Abstract
Many stroke survivors suffer from impaired hand function. Biomechanics of hand grip suggests that abnormally directed grip force can hamper gripping abilities and hand function. This study examined the relation between the ability to precisely direct fingertip force and clinical hand function scores among individuals affected by stroke. Specifically, clinical hand function tests of the Fugl-Meyer, Chedoke McMaster, and Box and Block Test were used, since they involve various hand movements required for activities of daily living. Digit force direction during static grip was recorded using multiaxial load cells. Data for 59 chronic stroke survivors were analyzed. We found that larger angular deviation of digit force from the normal direction was significantly associated with lower hand functional levels (p<.001 for all three clinical tests). Particularly, stroke survivors whose digit force deviated more than 21° from the normal direction could not achieve the normal level of Fugl-Meyer or Chedoke or move more than 4 blocks in a minute. The biomechanics of the way digit force direction affects hand grip function is described. In addition, underlying mechanisms for altered digit force direction post stroke are postulated, including impaired somatosensation and abnormal neural input to muscles. In summary, this study identifies a new biomechanical marker for hand functional level and recovery. Future interventions may focus on correcting digit force direction to improve hand functional outcome.
Keywords: Stroke, Hand grip, Rehabilitation, Digit, Finger, Direction, Control
1. Introduction
Many of 7 million stroke survivors in the U.S. (Roger et al., 2012) have impaired hand function (Trombly, 1989; Gray et al., 1990; Nakayama et al., 1994; Kamper et al., 2003). Stroke survivors’ attempt at grasping an object often leads to the object slipping out of the hand. Not only reduced strength (Boissy et al., 1999) but also impaired grip coordination can affect grip function in daily living for people after stroke (Nowak and Hermsdorfer, 2005; Blennerhassett et al., 2006). One of the aspects of impaired grip coordination is altered force direction (Cole, 2006).
Altered digit force direction may hamper gripping abilities via biomechanics of hand grip. To grip an object without slippage, digit force must be directed to the object within an allowed angle range defined by the cone of friction (Fig. 1) (Fikes et al., 1994; MacKenzie and Iberall, 1994). For instance, to grip a rubber-finished object, digit force cannot deviate more than 42° (Seo et al., 2010). When the digit force direction lies outside the cone of friction, shear force becomes greater than maximum allowable friction force and the finger would slip against the object thereby resulting in loss of grip. If digit force is directed near the cone of friction, small perturbation to the finger or grasped object may lead to changes in digit force direction and loss of grip.
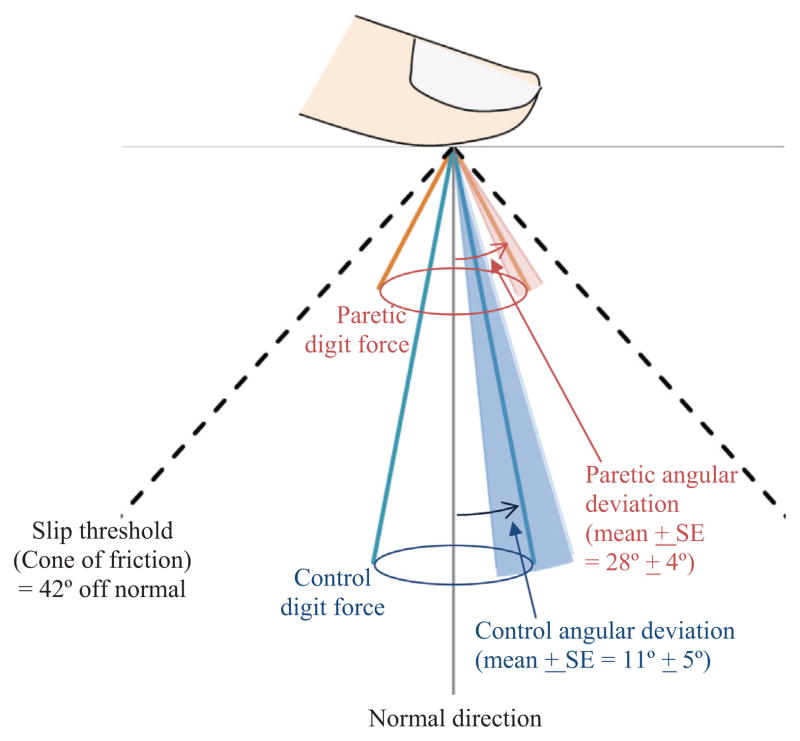
Fig. 1.
