Abstract
Identifying modifiers of glioma risk in patients with type 1 neurofibromatosis (NF1) could help support personalized tumor surveillance, advance understanding of gliomagenesis and potentially identify novel therapeutic targets. Here we report genetic polymorphisms in the human adenylate cyclase gene ADCY8 which correlate with glioma risk in NF1 in a sex-specific manner, elevating risk in females while reducing risk in males. This finding extends earlier evidence of a role for cAMP in gliomagenesis based on results in a genetically engineered mouse model (Nf1 GEM). Thus, sexually dimorphic cAMP signaling might render males and females differentially sensitive to variation in cAMP levels. Using male and female Nf1 GEM, we found significant sex differences exist in cAMP regulation and in the growth promoting effects of cAMP suppression. Overall, our results establish a sex-specific role for cAMP regulation in human gliomagenesis, specifically identifying ADCY8 as a modifier of glioma risk in NF1.
Introduction
Neurofibromatosis type 1 (NF1) is a common autosomal dominant cancer predisposition syndrome that affects males and females of all races and ethnicities, and variably results in multiple developmental abnormalities and neoplasias (1). Currently, the severity with which multiple body systems will be affected by complications of NF1 remains largely unpredictable, which significantly hinders the delivery of care (2). Controversies surrounding the management of optic pathway gliomas (OPG) in these patients illustrate this point. These NF1-associated brain tumors occur in approximately 20% of affected individuals, and in up to 50% of NF1 OPG cases, chemotherapy is initiated, usually prompted by vision loss (3). The unpredictable growth of OPGs has impeded the adoption of consensus guidelines for care and confounds assessments of treatment efficacy (4). Identifying biomarkers for OPG risk would transform our management of NF1 patients.
The majority of NF1-associated gliomas occur in the anterior optic pathway of young children (< 7 years old). Previously, we showed that alterations in cAMP levels could vary the stereotypical pattern of OPG formation, and that pharmacological elevation of cAMP levels could block OPG growth in an established genetically engineered mouse (GEM) model of NF1-associated OPG (5),(6, 7). These studies established the cAMP pathway as a candidate modifier of glioma risk in NF1. Here, we provide a measure of validation for these studies by showing that polymorphisms in adenylate cyclase 8 (ADCY8) modify NF1 glioma risk in a sex-specific fashion. Moreover, we found that sexual dimorphism in cAMP signaling and sex differences in cAMP-dependent growth regulation are well modeled in murine Nf1-/- astrocytes.
Materials and Methods
Ethics Statement
Animal studies
Animals were used in accordance with an Animal Studies Protocol (# 20120174) approved by the Animal Studies Committee of the Washington University School of Medicine per the recommendations of the Guide for the Care and Use of Laboratory Animals (National Institutes of Health).
Human Studies
DNA specimens acquired from individuals with NF1 were processed and are being reported in accordance Institutional Review Board (IRB)-approved Human Studies Protocols at each of the participating institutions.
Chemicals, Reagents, and Antibodies
All chemicals were obtained from Sigma-Aldrich (St Louis, MO) unless otherwise indicated.
Human DNA sample collection
Individuals with NF1 were recruited for this study from NF1 Clinical Programs at Washington University in St. Louis (WU), the University of Toronto (TORONTO), University of Utah (UTAH) and New York University (NYU). Those with and without OPG were identified from MRI scans. Criteria for OPG included clear optic nerve or chiasm enlargement or enhancement. Other optic nerve abnormalities such as tortuosity or dilated, fluid filled optic nerve sheaths did not qualify as OPGs (8). Patients without OPG had negative MRIs obtained after the age of ten. DNA was extracted from blood using Qiagen DNA Blood mini-kits (Valencia, CA) and from saliva using DNA Genotek Oragene DNA kits (Kanata, ON, CA) according to the manufacturers' instructions. Following quality checks and concentration optimization, DNA was hybridized to Affymetrix 6.0 single nucleotide polymorphism microarrays at The Genome Institute, Washington University or ARUP, Salt Lake City. Intensity scanning was performed in the same laboratories in which hybridization occurred. All data are accessible through the geo database, accession number GSE62215 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE62215).
