Abstract
Purpose
We aimed to identify serum metabolites as potential valuable biomarkers for lung cancer and to improve risk stratification in smokers.
Experimental Design
We performed global metabolomic profiling followed by targeted validation of individual metabolites in a case-control design of 386 lung cancer cases and 193 matched controls. We then validated bilirubin, which consistently showed significant differential levels in cases and controls, as a risk marker for lung cancer incidence and mortality in a large prospective cohort comprised of 425,660 participants.
Results
Through global metabolomic profiling and following targeted validation, bilirubin levels consistently showed a statistically significant difference among healthy controls and lung cancer cases. In the prospective cohort, the inverse association was only seen in male smokers, regardless of smoking pack-years and intensity. Compared with male smokers in the highest bilirubin group (>1 mg/dL), those in the lowest bilirubin group (<0.75 mg/dL) had 55% and 66% increase in risks of lung cancer incidence and mortality, respectively. For every 0.1 mg/dL decrease of bilirubin, the risks for lung cancer incidence and mortality increased by 5% and 6% in male smokers, respectively (both P < 0.001). There was a significant interaction between low serum bilirubin level and smoking on lung cancer risk (P for interaction = 0.001).
Conclusion
Low levels of serum bilirubin are associated with higher risks of lung cancer incidence and mortality in male smokers and can be used to identify higher risk smokers for lung cancer.
Keywords: lung cancer, smokers, metabolomics, bilirubin, cohort
Introduction
Lung cancer is the second most common cancer and the leading cause of cancer deaths in both men and women in the United States (1). Recent studies by the National Lung Screening Trial (NLST) have showed that low-dose computed tomography (LDCT) can reduce lung cancer mortality by 20% (2). Based on these findings, LDCT screening based on NLST selection criteria, i.e., current or former smokers aged 55–74 years with at least 30 pack-years of smoking history and no more than 15 years since quitting, has been recommended by the majority of professional organizations in the US (1, 3–5). Moreover, it has recently been reported that participants with the highest risk for lung cancer deaths accounted for the most screening-prevented lung-cancer deaths and benefitted most from LDCT (6). Although smoking is the predominant risk factor for lung cancer, considering smoking alone is not sufficient to identify the highest-risk individuals for lung cancer (3, 6). Therefore, novel biomarkers for lung cancer incidence and mortality, particularly among smokers, are urgently needed in the clinical setting to improve risk prediction and reduce false positives of LDCT screening.
Metabolomics is the systematic study of the unique chemical fingerprints generated by metabolic processes of an organism (7). Metabolomic profiling, emerging as an important tool to identify biomarkers, provides a functional readout of physiological and pathological characteristics (8). An increasing number of studies have utilized metabolomic profiling to reveal metabolic alterations associated with various cancers (8–16), including lung cancer (17–19). However, only a small number of metabolites have been examined and studies to date suffer from a lack of prospective validation (17–19).
To identify serum metabolites as novel biomarkers for lung cancer, we first performed metabolomic profiling followed by targeted metabolite validation in a lung cancer case-control study with three phases to identify top promising metabolites that differentiated lung cancer cases from healthy controls. Bilirubin emerged as the consistently significant metabolite. We then sought to validate bilirubin as a risk marker for lung cancer in a large prospective cohort study. During this validation stage, we prospectively analyzed serum bilirubin levels in a cohort of 425,660 subjects to assess its ability in identifying smokers with particularly high risk for lung cancer.
Materials and Methods
Study population
Stage 1: Case-control studies
The subjects are participants in an ongoing lung cancer case-control study at the University of Texas MD Anderson Cancer Center. Details of subject recruitment methods have been reported previously (20). Cases were newly diagnosed, histologically confirmed non-small cell lung cancer (NSCLC) patients previously untreated with chemotherapy or radiotherapy at MD Anderson Cancer Center. There was no restriction of age, sex, or ethnicity at study recruitment. Early stage NSCLC included stages I and II, while late stage NSCLC included stages III and IV. The healthy controls came from the Kelsey Seybold Clinics, the largest private multispecialty physician group practice in Houston. To control for the confounding effect of ethnicity, we only included Caucasians for our study. Twenty each of controls, early stage, and late stage lung cancer cases (hereafter referred to as “trio”) were used for metabolomic profiling. Promising metabolites identified from this profiling were examined in two additional sets of case-control samples, consisting of 50 trios and 123 trios, respectively. All participants completed an in-person interview using a structured questionnaire. Demographic characteristics, smoking history, family history of cancer, and exposure data were collected. After the interview, each participant donated 40ml blood sample for molecular analysis.
Stage 2: Prospective cohort study
The cohort consisted of 425,660 Taiwanese adults aged 20 years and older who participated in a standard medical screening program between 1994 and 2008. Details of this cohort have been reported (21, 22). Briefly, median follow-up time for the cohort is 8 years (interquartile range: 5–11 years) for male participants and 9 years (interquartile range: 5–11 years) for female participants. All participants completed a self-administered health history questionnaire and underwent a series of medical tests for blood, urine, physical examination, body measurements and functional tests. Overnight fasting blood was analyzed for a standard panel of markers, including serum bilirubin. The cohort members were followed through 2008 for cancer and vital status, which were assessed by linkage of the individual ID to the National Cancer Registry and National Death file.
The studies were approved by the Institutional Review Boards of the University of Texas MD Anderson Cancer Center and Kelsey Seybold Clinics, as well as the National Health Research Institutes, Taiwan. All participants provided written informed consent.
