Abstract
The objective of this study was to induce anterior cruciate ligament (ACL) and meniscal damage, via a single tibiofemoral compressive impact, in order to document articular cartilage and meniscal changes post impact. Tibiofemoral joints of Flemish Giant rabbits were subjected to a single blunt impact that ruptured the ACL and produced acute meniscal damage. Animals were allowed unrestricted cage activity for 12 weeks before euthanasia. India ink analysis of the articular cartilage revealed higher degrees of surface damage on the impacted tibias (p=0.018) and femurs (p<0.0001) compared to controls. Chronic meniscal damage was most prevalent in the medial central and medial posterior regions. Mechanical tests revealed an overall 19.4% increase in tibial plateau cartilage thickness (p=0.026), 34.8% increase in tibial plateau permeability (p=0.054), 40.8% increase in femoral condyle permeability (p=0.029), and 20.1% decrease in femoral condyle matrix modulus (p=0.012) in impacted joints compared to controls. Both the instantaneous and equilibrium moduli of the lateral and medial menisci were decreased compared to control (p<0.02). Histological analyses revealed significantly increased presence of fissures in the medial femur (p = 0.036). In both the meniscus and cartilage there was a significant decrease in GAG coverage for the impacted limbs. Based on these results it is clear that an unattended combined meniscal and ACL injury results in significant changes to the soft tissues in this experimental joint 12 weeks post injury. Such changes are consistent with a clinical description of mid to late stage PTOA of the knee.
Keywords: meniscus, impact, osteoarthritis, knee
1. Introduction
Osteoarthritis (OA) has been considered by the World Health Organization to be among the top 10 conditions representing a global disease burden (Lopez et al., 2006). While OA is often a result of joint degradation over time, early onset can be triggered by a number of factors (Felson et al., 2000; Lawrence et al., 2008) including joint trauma (Lohmander et al., 2007). With a high occurrence of knee injury (Hootman et al., 2002) and knee OA (Zhang et al., 2008), post-traumatic osteoarthritis (PTOA) of the knee has been of particular interest to researchers and clinicians.
It has been reported that as high as 78% of knee injuries involving ACL tears are due to “non-contact” type of injuries (Noyes et al., 1983). High compressive tibiofemoral loading, often experienced during jump landings (Boden et al., 2000; Meyer and Haut, 2005; Speer et al., 1995; Yeow et al., 2011) is one potential non-contact mode of ACL rupture. These compressive loads have been shown to cause acute damage to articular cartilage, bone, and the menisci both clinically and in a laboratory setting (Killian et al., 2010a; Levine et al., 2012; Meyer and Haut, 2005). Current models of knee PTOA have primarily used in vivo animal models with surgical transection of the ACL (ACLT) to study the mechanisms of damage to these joints (Batiste et al., 2004; Hellio Le Graverand et al., 2001; M Yoshioka et al., 1996). However, ACLT models do not account for occult and acute damages to the surrounding structures which are often present in ACL injuries (Isaac et al., 2010a; Rosen et al., 1991).
Menisci are often injured in conjunction with the ACL (Felson and Neogi, 2004; McDaniel and Dameron, 1980). The menisci act to distribute joint load and stabilize the knee. Their unique wedge-like shape and composition allow them to bear up to 75% of loads acting across the knee (Shrive et al., 1978). Meniscal damage can alter the contact loads acting on the adjacent articular cartilage, which may lead to further cartilage damage and the development of osteophytes (Crema et al., 2010). Additionally, over 80% of ACL injury cases note osteochondral lesions in the posterior-lateral and posterior-medial aspects of the tibia and/or anterior-lateral aspect of the femoral condyle (Atkinson et al., 2008). These lesions are associated with damage to overlying articular cartilage and chondrocytes, which may help precipitate degenerative changes to the joint (Frobell et al., 2009).
To recapitulate what is observed in non-contact ACL injury scenarios, a closed joint in vivo lapine tibiofemoral impact model has been utilized in the current study. Previous work with this tibiofemoral impact (ACLF) model (Isaac et al., 2010b; Killian et al., 2010a) has shown that, in addition to ACL rupture, the impact produces acute meniscal, cartilaginous, and bone damages. The objective of the current study was to assess the gross, mechanical, and histological changes to both articular cartilage and menisci 12 weeks after impact. It was hypothesized that any untreated acute damage resulting from the impact would exacerbate in the joint and lead to chronic changes in material and biochemical properties, consistent with the clinical description of mid-to late-stage PTOA.
2. Materials and Methods
2.1 ACLF Model
This study was approved by the All-University Committees on Animal Use and Care. Six skeletally mature Flemish Giant rabbits (5.7 ± 0.2 kg) were anesthetized (2% isoflurane and oxygen) and subjected to an impact of the right tibiofemoral joint. The animals were housed in individual cages (60 × 60 × 14 in), and were euthanized twelve weeks post injury.
Impact was delivered similar to a previous study (Isaac et al., 2008). In brief, the rabbits were placed in a supine position with the right tibiofemoral joint at 90° flexion. A 1.75 kg mass with a pre-crushed aluminum honeycomb interface (Hexweb, 3.76 MPa crush strength, Stamford, CT) head was dropped from a height of 70 cm, striking the distal femur. Magnetic resonance imaging (MRI) was performed to document any acute damage. Left, non-impacted, limbs served as paired controls. Gait abnormalities were assessed on a qualitative basis by a licensed veterinary technician (JA) during the duration of the study. The rabbits favored the non-impacted limb for 3–5 days, but showed no signs of gait abnormality for the remaining duration of the study. The rabbits were monitored and buprenorphine (0.3mL/kg BW) was administered for pain every 8 hours for 72 hours following impact.
2.2 Tissue Harvesting and Morphological Assessment
Tissue was harvested immediately after euthanasia and refrigerated until mechanical tests were performed (within 18 hours). The surfaces of the plateaus and condyles were stained with India ink to highlight surface fissures and other irregularities. Blinded gross morphological assessments from three graders were made with the following grading scale (Makoto Yoshioka et al., 1996): 1 = intact cartilage with the surface appearing normal, 2 = a few surface lesions that retain ink, 3 = moderate fibrillation retaining intense black patches of ink, and 4 = full thickness erosion exposing underlying bone.