Our previous study showed that chronic stroke survivors with severe hand impairment gripped with their paretic digit force directed further away from the normal/perpendicular direction compared to age-matched persons without stroke (control) (Seo et al., 2010). The mean angular deviation of digit force is illustrated as the width of the cone for each of the paretic and control hands. The paretic cone is shorter than the control cone, since the paretic hand is substantially weaker than the control hand, and thus produces less grip force. In addition to the mean values, ±1 standard error is shown as the shade around each cone. When the digit force deviation angle reaches the slip threshold (42° for rubber finish), the finger slips against the object surface (Fikes et al., 1994; MacKenzie and Iberall, 1994). The paretic digit force deviation angle is closer to the slip threshold compared to control, indicating greater likelihood of finger-object slippage, grip loss, and object drop.
Chronic stroke survivors with severe hand impairment were shown to apply grip force far off from the direction normal to the grip surface, compared to age-matched adults without stroke, as illustrated in Fig. 1 (Seo et al., 2010). This large digit force angular deviation was associated with frequent slip between the finger and grip surface, observed for 55% of trials (Seo et al., 2010), which can result in grip loss and object dropping. Given the biomechanical basis by which digit force direction affects grip abilities, the extent of digit force angular deviation may be closely related to the ability to perform activities of daily living using the hand.
However, the direct quantitative relationship between the extent of digit force angular deviation and clinical functional ability is currently unknown. The previous study involved stroke survivors with severe hand impairment only. A larger dataset involving stroke survivors with a wide range of hand functional levels is needed to determine the relationship between digit force direction and clinical hand function. Understanding this relationship would provide an insight for the way hand function is impacted by altered digit force direction post stroke, and enable development of assistive devices or therapeutic interventions that improve digit force direction and thus improve hand function for these individuals. Therefore, this study examined if the extent that digit force direction deviates from the normal direction relates to clinical hand function scores whose primary tasks involve dexterous and gross upper limb movements required by activities of daily living. The clinical hand function tests included: the Fugl-Meyer Assessment for the hand and wrist (Fugl-Meyer et al., 1975), Chedoke McMaster hand scale (Gowland et al., 1995), and Box and Block Test (Mathiowetz et al., 1985).
2. Method
2.1. Subjects
Fifty nine chronic stroke survivors’ data were analyzed. The mean age was 58 and standard deviation was 12 years. Time since stroke ranged 1–21 years. They were 36 males and 23 females. Seventeen subjects’ data were from previous studies (Seo et al., 2010, 2011a). Only data without any intervention was used from Seo et al. (2011a). The other 42 subjects’ data were newly obtained. The hand impairment level of the 59 subjects ranged from mild to severe, as seen by the Stages 1–7 of the Chedoke McMaster hand scale determined by a therapist: Chedoke Stage 1 implies flaccidity, while people who can perform active movements with and without facilitation belong to Stage 2 and 3, respectively; Simple and complex synergy movements are possible at Stage 4 and 5, respectively; Stage 6 implies limitation in more complex or faster movements than needed in daily activities, while Stage 7 represents no evidence of functional impairment (Miller et al., 2008). All subjects signed written consent approved by IRB.