Metabolomic profiling and quantification of individual metabolites
The metabolomic profiling analysis was carried out by Metabolon Inc, (Durham, NC) as described previously (23). Internal controls included injection, process, and alignment standards for quality assurance/quality control (QA/QC) procedures to control for experimental variability. Samples were kept at −80°C until assays were performed. For the series of validation studies, standard powders for two metabolites, i.e., λ-glutamylalanine and bilirubin, were purchased from Sigma-Aldrich (St. Louis, MO). Quantification of individual metabolite in serum was determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods using a 3200 QTRAP® MS/MS coupled by an Agilent 1200 Series HPLC system at Dr. Dong Liang’s laboratory. Standard curves for each compound were constructed by spiking known amount of the standard to a series of control plasma (Gulf Coast Blood Bank, Houston, TX). Serum bilirubin levels measured by both metabolomic profiling and LC-MS/MS were levels of unconjugated bilirubin in serum.
Statistical analysis
In the case-control analysis, the Pearson χ2 test was used to examine the differences in sex and smoking status between cases and controls. Student’s t test was used to test for differences in age and pack-years of smoking as continuous variables. For the metabolomic profiling analysis, missing metabolite measurements were imputed with the compound minimum (minimum value imputation). Only metabolites with detectable expression in at least 80% of the samples were kept for further analysis. For both metabolomics profiling and individual metabolite quantification, the nonparametric trend test was used to analyze the trend across the trios. Bonferroni correction was used to account for multiple comparisons from metabolomic profiling results, and a P value < 0.05/n (n = number of comparisons) was considered as the significance level to take into account multiple comparisons. Spearman’s correlation was used to assess the correlation between the two values measured by metabolomic profiling and individual metabolite quantification using LC-MS/MS.
For the prospective cohort validation study, lung cancer cases diagnosed within one year of recruitment into the cohort were excluded to minimize potential reverse causality. For lung cancer incidence, the event time was from the date of recruitment to the end of follow-up, or the date of lung cancer identification if earlier. For lung cancer mortality, the event time was from the date of recruitment to the end of follow-up, or the date of death due to lung cancer if earlier. Serum total bilirubin levels were divided into three groups with equal tertile. Cox proportional hazards models were used to assess the association of serum total bilirubin levels with lung cancer incidence and mortality. Hazard ratios were adjusted for age, educational level (middle school or lower, high school, junior college, or college or higher), body mass index (BMI) and pack-years of smoking in a multivariable model with continuous variables whenever appropriate. The proportional hazards assumption was assessed by plotting Schoenfeld residuals versus time and examining their correlation. Interaction between smoking and serum total bilirubin level on lung cancer risk was assessed by introducing the product of smoking and serum bilirubin level in the multivariable Cox regression model. All statistical tests were two-sided with the threshold for significance set at 0.05. Statistical analyses were performed using Stata 10.0 (StataCorp, College Station, TX).
Results
Characteristics of the study populations
In the case-control study, all three phases of lung cancer cases and healthy controls were Caucasians, matched on age and gender (Supplementary Table S1). In the prospective cohort study, there were 202,902 men and 222,758 women aged 20 years and older. Selected demographic characteristics and exposures of the cohort participants are shown in Table 1, presented by gender and tertiles of bilirubin level (<0.75, 0.75–1 and >1 mg/dL for men and <0.61, 0.61–0.82 and >0.82 mg/dL for women). Distribution of serum total bilirubin levels among the participants in the cohort is shown in Supplementary Fig. S1. Among male participants in the cohort, over half (52.1%) were smokers, with 25% of them being heavy smokers of ≥30 pack-years. In contrast, only 17,123 (8.3%) female participants were smokers, with 1,327 (8.3%) of them being heavy smokers. During the follow-up, there were 809 incident lung cancer cases and 614 lung cancer deaths among the males, and 524 lung cancer cases and 330 deaths among the females.
Table 1.
Characteristics of the participants in the prospective cohort by gender and serum total bilirubin levels a
| Characteristics | Men (N = 202,902), N (%)
|
Women (N = 222,758), N (%)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Total | Total bilirubin level (mg/dL)
|