High-density Affymetrix Genome-wide SNP Array Analysis
The Birdseed-v2 was used to make initial genotype calls. Samples with a genotyping call rate <95% and contrast QC<0.4 according to the Affymetrix genotyping console analysis were removed and genotypes were regenerated using the remaining samples. PLINK (9) was used for SNP QC to exclude those failing Hardy-Weinberg test (P≤1e-06) or missingness test (P<0.1) or with a MAF <0.05. A total of 680,187 SNPs were analyzed. The logistic regression model for glioma risk was modeled with a SNP, Sex, SNP × Sex interaction, biospecimen (saliva/blood) and cohort (WU/UTAH/TORONTO/NYU), as well as the first 4 principal components from principal component analysis (PCA) using linkage disequilibrium (LD) pruned SNPs to control for population stratification. The bioConductor package “SNPRelate” (10) was used for LD pruning (the maximum basepairs in the sliding window=10e06, LD threshold=0.2 and the “composite” method was adopted for LD metrics) and PCA analysis and “GWASTools” (11) was used for genome wide association analysis using logistic regression modeling under the dominant genetic model. The odds ratios (ORs) of male, female, ratio of the ORs for glioma risk between males and females (the SNP × Sex interaction) and the likelihood ratio (LR) P-values on the ratio which was obtained by comparing the full logistic regression model to the model leaving the interaction out were reported. To account for multiple comparisons, the permutation-adjusted P-values and the false discovery rate (FDR) adjusted P-value were calculated. Specifically, the case/control status was permuted (for 500 times) and the LR P-values of SNP × Sex corresponding to each permuted phenotype were calculated under the same full logistic regression model. The permutation-adjusted P-value was finally computed as the proportion of permutations with at least one SNP's permutated P-value ≤ the original LR P-value corresponding to the non-permuted status. Linkage disequilibrium analysis was conducted using PLINK and the LD measure R2 was reported. Major Allele Frequency (MAF) for SNPs in the general population was determined using 1093 total samples in the 1000 Genome phase I data released on May 2011 using ENGINES (SPSmart version 5.1.1 and dbSNP build 132) (12).
Primary Astrocyte Cultures
Animals were maintained on a C57Bl/6 background. Primary Nf1-/- astrocytes were isolated from the cortices of individual neonatal Nf1-CKO (Nf1flox/flox; GFAP-Cre) mice at postnatal day 1-2 as described (5). The sex of the newborn mice was determined by Jarid 1C/Jarid 1D PCR. Astrocytes of the same sex were combined and cryo-preserved. Wildtype (WT) astrocytes were similarly derived from neonatal Nf1flox/flox mice. Western blot analysis for neurofibromin expression was performed by standard methods utilizing rabbit anti-Nf1 antibody (1:200, Santa Cruz), mouse anti-β-actin antibody (1:30,000, Sigma), and IRdye680 or 800-conjugated donkey anti-mouse or rabbit IgG (1: 30,000, LI-COR). Only cells at low passage numbers (<6) were used. Each experiment included at least 4 separate cultures derived from at least two litters/sex.
Real-time PCR
RNA was extracted from WT and Nf1-/- astrocytes using the Qiagen RNeasy kit (Qiagen, Valenica, CA). cDNA was generated using the Superscript first-strand cDNA synthesis system (Invitrogen). Real-time quantitative PCR reactions were performed using Power Sybr Green PCR master mix (Applied Biosystems (Carlsbad, CA)) using primers as indicated in Supplemental Table 3. Triplicate measures were made for each sample and corresponding GAPDH control. PCR and data collection were done using the BioRad MiniOpticon Real Time PCR machine and Opticon Monitor 3 Software from BioRad (Hercules, CA). Relative transcript copy number was calculated using the delta-delta-C(t) method. The relative expression values of cAMP modulators in cells derived from female Nf1-/- astrocytes were normalized to those from male expression levels (n = 3-5 separate litters/genotype).