Any visible meniscal damage was morphologically scored similar to previous grading systems (Pauli et al., 2011). Scoring was performed by four blinded individuals and averaged. Blinded graders were asked to assign a score from 0–4 to three different regions (anterior, central, and posterior) (Killian et al., 2010b) for both medial and lateral menisci, with 0 = normal, 1= surface damage, 2 = un-displaced tears, 3 = displaced tears, and 4 = tissue maceration. Grading, as well as mechanical and histomorphological meniscal assessments, were performed regionally to account for previously identified regional variations.(Killian et al., 2010b; Sweigart et al., 2004)
2.2 Mechanical Analysis
Indentation relaxation testing was performed on both tissues in a room temperature 0.9% phosphate buffered saline bath. The articular cartilage was tested at four sites to account for both covered and uncovered tibial cartilage and weight bearing and non-weight bearing femoral cartilage (Figure 1). The thickness of each indentation site was estimated by inserting a needle at a location adjacent to each indentation site and observing when the force changed due to contact with calcified cartilage. A 1.59 mm diameter, spherical nonporous steel probe was then pressed into the cartilage to a depth of 30% of its estimated thickness for 180 seconds. After a 1200s rest time the probe was replaced with the needle and the thickness (t) was determined at the indentation site. The mechanical tests were simulated with a previously described fibril-reinforced biphasic cartilage model (Golenberg et al., 2009) and implemented in a finite element package (Abaqus v.6.3, Hibbet Karlsson & Sorensen, Inc., Pawtuket, RI, USA). The voids ratio was assumed linear and depth-dependent, increasing from 70% at the cartilage-bone interface to 85% at the superficial zone (Golenberg et al., 2009). The matrix modulus (Em), fiber modulus (Ef), and tissue permeability (k0) of cartilage were determined from this model with a custom-written, Gauss-Newton constrained nonlinear least square minimization procedure.
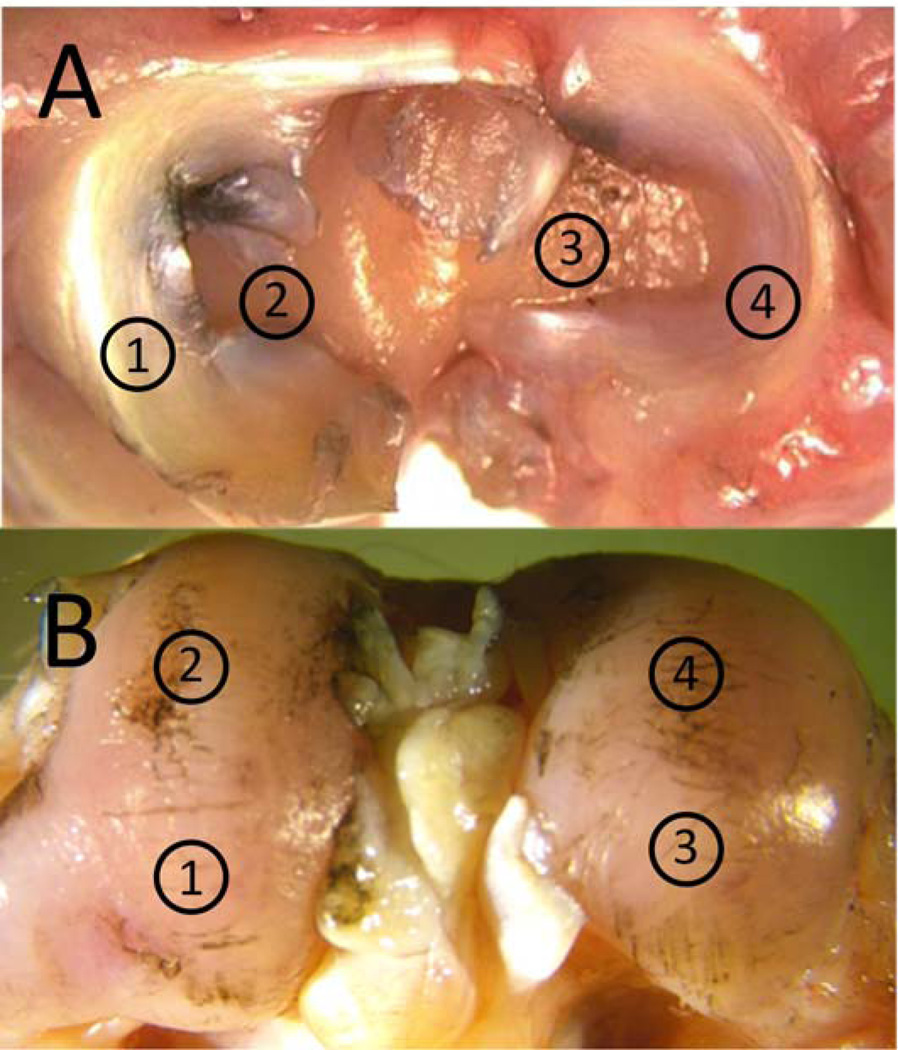
Figure 1.
Sites for indentation relaxation A) tibial sites 1 and 4 were covered by meniscus and sites 2 and 3 were uncovered. B) femoral sites 1 and 3 were sites that contacted the tibia during 90 degrees of flexion (impact orientation) and sites 2 and 4 were sites that contacted the tibia during normal gait.
A cylindrical steel 1.59 mm diameter indenter tip was used for load relaxation testing of the menisci (Fischenich et al., 2014; Li et al., 2006; Sweigart et al., 2004). An indention depth of 0.25 mm was applied, and held for 900 seconds to reach equilibrium. Similar to previous studies (Li et al., 2006) Hertzian contact was assumed between an elastic half space (meniscus), and a rigid sphere (indenter). A Poisson’s ratio of 0.01 was assigned to the menisci (Sweigart et al., 2004). The elastic modulus and Poisson’s ratio of the indenter were 210 GPa and 0.3 respectively.
2.3 Histological Analysis
All tissues were fixed in 10% formalin. The bones were then decalcified using 20% formic acid. Tibial plateaus were sectioned coronally at the midpoint in the anterior-posterior frame of reference. Each femoral condyle was sectioned sagittally at the midpoint of the condyle. Sectioned plateaus and condyles were embedded in paraffin and sectioned at 6 µm. The menisci were embedded in optimum cutting temperature medium (OCT, Pelco; Redding, CA), flash frozen using liquid nitrogen, and cut into 6µm slices. All sections were stained using Hematoxlyin, Safranin-O, and Fast Green.