Total | Total bilirubin level (mg/dL)
|
|||||
| >1 | 0.75–1 | <0.75 | >0.82 | 0.61–0.82 | <0.61 | |||
|
|
|
|
|
|||||
| Total | 202,902 | 67,841 (33.4) | 65,540 (32.3) | 69,521 (34.3) | 222,758 | 75,189 (33.8) | 72,207 (32.4) | 75,362 (33.8) |
| Age (y), mean (SD) | 41 (14) | 40 (14) | 42 (14) | 41 (14) | 41 (14) | 41 (14) | 42 (14) | 41 (13) |
| 20–39 | 112,584 (55.5) | 39,399 (35.0) | 35,270 (31.3) | 37,915 (33.7) | 119,946 (53.9) | 41,854 (34.9) | 37,510 (31.3) | 40,582 (33.8) |
| 40–59 | 63,447 (31.3) | 19,927 (31.4) | 21,201 (33.4) | 22,319 (35.2) | 76,087 (34.2) | 23,908 (31.4) | 25,500 (33.5) | 26,679 (35.1) |
| ≥60 | 26,871 (13.2) | 8,515 (31.7) | 9,069 (33.8) | 9,287 (34.6) | 26,725 (12) | 9,427 (35.3) | 9,197 (34.4) | 8,101 (30.3) |
| BMI (kg/m2), mean (SD) | 23.9 (3.4) | 23.5 (3.3) | 23.9 (3.3) | 24.2 (3.4) | 22.3 (3.6) | 21.8 (3.5) | 22.3 (3.6) | 22.8 (3.7) |
| <25 | 134,591 (66.4) | 47,499 (35.3) | 43,264 (32.1) | 43,828 (32.6) | 176,567 (79.3) | 62,056 (35.2) | 57,139 (32.4) | 57,372 (32.5) |
| 25–29.9 | 59,734 (29.5) | 18,062 (30.2) | 19,565 (32.8) | 22,107 (37.0) | 38,454 (17.3) | 11,040 (28.7) | 12,660 (32.9) | 14,754 (38.4) |
| ≥30 | 8,516 (4.2) | 2,264 (26.6) | 2,696 (31.7) | 3,556 (41.8) | 7,689 (3.5) | 2,080 (27.1) | 2,394 (31.1) | 3,215 (41.8) |
| Educational levels | ||||||||
| Middle school or lower | 40,499 (20.6) | 12,823 (31.7) | 13,379 (33.0) | 14,297 (35.3) | 70,385 (32.6) | 23,109 (32.8) | 23,546 (33.5) | 23,730 (33.7) |
| High school | 45,601 (23.2) | 14,665 (32.2) | 14,693 (32.2) | 16,243 (35.6) | 54,124 (25.1) | 17,238 (31.9) | 17,186 (31.8) | 19,700 (36.4) |
| Junior college | 45,367 (23.1) | 15,705 (34.6) | 14,526 (32.0) | 15,136 (33.4) | 42,941 (19.9) | 15,153 (35.3) | 13,525 (31.5) | 14,263 (33.2) |
| College or higher | 64,987 (33.1) | 22,603 (34.8) | 20,844 (32.1) | 21,540 (33.2) | 48,400 (22.4) | 17,428 (36.0) | 15,692 (32.4) | 15,280 (31.6) |
| Smoking status | ||||||||
| Non-smoker | 92,864 (47.9) | 35,175 (37.9) | 30,188 (32.5) | 27,501 (29.6) | 188,685 (91.7) | 64,488 (34.2) | 61,534 (32.6) | 62,663 (33.2) |
| Smoker | 101,092 (52.1) | 29,632 (29.3) | 32,451 (32.1) | 39,009 (38.6) | 17,123 (8.3) | 4,891 (28.6) | 5,228 (30.5) | 7,004 (40.9) |
| <30 pack-years | 72,153 (74.9) | 21,843 (30.3) | 23,084 (32.0) | 27,226 (37.7) | 14,662 (91.7) | 4,303 (29.4) | 4,403 (30.0) | 5,956 (40.6) |
| ≥30 pack-years | 24,146 (25.1) | 6,269 (26.0) | 7,777 (32.2) | 10,100 (41.8) | 1,327 (8.3) | 279 (21) | 434 (32.7) | 614 (46.3) |
| Lung cancer incidence | 809 (0.4) | 215 (26.6) | 270 (33.4) | 324 (40.1) | 524 (0.2) | 155 (29.6) | 187 (35.7) | 182 (34.7) |
| Lung cancer mortality | 614 (0.3) | 147 (23.9) | 214 (34.9) | 253 (41.2) | 330 (0.2) | 107 (32.4) | 115 (34.9) | 108 (32.7) |
Percentage may not total 100 because of rounding.
Global metabolomic profiling of lung cancer
Serum global metabolomic profiles of 40 lung cancer cases and 20 healthy controls (20 trios) were assessed in the initial case-control study and a total of 403 metabolites were identified. After exclusion of metabolites detected in less than 80% of samples, 306 (76%) metabolites remained. These metabolites were mapped to 8 super-pathways and 61 sub-pathways (Supplementary Table S2). Among these, 29 metabolites exhibited a significant trend of expression when comparing normal controls, early and late stage cases, 12 of which had P for trend values < 0.01 (Supplementary Table S3). After taking into account multiple comparisons, λ-glutamylalanine remained as the only metabolite meeting statistical significance after Bonferroni correction [P for trend < 1.63 × 10−4 (0.05/306)].
Target validation of individual metabolites
Metabolites exhibiting a significant trend in levels from normal individuals to early and late stage patients are also potential biomarkers for the detection and prognosis of lung cancer. Of the 29 metabolites with significant trends, bilirubin caught our most interest given its potent endogenous cytoprotective properties and more importantly, its inverse association with cardiovascular disease and respiratory disease in previous reports (24–27). Therefore, we selected bilirubin and λ-glutamylalanine, which showed the most significant trend from metabolomic profiling and after Bonferroni correction for further validation. We developed standard LC/MS-MS assays for these metabolites and used these assays to measure their levels in the 20 trios of cases and controls from phase I of the case-control study; we found excellent correlation with metabolomic profiling data (Supplementary Tables S4 and S5). We further examined levels of bilirubin and λ-glutamylalanine in additional 50 trios of serum samples (phase II) and 123 trios of serum samples (phase III) from controls, early and late-stage patients (Supplementary Table S5). Through this process, bilirubin emerged as a metabolite that consistently showed a statistically significant trend in all three sets of trio data.