Drug Treatments
For cAMP measurements, astrocytes were cultured in serum free DMEM/F12 media (24 hrs), and then treated with the ADCY activator, Forskolin (FSK, 10 μM) and the phosphodiesterase inhibitor, IBMX (1mM), FSK alone, or DMSO control as indicated. For cell number experiments, 75,000 cells per well were plated in 6-well plates. Twenty-four hours after plating, cells were serum starved for 24 hours, and then treated with dideoxyadenosine (DDA, 100 μM) or CXCL12 (0.1μg/ml (Peprotech)) in serum free DMEM/F12 as indicated. Cells cultured in DMEM/F12 + vehicle served as control. Cell number was determined by trypan blue exclusion.
cAMP ELISA
cAMP was measured by competitive immunoassay using a Correlated Enzyme Immunoassay Kit (Enzo Life Sciences, Farmingdale, NY) according to the manufacturer's instructions and as previously described (5).
Statistics
Baseline Data were analyzed using GraphPad Prism version 4.00 (GraphPad Software) or Stata10 (Stata). Specific statistical tests are as indicated in the text and figure legends. All tests were two-sided and a p-value < 0.05 was considered statistically significant.
Results
DNA samples were obtained from 243 individuals with NF1 and genotyped using Affymetrix whole genome human SNP array 6.0. Two hundred thirty-six specimens, 123 from individuals with OPG and 113 from individuals without OPG, passed quality control filtering (Supplemental Table 1). Both the tumor and non-tumor groups had equivalent numbers of males and females (P=0.90, Fisher's exact test). The average genotyping rate in the 236 individuals was 98.43%.
Our analysis focused on 2,761 unique SNPs in 22 key regulators of intracellular cAMP levels (Supplemental Table 2). Calculations for odds ratio (OR) for glioma between genotypes within males and females, the ratio of the male OR to female OR, and corrections for multiple comparisons were calculated as described in Materials and Methods. At the 5% statistical significance level on the FDR-adjusted P-values, we identified three SNPs in adenylate cyclase 8 (ADCY8: rs724365, FDR P=0.014; rs4736688, FDR P=0.014; rs1435446, FDR P=0.043) (Table 1). Linkage disequilibrium (LD) analysis indicated that recombination rarely occurred between rs724365 and rs1435446 in the population (R2=0.92) while the LDs between each of the loci with rs4736688 were medium with both R2 slightly above 0.5. Additionally, SNPs in CXCR7 (rs2568554) and ADCYAP1 (rs16952813) were nearly significant at the 10% significance level (Table 1).
Table 1. SNPs with significant association with optic glioma risk in individuals with NF1.
| SNP | rs724365 | rs4736688 | rs1435446 | rs2568554 | rs16952813 | |
|---|---|---|---|---|---|---|
|
|
||||||
| SNP identifiers | Gene Symbol | ADCY8 | ADCY8 | ADCY8 | CXCR7 | ADCYAP1 |
|
|
||||||
| Chr | 8 | 8 | 8 | 2 | 18 | |
|
|
||||||
| Major Allele frequency (MAF) | Population* | 0.263 | 0.39 | 0.241 | 0.2 | 0.14 |
| NF1 dataset | 0.242 | 0.361 | 0.239 | 0.11 | 0.102 | |
|
|
||||||
| Odds Ratio (OR) | Female | 4.09 | 2.59 | 3.13 | 4.79 | 1.88 |
| Male | 0.31 | 0.19 | 0.31 | 0.31 | 0.1 | |
|
|
||||||
| Male/Female OR | Estimate | 0.0746 | 0.07474 | 0.0982 | 0.0638 | 0.0524 |
| 95% CI | 0.0233∼0.239 | 0.0227∼0.2457 | 0.0313∼0.3076 | 0.0138∼0.296 | 0.0088∼0.3128 | |
| LR P+ | 6.35E-06 | 9.31E-06 | 4.33E-05 | 0.0002 | 0.0002 | |
| Permutated LR P& | 0.018 | 0.03 | 0.138 | 0.446 | 0.472 | |
| FDR LR P& | 0.014 | 0.014 | 0.043 | 0.102 | 0.102 | |
|
|
||||||
| SNP ranking | LR P | 5 | 9 | 40 | 177 | 185 |
| FDR LR P | 4 | 4 | 26 | 99 | 99 | |
MAF in the population was determined in 1093 total samples from the 1000 Genome phase I data, including AFRICA (N=246), EUROPE (N=380), EAST ASIA (N=286), AMERICA (N=181).
