Abstract
The formation of a neurite, the basis for axons and dendrites, begins with a concerted accumulation and organization of actin and microtubules. While much is known about proteins that play a role in these processes, as they perform similar functions in axon branching and filopodia formation, much remains to be discovered how individual cytoskeletal regulators interact to form a neurite. Here we review the literature regarding different models of filopodial formation and how proteins that control actin organization and polymerization induce neurite formation. While several different regulators of actin polymerization play roles in neurite initiation there is redundancy between these regulators, as the effects of loss of a single regulator can be mitigated by addition of neurite promoting substrates and proteins. Similar to actin dynamics, both microtubule stabilizing and destabilizing proteins play a role in neurite initiation. Furthermore, interactions between the actin and microtubule cytoskeleton are required for neurite formation. Several lines of evidence indicate that the interactions between these two components of the cytoskeleton are needed for force generation as well as localization of microtubules at sites of nascent neurites. The general theme that emerges is the existence of several central regulatory pathways that extracellular cues converge on which can control and organize both actin and microtubules to induce the formation of neurites.
Keywords: neurite initiation, neuritogenesis, actin, microtubules, filopodia
Introduction
Neurite extension is the essential first step in the formation of axons and dendrites, necessary components to develop a functional neuronal network. While extracellular cues influence neurite outgrowth, it is the reorganization of the actin cytoskeleton which ultimately drives morphological change, and thus this review focuses on the cytoskeleton in the context of neurite formation (reviewed in da Silva and Dotti, 2002). A neurite is formed by regulating the cytoskeleton within the neuronal cell body to produce filopodia, which serve as the first step in neurite formation. Historically it is thought that this is accomplished through actin and microtubule networks fulfilling separate, independent roles, but several adaptor proteins that link the two networks have demonstrated that these two networks act in concert to form a neurite. Neurofilaments are neuron specific intermediate filaments that regulate the cytoskeleton and axon transport (Yuan et al., 2012). While nerve growth factor (NGF) induced neurite formation is associated with an increase in neurofilament expression, knockdown and antibodies against specific neurofilaments prevents the differentiation of neurites into axons but does not appear to not play a major role in the initiation of neurites, thus this review will focus on the actin and microtubule cytoskeleton (Lin and Szaro, 1996; Lindenbaum et al., 1988; Shea and Beermann, 1999; Szaro et al., 1991). The current concepts for understanding how neurites form are summarized in Figure 1, and discussed in later sections of this review.
Fig. 1.
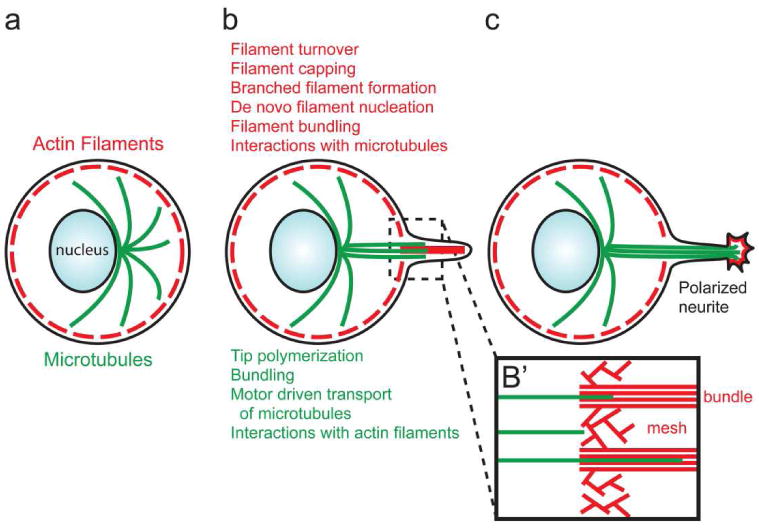
Overview of cytoskeletal dynamics and organization during neurite formation. (a) Prior to the emergence of neurites the neuronal cell body exhibits a submembranous actin filament (red) cytoskeleton that does not engage in protrusive activity. Microtubules (green) are formed at the perinuclear centrosome and emanate toward the periphery. (b) One of the major events of neurite formation is the initiation of actin filament based protrusions from the periphery of the neuronal cell body. Microtubules and actin filaments are coordinated during this phase leading to the formation of a neurite. (b') Microtubules exhibit a preference for extending along filopodial bundles of actin filaments, relative to the more geometrically complex mesh of interconnected filaments characteristic of lamellipodia. Thus, filopodial actin filament bundles serve as guides for the orchestration of these two components of the cytoskeleton. (c) Following the invasion of the filopodium by microtubules, the filopodium then develops polarity (i.e., develops a protrusive growth cone at its tip) and is now considered a neurite. The entry of microtubules allows for the transport of a multitude of molecular cargoes and organelles into the nascent neurite.
The in vivo observation of neurite initiation has proven difficult due to the experimental requirements. Thus, much of the available data utilize in vitro dissociated cultures which enable close spatial and temporal observation. Primary dissociated hippocampal and cortical cultures replicate many of the neuronal structures seen during development, permitting observation of axon and dendrite formation and later, synapse formation (Banker and Goslin, 1988). Manipulation of these cultures is often technically challenging, thus for the study of neurite formation, neuroblastoma cells or NGF treated PC12 cells, a cell line derived from a pheochromocytoma of the rat adrenal medulla, are often used. These alternative model systems initiate neurites that resemble precursors of axons and dendrites, with similar but not identical cytoskeletal organization, but do not develop further into mature axons and dendrites (Dotti et al., 1988).
Actin dynamics during neurite initiation
The initiation of a neurite begins with the formation of an actin rich filopodia from the neuronal cell body, followed by a broadening of the filopodia into a neurite. However, the specific mechanism that coordinates the actin cytoskeleton to form filopodia remain unclear (Faix et al., 2009; Mattila and Lappalainen, 2008; Mellor, 2010; Svitkina et al., 2003; Vignjevic et al., 2006). The two prevailing models for the initiation of filopodia are the convergent elongation model and the de novo nucleation model. The convergent elongation model proposes that branched actin filament networks formed by the Arp2/3 complex within lamellipodia are elongated by factors such as anti-capping proteins and then bundled into filopodia through proteins such as fascin (Svitkina et al., 2003). The de novo nucleation model proposes that filopodia are formed through factors that nucleate actin and elongate actin fibers in a single direction which are then crosslinked into filopodial actin bundles (Vignjevic et al., 2006). These two models of filopodial formation are not necessarily exclusive, and the mechanisms for filopodial formation in specific neuronal populations may depend on the cell type and environment.