Bone/cartilage sections were imaged and the articular cartilage was histologically graded in three categories, using a modified Mankin scale (Table 1). Meniscal slides were imaged and GAG staining intensity was evaluated similar to previous studies (Fischenich et al., 2014): 0 = no staining, slight staining = 1, moderate staining =2, and strong staining =3. Stain intensity evaluations were performed by 4 individuals and the data from each specimen and location were averaged across individuals. Meniscal GAG coverage was analyzed quantitatively using Image J (NIH, Bethesda, MD) with FIJI package. First, the total area of tissue was determined using the analyze particle tool in Image J. Color Deconvolution was then applied to isolate the red associated with GAG from the blue/green of the cytoplasm. Once the blue/green was removed the area of GAG coverage was determined as a percent of the total area of the tissue of interest.
Table 1.
Modified Mankin grading scale
| Measure | Score 0 |
1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
|
GAG Staining |
Uniform | Loss of staining in superficial zone <50% of length of condyle or plateau |
Loss of staining in superficial zone >50% of length of condyle or plateau |
Loss of staining in upper 2/3 < 50% of length of condyle or plateau |
Loss of staining in upper > 50% of length of condyle or plateau |
Loss of staining in full depth of cartilage <50% of length of condyle or plateau |
Loss of staining in full depth of cartilage >50% of length of condyle or plateau |
| Fissures | Absent | Surface fibrillation <50% of length of condyle or plateau |
Surface fibrillation >50% of length of condyle or plateau |
1–2 midzone fissures |
3–5 midzone fissures |
Full depth fissures or 5 + midzone |
Large segments of cartilage eroded with full depth fissures |
|
Tidemark Integrity |
Normal | Multiple | Focal loss | Diffuse loss | Total loss | NA | NA |
2.4 Statistical Analysis
Two factor (site, group) repeated measures analysis of variance (ANOVA) with Tukey’s post-hoc tests were conducted on mechanical and thickness data from the articular cartilage test sites. The morphological grades of the articular cartilage were subjected to a two factor (site, group) ANOVA on ranks to compare overall values. Histology results of the articular cartilage and all meniscal morphology and GAG intensity were analyzed using a Wilcoxon signed-rank. Meniscal mechanics and GAG coverage data were subjected to a one way repeated measures ANOVA with post hoc Tukey tests to compare each scoring category between the impacted and contralateral control limb. Significance was taken to be p < 0.05 for all metrics.
3. Results
3.1 Morphology
Acutely, all impacted joints experienced ACL rupture and 8 of the 12 menisci experienced damage, as confirmed with MRI. At twelve weeks post impact all impacted joints demonstrated some level of osteoarthritic change including osteophyte formation, articular cartilage fibrillation, and synovial inflammation.
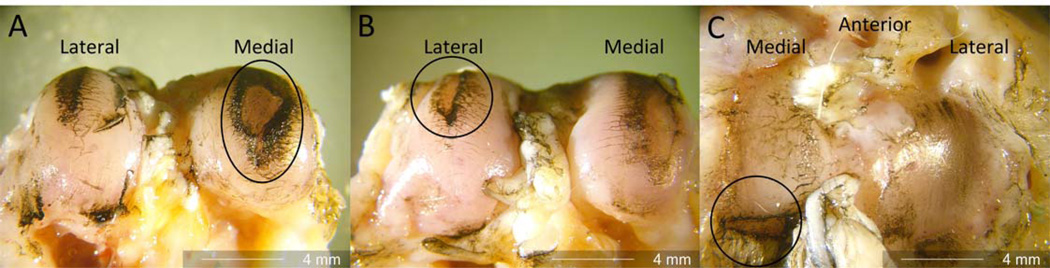
Gross morphological analyses at 12 weeks post impact indicated that the impacted tibial plateaus had a higher degree of articular surface damage than the controls (p=0.018). The femurs also showed a higher degree of surface damage in the impacted limbs (p<0.001) (Table 2). Figure 2 shows representative images of cartilage damage including full thickness erosion in 4 of 6 medial femoral condyles (2a), 1 of 6 lateral femoral condyles (2b), and 5 of 6 medial tibial plateaus (2c).
Table 2.
Average articular cartilage morphology and mechanics of tibia plateau and femoral condyles (mean with standard deviation)
| Femur | Tibia | |||
|---|---|---|---|---|
| Control | ACLF | Control | ACLF | |
| Morphology score | 1.43 (0.444) | 2.32 (1.07)* | 1.43 (.663) | 1.96 (0.989)* |
| t (mm) | 0.47 (0.16) | 0.53 (0.14) | 0.63 (0.27) | 0.75 (0.31)* |
| Em (MPa) | 1.21 (0.38) | 0.97 (0.51)* | 1.09 (0.81) | 0.87 (0.44) |
| Ef (MPa) | 9.35 (7.25) | 13.5 (16.1) | 8.30 (10.5) | 11.3 (16.2) |
| k0 (10−14 m4/Ns) | 9.68 (6.89) | 17.20 (14.36)* | 19.9 (18.6) | 26.7 (16.4)* |
denotes significant difference of p<0.05 between control and ACLF.
Figure 2.
Full thickness cartilage erosion was noted on the medial femoral condyles (a), on the lateral femoral condyle (b), and full thickness erosion was noted in the medial-posterior aspect of the tibia (c).
Twelve weeks post injury, statistically significant increases (p<0.05) in damage between impacted and contralateral limbs were observed for all medial meniscal regions (Figure 3). All lateral meniscal regions had an increase in damage, although not significant (p<0.1). Damage was most severe in the posterior meniscal region of both hemijoints and the central region of the medial meniscus. Tearing was seen chronically in all 6 medial menisci and 4 of the lateral menisci (Table 3). The medial meniscus sustained more extensive damage than the lateral (a score ≥ 2.5 in 11/18 medial regions and only 6/18 lateral regions).