2.2. Procedure
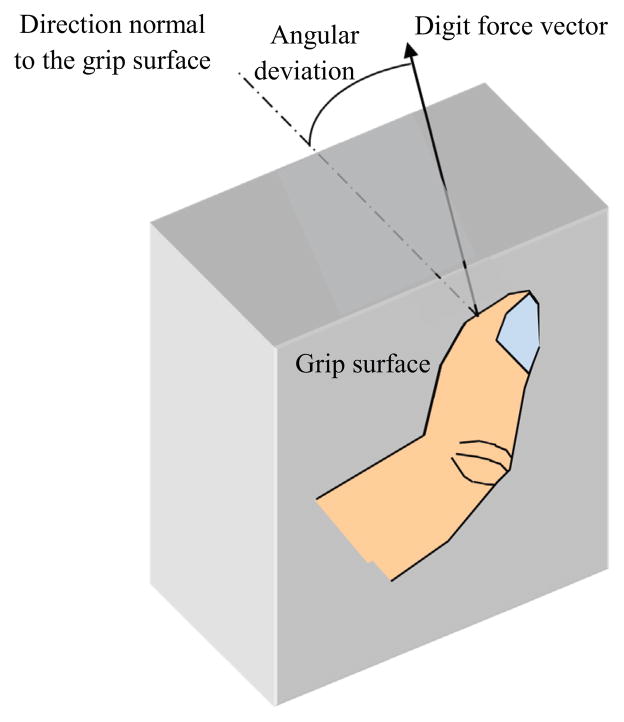
This study quantified digit force angular deviation from the normal direction during static grip for each subject (Fig. 2). Subjects rested their forearm on a table and placed the thumb and index finger on two fixed flat rubber surfaces. For subjects with severe impairment, the experimenter assisted with placing their fingers on the grip surfaces. Subjects were instructed to grip against the fixed surfaces for 5 s at the maximum effort, 5 N, and 2 N, at least three times each. The surfaces were instrumented with 6-axis load cells (ATI Industrial Automation, Apex, NC) to record normal and shear forces from each finger. For submaximal grips, visual feedback for normal force was provided to help subjects match their grip force to the target. The digit force deviation angle was computed as the arctangent of the ratio of total shear force to normal force over a one-second period in which the average normal force was the greatest for maximal grips or closest to the target for the 5 N or 2 N grips (Seo et al., 2010). As the deviation angle variation by digits or force levels within a person is slight compared to between-subject variance (Seo et al., 2010), an average deviation angle across both digits and force levels was used to characterize digit force direction for each subject for the correlation analysis.
Fig. 2.
The angle by which the digit force vector is deviated from the normal direction was computed as the arctangent of the ratio of total shear force to normal force.
In addition, clinical functional scores including the Fugl-Meyer for the hand and wrist (out of 24), Chedoke McMaster hand scale (out of 7), and Box and Block Test (number of blocks moved in 60 s) were recorded. Since this study involves retrospective data analysis, missing data exist. The Chedoke McMaster score was obtained for 59 subjects, whereas the Fugl-Meyer score was obtained for 46 subjects and the Box and Block Test score for 37 subjects. All data were for the affected hand.
2.3. Analysis
Pearson correlation was used to examine correlations between the deviation angle and the Fugl-Meyer upper extremity score, the deviation angle and the Chedoke McMaster hand score, and the deviation angle and the Box and Block Test. Three correlation analyses were used, since the functional scores were correlated with each other and thus cannot be combined in a single regression. Time since stroke, age, and gender did not influence the results of the regression analysis probably because all subjects were in the chronic stage (>9 months post stroke) and the age/gender effects, if there are any, are not as prominent as the effect of post-stroke impairment levels. Thus, the results without controlling for age, gender, and time since stroke are presented.
3. Results
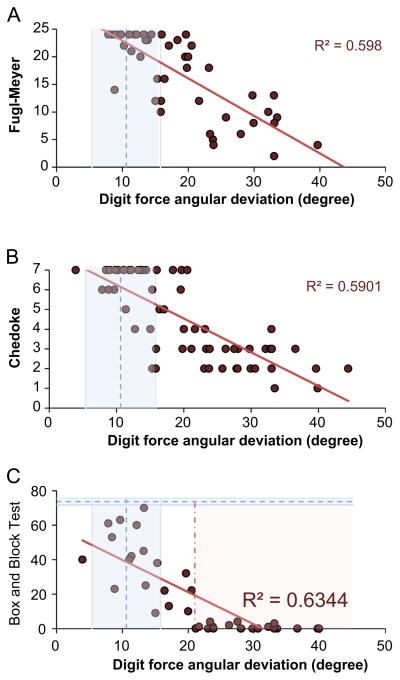
The extent to which digit force direction deviated from the normal direction was negatively correlated with the Fugl-Meyer upper extremity score, Chedoke McMaster hand score, and Box and Block Test score as shown in Fig. 3A–C, respectively (p<.001, R2≈0.6 for all). Greater digit force deviation was associated with worse hand function seen by a lower Fugl-Meyer score, lower Chedoke score, and lower Box and Block Test score.