Actin binding proteins regulate actin polymerization and organization
The actin cytoskeleton that gives rise to filopodia is regulated by a large array of actin-binding proteins, which control the nucleation, polymerization and organization of actin within the cell. The Arp2/3 complex, a major component of the convergent elongation model, is an actin nucleating complex that binds to existing actin filaments and nucleates new filaments resulting in a branched actin filament network (Dotti et al., 1988; Yang and Svitkina, 2011). Interestingly, Dip1 a novel activator of the Arp2/3 complex can give rise to single filaments without the need of other actin filaments and does not generate branched filaments potentially blurring the line between the “orthodox” forms of the de novo nucleation and convergent elongation models (Wagner et al., 2013). The role of the Arp2/3 complex with respect to neurite formation is unclear. Several observations using DIC microscopy on primary hippocampal neurons suggest that the major force driving filopodial formation in neurites is through convergent elongation. These studies clearly demonstrate that broad lamellipodia form along the cell body, which then segment at certain locations, and are followed by an accumulation of microtubules in an ordered array and an extension away from the cell body (Dehmelt et al., 2003; Dotti et al., 1988; Tang and Goldberg, 2000; Yu et al., 2001). However, loss of function of the Arp2/3 complex in hippocampal neurons, through siRNA mediated knockdown of the Arp3 and p34-Arc subunits, does not inhibit neurite formation, instead there is an increase in irregular neurites which are shorter and broader (Korobova and Svitkina, 2008). In contrast, overexpression of the Arp2/3 complex activator N-WASP in hippocampal cultures increases the total number of neurites (Pinyol et al., 2007). In a third study, expression of peptides that prevent activation of the Arp2/3 complex had no effect on hippocampal neuron axon formation or total dendrite number (Strasser et al., 2004). While the specific role of the Arp2/3 complex in neurite initiation remains unclear, these data suggest that neurite initiation by the Arp2/3 complex may be dependent on precise levels of the nucleation of a branched actin network. This is supported by data in non-neuronal cultures demonstrating that actin capping proteins, which prevent polymerization at the barbed end of actin, can contribute to Arp2/3 mediated filament branching (Bear and Gertler, 2009; Skoble et al., 2001). It is thought that when actin filaments are capped, more G-actin is available for Arp2/3 nucleation instead of filament elongation, which leads to the preferential formation of branched actin networks, however if and to what extent anti-capping influences Arp2/3 mediated branching in neurons remains unknown (Akin and Mullins, 2008; Korobova and Svitkina, 2008; Mogilner and Rubinstein, 2005).
F-BAR proteins have primarily been studied for their role in endocytosis, but in recent years several F-BAR proteins including Cdc42-interacting protein 4 (CIP4) have also been shown to play a role in filopodial and lamellipodial protrusion (Carlson et al., 2011; Guerrier et al., 2009; Lee et al., 2010; Saengsawang et al., 2012). CIP4 is an adaptor protein that links negatively-charged membrane phosopholipids through its F-BAR domain, binds active Cdc42 through its HR1 domain and other actin associated proteins through its SH3 domain (Aspenstrom, 2009; Carnahan and Gould, 2003; Heath and Insall, 2008; Roberts-Galbraith and Gould, 2010). CIP4 overexpression in cortical neurons results in lamellipodial formation around the cell body and inhibits neurite formation, whereas CIP4 null cortical neurons initiate neurites twice as fast as controls (Saengsawang et al., 2012). This effect is dependent on both the F-BAR domain to localize CIP4 to the leading edge of lamellipodia and the SH3 domain, which does not function through Arp2/3 complex, and may act through formins to nucleate and elongate actin or Ena/VASP anticapping proteins (Saengsawang et al., 2013). These data demonstrate that contrary to its role in non-neuronal cells, in neuronal cells CIP4 appears to decrease neurite formation through controlling lamellipodia and not through regulation of endocytosis or filopodia formation.
Controlling actin polymerization and depolymerization in neurite formation
The actin cytoskeleton is also modified by controlling actin barbed end polymerization, forming long actin filaments through anticapping proteins such as Ena/VASP (Reviewed in Menna et al., 2011). Actin filament polymerization occurs through addition of G-actin to the barbed end, thus capping of the barbed end prevents polymerization (Menna et al., 2011). In the de novo nucleation model, Ena/VASP, which is localized to the plasma membrane, initiates the formation of a filopodia by encouraging actin filament growth at the membrane outward into a long extension, (Breitsprecher et al., 2008). In the convergent elongation model, branched actin networks are reorganized into a bundle, and filaments within this bundle are protected from capping by Ena/VASP to form a filopodia (Svitkina et al., 2003). Interestingly, the actin bundling protein fascin which in and of itself is dispensable for neurite formation, can increase the efficiency of Ena/VASP associated actin polymerization, indicating a possible cooperative role between actin organization and actin filament polymerization in neurite initiation (Winkelman et al., 2014; Yamakita et al., 2009). Cultured Ena/VASP deficient cortical neurons produce no filopodia, and subsequently no neurites, and instead exhibit broad lamellipodia (Kwiatkowski et al., 2007). While loss of Ena/VASP expression prevents the formation of neurites, these effects can be mitigated by culturing neurons on filopodia promoting substrates such on laminin and fibroblasts or expressing other anticapping proteins such as mDia2, or expression of myosin X, an unconventional tip-complex protein (Dent et al., 2007). These data demonstrate that there are several mechanisms that can be used in the generation of the filopodial actin cytoskeleton, indicating that the net regulation of localized actin polymerization and nucleation is the guiding principle, and multiple actin regulatory proteins or pathways can achieve the same endpoint.