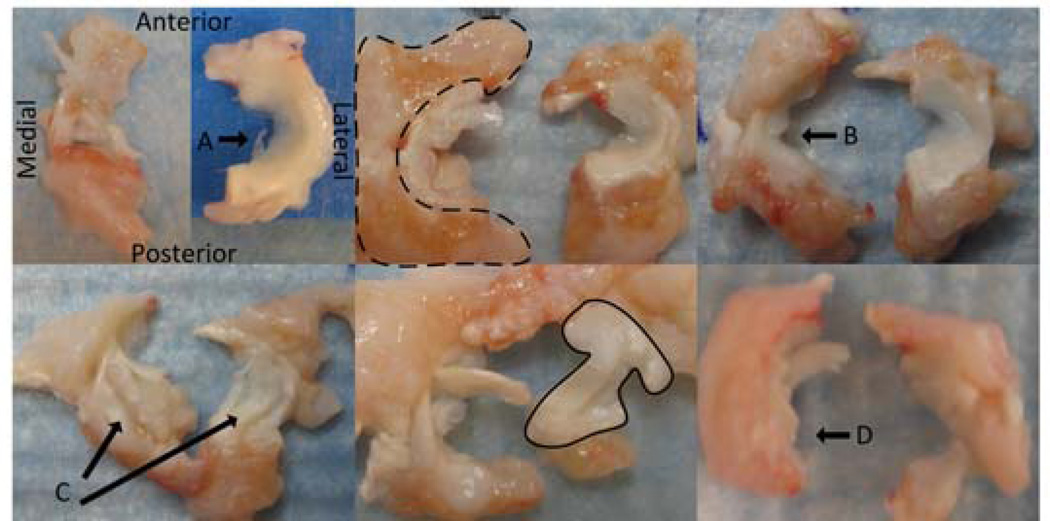
Figure 3.
ACLF Menisci 12 weeks post-impact (animals 1–6 left to right and top to bottom, all specimens are oriented identical to the first image). Dashed line outlines example synovium, Solid line outlines example of meniscal tissue, displaced parrot beak tear (a), tissue maceration to central region (b), complex tearing of both the medial and lateral menisci (c), and tissue maceration of the posterior horn (d).
Table 3.
Meniscal Morphological Scores at 12 Weeks (mean with standard deviation)
| Rabbit | Lateral | Medial | ||||
|---|---|---|---|---|---|---|
| A+ | C+ | P+ | A* | C* | P* | |
| 1 | 0 (0) | 0 (0) | 2.3 (0.8) | 1.3 (0.4) | 3.0 (0) | 3.5 (0.5) |
| 2 | 0.3 (0.4) | 0.3 (0.4) | 0.3 (0.4) | 2.3 (0.4) | 3.0 (0) | 3.5 (0.5) |
| 3 | 0 (0) | 0.3 (0.4) | 0 (0) | 0.3 (0.4) | 3.5 (0.5) | 0.3 (0.4) |
| 4 | 2.0 (0) | 2.8 (0.4) | 3.0 (0) | 1.5 (0.5) | 3.0 (0) | 3.3 (0.4) |
| 5 | 0.5 (0.5) | 3.0 (0) | 3.0 (0.7) | 1.0 (1.2) | 3.3 (0.4) | 3.0 (0.7) |
| 6 | 1.8 (0.4) | 3.8 (0.4) | 3.8 (0.4) | 1.0 (0) | 2.8 (0.4) | 3.5 (0.5) |
denotes p<0.05 and
denotes p<0.1 between control and ACLF.
All control menisci where normal.
3.2 Mechanical Analysis
Extensive tissue damage and maceration limited mechanical testing at numerous sites. Full thickness erosion was found in 4 of 6 medial femoral condyles, so only n=2 mechanical tests could be performed at this site. Despite that, there was an overall 40.8% increase in permeability (p=0.029) and a 20.1% decrease in Em (p=0.012) of femoral articular cartilage when comparing injured to control limbs. Likewise, the tibial plateau data showed an overall increased cartilage thickness of 19.4% (p=0.026) and an increased tissue permeability of 34.8% (p=0.054) (Table 2).
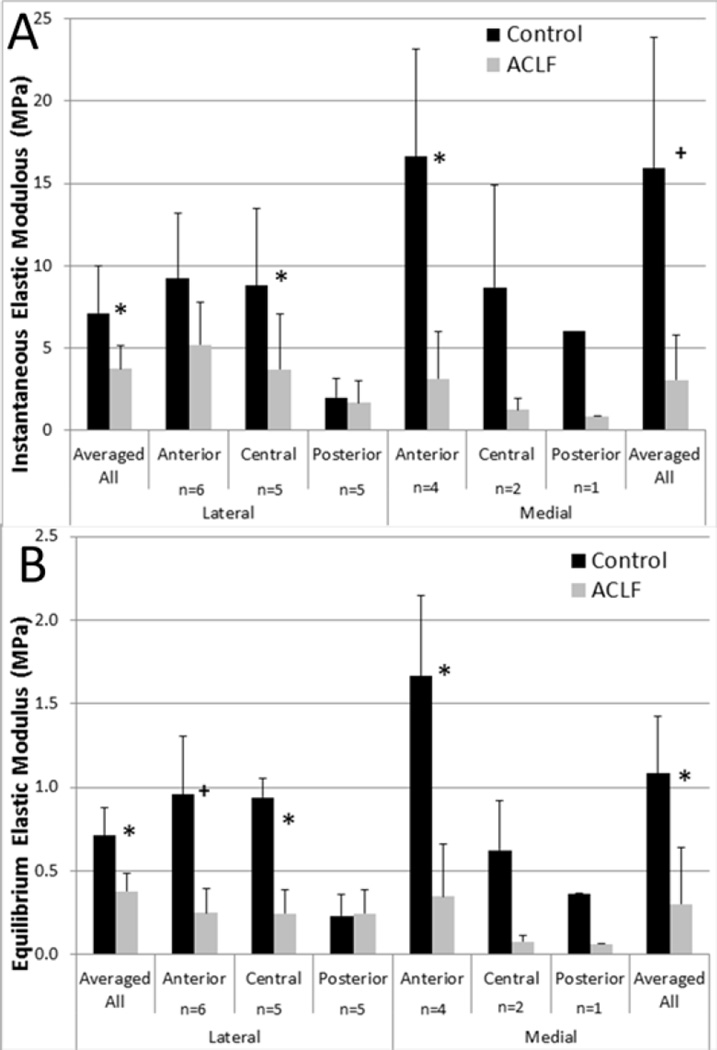
The lateral anterior region of the menisci was the only full sample set of n=6. The remaining regions were as follows: lateral central and lateral posterior n=5, medial anterior n=4, medial central n=2, and medial posterior n=1. Because statistical analysis could not be performed on the medial central and medial posterior meniscal regions due to small sample sizes, the regional data were averaged for a given hemijoint and specimen (Figure 4a and 4b). Significant decreases (p<0.05) in the instantaneous and equilibrium elastic moduli compared to control limbs were documented in the lateral menisci. The medial meniscus indicated the largest average percentage decrease from control to impacted limb in both the instantaneous and equilibrium elastic modulus, but only the data collected at equilibrium showed a significant decrease (p<0.05). Gross damage to the medial central and posterior regions reduced the number of mechanically testable samples. However, of the remaining four regions (lateral anterior, central, and posterior and medial anterior) significant decreases (p<0.05) were documented in the lateral central, and medial anterior both instantaneously and at equilibrium (Figure 4a and 4b).