Fig. 3.
A significant relationship was observed (A) between the digit force angular deviation and the Fugl-Meyer upper extremity score (out of 24), (B) between the digit force angular deviation and the Chedoke McMaster hand score (out of 7), and (C) between the digit force angular deviation and the Box and Block Test (p<0.001 and R2≈0.6 for all). As a benchmark, the mean±1 standard error digit force angular deviation for age-matched control subjects (11° ±5°) obtained in the previous study (Seo et al., 2010) is shown with a vertical dash line and shades around that dash line. For the Box and Block Test, the mean±1 standard error normative score (74±2) for 55–59 years old healthy adults from a previous study (Mathiowetz et al., 1985) is noted with a horizontal dash line and shades around that dash line (C). In addition, the vertical dash dot line at 21° represents the digit force deviation above which no more than four blocks could be moved (C).
The majority of stroke survivors whose digit force direction was comparable to the age-matched stroke-free control data (11° ±5°) obtained in the previous study (Seo et al., 2010) had the maximum possible scores for the Fugl-Meyer and Chedoke (24 and 7, respectively, Fig. 3A and B). Unlike Fugl-Meyer and Chedoke, the Box and Block Test did not have a ceiling effect. Stroke survivors with smaller digit force deviation scored higher on the Box and Block Test (Fig. 3C). Instead, the Box and Block Test had a floor effect in which stroke survivors whose digit force direction was off the normal direction by more than 21° could not move more than 4 blocks in a minute. None of the stroke survivors with digit force deviation greater than 21° were able to achieve the maximum Fugl-Meyer or Chedoke score.
4. Discussion
This study demonstrates that larger digit force angular deviation from the normal direction during grip is associated with worse hand function. Specifically, for stroke survivors whose digit force direction was off the normal direction by more than 21°, none of them achieved normal Fugl-Meyer or Chedoke scores or moved more than four blocks in a minute (Fig. 3). On the contrary, for stroke survivors with digit force angular deviation less than 16°, comparable to age-matched control (Seo et al., 2010), the majority of them had normal Fugl-Meyer and Chedoke scores, and they moved, on average, 44 blocks in a minute. While substantially better than those with large digit force angular deviation, 44 blocks a minute is less than two thirds of the normative score for age-matched control (Mathiowetz et al., 1985), possibly due to reduced strength and slowness in movement.
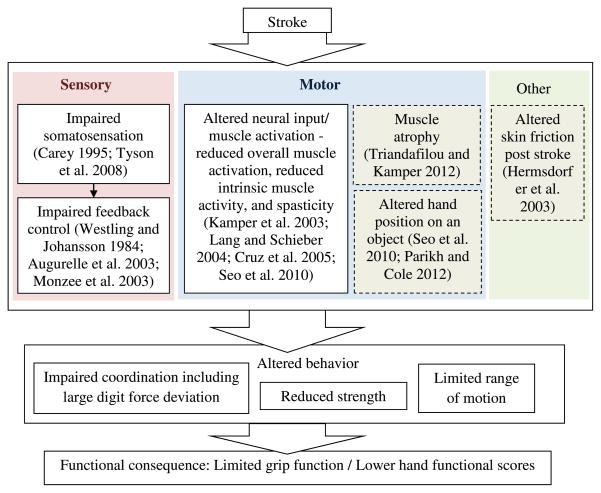
The result of this study extends the previous finding by showing that altered digit force direction is not only present for chronic stroke survivors (Seo et al., 2010), but also directly related to the severity of their hand impairment level. This result suggests that hand functional impairment after stroke is attributable not only to reduced grip strength (Boissy et al., 1999) and other grip coordination issues (Nowak et al., 2003; Nowak and Hermsdorfer, 2005; Blennerhassett et al., 2006), but also to altered digit force direction (Fig. 4). Specifically, large angular deviation of digit force from the normal direction post stroke may hamper grip abilities and negatively impact hand functional scores involving various grips required by activities of daily living, as detailed below.