The actin cytoskeleton is a dynamic structure and studies of the roles of additional actin regulatory proteins illustrate how the balance of actin polymerization may lead to the formation of neurites. Knockdown of actin depolymerizing factor (ADF)/cofilin, which promotes the pointed end depolymerization of actin filaments and can also sever filaments, inhibits neurite extension in both PC12 cells and chick dorsal root gangla (DRG) neurons (Endo et al., 2007). The current model is that ADF/cofilin increases actin turnover, the rate at which filaments are recycled into monomers. Observations in non-neuronal cells demonstrating that ADF/cofilin is localized at the base of branched actin arrays during lamellipodia protrusion, which would disassemble actin continuously toward the rear while actin is added to the leading edge, provide a mechanism for how ADF/cofilin may generate a neurite (Svitkina and Borisy, 1999). Consistently, genetic ablation of ADF/cofilin greatly impairs the emergence of neurites from cortical neurons in vitro and in vivo (Flynn et al., 2012). This study also provided evidence that the actin filament severing activity of ADF/cofilin is more relevant to its regulation of neurite formation than its role in promoting filament pointed end depolymerization. Loss of ADF/cofilin activity also has consequences for the ability of microtubules to penetrate the periphery of the neuronal cell body and give rise to the backbone of the emergent neurite, likely through the regulation of actin filament organization in the periphery of the neuronal cell body. Thus, through the regulation of actin filament dynamics during early stages of neurite formation, ADF/cofilin contributes to the orchestration of the actin filament and microtubule reorganization required for neurite formation.
Opposite in function to cofilin is profilin, an actin monomer binding protein that promotes actin filament polymerization in a variety of cell types (Haarer et al., 1990; Haugwitz et al., 1994; Kang et al., 1999; Pantaloni and Carlier, 1993; Verheyen and Cooley, 1994). Cultured hippocampal neurons from the profilin lla knockout mice exhibit more neurites, and overexpression produces the opposite effects, indicating that profilin is a negative regulator of neurite initiation and elongation (Da Silva et al., 2003). The knockout mice also display increased levels of G-actin and less F-actin and the converse is true in profilin overexpressing cells (Da Silva et al., 2003). These data on cofilin and profilin all serve to illustrate that the balance of actin polymerization and depolymerization contributes to neurite formation, possibly in a localized manner around the neuronal cell body.
Tropomyosins, which form polymers along the major groove of actin filaments, can induce neurite formation in neuroblastoma cells as well as interact with several actin binding proteins suggesting a possible mechanism of neurite regulation (Curthoys et al., 2013). In mammalian cells there are over 40 different isoforms of tropomyosin, several of which can compete with actin binding proteins such as fascin, which bundles actin and promotes actin polymerization, or increase the inactive fraction of ADF/cofilin when overexpressed in neuroblastoma cells (Bryce et al., 2003; Creed et al., 2011; Gunning, 2008).
Myosin II is an actin filament binding force generating motor protein which can inhibit or promote the extension of differentiated axons in a context dependent manner (Ketschek et al., 2007). Inhibition of myosin II function in cultured chicken forebrain neurons promotes the elongation of neurites from neuronal cell bodies, but the neurons generate approximately 20% fewer neurites (Kollins et al., 2009). In the same study, inhibition of RhoA activity, which drives myosin II contractility as well as multiple other effector systems, increased both the number of neurites and their lengths. These observations indicate that functional outcome of individual final effectors (e.g., myosin II) may yield different results when compared to the inactivation of the effectors when upstream positive regulators are also inhibited (e.g., RhoA), which instead result in a coordinated change in the function of multiple effector systems.
While much remains to be discovered about the function of these actin regulatory proteins and how they specifically control actin dynamics during neurite formation, evidence indicates that their activity plays a role in the balance of actin-based neurite extension. Overall, the literature indicates that neurons can utilize a variety of actin filament regulatory proteins to orchestrate the initial stages of neurite formation. However, the underlying theme is one of localized differences in actin filament dynamics and organization leading to the localized emergence of the nascent neurite. A major missing component of the story is the dynamics and organization of actin filaments in neuronal cell bodies prior to the emergence of protrusive structures (e.g., filopodia). Recent super-resolution microscopic studies have unveiled novel forms of actin filament organization in mature axons, and the application of these modern methods will likely provide important insights into the actin filament cytoskeleton of the neuronal cell body prior to and during the formation of neurites (Xu et al., 2013).
Regulation of microtubules during neuritogenesis
Mature neurites, both axons and dendrites, are supported by a microtubule cytoskeleton consisting of bundled microtubules. The extension of microtubules into actin rich filopodia is a fundamental step in the formation of a neurite. The primary subunit of microtubules is a heterodimer of one α and one β-tubulin polypeptide and several isoforms of α and β-tubulin have an increased expression during neurite formation and NGF induced neurite initiation (Joshi and Cleveland, 1989; Knoops and Octave, 1997; Sullivan, 1988). Type III β-tubulin is the only neuron specific isoform and it has also been shown to be phosphorylated following NGF induced neurite formation, but the function of this phosphorylation is unclear (Aletta, 1996).
Microtubules can be extended into the filopodia by distributing stabilized microtubules into the actin rich protrusions or through polymerization, both of which contribute to the maturation of filopodia into neurites in both cortical and sympathetic neuronal cultures (Dent et al., 2007; Smith, 1994). Expression of microtubule based motor proteins that can move filaments, such as the + end directed kinesin, is sufficient to elicit formation of neurite-like structures in cultured insect Sf9 cells, and the emergent neurites from these experiments have microtubule polarity resembling that of dendrites and axons depending on the motor protein (Sharp et al., 1996; Sharp et al., 1997). Similarly, the microtubule associated protein 2c (MAP2c) induced formation of neurites is dependent on the - end directed motor protein dynein, and function blocking dynein antibodies in neuro2a cells significantly inhibit neurite-like protrusions (Dehmelt et al., 2006). These data in conjunction with the observation that the maximal effect of taxol, a drug that stabilizes microtubules, is a 50% inhibition of neurite formation suggest that motor dependent rearrangement of stable microtubules likely plays an important role in neurite formation (Letourneau and Ressler, 1984).
Regulation of microtubules by MAPs
Similar to actin dynamics, both microtubule stabilizing proteins, such as MAP1b, and destabilizing proteins, such as stathmin-like 2, play a role in neurite initiation (Dehmelt et al., 2003; Li et al., 2009; Riederer, 2007; Teng et al., 2001; Vandecandelaere et al., 1996). MAP1b, promotes microtubule nucleation, polymerization and stabilization both in vitro and in vivo, and siRNA knockdown in PC12 cells inhibits NGF induced neurite initiation (Brugg et al., 1993; Pedrotti and Islam, 1995; Takemura et al., 1992; Vandecandelaere et al., 1996). Interestingly, the neuron specific protein stathmin-like 2, which can bind to microtubules, inhibit their assembly and induce microtubule disassembly, when overexpressed in PC12 cells increases the number of neurites and when knocked down in hippocampal cells result in axons less likely to form, indicating that a balance of polymerization and depolymerization is required (Riederer et al., 1997).