Validation of bilirubin as a lung cancer marker in a large cohort
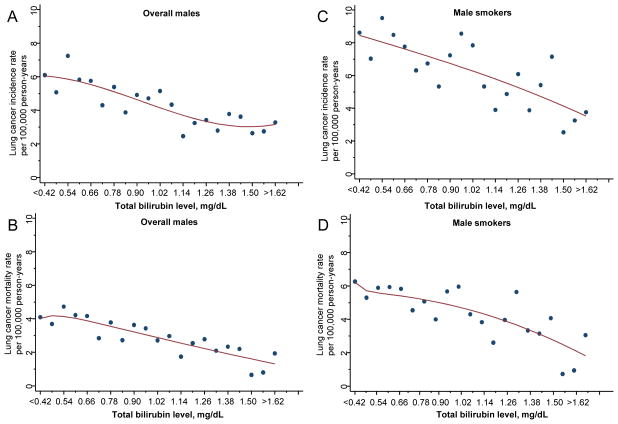
Since bilirubin is a routine blood test in health examination, we next assessed the association of blood test serum total bilirubin levels with lung cancer incidence and mortality using a large prospective cohort in Taiwan. As expected, there was a strong dose response relationship between lung cancer risk/mortality and pack-years of smoking or smoking intensity in this cohort (Tables 2 and 3). Furthermore, among males, using non-smokers with the highest tertile of bilirubin levels (>1 mg/dL) as reference, smokers in the lowest tertile of bilirubin levels (<0.75 mg/dL) had a 2.86-fold increased risk of developing lung cancer (Table 2). Smokers with <30 and ≥30 pack-years of smoking in the lowest tertile of bilirubin levels had HRs of 1.40 and 4.14 respectively (Table 2 and Supplementary Fig. S2). Similarly, smokers in the lowest tertile of bilirubin levels who smoked <10, 10–19 and ≥20 cigarettes per day had HRs of 1.85, 2.70 and 4.32, respectively (Table 2 and Supplementary Fig. S2). Similar results were found for lung cancer mortality (Table 3 and Supplementary Fig. S2). In contrast, among females, lower serum bilirubin levels were not significantly associated with lung cancer incidence or mortality overall, in female smokers or in female non-smokers (Supplementary Table S6). Table 4 presents the rates of lung cancer incidence and mortality stratified by tertiles of serum bilirubin levels and corresponding risk estimates in males. The incidence rate of lung cancer per 10,000 person-years was 6.93 (95% CI, 6.20–7.75) in the lowest tertile compared to 4.27 (95% CI, 3.71–4.90) in the highest tertile of bilirubin levels, which translated to a 52% increased risk of lung cancer for the low bilirubin group (P < 0.001). The corresponding lung cancer specific mortality rate was 4.88 (95% CI, 4.32–5.52) in the lowest tertile compared to 2.70 (95% CI, 2.30–3.17) in the highest tertile, a 71% increased risk in lung cancer specific mortality for the low bilirubin group (P < 0.001) (Table 4). We plotted the lung cancer incidence rates against subgroups of bilirubin levels and introduced a best-fit model. Those with bilirubin levels <0.42 mg/dL showed more than 80% increase in lung cancer incidence rate (6.1 vs 3.27 per 100,000 person-years, Fig. 1A) and over two folds increase in mortality rate (4.09 vs 1.94 per 100,000 person-years, Fig. 1B) compared to the subgroup with bilirubin levels >1.62 mg/dL.
Table 2.
Relationship among smoking quantity, bilirubin levels and risk for lung cancer incidence in male participants the prospective cohort study
| Men (N=202,902)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Total bilirubin level (mg/dL)
|
|||||||||||
| >1 | 0.75–1 | <0.75 | ||||||||||
|
|
|
|
|
|||||||||
| No. | No. of incidence |
HRa (95% CI) | No. | No. of incidence |
HRa (95% CI) | No. | No. of incidence |
HRa (95% CI) | No. | No. of incidence |
HRa (95% CI) | |
| Non-smoker | 92,864 | 156 | 1 (Ref) | 35,175 | 64 | 1 (Ref) | 30,188 | 50 | 0.87 (0.59–1.27) | 27,501 | 42 | 0.85 (0.56–1.27) |
| Total smokers | 101,092 | 603 | 2.64 (2.19–3.18) | 29,632 | 139 | 1.84 (1.35–2.51) | 32,451 | 202 | 2.38 (1.77–3.19) | 39,009 | 262 | 2.86 (2.15–3.81) |
| Pack-year | ||||||||||||
| <30 pack-years | 72,153 | 123 | 1.31 (1.02–1.69) | 21,843 | 27 | 0.80 (0.50–1.29) | 23,084 | 47 | 1.37 (0.92–2.03) | 27,226 | 49 | 1.40 (0.94–2.07) |
| ≥30 pack-years | 24,146 | 454 | 4.01 (3.27–4.91) | 6,269 | 108 | 3.14 (2.25–4.36) | 7,777 | 145 | 3.48 (2.54–4.77) | 10,100 | 201 | 4.14 (3.06–5.60) |
| # of Cigarettes per day | ||||||||||||
| <10 | 31,520 | 106 | 1.55 (1.20–2.01) | 10,602 | 23 | 0.96 (0.58–1.57) | 10,270 | 37 | 1.39 (0.91–2.13) | 10,648 | 46 | 1.85 (1.24–2.74) |
| 10–19 | 38,866 | 261 | 2.71 (2.19–3.34) | 11,031 | 62 | 1.97 (1.36–2.84) | 12,557 | 91 | 2.58 (1.84–3.60) | 15,278 | 108 | 2.70 (1.95–3.72) |
| ≥20 | 26,879 | 221 | 4.29 (3.46–5.33) | 6,759 | 52 | 3.39 (2.31–4.96) | 8,374 | 70 | 3.72 (2.61–5.30) | 11,746 | 99 | 4.32 (3.11–5.99) |
Adjusted for age, educational level and body mass index
Table 3.