Fig. 4.
Potential contributors for large digit force deviation and limited hand function post stroke.
While correlation does not prove a causal relationship, it is plausible that altered digit force direction hampers gripping abilities due to the unique biomechanics of hand grip. To successfully grasp an object, the digits must apply properly scaled and directed grip forces to the object (Johansson and Westling, 1984; Gordon et al., 1991). Digit force directed outside the cone of friction leads to slippage against the object to be grasped (Fikes et al., 1994; MacKenzie and Iberall, 1994). To minimize the likelihood of finger-object slippage from perturbation or inherent noise in the sensorimotor system, healthy young adults typically grip in the near-normal direction (Seo et al., 2011b). Despite this straight-forward biomechanics of the way digit force direction affects grip, the relationship between digit force direction and grip function has not been substantially studied. To our best knowledge, the present study represents the first to demonstrate significant correlations between digit force direction and clinical hand functional scores.
Two main underlying reasons for large angular deviation of digit force from the normal direction post stroke may be impaired somatosensation and altered neural input to muscles (Fig. 4). First, post-stroke impairment in somatosensory perception is common (Carey, 1995; Tyson et al., 2008). Somatosensory feedback is critical in digit force control (Johansson and Westling, 1984; Gordon et al., 1991). Reduced somatosensation with digital anesthesia or with nerve compression leads to inappropriate grip force coordination, frequent object dropping, and clumsiness (Westling and Johansson, 1984; Augurelle et al., 2003; Monzee et al., 2003; Keith et al., 2009). Similarly, damage to somatosensory afferents in the brain or impaired cortical somatosensory processing post stroke may fail to adequately inform the central nervous system of skin deformation, strain, kinesthesia, and proprioception, thereby hindering feedback control to adjust digit force and resulting in large digit force angular deviation from the normal direction.
Second, altered neural input is well documented to lead to abnormal muscle activation patterns including spasticity (Mottram et al., 2009) and abnormal muscle synergies (Brunnstrom, 1970; Dewald et al., 1995). Post-stroke abnormal muscle activation pattern for the hand muscles is characterized by hypoactive intrinsic and extensor muscles and hyperactive extrinsic flexor muscles (Kamper et al., 2003; Lang and Schieber, 2004; Cruz et al., 2005; Seo et al., 2010), likely due to distal muscles’ greater reliance on corticospinal drive (Palmer and Ashby, 1992; Turton and Lemon, 1999) and vulnerability to stroke (Cruz et al., 2005). This is problematic since every single muscle’s action is important when it comes to precise directional control of the digit force (Kutch and Valero-Cuevas, 2011). In particular, intrinsic muscles play a critical role in directional control of the digit force, with the first palmar and dorsal interosseous muscles reducing the possible force production in specific directions by 80–90% on average (Kutch and Valero-Cuevas, 2011). As such, altered muscle activation pattern with underactivated intrinsic muscles impairs the delicate directional control of digit force, leading to large deviation from the normal direction (Valero-Cuevas et al., 2000; Milner and Dhaliwal, 2002; Cole, 2006). The critical role of each muscle’s action in directional control of digit force also suggests that if individual muscle weakness persists, sensory intervention alone cannot sufficiently correct the altered digit force direction.