LR P was derived from likelihood ratio (LR) test on the SNPxSex interaction term in logistic regression models.
Permutation and FDR adjustment was separately conducted on the SNPs on the cAMP pathway.
SNP ranking based on raw LR P and FDR-adjusted LR P (on all SNPs) compared the performance of each SNP in the cAMP pathway to all SNPs on the array.
Unexpectedly, associations between ADCY8 SNPs and glioma risk were sex-dependent. The minor alleles of each ADCY8 SNP elevated glioma risk in females and decreased risk in males (Table 1). The resulting SNP × Sex interaction effects were highly significant, indicating that sequence variants in ADCY8 are potential sex-specific modifiers of glioma risk in NF1.
As the SNPs had sex-specific effects, we reviewed NF1 OPG case series for evidence of sex disparity. We found 543 OPG cases diagnosed from both, routine surveillance scans of asymptomatic individuals and scans obtained to evaluate symptoms. Six series reported higher frequency of OPG in females, four reported higher frequency in males, and three reported equal incidence. Overall, 297 or 55% of cases occurred in females (Table 2), suggesting a slight female predominance. However, not all cited studies were population-based, and in those series that include scans for symptoms the results may be skewed towards increased rates in females as sex differences in glioma-associated symptoms have been reported (13).
Table 2. Frequency of Optic Pathway Gliomas in Males and Females with NF1.
| Study | Location | Total | M | F |
|---|---|---|---|---|
| Listernick et al. (1995) | Chicago, IL | 17 | 5 | 12 |
| Chateil et al. (2001 | Cedex, FRA | 14 | 7 | 7 |
| Czyzyk et al. (2003) | Warsaw, POL | 51 | 19 | 32 |
| Thiagalingam et al. (2004) | Sydney, AUS | 54 | 27 | 27 |
| Blazo et al. (2004) | TX | 24 | 10 | 14 |
| Pascual-Castroviejo I et al. (2008) | Madrid, SPN | 80 | 22 | 58 |
| Nicolin et al. (2009) | Toronto, CA | 78 | 48 | 30 |
| Segal et al. (2010) | Montreal, CN | 44 | 24 | 20 |
| de Blank et al. (2013) | Philadelphia, PA | 50 | 25 | 25 |
| Incecik et al. (2013) | Turkey | 9 | 5 | 4 |
| Gooden et al. (2014) | Liverpool, UK | 19 | 11 | 8 |
| Kalin-Hajdu et al. (2014) | Montreal, CA | 7 | 3 | 4 |
| Diggs-Andrews et al. (2014) | St Louis, MO | 96 | 40 | 56 |
|
| ||||
| Total % | 543 |
246 (45%) |
297 55%) |
|
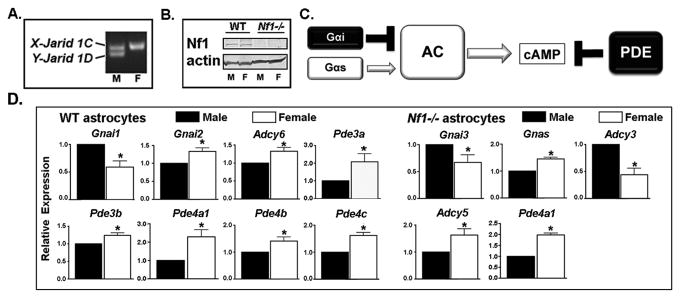
Prior murine studies suggested that spatiotemporal regulation of CXCL12 and intracellular cAMP during development could influence the pattern of tumorigenesis in NF1 (5, 7). Identification of ADCY8 as a sex-specific modifier of glioma risk in NF1 potentially provides important human validation for these studies. To examine whether cAMP exerts a sex-specific effect on tumorigenesis, we established primary cultures of male and female post-natal day one forebrain astrocytes from wildtype (WT) and Nf1flox/flox;GFAPcre (Nf1-/-) mice based on expression of X and Y chromosome encoded paralogs Jarid 1C and Jarid 1D (14) (Figure 1A) and verified equivalent deletion of neurofibromin (Figure 1B).