Relationship among smoking quantity, bilirubin levels and risk for lung cancer mortality in male participants the prospective cohort study
| Men (N = 202,902)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Total bilirubin level (mg/dL)
|
|||||||||||
| >1 | 0.75–1 | <0.75 | ||||||||||
|
|
|
|
|
|||||||||
| No. | No. of mortality |
HRa (95% CI) | No. | No. of mortality |
HRa (95% CI) | No. | No. of mortality |
HRa (95% CI) | No. | No. of mortality |
HRa (95% CI) | |
| Non-smoker | 92,864 | 98 | 1 (Ref) | 35,175 | 36 | 1 (Ref) | 30,188 | 34 | 1.04 (0.65–1.66) | 27,501 | 28 | 0.99 (0.60–1.63) |
| Total smokers | 101,092 | 478 | 3.24 (2.60–4.05) | 29,632 | 104 | 2.39 (1.63–3.50) | 32,451 | 165 | 3.28 (2.28–4.72) | 39,009 | 209 | 3.96 (2.77–5.65) |
| Pack-year | ||||||||||||
| <30 pack-years | 72,153 | 90 | 1.62 (1.20–2.18) | 21,843 | 14 | 0.80 (0.43–1.48) | 23,084 | 39 | 2.08 (1.31–3.30) | 27,226 | 37 | 2.01 (1.26–3.20) |
| ≥30 pack-years | 24,146 | 370 | 4.78 (3.77–6.05) | 6,269 | 89 | 4.18 (2.81–6.22) | 7,777 | 119 | 4.56 (3.11–6.69) | 10,100 | 162 | 5.52 (3.81–7.99) |
| # of Cigarettes per day | ||||||||||||
| <10 | 31,520 | 84 | 1.95 (1.45–2.62) | 10,602 | 14 | 1.05 (0.56–1.94) | 10,270 | 29 | 1.91 (1.17–3.14) | 10,648 | 41 | 2.88 (1.83–4.52) |
| 10–19 | 38,866 | 211 | 3.38 (2.65–4.32) | 11,031 | 48 | 2.65 (1.71–4.09) | 12,557 | 77 | 3.65 (2.45–5.45) | 15,278 | 86 | 3.75 (2.53–5.55) |
| ≥20 | 26,879 | 172 | 5.16 (4.01–6.65) | 6,759 | 42 | 4.64 (2.96–7.27) | 8,374 | 55 | 4.95 (3.24–7.57) | 11,746 | 75 | 5.74 (3.85–8.56) |
Adjusted for age, educational level and body mass index
Table 4.
Lung cancer incidence and mortality rates and adjusted HR per tertile of serum total bilirubin level among the male participants in the prospective cohort study by smoking status and smoking intensity
| Characteristics | Men (N=202,902)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of lung cancer incidence
|
Adjusted HRa (95% CI)
|
Incidence Rate Per 10 000 Person-Years (95% CI)
|
|||||||
| Total bilirubin level (mg/dL)
|
Total bilirubin level (mg/dL)
|
Total bilirubin level (mg/dL)
|
|||||||
| >1 | 0.75–1 | <0.75 | >1 | 0.75–1 | <0.75 | >1 | 0.75–1 | <0.75 | |
|
|
|
|
|||||||
| Total | 215 | 270 | 324 | 1 (Ref) | 1.24 (1.03–1.51) | 1.52 (1.26–1.82) | 4.27 (3.71–4.90) | 5.79 (5.12–6.54) | 6.93 (6.20–7.75) |
| Non-smoker | 64 | 50 | 42 | 1 (Ref) | 0.86 (0.59–1.27) | 0.84 (0.56–1.26) | 2.56 (1.98–3.30) | 2.46 (1.86–3.27) | 2.35 (1.72–3.22) |
| Total smokers | 139 | 202 | 262 | 1 (Ref) | 1.29 (1.03–1.62) | 1.55 (1.25–1.92) | 6.05 (5.09–7.18) | 8.37 (7.27–9.64) | 9.75 (8.61–11.03) |
| <30 pack-years | 27 | 47 | 49 | 1 (Ref) | 1.71 (1.04–2.79) | 1.77 (1.09–2.89) | 1.57 (1.06–2.33) | 2.74 (2.05–3.67) | 2.61 (1.95–3.48) |
| ≥30 pack-years | 108 | 145 | 201 | 1 (Ref) | 1.10 (0.85–1.43) | 1.31 (1.03–1.67) | 22.41 (18.46–27.21) | 24.85 (21.05–29.35) | 27.79 (24.11–32.02) |
| No. of lung cancer mortality
|
Adjusted HRa (95% CI)
|
Mortality Rate Per 10 000 Person-Years (95% CI)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total bilirubin level (mg/dL)
|
Total bilirubin level (mg/dL)
|
Total bilirubin level (mg/dL)
|
|||||||
| >1 | 0.75–1 | <0.75 | >1 | 0.75–1 | <0.75 | >1 | 0.75–1 | <0.75 | |
|
|
|
|
|||||||
| Total | 147 | 214 | 253 | 1 (Ref) | 1.39 (1.12–1.72) | 1.71 (1.39–2.10) | 2.70 (2.30–3.17) | 4.11 (3.59–4.69) | 4.88 (4.32–5.52) |
| Non-smoker | 36 | 34 | 28 | 1 (Ref) | 1.03 (0.65–1.65) | 0.98 (0.59–1.61) | 1.34 (0.96–1.85) | 1.49 (1.06–2.09) | 1.43 (0.99–2.07) |
| Total smokers | 104 | 165 | 209 | 1 (Ref) | 1.37 (1.07–1.76) | 1.66 (1.31–2.10) | 4.18 (3.45–5.07) | 6.17 (5.30–7.19) | 7.02 (6.13–8.04) |
| <30 pack-years | 14 | 39 | 37 | 1 (Ref) | 2.60 (1.41–4.80) | 2.56 (1.38–4.74) | 0.77 (0.45–1.30) | 2.06 (1.51–2.82) | 1.80 (1.31–2.49) |
| ≥30 pack-years | 89 | 119 | 162 | 1 (Ref) | 1.09 (0.82–1.44) | 1.32 (1.01–1.71) | 16.54 (13.44–20.36) | 18.02 (15.06–21.57) | 20.13 (17.26–23.48) |
Adjusted for age, educational level and body mass index
Figure 1.