Other factors were considered but not thought as primary reasons for large digit force deviation post stroke. For instance, muscle atrophy as much as 15% reduction in cross-sectional areas exists in chronic stroke survivors, but the extent of reduction in the muscle size was found to be uniform across all hand muscles (Triandafilou and Kamper, 2012). Muscle atrophy but no difference in the relative atrophy across the hand muscles results in a scaling-down of digit force, but no changes in the direction (Valero-Cuevas et al., 2000). Additionally, altered orientation of the finger relative to an object was found not to be related to digit force angular deviation (Seo et al., 2010). Similarly, the possibility that large digit force angular deviation may be to compensate for misalignment of the two digits to minimize unwanted moment applied to a virtual object (Parikh and Cole, 2012) was considered. However, stroke survivors with severe impairment appear to have large digit force deviation not necessarily in the direction toward the opposite digit to minimize moment (analysis not reported here). Lastly, change in skin friction, possibly due to abnormal perspiration in case stroke affects autonomic nervous system function, could result in a different size of the cone of friction (Naylor, 1955; Smith et al., 1997) and allow large angular deviation of digit force from the normal direction for stroke survivors. However, no evidence exists to suggest post-stroke changes in skin friction (Hermsdorfer et al., 2003).
The significance of this study is that a new biomechanical marker was identified to be correlated with clinical hand function, with an insight for the way hand function is impacted by altered digit force direction post stroke. This knowledge encourages development of assistive devices or targeted therapies to improve digit force direction and thus hand function. For instance, to compensate for impaired somatosensation post stroke, sensory enhancement techniques (Conforto et al., 2007; Enders et al., 2013; Kurita et al., 2013) or visual feedback (Ellis et al., 2005; Seo et al., 2011a) may be used to improve hand function. Correcting digit force direction or muscle activation pattern using neuromuscular electrical stimulation and muscle strengthening (Santos et al., 2006; Dean et al., 2007) may directly help improve hand grip function. Alternatively, daily objects may be modified with high-friction surfaces (Seo and Enders, 2012; Slota et al., 2014) or adaptive shapes to accommodate stroke survivors’ altered digit force direction and reduce finger-object slips, thereby improving hand function.
Acknowledgments
This publication was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055, the Wisconsin Women’s Health Foundation, and the University of Wisconsin-Milwaukee Office of Undergraduate Research. Its contents are solely the responsibility of the author and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
References
- Augurelle AS, Smith AM, Lejeune T, Thonnard JL. Importance of cutaneous feedback in maintaining a secure grip during manipulation of hand-held objects. J Neurophysiol. 2003;89:665–671. doi: 10.1152/jn.00249.2002. [DOI] [PubMed] [Google Scholar]
- Blennerhassett JM, Carey LM, Matyas TA. Grip force regulation during pinch grip lifts under somatosensory guidance: comparison between people with stroke and healthy controls. Arch Phys Med Rehabil. 2006;87:418–429. doi: 10.1016/j.apmr.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Boissy P, Bourbonnais D, Carlotti MM, Gravel D, Arsenault BA. Maximal grip force in chronic stroke subjects and its relationship to global upper extremity function. Clin Rehabil. 1999;13:354–362. doi: 10.1191/026921599676433080. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Movement Therapy in Hemiplegia: A Neurophysiological Approach. Medical Dept. Harper & Row; New York, NY: 1970. [Google Scholar]
- Carey LM. Somatosensory loss after stroke. Crit Rev Phys Rehabil Med. 1995;7:51–91. [Google Scholar]
- Cole KJ. Age-related directional bias of fingertip force. Exp Brain Res. 2006;175:285–291. doi: 10.1007/s00221-006-0553-0. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Cohen LG, dos Santos RL, Scaff M, Marie SK. Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. J Neurol. 2007;254:333–339. doi: 10.1007/s00415-006-0364-z. [DOI] [PubMed] [Google Scholar]