Figure 4.
A) Instantaneous elastic modulus of the B) Equilibrium elastic modulus by region of the menisci (mean with standard deviation) * denotes p<0.05 and + denotes p<0.1 between control and ACLF.
3.3 Histological Analysis
Using a modified Mankin grading system the medial femur was found to have significant changes (p<0.05) in GAG content and articular cartilage fissures as compared to the control limb. Although not significant the tidemark integrity decreased (p<0.1) in both the medial femur and tibia (Table 4). No significant differences were found in the lateral hemijoint. Damage was primarily characterized by a loss of GAG in the femur and increased articular cartilage fissuring.
Table 4.
Average Mankin score for the measures of interest (mean and standard deviation)
| Femur | Tibia | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | ACLF | Control | ACLF | |||||
| Lateral | Medial | Lateral | Medial | Lateral | Medial | Lateral | Medial | |
|
GAG Staining |
3.71 (1.76) | 1.67 (0.58) | 4.46 (1.30) | 4.88 (1.30)* | 1.71 (0.43) | 1.96 (0.71) | 2.63 (1.35) | 3.58 (1.49) |
| Fissures | 2.38 (0.56) | 1.58 (1.44) | 3.04 (2.08) | 4.79 (1.11)* | 1.54 (2.02) | 2.13 (1.49) | 1.25 (1.12) | 2.71 (2.05) |
|
Tidemark Integrity |
1.67 (1.51) | 0.63 (0.80) | 2.46 (0.99) | 2.79 (1.48)+ | 1.04 (0.91) | 0.71 (0.70) | 1.29 (1.03) | 1.88 (1.47)+ |
denotes p<0.05 and
denotes p<0.1 between control and ACLF.
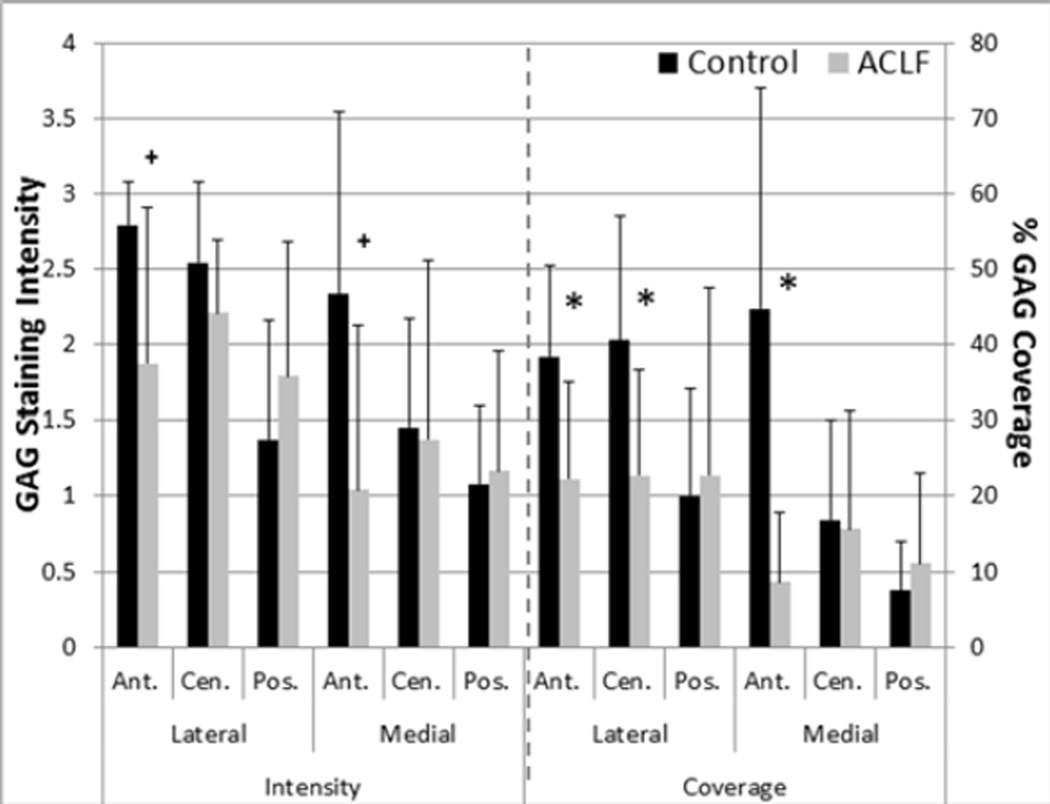
Average meniscal GAG coverage and stain intensity were well correlated (R2=0.85, n=6 in all regions). Qualitatively assessing stain intensity showed large percentage decreases in average grades of intensity in anterior meniscal regions, but no significant differences (Figure 5). Quantitative GAG coverage assessments, however, did significantly decrease in the anterior regions of both the lateral (p<0.02) and medial (p<0.04) meniscus of the impacted limb, as well as the lateral central region (p<0.04). The greatest decreases were seen in the anterior regions with an average 39.3% decrease in the lateral anterior region and a 74.3% decrease in the medial anterior meniscal regions between control and impacted limbs.
Figure 5.
GAG intensity grading and percent area of GAG coverage in the menisci (mean with standard deviation) * denotes p<0.05 and + denotes p<0.1 between control and ACLF.
4. Discussion
Using an in vivo closed joint traumatic impact model we were able to rupture the ACL as well as create acute articular cartilage and meniscal damage with a single tibiofemoral compressive impact. Damage was not assessed at time zero beyond MRI, as a previous impact study showed no acute change in tissue mechanical properties following impact (Ewers et al., 2002). More damage was seen at dissection in the medial hemijoint than the lateral, which is consistent with clinical studies on OA patients (Bellabarba et al., 1997; McDaniel and Dameron, 1980; Smith and Barrett, 2001). To the authors knowledge this is the first study to look at whole body meniscal mechanical properties in both healthy and impacted tissue.