Serum total bilirubin levels and lung cancer incidence rates in overall males (A) and male smokers (C); and lung cancer mortality rates in overall males (B) and male smokers (D) of the prospective cohort study.
The ability of bilirubin in identifying smokers with higher risk of lung cancer
We then assessed the association between bilirubin levels and lung cancer incidence or mortality rate stratified by smoking status. Among females, neither non-smokers or smokers showed significant association, as only 17,123 (8.3%) participants were smokers and there were only 37 lung cancer cases among them. Among males, the association was only present in smokers and there was a significant interaction between low serum bilirubin level and smoking on lung cancer risk (P for interaction = 0.001). Compared to smokers with bilirubin levels in the highest tertile, smokers with bilirubin levels in the middle and lowest tertiles had significantly increased lung cancer risk (HRs, 1.29 and 1.55) and mortality (HRs, 1.37 and 1.66) (Table 4). The risk appeared to be stronger in light smokers: the HRs for the lowest tertile of bilirubin compared to the highest tertile were 1.77 for incidence and 2.56 for mortality in smokers of <30 pack years and 1.31 for incidence and 1.32 for mortality in smokers of ≥30 pack years, respectively (Table 4). We also plotted the lung cancer incidence and mortality rates against subgroups of bilirubin levels in smokers and introduced a best fit model, those with bilirubin levels <0.42 mg/dL showed more than two folds increase in both lung cancer incidence rate (8.62 vs 3.76 per 100,000 person-years, Fig. 1C) and mortality rate (6.27 vs 3.05 per 100,000 person-years, Fig. 1D) compared to the subgroup with bilirubin levels >1.62 mg/dL. The logistic regression model showed a 5% (95% CI, 3%–8%, P < 0.001) increase in lung cancer incidence and 6% (95% CI, 3%–9%, P < 0.001) increase in lung cancer mortality per 0.1 mg/dL decrease in bilirubin level, after adjusting for age, BMI and educational level.
Discussion
The purpose of this study is to identify biomarkers among serum metabolites to assist in identifying high-risk individuals for lung cancer development. Through this multi-stage study, we have identified and validated serum bilirubin as a risk predictor for lung cancer incidence as well as mortality in male smokers. While smoking is a strong risk factor for lung cancer and shows a dose-response relationship, the smoking-related risk is particularly high among male smokers with low levels of serum bilirubin, a 55% increase among those with bilirubin <0.75 mg/dL. Among males, smokers with ≥30 pack years had a 4-fold increase in lung cancer risk, and within this group, those with bilirubin level <0.75 mg/dL had a 31% higher risk compared to those with bilirubin level >1 mg/dL. The potential of using serum bilirubin to identify smokers at particularly high-risk for lung cancer, over and above the risk associated with heavy smoking, is an important observation. The inverse relationship between bilirubin levels and lung cancer can be translated into a 5% increase in lung cancer risk and a 6% increase in lung cancer mortality for each 0.1 mg/dL decrease in bilirubin levels. In most clinical settings, emphasis is placed on elevated bilirubin for diagnosis of liver diseases, therefore low values of bilirubin are generally ignored. Making use of low serum bilirubin values to counsel heavy smokers who are at particularly high risk for lung cancer about smoking cessation can be carried out easily in many clinic settings.
Elevated levels of serum bilirubin have been associated with a lower risk of respiratory diseases and lung cancer (24, 27). The mechanism of this association was credited to the antioxidant and anti-inflammatory properties of bilirubin. As bilirubin is a commonly ordered laboratory test, uncovering this potentially protective relationship is intriguing. This study, while in line with the reported conclusion, is the first to study the role of bilirubin as a risk factor for lung cancer mortality, to focus on the analysis in smokers in detail, and to quantify the hazards of low bilirubin.
It has been shown that smoking is associated with lower serum bilirubin levels (27–29). In our study, we have also found that serum bilirubin levels were lower in smokers compared to non-smokers among participants in the cohort. However, the inverse association between serum bilirubin levels and lung cancer incidence/mortality remained significant after we adjusted for smoking status/pack-years among overall male participants in the cohort. We also found that lower bilirubin was associated with higher risks of lung cancer and mortality among male smokers overall, and among male smokers with similar pack-years of smoking through our stratified analyses, suggesting that bilirubin level is associated with lung cancer risk at least partially independent of smoking status/quantity. In addition, we have also found a significant interaction between low serum bilirubin level and smoking on lung cancer risk (P for interaction = 0.001), suggesting that bilirubin may exert its function by interacting with smoking and lowering lung cancer risk among smokers who have higher oxidative stress and inflammation (30).