- Cruz EG, Waldinger HC, Kamper DG. Kinetic and kinematic workspaces of the index finger following stroke. Brain. 2005;128:1112–1121. doi: 10.1093/brain/awh432. [DOI] [PubMed] [Google Scholar]
- Dean JC, Yates LM, Collins DF. Turning on the central contribution to contractions evoked by neuromuscular electrical stimulation. J Appl Physiol. 2007;103:170–176. doi: 10.1152/japplphysiol.01361.2006. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Ellis MD, Holubar BG, Acosta AM, Beer RF, Dewald JP. Modifiability of abnormal isometric elbow and shoulder joint torque coupling after stroke. Muscle Nerve. 2005;32:170–178. doi: 10.1002/mus.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders LR, Hur P, Johnson MJ, Seo NJ. Remote vibrotactile noise improves light touch sensation in stroke survivors’ fingertips via stochastic resonance. J Neuroeng Rehabil. 2013;10:105. doi: 10.1186/1743-0003-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikes TG, Klatzky RL, Lederman SJ. Effects of object texture on precontact movement time in human prehension. J Mot Behav. 1994;26:325–332. doi: 10.1080/00222895.1994.9941688. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1 A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gordon AM, Forssberg H, Johansson RS, Westling G. Integration of sensory information during the programming of precision grip: comments on the contributions of size cues. Exp Brain Res. 1991;85:226–229. doi: 10.1007/BF00230004. [DOI] [PubMed] [Google Scholar]
- Gowland C, VanHullenaar S, Torresin W, Moreland J, Vanspall B, Barrecca S, Ward M, Huijbregts M, Stratford P, Barclay-Goddard R. Chedoke-McMaster Stroke Assessment: Development, Validation and Administration Manual. Chedoke-McMaster Hospitals and McMaster University; Hamilton, Canada: 1995. [Google Scholar]
- Gray CS, French JM, Bates D, Cartlidge NE, James OF, Venables G. Motor recovery following acute stroke. Age Ageing. 1990;19:179–184. doi: 10.1093/ageing/19.3.179. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Hagl E, Nowak DA, Marquardt C. Grip force control during object manipulation in cerebral stroke. Clin Neurophysiol. 2003;114:915–929. doi: 10.1016/s1388-2457(03)00042-7. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56:550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- Kamper DG, Harvey RL, Suresh S, Rymer WZ. Relative contributions of neural mechanisms versus muscle mechanics in promoting finger extension deficits following stroke. Muscle Nerve. 2003;28:309–318. doi: 10.1002/mus.10443. [DOI] [PubMed] [Google Scholar]
- Keith MW, Masear V, Chung K, Maupin K, Andary M, Amadio PC, Barth RW, Watters WC, 3rd, Goldberg MJ, Haralson RH, 3rd, Turkelson CM, Wies JL. Diagnosis of carpal tunnel syndrome. J Am Acad Orthop Surg. 2009;17:389–396. doi: 10.5435/00124635-200906000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita Y, Shinohara M, Ueda J. Wearable sensorimotor enhancer for fingertip based on stochastic resonance effect. IEEE Trans Hum Mach Syst. 2013;43:333–337. [Google Scholar]
- Kutch JJ, Valero-Cuevas FJ. Muscle redundancy does not imply robustness to muscle dysfunction. J Biomech. 2011;44:1264–1270. doi: 10.1016/j.jbiomech.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J Neurophysiol. 2004;91:1722–1733. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- MacKenzie CL, Iberall T. The Grasping Hand. 1994. [Google Scholar]
- Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the box and block test of manual dexterity. Am J Occup Ther. 1985;39:386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- Miller P, Huijbregts M, Gowland C, Barreca S, Torresin W, Moreland J, Dunkley M, Griffiths J, VanHullenaar S, Vanspall B, Ward M, Stratford P, Barclay-Goddard R. Chedoke-McMaster Stroke Assessment: Development, Validation and Administration Manual. Chedoke-McMaster Hospitals and McMaster University; Hamilton, Canada: 2008. [Google Scholar]
- Milner TE, Dhaliwal SS. Activation of intrinsic and extrinsic finger muscles in relation to the fingertip force vector. Exp Brain Res. 2002;146:197–204. doi: 10.1007/s00221-002-1177-7. [DOI] [PubMed] [Google Scholar]