Figure 1. Sex differences in cAMP regulator expression in WT and Nf1-/- astrocytes.
(A) Neonatal mice were sexed by Jarid 1C/Jarid 1D PCR. “M” indicates male and “F” indicates female derived samples. (B) Western blot analysis confirmed loss of neurofibromin expression in Nf1-/- astrocytes. Actin serves as loading control. “M” indicates male and “F” indicates female derived samples. (C) Schematic of cAMP regulation indicating the different families of regulators whose expression was evaluated. (D) Significant differences in expression were detected for cAMP regulators in WT and Nf1-/- astrocytes as indicated. Shown are the mean and SEM of expression in female cells relative to expression in male cells from 3-5 separate litters per genotype. *p≤0.05, as detailed in the text.
We first looked for sex differences in cAMP regulator expression (Figure 1C) and in intracellular cAMP levels. While there were no sex differences in Adcy8 expression, there were clear effects of sex and neurofibromin loss on the expression of multiple other components of the cAMP pathway (Figure 1D). Intracellular cAMP levels were consistently lower in male compared to female Nf1-/- astrocytes (Male: 6.97 +/- 1.5, Female: 10 +/- 0.89 pmol/mg protein, (P=0.03, t test, n=3 independent litters)), indicating that cell intrinsic sexual dimorphism in cAMP regulation exists in Nf1-/- astrocytes.
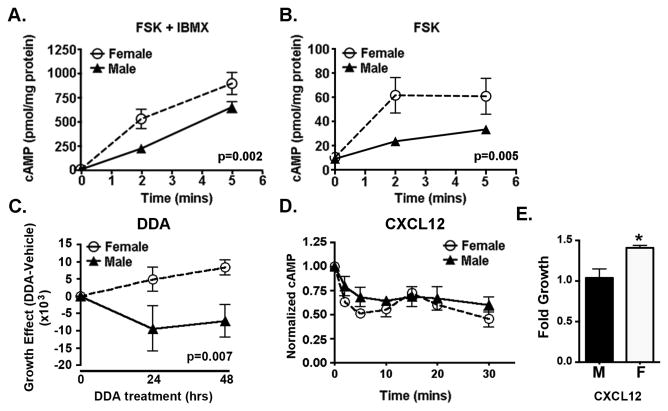
Next we looked for functional differences in cAMP synthesis and degradation. We assessed differences in synthesis (ADCY activity) by treating male and female with the pan-ADCY activator, forskolin (FSK), in the presence of complete inhibition of cAMP degradation by the pan-phosphodiesterase (PDE) inhibitor, IBMX (Figure 2A). Under these conditions, differences in cAMP levels reflect differences in synthetic capacity and not differences in degradation. Cyclic AMP levels rose to 650 and 900 pmol/mg protein in male and female Nf1-/- astrocytes, respectively, indicating greater cAMP synthetic capacity in female Nf1-/- astrocytes.
Figure 2. Sexual dimorphism in cAMP regulation in Nf1-/- astrocytes.