Our findings may also have implications for the LDCT screening for lung cancer. It has been reported that LDCT screening prevented the most deaths from lung cancer among participants with the highest risk for lung cancer deaths 60% of participants at the highest risk accounted for 88% of prevented lung-cancer deaths (7). Based on our results, male smokers with bilirubin level <0.75 mg/dL have a 66% increased risk for lung cancer mortality compared to those with bilirubin level >1 mg/dL, and for heavy smokers of ≥30 pack-years, the hazard ratio is smaller, but still significant (HR = 1.32, P < 0.001). Consideration of bilirubin levels might improve identifying participants with the highest risk for lung cancer mortality who would benefit the most from the screening, and help improve the specificity of LDCT screening. Furthermore, bilirubin results could be used to target and motivate both light and heavy smokers for smoking cessation. Indeed, the ability of low bilirubin in predicting high risk of lung cancer was not limited to male smokers with ≥30 pack-years in our study. The relationship was seen for all male smokers, regardless of pack years of smoking.
We conducted a series of sensitivity analyses to strengthen our conclusion. We excluded participants with lung cancer diagnosed within 3 years of cohort enrollment. We restricted bilirubin levels within normal range, excluding participants with abnormal liver enzymes or blood counts. Additional variables (drinking status, physical activity and systolic blood pressure) were adjusted in the multivariable models. Results essentially remained unchanged after all of the above sensitivity analyses were carried out.
Recently, several research groups had applied metabolomic profiling of serum/plasma to unveil metabolic alterations associated with lung cancer, but all were limited by the small number of metabolites detected. Hori et al. study detected a total of only 58 metabolites in serum using gas chromatography/mass spectrometry (GC/MS) and found 23 with differential detection in lung cancer patients compared to healthy controls in a Japanese population (17). In another Japanese study, Maeda et al. studied 21 plasma amino acids in NSCLC patients by liquid chromatography/mass spectrometry (LC/MS) and showed that differences in the amino acid profiles may be used for screening NSCLC (19). Jordan and colleagues used nuclear magnetic resonance (NMR) to measure 21 metabolites and showed the potential of serum metabolomics to differentiate between lung cancer subtypes and between patients and controls (18). These studies were limited by the small number of metabolites detected. Our global unbiased metabolomic profiling approach identified 403 known metabolites from different stages of lung cancer, yielding a comprehensive picture of the metabolic profile changes associated with cancer progression. Validated with two additional study sets, bilirubin was found and confirmed as the consistently significant biomarker for lung cancer, which was further validated prospectively in a large cohort.
A few potential limitations should be considered in the interpretation of our findings. First, while we observed significant inverse associations between serum bilirubin levels and lung cancer in male smokers, the associations were not statistically significant in female smokers, which was most likely due to the lack of power resulting from a small number of female smokers (8.3% of total females) and very few number of lung cancer cases (n=37) among them. Second, although we observed an inverse relationship between bilirubin levels and lung cancer risk, the causality of the association remains unclear. Low bilirubin level could be a consequence of cancer rather than a predisposing factor. It is noteworthy that the significant risk remained after we excluded lung cancer occurring within three years of the bilirubin tests. Third, only the bilirubin data at the time of enrollment were analyzed. In a subset of subjects that had two bilirubin tests performed longitudinally, we found highly correlative data, implying the stability of total bilirubin results over time.
In summary, low levels of serum bilirubin are associated with higher risk for lung cancer incidence and mortality in male smokers and can be used to identify higher risk smokers for lung cancer development and mortality. Future prospective studies that incorporate this variable into NLST selection criteria to fully assess its potential use for LDCT screening are warranted.
Supplementary Material
Translational relevance.
Through this multi-phase study comprised of global metabolomic profiling and prospective validation in a large cohort, we have identified and validated bilirubin as a risk predictor for lung cancer incidence as well as mortality in smokers. For every 0.1 mg/dL decrease of bilirubin, the risks for lung cancer incidence and mortality increased by 5% and 6% in male smokers, respectively (both P < 0.001). Smokers with ≥30 pack years had a 4-fold increase in lung cancer risk, but within this group, those with bilirubin of <0.75 mg/dL compared to >1 mg/dL had a 31% higher risk. Addition of this variable into National Lung Screening Trial (NLST) selection criteria for low-dose computed tomography (LDCT) screening might help identify higher risk smokers who would benefit more from LDCT screening and reduce false positives.
Acknowledgments
Grant Support
This work was supported by the National Cancer Institute (P50 CA070907 Project 2 to XW); MD Anderson Research Trust and MD Anderson Institutional support for the Center for Translational and Public Health Genomics (to XW); and Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH102-TD-B-111-004 to CPW).
Footnotes
Conflicts of interest: We declare that we have no conflicts of interest.
Contributors
XW, CPW, and SML designed the study. CPW, FZ, DL, JG, YY, XP, MAH, MH, MKT, SML, and XW analyzed and interpreted the data. CPW, FZ and XW wrote the manuscript and submitted the paper for publication. CW, HS, WHC, CHC, CAH, and SML critically revised the article for important intellectual content. All authors had final approval of the article. CKT provided study materials from the cohort and was responsible for quality control of cohort data. CKT was responsible for collection and assembly of cohort data. SML was clinical collaborator for this study.