In addition to their role in microtubule stabilization, MAPs can also play a role in their transport. The embryonic isoform of MAP2, MAP2c, is expressed in neuroblasts preceding neurite formation and triggers neurite initiation (Dehmelt et al., 2003). Live imaging in these neuro-2a cells shows that MAP2c triggers neurite formation through rapid accumulation and bundling of stable MAP2c bound microtubules, and in hippocampal neurons loss of either the MAP2c microtubule binding domain or the protein kinase A binding domain of MAP2c impairs neurite formation (Dehmelt et al., 2006). The expression of the higher molecular weight MAP2a and MAP2b isoforms generally occurs after neurite growth and during neuron maturation, however their expression has been observed in developing neuroblasts in the optic tectum preceding differentiation into multipolar cells, but the roles of MAP1a and MAP2b with respect to neurite initiation in this case are unknown (Matus et al., 1990; Schoenfeld and Obar, 1994; Yasuda and Fujita, 2003).
Coordinating microtubules and actin during neurite formation
MAP2c also binds actin filaments through its microtubule binding domain, which is necessary to promote neurite formation, suggesting that it may physically link both the actin and microtubule cytoskeleton (reviewed in Dehmelt and Halpain, 2004; Kim et al., 1979; Roger et al., 2004). Based on data from non-neuronal cells and Aplysia growth cones, these interactions suggest that by linking microtubules to the actin filament network, it may be possible for motors such as dynein or kinesin, which play a role in neurite formation, to use actin as a scaffold to move microtubules and generate force to push the cell membrane outward (Dehmelt et al., 2006; Lu et al., 2013; Salmon et al., 2002; Schaefer et al., 2002). Microtubules have also been observed to align with actin filament bundles in Aplysia growth cones, suggesting that filopodial actin filament bundles may serve to capture and guide microtubules during the early steps of neurite formation (Schaefer et al., 2002). Following axotomy, microtubules can push on the membrane and give rise to filopodia-like protrusions, and this process also occurs in the presence of actin polymerization blocking drugs (Goldberg and Burmeister, 1992). Furthermore, neurite initiation can occur in the presence of actin depolymerizing drugs (Goldberg and Burmeister, 1992; Lu et al., 2013). These data indicate that if actin does provide a scaffold for motors to move microtubules against the cell membrane there is functional redundancy, such as using microtubule on microtubule sliding to generate force, to move the membrane and form neurites (Lu et al., 2013).
In a similar vein Drebrin, an actin filament binding protein, regulates both actin and microtubule dynamics (Geraldo et al., 2008). Drebrin is a major actin-binding protein expressed in the brain during neuronal development that plays a role in neuronal migration and formation of neurite-like processes (Dun et al., 2012; Dun and Chilton, 2010; Hayashi et al., 1996; Mizui et al., 2009). Drebrin binds to the side of single actin filaments where it competes with actin filament binding proteins such as α-actinin, tropomyosin and fascin in vitro and in vivo (Biou et al., 2008; Ishikawa et al., 1994; Sasaki et al., 1996). Interestingly, while Drebrin appears to be a positive regulator of neurite formation, several of the actin filament binding proteins it competes with also appear to positively regulate neurite initiation, such as α-actinin, which when knocked down in neuroblastoma cells inhibits neurite formation and tropomyosin isoforms which when overexpressed induce neurite formation (Curthoys et al., 2013; Torii et al., 2012). In addition to this activity, Drebrin also binds the microtubule regulator EB3, a protein that binds microtubule + ends, suggesting that by linking EB3 to actin, Drebrin may also target microtubule tips to actin rich filopodia (Bazellieres et al., 2012; Geraldo et al., 2008). While much remains to be learned about how the actin and microtubule cytoskeletal systems interact, these studies indicate that adaptor molecules linking microtubules to actin filaments represent a major regulatory point to be considered in addition to polymerization and organization.
Collectively, these studies indicate that microtubule polymerization and dynamics, their organization (e.g., bundling) and motor protein driven processes are coordinated to contribute to the microtubule-based component of neurite formation. As with actin filaments, a thorough understanding of the organization of microtubules in the neuronal cell body and during the initial steps of the process of neurite formation is lacking. 3-dimensional reconstructions of cultured sensory neurons with established axons demonstrate this complex organization of microtubules in the cell body (Letourneau and Wire, 1995). These studies demonstrate that microtubules form a cage around the nucleus and serve as potential organizers of the Golgi apparatus within the cell body. Microtubules associated with the Golgi also give rise to the bundles entering neurites, possibly providing a direct substratum for transport based mechanisms into the neurites. However, the dynamics and reorganization of microtubules in the transition from a round cell body to one exhibiting neurites largely remain to be determined.
Extrinsic cues and the signaling pathways underlying neurite formation
Extracellular cues, such as NGF and brain-derived neurotrophic factor (BDNF) regulate filopodia, lamellipodia and neurite formation though several common signaling pathways that control the cytoskeleton (Reviewed in Gonzalez-Billault et al., 2012; Jackson et al., 1996; Mai et al., 2009). Local calcium and cAMP/cGMP gradients can mediate filopodia formation and neurite growth in response to these cues (Emery et al., 2014; Gysbers et al., 2000; Mai et al., 2009; Shelly et al., 2010; Zheng and Poo, 2007). Hippocampal cells cultured on stripes of membrane permeable fluorescent analogs of cAMP or cGMP demonstrate that localized cAMP and cGMP activities were sufficient to induce formation of axons and dendrites, respectively (Shelly et al., 2010). GTP enhances NGF dependent neurite formation in PC12 cells and increases intracellular calcium (Gysbers et al., 2000; Gysbers and Rathbone, 1996a, b). This neurite enhancing effect is attenuated by blocking L-type calcium channels or preventing intracellular calcium store release, indicating that calcium plays a role in neurite initiation (Gysbers et al., 2000).