- Monzee J, Lamarre Y, Smith AM. The effects of digital anesthesia on force control using a precision grip. J Neurophysiol. 2003;89:672–683. doi: 10.1152/jn.00434.2001. [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Suresh NL, Heckman C, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic–paretic stroke survivors. J Neurophysiol. 2009 doi: 10.1152/jn.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75:394–398. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Naylor PF. The skin surface and friction. Br J Dermatol. 1955;67:239–246. doi: 10.1111/j.1365-2133.1955.tb12729.x. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J. Grip force behavior during object manipulation in neurological disorders: toward an objective evaluation of manual performance deficits. Mov Disord. 2005;20:11–25. doi: 10.1002/mds.20299. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J, Topka H. Deficits of predictive grip force control during object manipulation in acute stroke. J Neurol. 2003;250:850–860. doi: 10.1007/s00415-003-1095-z. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Evidence that a long latency stretch reflex in humans is transcortical. J Physiol. 1992;449:429–440. doi: 10.1113/jphysiol.1992.sp019094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh PJ, Cole KJ. Handling objects in old age: forces and moments acting on the object. J Appl Physiol. 2012;112:1095–1104. doi: 10.1152/japplphysiol.01385.2011. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M, Zahner LH, McKiernan BJ, Mahnken JD, Quaney B. Neuromuscular electrical stimulation improves severe hand dysfunction for individuals with chronic stroke: a pilot study. J Neurol Phys Ther. 2006;30:175–183. doi: 10.1097/01.npt.0000281254.33045.e4. [DOI] [PubMed] [Google Scholar]
- Seo NJ, Enders LR. Hand grip function assessed by the box and block test is affected by object surfaces. J Hand Ther. 2012;25:397–404. doi: 10.1016/j.jht.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Seo NJ, Fischer HW, Bogey RA, Rymer WZ, Kamper DG. Use of visual force feedback to improve digit force direction during pinch grip in persons with stroke: a pilot study. Arch Phys Med Rehabil. 2011a;92:24–30. doi: 10.1016/j.apmr.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Seo NJ, Rymer WZ, Kamper DG. Altered digit force direction during pinch grip following stroke. Exp Brain Res. 2010;202:891–901. doi: 10.1007/s00221-010-2193-7. [DOI] [PubMed] [Google Scholar]
- Seo NJ, Shim JK, Engel AK, Enders LR. Grip surface affects maximum pinch force. Hum Factors. 2011b;53:740–748. doi: 10.1177/0018720811420256. (The Journal of the Human Factors and Ergonomics Society) [DOI] [PubMed] [Google Scholar]
- Slota GP, Enders LR, Seo NJ. Improvement of hand function using different surfaces and identification of difficult movement post stroke in the Box and Block Test. Appl Ergon. 2014;45:833–838. doi: 10.1016/j.apergo.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Smith AM, Cadoret G, St-Amour D. Scopolamine increases prehensile force during object manipulation by reducing palmar sweating and decreasing skin friction. Exp Brain Res. 1997;114:578–583. doi: 10.1007/pl00005666. [DOI] [PubMed] [Google Scholar]
- Triandafilou KM, Kamper DG. Investigation of hand muscle atrophy in stroke survivors. Clin Biomech (Bristol, Avon) 2012;27:268–272. doi: 10.1016/j.clinbiomech.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombly CA. Occupational Therapy for Physical Dysfunction. Williams & Wilkins; Baltimore: 1989. Stroke; pp. 454–471. [Google Scholar]
- Turton A, Lemon RN. The contribution of fast corticospinal input to the voluntary activation of proximal muscles in normal subjects and in stroke patients. Exp Brain Res. 1999;129:559–572. doi: 10.1007/s002210050926. [DOI] [PubMed] [Google Scholar]
- Tyson SF, Hanley M, Chillala J, Selley AB, Tallis RC. Sensory loss in hospital-admitted people with stroke: characteristics, associated factors, and relationship with function. Neurorehabil Neural Repair. 2008;22:166–172. doi: 10.1177/1545968307305523. [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Towles JD, Hentz VR. Quantification of fingertip force reduction in the forefinger following simulated paralysis of extensor and intrinsic muscles. J Biomech. 2000;33:1601–1609. doi: 10.1016/s0021-9290(00)00131-7. [DOI] [PubMed] [Google Scholar]
- Westling G, Johansson RS. Factors influencing the force control during precision grip. Exp Brain Res. 1984;53:277–284. doi: 10.1007/BF00238156. [DOI] [PubMed] [Google Scholar]