The irregular changes in GAG coverage of the impacted menisci are of particular interest. Coverage was shown to decrease in anterior regions despite these being locations of less damage. Immobilization has been shown to decrease GAG content in the meniscus (Djurasovic et al., 1998), and dynamic overloading under specific conditions has been shown to up-regulate aggrecan production (Zielinska et al., 2009). In the current study it is unknown how the loading pattern changed as a result of the initial acute damage in the joint. However, in the ACL deficient knee it has been shown that there is a significant anterior translation of the tibia. If this is the case in our model, it may suggest there was a decrease in load experienced in the anterior region of the meniscus post trauma. The unloading of the anterior region might help explain GAG loss in that region, while less of a change was observed in the posterior regions despite heavy damage. Furthermore, a previous canine ACL transection study (Adams et al., 1983) documented initial decreases in meniscal GAG at 1 week post injury with levels returning to normal or above normal after 15–18 months. This initial decrease in GAG could be due to an inflammatory response, as numerous studies (Lemke et al., 2010; McNulty et al., 2010) have demonstrated GAG degradation under inflammatory conditions. We were limited in our assessment of GAG changes, as they may be temporal and this study is presenting results from a single time point of 12 weeks.
The main tear orientation of the acutely damaged menisci in this model was longitudinal vertical tears, which have been reported as the most common in clinical injury scenarios (Lewandrowski et al., 1997; Tandogan et al., 2004). Meniscal damage in this model was mostly seen in the posterior region, which is also in agreement with clinical findings (Greis et al., 2002; Smith and Barrett, 2001). When compared to previous ACLT models (Adams et al., 1983; Brophy et al., 2012; Hellio Le Graverand et al., 2001; Smith and Barrett, 2001; M Yoshioka et al., 1996) the chronic gross meniscal damage observed in this ACLF model showed a higher incidence of tearing and more extensive tear patterns. ACLT models have documented chronic damage primarily as incomplete tears and the occasional bucket handle tear (Adams et al., 1983; Hellio Le Graverand et al., 2001; Smith and Barrett, 2001). The ACLF model produced full thickness, displaced, and complex tearing of meniscal tissue chronically. The difference between models suggests that when acute damage is present the pattern and progression of meniscal damage is affected.
In addition to gross meniscal damage, changes were also observed in the underlying cartilage. Mechanical data from indentation-relaxation tests revealed increases in cartilage permeability and decreases in matrix stiffness in the impacted versus contralateral control joints. The changes in permeability may be a result of significant increases in surface damage in both the tibia and femur. This would be consistent with a previous study by Setton et al. (Setton et al., 1993) suggesting that surface fibrillation increases tissue permeability, potentially increasing loads on the solid matrix. Increases in solid matrix stresses may also help explain the decreases in femur Em and the trend for a decrease in Em on the tibial plateau observed in the current study.
There were also increases in thickness of the tibial plateau cartilage. This was likely a result of tissue swelling due to damage to the network of collagen fibers, which has previously been observed in canine models (Adams and Brandt, 1991; Brandt, et al., 1991). However, these thickness changes were not associated with corresponding statistically significant changes in the matrix fiber modulus, as might be expected. One shortcoming of the current study was that the fibril-reinforced biphasic model did not account for potential changes in the water content (voids ratio) and the orientation of fibers that may be present in diseased cartilage. Future studies may need to adjust for these changes when modeling potentially damaged cartilage. Another shortcoming of the current study was the initial observation of altered gait during the first 3–5 days following impact, potentially raising concerns over using the contralateral limb as the control. Additionally, due to the qualitative nature of assessing gait, there may have been additional subtle changes in joint loading at later time points that were not observable to the veterinary technician. Future studies may want to quantitatively evaluate gait over time, perhaps using a force plate treadmill, in order to better assess any abnormal loading.
Previous studies (Donohue et al., 1983; Isaac et al., 2010b; Milentijevic et al., 2005) have investigated articular cartilage changes following impact using biochemical analysis. Substantial loss of GAG through the depth of the impacted cartilage was observed as early as 3 weeks post injury by Milentijevic et al. (Milentijevic et al., 2005). In a longer study investigating tibial plateau changes, Issac et al. (Isaac et al., 2010b) reported significant increases in fissuring and loss of GAG at 6 and 12 months. While not all of these models included ACL failure, outcomes from this impact-induced ACL failure model are in agreement with these previous studies (Saito et al., 2012; Makoto Yoshioka et al., 1996). Significant loss of GAG and an increase of surface fissuring were documented in the medial femur. Greater GAG depletion was also observed in the medial compartment where fissuring and erosion occurred more frequently, with greater GAG depletion documented in the femoral condyle versus the tibial plateau.
Significant increases in meniscal damage and decreases in mechanical properties likely contributed to the overloading and erosion of the articular cartilage. Meniscal morphological damage, as well as the permeability of the tibial cartilage, significantly increased after traumatic loading of the joint. It is likely that the acute injury to the meniscus may have caused exacerbated chronic damage to underlying cartilage, just as a recent human cadaver study has shown damage and depression in the sub-meniscal cartilage after a combination of ACL rupture with a meniscal tear (Levine et al., 2012). The medial hemijoint served as the site of the highest degree of overall change in both the menisci and articular cartilage. Meniscal damage was most common in the central and posterior regions of the medial meniscus while histological and morphological analyses identified the proximal and distal medial femoral condyle as the most affected site for articular cartilage. Further analysis is necessary to determine the relationship between the tissues’ degradation times, but it seems clear that there may exist some relationship between meniscal and articular cartilage damages in the chronic setting after blunt force trauma to the joint. An additional study involving an impact to the joint resulting in acute and occult damages in the absence of an ACL rupture would be necessary to determine if acute meniscal injury and overloading of the joint alone would cause similar damage.
Traditional ACLT models have shown that destabilization of the joint can lead to early development of OA, but they fail to address damage to surrounding structures often documented following traumatic injury. In this regard, the current ACLF model may help provide a more complete understanding of whole joint changes after blunt force trauma. This model has shown significant chronic damage to the joint, including damage to the cartilage surface and its matrix as well as significant meniscal damage. This is the first study to document changes to the menisci following a severe impact loading of the joint. It is clear from the current study that significant changes occur in the menisci, possibly correlating with articular cartilage damage. These damages should be considered in future PTOA studies on the knee joint. Thus, the current in vivo, small animal model for the study of PTOA may ultimately aid in the development of future intervention strategies to help limit or mitigate joint degeneration in this trauma patient.