Adding NGF to PC12 cells induces transient activation of the GTPases Rac1 and Cdc42 at the cell periphery and localized cycling of activity at the motile tips of filopodia (Aoki et al., 2004). Expressing constitutively active Rac1 promotes neurite formation and inactivation of Rac inhibits neurites in several cell types, including chick primary retinal neurons and rat hippocampal neurons (Albertinazzi et al., 1998; Schwamborn and Puschel, 2004). Recruitment and activation of Rac1 to actin filament rich protrusions is also associated with a decrease in the GTPase RhoA, that when constitutively active can prevent BDNF dependent neurite initiation and induce neurite retraction (Da Silva et al., 2003; Izawa et al., 1998; Katoh et al., 1998; Sebok et al., 1999; Yamaguchi et al., 2001). It should be noted that these signaling pathways are highly regulated and can produce different cellular responses under different cellular conditions. For example, both constitutively active Rac1 and Cdc42 expressed in Drosophila giant fiber neurons inhibit neurite outgrowth and dominant negative Rac1 can promote neurite formation in chicken DRG cells (Allen et al., 2000; Fournier et al., 2003). These data suggest that the ideal conditions for neurite initiation may not solely be the product of constitutively “on” or “off” GTPases, rather ideal neurite initiation may require specific localization of GTPases or the temporal cycling of GTPases “on and off”, which can for example cause Cdc42 to induce supernumerary axons in hippocampal cells (Schwamborn and Puschel, 2004). These studies indicate that neurite promoting signals converge on several common pathways, Rac and Cdc42 being central regulators of neurite initiation and Rho inhibiting neurite initiation and inducing neurite retraction, but the functional outcome of the activity of these GTPases has to be considered in the context of the additional consideration such as the temporal control of activation, cell type, substratum, and growth factors.
A likely mechanism for how neurotrophin signaling can control Rac1, Cdc42 and RhoA is suggested by live imaging of filopodia formation on chicken DRG axons, which demonstrates that NGF increases the formation of microdomains of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which colocalizes with fluorescently labeled actin patches from which filopodia emerge (Ketschek and Gallo, 2010). TrkA is the tyrosine receptor kinase that activates PI 3-kinase (PI3K), which is required for NGF activation of both Rac1 and Cdc42 in PC12 cells as well as inhibition of RhoA (Aoki et al., 2004; Nusser et al., 2002). Likewise, BDNF signaling through the TrkB receptor activates PI3K and causes in vivo accumulation of PIP3 and increases dendritic filopodial motility and number in hippocampal dendrites (Luikart et al., 2008). PI3K signaling can activate Rac and Cdc42 which are upstream targets of several cytoskeleton modifying proteins such as the Arp2/3 complex, which as discussed earlier may play a role in neurite formation (Derivery and Gautreau, 2010; Gallo, 2010). In summary, the proposed cascade for how neurotrophins can induce neurites consists of: NGF/BDNF, TrkA/TrkB, PI3K, increased local PIP3, and an increase in Cdc42 and Rac with a decrease in RhoA signaling. While roles for Rac1, Cdc42 and RhoA as major regulatory points in the initiation of neurites are overall well established, much remains to be determined as to how they serve to specifically regulate the cytoskeleton into initiating a neurite. Nakamura et al. (2008) have merged multiple lines of evidence regarding the spatio-temporal patterns of PIP3, Rac1 and its activator Vav, with the SHIP2 phosphatase in PC12 cell line to generate a quantitative model consistent with biological data for the determining the sites of neurite emergence (Figure 2). In this model, activation of TrkA, the receptor for NGF, results in the formation of PI3K-Vav-Rac1 domains of activity which are initially homogenous around the perimeter of the cell. However, through a Turing reaction-diffusion system involving a SHIP2 phosphatase negative feedback loop, PIP3 is converted to PI(3,4)P2 in long lateral domains, allowing localized domains of PIP3-Rac1 activity to form at sites of the lower diffusing Vav. Experimental analysis of this model using subcellularly localized activation/inactivation approaches targeting the proposed pathway will be required to test this intriguing model.
Fig. 2.
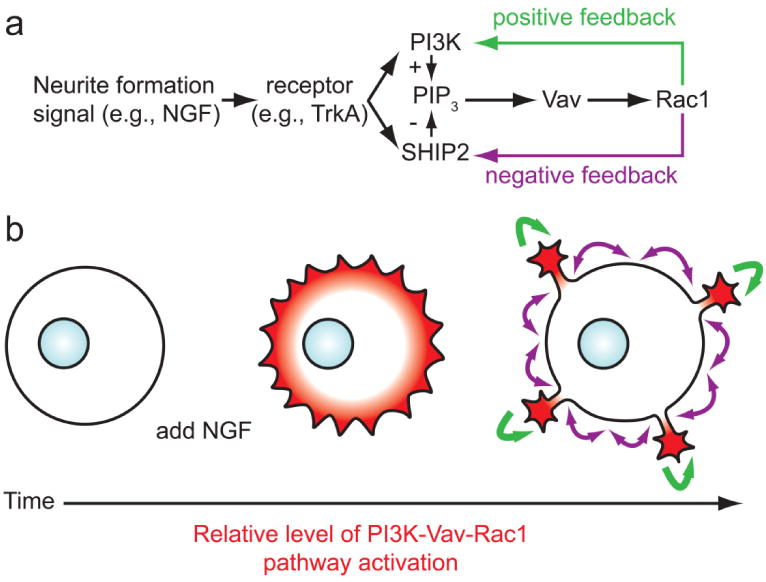
Hypothetical model for the formation of localized domains of PI3K-Rac1 signaling that determine sites of neurite formation from the cell body. (a) This model is derived from studies of NGF-induced neurite formation from PC12 cells, for further details on this model please see the paper initially describing it by Nakamura et al. (2008). The model is based on the activation of PI3K and the antagonistic phosphatase SHIP2 by NGF. Initially, PI3K driven increases in PIP3 levels at the membrane drive the activation of Rac1 by Vav. Rac1 then signals back to PI3K and SHIP2 through positive (PI3K) and negative (SHIP2) feedback loops. The diffusion coefficient of SHIP2 is greater than Vav, consistent with the requirement for the negative and positive feedback loops in the context of a Turing's reaction-diffusion system (Aoki et al., 2005). (b) The function of the mechanism in panel (A) is to drive the initially homogenous activation of the PI3K-Rac1 pathway (red) at the perimeter of the neuronal cell body into discrete domains of activation. The discrete domains are then able to locally drive the formation of neurites through the positive feedback loop, while suppressing formation elsewhere along the perimeter through the negative feedback loop.