Cyclic AMP levels were measured by ELISA in male and female Nf1-/- astrocytes treated with (A) the ADCY activator, FSK (10 μM) and the pan-PDE inhibitor, IBMX (1 mM), or (B) FSK (10 μM) alone for the times indicated. Shown are the means and SEM of cAMP levels from four independent experiments. P values are as indicated and were determined by two way ANOVA. (C) Cell number was measured by Trypan Blue exclusion in male and female Nf1-/- astrocytes treated with vehicle or dideoxyadenosine (DDA, 100 μM). Shown are the means and SEM of the differences between DDA and vehicle treated male and female cultures derived from three independent experiments. P value is as indicated and was determined by two way ANOVA. (D) Cyclic AMP levels were measured by ELISA in male and female Nf1-/- astrocytes treated with CXCL12 (0.1μg/ml) for the times indicated. Shown are the means and SEM of three independent experiments measuring the responses of male and female Nf1-/- astrocytes normalized to their basal values. (E) Cell number was measured by Trypan Blue exclusion in male and female Nf1-/- astrocytes treated with vehicle or CXCL12 (0.1μg/ml, 48 hrs). Shown are the means and SEM of the ratio of cell numbers in CXCL12-treated/vehicle-treated cultures derived from three independent experiments. *=p<0.05 as determined by two tailed t-test.
To detect sex differences in degradative (PDE) capacity, we treated with FSK alone (Figure 2B). Under these conditions cAMP levels are determined by total ADCY capacity and counter-regulatory increases in PDE activity. Cyclic AMP levels reached a plateau at approximately six-fold and three-fold above baseline in female and male astrocytes, respectively, indicating male astrocytes have greater capacity to upregulate PDE activity.
The SNP array data suggested that variation in ADCY activity has a sexually dimorphic effect on glioma risk. Previously, we showed that ADCY inhibition with dideoxyadenosine (DDA) promotes astrocyte growth (5). Here, we looked for sex differences in DDA effects. We found that, paralleling the human data, inhibition of ADCY activity promoted growth of female astrocytes but suppressed the growth of male astrocytes (Figure 2C).
The effect of DDA on Nf1-/- astrocytes was previously shown to phenocopy the growth-promoting effects of CXCL12 (5). Here, CXCL12 treatment suppressed cAMP levels in both male and female Nf1-/- astrocytes (Figure 2D), but only the female astrocytes exhibited a growth response (Figure 2E). Together these observations identify sex differences in the growth promoting effects of ADCY inhibition and cAMP suppression.
Discussion
Sex is a significant determinant of many human diseases (15) and has been shown to interact with genetic modifier loci to determine risk in a mouse model of high–grade glioma associated with combined loss of Nf1 and p53 (16, 17). This however, is the first study to confirm a role for cAMP regulation in human gliomagenesis and to report that cAMP's effect is modified by sex.
Two lines of evidence suggest that sexually dimorphic growth responses to ADCY activity are relevant. First, inhibition of ADCY by DDA had opposing effects on the growth of female and male Nf1-/- astrocytes. Second, despite comparable suppression of cAMP levels, CXCL12 promoted the growth of female but not male astrocytes. While not demonstrated to specifically involve ADCY8, the close parallel between these results and the effect of polymorphisms in ADCY8 on glioma risk in males and females with NF1 suggest that these mechanisms are relevant to human disease and that this Nf1 GEM will be an important model for studying sexual dimorphism in the cAMP pathway.
Despite dramatically opposing effects of ADCY8 SNPs on OPG risk in males and females, there is little sex disparity in OPG incidence. Thus, we hypothesize that OPGs that arise through variation in ADCY may represent a subset of disease that is more common in females. Sex disparities limited to molecular subsets of brain tumors are established in other childhood brain tumors like medulloblastoma (18).
Finally, these observations provide a rationale for clinical evaluation of personalized glioma surveillance in NF1 using SNP-based tools to identify those at the greatest risk.
Supplementary Material
Acknowledgments
We thank Clint C. Mason for sample selections/randomizations. This work was supported by grants from The Children's Discovery Institute of Washington University (JBR&DHG), the NCI RO1-CA136573 (JBR&DHG), UO1-CA141549 (DHG), the NIH UL1RR025764 (DAS), The DOD W81XWH-11-1-0250 (DAS), Children's Tumor Foundation Young Investigator Award (TS), IRP ZIA BC 010539 of the NIH, NCI (KMR), The Hospital for Sick Children Research Institute (PP), the University of Utah Clinical Genetics Research Program (DAS). DRS was supported in part by the intramural program of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute. The Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine helped with genomic analysis and is partially supported by NCI Cancer Center Support Grant #P30 CA91842 to the Siteman Cancer Center and by ICTS/CTSA Grant# UL1TR000448 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Government.