Acknowledgements
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R21 AR060464. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors acknowledge the help of Mr. Clifford Beckett in the mechanical tests, and Ms. Jean Atkinson for care of the animals during the blunt traumas and in the chronic aspects of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article
References
- Adams ME, Billingham ME, Muir H. The glycosaminoglycans in menisci in experimental and natural osteoarthritis. Arthritis Rheum. 1983;26:69–76. doi: 10.1002/art.1780260111. [DOI] [PubMed] [Google Scholar]
- Atkinson PJ, Cooper TG, Anseth S, Walter NE, Kargus R, Haut RC. Association of knee bone bruise frequency with time postinjury and type of soft tissue injury. Orthopedics. 2008;31:440. doi: 10.3928/01477447-20080501-01. [DOI] [PubMed] [Google Scholar]
- Batiste DL, Kirkley A, Laverty S, Thain LMF, Spouge AR, Holdsworth DW. Ex vivo characterization of articular cartilage and bone lesions in a rabbit ACL transection model of osteoarthritis using MRI and micro-CT. Osteoarthritis Cartilage. 2004;12:986–996. doi: 10.1016/j.joca.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Bellabarba C, Bush-Joseph CA, Bach BR. Patterns of meniscal injury in the anterior cruciate-deficient knee: a review of the literature. Am. J. Orthop. (Belle Mead. NJ) 1997;26:18–23. [PubMed] [Google Scholar]
- Boden BP, Dean GS, Feagin JA, Garrett WE. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23:573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- Brophy RH, Martinez M, Borrelli J, Silva MJ. Effect of combined traumatic impact and radial transection of medial meniscus on knee articular cartilage in a rabbit in vivo model. Arthroscopy. 2012;28:1490–1496. doi: 10.1016/j.arthro.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crema MD, Guermazi a, Li L, Nogueira-Barbosa MH, Marra MD, Roemer FW, Eckstein F, Le Graverand MPH, Wyman BT, Hunter DJ. The association of prevalent medial meniscal pathology with cartilage loss in the medial tibiofemoral compartment over a 2-year period. Osteoarthritis Cartilage. 2010;18:336–343. doi: 10.1016/j.joca.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Djurasovic M, Aldridge JW, Grumbles R, Rosenwasser MP, Howell D, Ratcliffe a. Knee joint immobilization decreases aggrecan gene expression in the meniscus. Am. J. Sports Med. 1998;26:460–466. doi: 10.1177/03635465980260032101. [DOI] [PubMed] [Google Scholar]
- Donohue JM, Buss D, Oegema TR, Thompson RC. The effects of indirect blunt trauma on adult canine articular cartilage. J. Bone Joint Surg. Am. 1983;65:948–957. [PubMed] [Google Scholar]
- Ewers BJ, Jayaraman VM, Banglmaier RF, Haut RC. Rate of blunt impact loading affects changes in retropatellar cartilage and underlying bone in the rabbit patella. J. Biomech. 2002;35:747–755. doi: 10.1016/s0021-9290(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann. Intern. Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- Felson DT, Neogi T. Osteoarthritis: is it a disease of cartilage or of bone? Arthritis Rheum. 2004;50:341–344. doi: 10.1002/art.20051. [DOI] [PubMed] [Google Scholar]
- Fischenich KM, Coatney Ga, Haverkamp JH, Button KD, DeCamp C, Haut RC, Haut Donahue TL. Evaluation of meniscal mechanics and proteoglycan content in a modified anterior cruciate ligament transection model. J. Biomech. Eng. 2014;136:1–8. doi: 10.1115/1.4027468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frobell RB, Le Graverand M-P, Buck R, Roos EM, Roos HP, Tamez-Pena J, Totterman S, Lohmander LS. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage. 2009;17:161–167. doi: 10.1016/j.joca.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Golenberg N, Kepich E, Haut RC. Histomorphological and mechanical property correlations in rabbit tibial plateau cartilage based on a fibril-reinforced biphasic mode. Int. J. Exp. Comput. Biomech. 2009;1:58–75. [Google Scholar]
- Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J. Am. Acad. Orthop. Surg. 2002;10:168–176. doi: 10.5435/00124635-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Hellio Le Graverand MP, Vignon E, Otterness IG, Hart Da. Early changes in lapine menisci during osteoarthritis development: Part I: cellular and matrix alterations. Osteoarthritis Cartilage. 2001;9:56–64. doi: 10.1053/joca.2000.0350. [DOI] [PubMed] [Google Scholar]
- Hootman JM, Macera CA, Ainsworth BE, Addy CL, Martin M, Blair SN. Epidemiology of musculoskeletal injuries among sedentary and physically active adults. Med. Sci. Sports Exerc. 2002;34:838–844. doi: 10.1097/00005768-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Isaac DI, Meyer EG, Haut RC. Chondrocyte damage and contact pressures following impact on the rabbit tibiofemoral joint. J. Biomech. Eng. 2008;130:041018. doi: 10.1115/1.2948403. [DOI] [PubMed] [Google Scholar]
- Isaac DI, Meyer EG, Haut RC. Development of a traumatic anterior cruciate ligament and meniscal rupture model with a pilot in vivo study. J. Biomech. Eng. 2010a;132:064501. doi: 10.1115/1.4001111. [DOI] [PubMed] [Google Scholar]
- Isaac DI, Meyer EG, Kopke KS, Haut RC. Chronic changes in the rabbit tibial plateau following blunt trauma to the tibiofemoral joint. J. Biomech. 2010b;43:1682–1688. doi: 10.1016/j.jbiomech.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Killian ML, Isaac DI, Haut RC, Déjardin LM, Leetun D, Donahue TLH. Traumatic anterior cruciate ligament tear and its implications on meniscal degradation: a preliminary novel lapine osteoarthritis model. J. Surg. Res. 2010a;164:234–241. doi: 10.1016/j.jss.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Killian ML, Lepinski NM, Haut RC, Haut Donahue TL. Regional and zonal histomorphological characteristics of the lapine menisci. Anat. Rec. (Hoboken) 2010b;293:1991–2000. doi: 10.1002/ar.21296. [DOI] [PubMed] [Google Scholar]
