi.00189.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duval EL, Kavelaars A, Veenhuizen L, van Vught AJ, van de Wal HJ, Heijnen CJ. Pro- and anti-inflammatory cytokine patterns during and after cardiac surgery in young children. EurJPediatr. 1999;158:387–393. doi: 10.1007/s004310051098. [DOI] [PubMed] [Google Scholar]
- 35.Guy-Grand D, DiSanto JP, Henchoz P, Malassis-Seris M, Vassalli P. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-gamma, TNF) in the induction of epithelial cell death and renewal. European journal of immunology. 1998;28:730–744. doi: 10.1002/(SICI)1521-4141(199802)28:02<730::AID-IMMU730>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 36.Sun X, Yang H, Nose K, et al. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. AmJPhysiol GastrointestLiver Physiol. 2008;294:G139–G147. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- 37.van Waardenburg DA, de Betue CT, Luiking YC, Engel M, Deutz NE. Plasma arginine and citrulline concentrations in critically ill children: strong relation with inflammation. The American journal of clinical nutrition. 2007;86:1438–1444. doi: 10.1093/ajcn/86.5.1438. [DOI] [PubMed] [Google Scholar]
- 38.de Betue CT, Deutz NE. Changes in Arginine metabolism during sepsis and critical illness in children. Nestle Nutrition Institute workshop series. 2013;77:17–28. doi: 10.1159/000351370. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell LA, Overgaard CE, Ward C, Margulies SS, Koval M. Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. American journal of physiology Lung cellular and molecular physiology. 2011;301:L40–L49. doi: 10.1152/ajplung.00299.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oudemans-van Straaten HM, van der Voort PJ, Hoek FJ, Bosman RJ, van der Spoel JI, Zandstra DF. Pitfalls in gastrointestinal permeability measurement in ICU patients with multiple organ failure using differential sugar absorption. Intensive care medicine. 2002;28:130–138. doi: 10.1007/s00134-001-1140-2. [DOI] [PubMed] [Google Scholar]