Organelle distribution during neurite formation
Mitochondria generate ATP which can be used locally in the vicinity of the mitochondrion or diffuse throughout the cytoplasm to serve the high ATP demands of neuronal development, such as that of actin polymerization (Bernstein and Bamburg, 2003; Jones, 1986). Recent work has identified localized functions of mitochondria along axons during development and a similar role for mitochondria may be at work in the cell body, contributing to the determination of the sites of neurite formation (Courchet et al., 2013; Spillane et al., 2013; Sun et al., 2013; Tao et al., 2013). Stalled mitochondria and their respiration, contribute to defining sites of the axon at which collateral branches will emerge (Courchet et al., 2013; Spillane et al., 2013; Tao et al., 2013). The formation of a collateral branch, similar to the initiation of a neurite from the cell body, is initiated by the emergence of an axonal filopodium (Gallo, 2010). The sites of formation of axonal filopodia are in large part determined by mitochondria positioning along the axon and their respiration (Ketschek and Gallo, 2010; Spillane et al., 2013; Tao et al., 2013). While the role of mitochondria positioning within the cell body during initial neurite formation has not been thoroughly investigated, in cultured hippocampal neurons mitochondria have been reported to be positioned at the base of the neurite destined to mature into an axon, at the expense of mitochondria targeting into neurites that will differentiate into dendrites (Mattson and Partin, 1999). In another study, preferential targeting of mitochondria into the nascent axon was observed in the same neuronal population, but not aggregation at the base of the axon (Ruthel and Hollenbeck, 2003). It will thus be of interest to further determine the possible contributions of mitochondria to the emergence and differentiation of neurites.
Similar to mitochondria, the repositioning of the centrosome and golgi apparatus have been found to correlate with neurite formation in some studies, but a relationship between these organelles and sites of neurite emergence has not been found (Caceres et al., 2012). Additionally, the endoplasmic reticulum might play a role in neurite formation by locally delivering signaling components, similar to how Rac1 activity induces focal release of calcium from the endoplasmic reticulum in Aplysia growthcones, (Zhang and Forscher, 2009). Blocking calcium release from intracellular stores inhibits neurite outgrowth, and endoplasmic reticulum related vesicle trafficking may provide spatial specificity in delivering calcium stores to sites of neurite initiation (Gysbers et al., 2000). It will be of interest to determine how neurite formation induced by different extracellular signals may differ in these responses at the cytological level as suggested by a study on N-cadherin mediated neurite formation in hippocampal cultures, which correlates centrosome and golgi repositioning and neurite formation with contact with N-cadherin extracellular domain coated beads but not Tenascin C beads (Gartner et al., 2012). Although a clear picture of how organelle distribution may be related to the site of neurite formation has yet to emerge, this will likely be an interesting venue for continued analysis.
Conclusions and perspectives
All together the available information indicates the need for considering the initiation of neurite formation at the systems level. Investigation of specific proteins will yield potential components of the mechanism of neurite initiation, but as a system, the mechanism can likely utilize different proteins with redundant functions. While much is known about how single molecules control actin or microtubules, the next step will be to understand how these molecules act in concert to control neurite initiation. Several adaptors physically link actin filaments and microtubules and are necessary for neurite formation. However, much remains to be understood about interplay between these two components of the cytoskeleton with regards to neurite formation, but imparting mechanical forces for growing or moving microtubules and the flow of actin filaments are likely mechanisms. Several extracellular cues converge on central pathways mediated by Rho GTPases. The GTPases Rac, Cdc42 and Rho control and organize both actin and microtubules to induce the formation of neurites, and the next step is to determine how these GTPases lead to the localized activation of key cytoskeletal regulators to initiate a neurite, and to further analyze the reaction-diffusion model proposed by Nakamura et al. (2008). Ultimately, our understanding of the mechanisms which determine how microtubule and actin filament based traffic regulate the early stages of neurite formation and coordinate with the underlying cytoskeletal remodeling will also need to be integrated into the spatio-temporal aspects of relevant signaling pathways (Reviewed by Villarroel-Campos et al., 2014). As noted in the main text, our understanding of the cytoskeletal organization of the neuronal cell body prior to neurite formation is lagging relative to neurons with established neurites. This information will be required in order to generate relevant models for how individual molecules, signaling pathways, and molecular modules operate to transform the periphery and center of the neuronal cell body during neurite formation. In addition to this, further investigation is needed to understand the role of membrane trafficking in neurite initiation, which can use actin based Ena/VASP or Arp2/3 exocytosis mechanisms to drive neurite formation, and may turn out to be a major requirement for neurite initiation (Gupton and Gertler, 2010). Understanding how the cytoskeleton is regulated at multiple levels, and how it is integrated with cytoplasmic reorganization, may help us understand not just how neurites are formed but will likely also provide insights into the greater context of growth cone turning, axon branching and possibly the restoration of neurites after injury.
Acknowledgments
This work was supported by an NIH award to GG (NS078030).