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo Ra, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke AK, Sandy JD, Voigt H, Dreier R, Lee JH, Grodzinsky AJ, Mentlein R, Fay J, Schünke M, Kurz B. Interleukin-1alpha treatment of meniscal explants stimulates the production and release of aggrecanase-generated, GAG-substituted aggrecan products and also the release of pre-formed, aggrecanase-generated G1 and m-calpain-generated G1-G2. Cell Tissue Res. 2010;340:179–188. doi: 10.1007/s00441-010-0941-4. [DOI] [PubMed] [Google Scholar]
- Levine JW, Kiapour AM, Quatman CE, Wordeman SC, Goel VK, Hewett TE, Demetropoulos CK. Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am. J. Sports Med. 2012;41:385–395. doi: 10.1177/0363546512465167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandrowski KU, Müller J, Schollmeier G. Concomitant meniscal and articular cartilage lesions in the femorotibial joint. Am. J. Sports Med. 1997;25:486–494. doi: 10.1177/036354659702500411. [DOI] [PubMed] [Google Scholar]
- Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J. Bone Joint Surg. Am. 2006;88:1826–1834. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am. J. Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- McDaniel WJ, Dameron TB. Untreated ruptures of the anterior cruciate ligament. A follow-up study. J. Bone Joint Surg. Am. 1980;62:696–705. [PubMed] [Google Scholar]
- McNulty aL, Estes BT, Wilusz RE, Weinberg JB, Guilak F. Dynamic loading enhances integrative meniscal repair in the presence of interleukin-1. Osteoarthritis Cartilage. 2010;18:830–838. doi: 10.1016/j.joca.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EG, Haut RC. Excessive compression of the human tibio-femoral joint causes ACL rupture. J. Biomech. 2005;38:2311–2316. doi: 10.1016/j.jbiomech.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Milentijevic D, Rubel IF, Liew ASL, Helfet DL, Torzilli Pa. An in vivo rabbit model for cartilage trauma: a preliminary study of the influence of impact stress magnitude on chondrocyte death and matrix damage. J. Orthop. Trauma. 2005;19:466–473. doi: 10.1097/01.bot.0000162768.83772.18. [DOI] [PubMed] [Google Scholar]
- Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J. Bone Joint Surg. Am. 1983;65:154–162. doi: 10.2106/00004623-198365020-00003. [DOI] [PubMed] [Google Scholar]
- Pauli C, Grogan SP, Patil S, Otsuki S, Hasegawa a, Koziol J, Lotz MK, D’Lima DD. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19:1132–1141. doi: 10.1016/j.joca.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen Ma, Jackson DW, Berger PE. Occult osseous lesions documented by magnetic resonance imaging associated with anterior cruciate ligament ruptures. Arthroscopy. 1991;7:45–51. doi: 10.1016/0749-8063(91)90077-b. [DOI] [PubMed] [Google Scholar]
- Saito M, Sasho T, Yamaguchi S, Ikegawa N, Akagi R, Muramatsu Y, Mukoyama S, Ochiai N, Nakamura J, Nakagawa K, Nakajima a, Takahashi K. Angiogenic activity of subchondral bone during the progression of osteoarthritis in a rabbit anterior cruciate ligament transection model. Osteoarthritis Cartilage. 2012;20:1574–1582. doi: 10.1016/j.joca.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Setton La, Zhu W, Mow VC. The biphasic poroviscoelastic behavior of articular cartilage: role of the surface zone in governing the compressive behavior. J. Biomech. 1993;26:581–592. doi: 10.1016/0021-9290(93)90019-b. [DOI] [PubMed] [Google Scholar]
- Shrive NG, O’Connor JJ, Goodfellow JW. Load-bearing in the knee joint. Clin. Orthop. Relat. Res. 1978:279–287. [PubMed] [Google Scholar]
- Smith JP, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament-deficient knees. A prospective analysis of 575 tears. Am. J. Sports Med. 2001;29:415–419. doi: 10.1177/03635465010290040501. [DOI] [PubMed] [Google Scholar]
- Speer KP, Warren RF, Wickiewicz TL, Horowitz L, Henderson L. Observations on the injury mechanism of anterior cruciate ligament tears in skiers. Am. J. Sports Med. 1995;23:77–81. doi: 10.1177/036354659502300113. [DOI] [PubMed] [Google Scholar]
- Sweigart Ma, Zhu CF, Burt DM, DeHoll PD, Agrawal CM, Clanton TO, Athanasiou Ka. Intraspecies and interspecies comparison of the compressive properties of the medial meniscus. Ann. Biomed. Eng. 2004;32:1569–1579. doi: 10.1114/b:abme.0000049040.70767.5c. [DOI] [PubMed] [Google Scholar]
- Tandogan RN, Taşer O, Kayaalp A, Taşkiran E, Pinar H, Alparslan B, Alturfan A. Analysis of meniscal and chondral lesions accompanying anterior cruciate ligament tears: relationship with age, time from injury, and level of sport. Knee Surg. Sports Traumatol. Arthrosc. 2004;12:262–270. doi: 10.1007/s00167-003-0398-z. [DOI] [PubMed] [Google Scholar]
- Yeow C-H, Kong C-Y, Lee PV-S, Goh JC-H. Correlation of axial impact forces with knee joint forces and kinematics during simulated ski-landing. J. Sports Sci. 2011;29:1143–1151. doi: 10.1080/02640414.2011.578146. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Coutts RD, Amiel D, Hacker SA. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1996;4:87–98. doi: 10.1016/s1063-4584(05)80318-8. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Coutts RD, Amiel D, Hacker SA. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthr. Cartil. 1996;4:87–98. doi: 10.1016/s1063-4584(05)80318-8. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Wang XH, Wei XC, Chen WY. Characterization of viscoelastic properties of normal and osteoarthritic chondrocytes in experimental rabbit model. Osteoarthritis Cartilage. 2008;16:837–840. doi: 10.1016/j.joca.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Zielinska B, Killian M, Kadmiel M, Nelsen M, Haut Donahue TL. Meniscal tissue explants response depends on level of dynamic compressive strain. Osteoarthritis Cartilage. 2009;17:754–760. doi: 10.1016/j.joca.2008.11.018. [DOI] [PubMed] [Google Scholar]