References
- 1.Wender R, Fontham ET, Barrera E, Jr, Colditz GA, Church TR, Ettinger DS, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63:107–17. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–29. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood DE, Eapen GA, Ettinger DS, Hou L, Jackman D, Kazerooni E, et al. Lung cancer screening. Journal of the National Comprehensive Cancer Network: JNCCN. 2012;10:240–65. doi: 10.6004/jnccn.2012.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaklitsch MT, Jacobson FL, Austin JH, Field JK, Jett JR, Keshavjee S, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. The Journal of thoracic and cardiovascular surgery. 2012;144:33–8. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 6.Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. The New England journal of medicine. 2013;369:245–54. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.2020 visions. Nature. 2010;463:26–32. doi: 10.1038/463026a. [DOI] [PubMed] [Google Scholar]
- 8.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–4. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 9.Gu H, Pan Z, Xi B, Asiago V, Musselman B, Raftery D. Principal component directed partial least squares analysis for combining nuclear magnetic resonance and mass spectrometry data in metabolomics: application to the detection of breast cancer. Anal Chim Acta. 2011;686:57–63. doi: 10.1016/j.aca.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritchie SA, Ahiahonu PW, Jayasinghe D, Heath D, Liu J, Lu Y, et al. Reduced levels of hydroxylated, polyunsaturated ultra long-chain fatty acids in the serum of colorectal cancer patients: implications for early screening and detection. BMC Med. 2010;8:13. doi: 10.1186/1741-7015-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Liu L, Wei S, Nagana Gowda GA, Hammoud Z, Kesler KA, et al. Metabolomics study of esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2011;141:469–75. 75 e1–4. doi: 10.1016/j.jtcvs.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda A, Nishiumi S, Shinohara M, Yoshie T, Hatano N, Okuno T, et al. Serum metabolomics as a novel diagnostic approach for gastrointestinal cancer. Biomed Chromatogr. 2011 doi: 10.1002/bmc.1671. [DOI] [PubMed] [Google Scholar]
- 13.Xue R, Lin Z, Deng C, Dong L, Liu T, Wang J, et al. A serum metabolomic investigation on hepatocellular carcinoma patients by chemical derivatization followed by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:3061–8. doi: 10.1002/rcm.3708. [DOI] [PubMed] [Google Scholar]
- 14.Gao H, Dong B, Liu X, Xuan H, Huang Y, Lin D. Metabonomic profiling of renal cell carcinoma: high-resolution proton nuclear magnetic resonance spectroscopy of human serum with multivariate data analysis. Anal Chim Acta. 2008;624:269–77. doi: 10.1016/j.aca.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Xu B, Huang J, Jia X, Xue J, Shi X, et al. 1H NMR-based metabonomic and pattern recognition analysis for detection of oral squamous cell carcinoma. Clin Chim Acta. 2009;401:8–13. doi: 10.1016/j.cca.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Bathe OF, Shaykhutdinov R, Kopciuk K, Weljie AM, McKay A, Sutherland FR, et al. Feasibility of identifying pancreatic cancer based on serum metabolomics. Cancer Epidemiol Biomarkers Prev. 2011;20:140–7. doi: 10.1158/1055-9965.EPI-10-0712. [DOI] [PubMed] [Google Scholar]
- 17.Hori S, Nishiumi S, Kobayashi K, Shinohara M, Hatakeyama Y, Kotani Y, et al. A metabolomic approach to lung cancer. Lung Cancer. 2011 doi: 10.1016/j.lungcan.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Jordan KW, Adkins CB, Su L, Halpern EF, Mark EJ, Christiani DC, et al. Comparison of squamous cell carcinoma and adenocarcinoma of the lung by metabolomic analysis of tissue-serum pairs. Lung Cancer. 2010;68:44–50. doi: 10.1016/j.lungcan.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda J, Higashiyama M, Imaizumi A, Nakayama T, Yamamoto H, Daimon T, et al. Possibility of multivariate function composed of plasma amino acid profiles as a novel screening index for non-small cell lung cancer: a case control study. BMC Cancer. 2010;10:690. doi: 10.1186/1471-2407-10-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spitz MR, Hong WK, Amos CI, Wu X, Schabath MB, Dong Q, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst. 2007;99:715–26. doi: 10.1093/jnci/djk153. [DOI] [PubMed] [Google Scholar]
- 21.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–82. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 22.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–53. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 23.Lawton KA, Berger A, Mitchell M, Milgram KE, Evans AM, Guo L, et al. Analysis of the adult human plasma metabolome. Pharmacogenomics. 2008;9:383–97. doi: 10.2217/14622416.9.4.383. [DOI] [PubMed] [Google Scholar]
- 24.Ryter SW, Morse D, Choi AM. Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am J Respir Cell Mol Biol. 2007;36:175–82. doi: 10.1165/rcmb.2006-0333TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin JP, O’Donnell CJ, Schwaiger JP, Cupples LA, Lingenhel A, Hunt SC, et al. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation. 2006;114:1476–81. doi: 10.1161/CIRCULATIONAHA.106.633206. [DOI] [PubMed] [Google Scholar]
- 26.Novotny L, Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Exp Biol Med (Maywood) 2003;228:568–71. doi: 10.1177/15353702-0322805-29. [DOI] [PubMed] [Google Scholar]
- 27.Horsfall LJ, Rait G, Walters K, Swallow DM, Pereira SP, Nazareth I, et al. Serum bilirubin and risk of respiratory disease and death. JAMA: the journal of the American Medical Association. 2011;305:691–7. doi: 10.1001/jama.2011.124. [DOI] [PubMed] [Google Scholar]
- 28.O’Malley SS, Wu R, Mayne ST, Jatlow PI. Smoking Cessation Is Followed by Increases in Serum Bilirubin, an Endogenous Antioxidant Associated With Lower Risk of Lung Cancer and Cardiovascular Disease. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2014 doi: 10.1093/ntr/ntu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frost-Pineda K, Liang Q, Liu J, Rimmer L, Jin Y, Feng S, et al. Biomarkers of potential harm among adult smokers and nonsmokers in the total exposure study. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2011;13:182–93. doi: 10.1093/ntr/ntq235. [DOI] [PubMed] [Google Scholar]
- 30.How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): 2010. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.