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Rubin JB, Gutmann DH. Neurofibromatosis type I - a model for nervous system tumour formation? Nature Cancer Reviews. 2005;5:557–64. doi: 10.1038/nrc1653. [DOI] [PubMed] [Google Scholar]
- 2.Ferner RE, Huson SM, Thomas N, Moss C, Willshaw H, Evans DG, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. Journal of medical genetics. 2007;44:81–8. doi: 10.1136/jmg.2006.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Annals of neurology. 2007;61:189–98. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolin G, Parkin P, Mabbott D, Hargrave D, Bartels U, Tabori U, et al. Natural history and outcome of optic pathway gliomas in children. Pediatr Blood Cancer. 2009;53:1231–7. doi: 10.1002/pbc.22198. [DOI] [PubMed] [Google Scholar]
- 5.Warrington NM, Woerner BM, Daginakatte GC, Dasgupta B, Perry A, Gutmann DH, et al. Spatiotemporal differences in CXCL12 expression and cyclic AMP underlie the unique pattern of optic glioma growth in neurofibromatosis type 1. Cancer Res. 2007;67:8588–95. doi: 10.1158/0008-5472.CAN-06-2220. [DOI] [PubMed] [Google Scholar]
- 6.Bajenaru ML, Hernandez MR, Perry A, Zhu Y, Parada LF, Garbow JR, et al. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–7. [PubMed] [Google Scholar]
- 7.Warrington NM, Gianino SM, Jackson E, Goldhoff P, Garbow JR, Piwnica-Worms D, et al. Cyclic AMP supppression is sufficient to induce gliomagenesis in a mouse model of Neurofibromatosis-1. Cancer Research. 2010;70:5717–27. doi: 10.1158/0008-5472.CAN-09-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji J, Shimony J, Gao F, McKinstry RC, Gutmann DH. Optic nerve tortuosity in children with neurofibromatosis type 1. Pediatric radiology. 2013;43:1336–43. doi: 10.1007/s00247-013-2694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X, Levine D, Shen J, Gogarten SM, Laurie C, Weir BS. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics. 2012;28:3326–8. doi: 10.1093/bioinformatics/bts606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gogarten SM, Bhangale T, Conomos MP, Laurie CA, McHugh CP, Painter I, et al. GWASTools: an R/Bioconductor package for quality control and analysis of genome-wide association studies. Bioinformatics. 2012;28:3329–31. doi: 10.1093/bioinformatics/bts610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amigo J, Salas A, Phillips C. ENGINES: exploring single nucleotide variation in entire human genomes. BMC bioinformatics. 2011;12:105. doi: 10.1186/1471-2105-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diggs-Andrews KA, Brown JA, Gianino SM, Rubin JB, Wozniak DF, Gutmann DH. Sex Is a major determinant of neuronal dysfunction in neurofibromatosis type 1. Annals of neurology. 2014;75:309–16. doi: 10.1002/ana.24093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PloS one. 2008;3:e2553. doi: 10.1371/journal.pone.0002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9:911–22. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amlin-Van Schaick JC, Kim S, DiFabio C, Lee MH, Broman KW, Reilly KM. Arlm1 is a male-specific modifier of astrocytoma resistance on mouse Chr 12. Neuro Oncol. 2012;14:160–74. doi: 10.1093/neuonc/nor206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walrath JC, Fox K, Truffer E, Gregory Alvord W, Quinones OA, Reilly KM. Chr 19(A/J) modifies tumor resistance in a sex- and parent-of-origin-specific manner. Mamm Genome. 2009;20:214–23. doi: 10.1007/s00335-009-9179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–72. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.