References
- Akin O, Mullins RD. Capping protein increases the rate of actin-based motility by promoting filament nucleation by the Arp2/3 complex. Cell. 2008;133:841–851. doi: 10.1016/j.cell.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertinazzi C, Gilardelli D, Paris S, Longhi R, de Curtis I. Overexpression of a neural-specific rho family GTPase, cRac1B, selectively induces enhanced neuritogenesis and neurite branching in primary neurons. The Journal of cell biology. 1998;142:815–825. doi: 10.1083/jcb.142.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aletta JM. Phosphorylation of type III beta-tubulin PC12 cell neurites during NGF-induced process outgrowth. Journal of neurobiology. 1996;31:461–475. doi: 10.1002/(SICI)1097-4695(199612)31:4<461::AID-NEU6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Shan X, Murphey RK. A role for Drosophila Drac1 in neurite outgrowth and synaptogenesis in the giant fiber system. Molecular and cellular neurosciences. 2000;16:754–765. doi: 10.1006/mcne.2000.0903. [DOI] [PubMed] [Google Scholar]
- Aoki K, Nakamura T, Fujikawa K, Matsuda M. Local phosphatidylinositol 3,4,5-trisphosphate accumulation recruits Vav2 and Vav3 to activate Rac1/Cdc42 and initiate neurite outgrowth in nerve growth factor-stimulated PC12 cells. Molecular biology of the cell. 2005;16:2207–2217. doi: 10.1091/mbc.E04-10-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Nakamura T, Matsuda M. Spatio-temporal regulation of Rac1 and Cdc42 activity during nerve growth factor-induced neurite outgrowth in PC12 cells. The Journal of biological chemistry. 2004;279:713–719. doi: 10.1074/jbc.M306382200. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P. Roles of F-BAR/PCH proteins in the regulation of membrane dynamics and actin reorganization. International review of cell and molecular biology. 2009;272:1–31. doi: 10.1016/S1937-6448(08)01601-8. [DOI] [PubMed] [Google Scholar]
- Banker G, Goslin K. Developments in neuronal cell culture. Nature. 1988;336:185–186. doi: 10.1038/336185a0. [DOI] [PubMed] [Google Scholar]
- Bazellieres E, Massey-Harroche D, Barthelemy-Requin M, Richard F, Arsanto JP, Le Bivic A. Apico-basal elongation requires a drebrin-E-EB3 complex in columnar human epithelial cells. Journal of cell science. 2012;125:919–931. doi: 10.1242/jcs.092676. [DOI] [PubMed] [Google Scholar]
- Bear JE, Gertler FB. Ena/VASP: towards resolving a pointed controversy at the barbed end. Journal of cell science. 2009;122:1947–1953. doi: 10.1242/jcs.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Actin-ATP hydrolysis is a major energy drain for neurons. J Neurosci. 2003;23:1–6. doi: 10.1523/JNEUROSCI.23-01-00002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biou V, Brinkhaus H, Malenka RC, Matus A. Interactions between drebrin and Ras regulate dendritic spine plasticity. The European journal of neuroscience. 2008;27:2847–2859. doi: 10.1111/j.1460-9568.2008.06269.x. [DOI] [PubMed] [Google Scholar]
- Breitsprecher D, Kiesewetter AK, Linkner J, Urbanke C, Resch GP, Small JV, Faix J. Clustering of VASP actively drives processive, WH2 domain-mediated actin filament elongation. The EMBO journal. 2008;27:2943–2954. doi: 10.1038/emboj.2008.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugg B, Reddy D, Matus A. Attenuation of microtubule-associated protein 1B expression by antisense oligodeoxynucleotides inhibits initiation of neurite outgrowth. Neuroscience. 1993;52:489–496. doi: 10.1016/0306-4522(93)90401-z. [DOI] [PubMed] [Google Scholar]
- Bryce NS, Schevzov G, Ferguson V, Percival JM, Lin JJ, Matsumura F, Bamburg JR, Jeffrey PL, Hardeman EC, Gunning P, et al. Specification of actin filament function and molecular composition by tropomyosin isoforms. Molecular biology of the cell. 2003;14:1002–1016. doi: 10.1091/mbc.E02-04-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A, Ye B, Dotti CG. Neuronal polarity: demarcation, growth and commitment. Current opinion in cell biology. 2012;24:547–553. doi: 10.1016/j.ceb.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BR, Lloyd KE, Kruszewski A, Kim IH, Rodriguiz RM, Heindel C, Faytell M, Dudek SM, Wetsel WC, Soderling SH. WRP/srGAP3 facilitates the initiation of spine development by an inverse F-BAR domain, and its loss impairs long-term memory. J Neurosci. 2011;31:2447–2460. doi: 10.1523/JNEUROSCI.4433-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan RH, Gould KL. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. The Journal of cell biology. 2003;162:851–862. doi: 10.1083/jcb.200305012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchet J, Lewis TL, Jr, Lee S, Courchet V, Liou DY, Aizawa S, Polleux F. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell. 2013;153:1510–1525. doi: 10.1016/j.cell.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed SJ, Desouza M, Bamburg JR, Gunning P, Stehn J. Tropomyosin isoform 3 promotes the formation of filopodia by regulating the recruitment of actin-binding proteins to actin filaments. Experimental cell research. 2011;317:249–261. doi: 10.1016/j.yexcr.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Curthoys NM, Freittag H, Connor A, Desouza M, Brettle M, Poljak A, Hall A, Hardeman E, Schevzov G, Gunning PW, et al. Tropomyosins induce neuritogenesis and determine neurite branching patterns in B35 neuroblastoma cells. Molecular and cellular neurosciences. 2013;58C:11–21. doi: 10.1016/j.mcn.2013.10.011. [DOI] [PubMed] [Google Scholar]
- da Silva JS, Dotti CG. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nature reviews Neuroscience. 2002;3:694–704. doi: 10.1038/nrn918. [DOI] [PubMed] [Google Scholar]
- Da Silva JS, Medina M, Zuliani C, Di Nardo A, Witke W, Dotti CG. RhoA/ROCK regulation of neuritogenesis via profilin IIa-mediated control of actin stability. The Journal of cell biology. 2003;162:1267–1279. doi: 10.1083/jcb.200304021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. Actin and microtubules in neurite initiation: are MAPs the missing link? Journal of neurobiology. 2004;58:18–33. doi: 10.1002/neu.10284. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Nalbant P, Steffen W, Halpain S. A microtubule-based, dynein-dependent force induces local cell protrusions: Implications for neurite initiation. Brain cell biology. 2006;35:39–56. doi: 10.1007/s11068-006-9001-0. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Smart FM, Ozer RS, Halpain S. The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J Neurosci. 2003;23:9479–9490. doi: 10.1523/JNEUROSCI.23-29-09479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Kwiatkowski AV, Mebane LM, Philippar U, Barzik M, Rubinson DA, Gupton S, Van Veen JE, Furman C, Zhang J, et al. Filopodia are required for cortical neurite initiation. Nature cell biology. 2007;9:1347–1359. doi: 10.1038/ncb1654. [DOI] [PubMed] [Google Scholar]
- Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. BioEssays : news and reviews in molecular, cellular and developmental biology. 2010;32:119–131. doi: 10.1002/bies.200900123. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun XP, Bandeira de Lima T, Allen J, Geraldo S, Gordon-Weeks P, Chilton JK. Drebrin controls neuronal migration through the formation and alignment of the leading process. Molecular and cellular neurosciences. 2012;49:341–350. doi: 10.1016/j.mcn.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun XP, Chilton JK. Control of cell shape and plasticity during development and disease by the actin-binding protein Drebrin. Histology and histopathology. 2010;25:533–540. doi: 10.14670/HH-25.533. [DOI] [PubMed] [Google Scholar]
- Emery AC, Eiden MV, Eiden LE. Separate Cyclic AMP Sensors for Neuritogenesis, Growth Arrest, and Survival of Neuroendocrine Cells. The Journal of biological chemistry. 2014;289:10126–10139. doi: 10.1074/jbc.M113.529321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Ohashi K, Mizuno K. LIM kinase and slingshot are critical for neurite extension. The Journal of biological chemistry. 2007;282:13692–13702. doi: 10.1074/jbc.M610873200. [DOI] [PubMed] [Google Scholar]
- Faix J, Breitsprecher D, Stradal TE, Rottner K. Filopodia: Complex models for simple rods. The international journal of biochemistry & cell biology. 2009;41:1656–1664. doi: 10.1016/j.biocel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Flynn KC, Hellal F, Neukirchen D, Jacob S, Tahirovic S, Dupraz S, Stern S, Garvalov BK, Gurniak C, Shaw AE, et al. ADF/cofilin-mediated actin retrograde flow directs neurite formation in the developing brain. Neuron. 2012;76:1091–1107. doi: 10.1016/j.neuron.2012.09.038. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. The cytoskeletal and signaling mechanisms of axon collateral branching. Developmental neurobiology. 2010;71:201–220. doi: 10.1002/dneu.20852. [DOI] [PubMed] [Google Scholar]
- Gartner A, Fornasiero EF, Munck S, Vennekens K, Seuntjens E, Huttner WB, Valtorta F, Dotti CG. N-cadherin specifies first asymmetry in developing neurons. The EMBO journal. 2012;31:1893–1903. doi: 10.1038/emboj.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldo S, Khanzada UK, Parsons M, Chilton JK, Gordon-Weeks PR. Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nature cell biology. 2008;10:1181–1189. doi: 10.1038/ncb1778. [DOI] [PubMed] [Google Scholar]
- Goldberg DJ, Burmeister DW. Microtubule-based filopodium-like protrusions form after axotomy. J Neurosci. 1992;12:4800–4807. doi: 10.1523/JNEUROSCI.12-12-04800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Billault C, Munoz-Llancao P, Henriquez DR, Wojnacki J, Conde C, Caceres A. The role of small GTPases in neuronal morphogenesis and polarity. Cytoskeleton (Hoboken, NJ) 2012;69:464–485. doi: 10.1002/cm.21034. [DOI] [PubMed] [Google Scholar]
- Guerrier S, Coutinho-Budd J, Sassa T, Gresset A, Jordan NV, Chen K, Jin WL, Frost A, Polleux F. The F-BAR domain of srGAP2 induces membrane protrusions required for neuronal migration and morphogenesis. Cell. 2009;138:990–1004. doi: 10.1016/j.cell.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P. Emerging issues for tropomyosin structure, regulation, function and pathology. Advances in experimental medicine and biology. 2008;644:293–298. doi: 10.1007/978-0-387-85766-4_22. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Developmental cell. 2010;18:725–736. doi: 10.1016/j.devcel.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysbers JW, Guarnieri S, Mariggio MA, Pietrangelo T, Fano G, Rathbone MP. Extracellular guanosine 5′ triphosphate enhances nerve growth factor-induced neurite outgrowth via increases in intracellular calcium. Neuroscience. 2000;96:817–824. doi: 10.1016/s0306-4522(99)00588-6. [DOI] [PubMed] [Google Scholar]
- Gysbers JW, Rathbone MP. GTP and guanosine synergistically enhance NGF-induced neurite outgrowth from PC12 cells. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 1996a;14:19–34. doi: 10.1016/0736-5748(95)00083-6. [DOI] [PubMed] [Google Scholar]
- Gysbers JW, Rathbone MP. Neurite outgrowth in PC12 cells is enhanced by guanosine through both cAMP-dependent and -independent mechanisms. Neuroscience letters. 1996b;220:175–178. doi: 10.1016/s0304-3940(96)13253-5. [DOI] [PubMed] [Google Scholar]
- Haarer BK, Lillie SH, Adams AE, Magdolen V, Bandlow W, Brown SS. Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. The Journal of cell biology. 1990;110:105–114. doi: 10.1083/jcb.110.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugwitz M, Noegel AA, Karakesisoglou J, Schleicher M. Dictyostelium amoebae that lack G-actin-sequestering profilins show defects in F-actin content, cytokinesis, and development. Cell. 1994;79:303–314. doi: 10.1016/0092-8674(94)90199-6. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ishikawa R, Ye LH, He XL, Takata K, Kohama K, Shirao T. Modulatory role of drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex. J Neurosci. 1996;16:7161–7170. doi: 10.1523/JNEUROSCI.16-22-07161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RJ, Insall RH. F-BAR domains: multifunctional regulators of membrane curvature. Journal of cell science. 2008;121:1951–1954. doi: 10.1242/jcs.023895. [DOI] [PubMed] [Google Scholar]
- Ishikawa R, Hayashi K, Shirao T, Xue Y, Takagi T, Sasaki Y, Kohama K. Drebrin, a development-associated brain protein from rat embryo, causes the dissociation of tropomyosin from actin filaments. The Journal of biological chemistry. 1994;269:29928–29933. [PubMed] [Google Scholar]
- Izawa I, Amano M, Chihara K, Yamamoto T, Kaibuchi K. Possible involvement of the inactivation of the Rho-Rho-kinase pathway in oncogenic Ras-induced transformation. Oncogene. 1998;17:2863–2871. doi: 10.1038/sj.onc.1202213. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Blader IJ, Hammonds-Odie LP, Burga CR, Cooke F, Hawkins PT, Wolf AG, Heldman KA, Theibert AB. Initiation and maintenance of NGF-stimulated neurite outgrowth requires activation of a phosphoinositide 3-kinase. Journal of cell science. 1996;109(Pt 2):289–300. doi: 10.1242/jcs.109.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. Intracellular diffusion gradients of O2 and ATP. The American journal of physiology. 1986;250:C663–675. doi: 10.1152/ajpcell.1986.250.5.C663. [DOI] [PubMed] [Google Scholar]
- Joshi HC, Cleveland DW. Differential utilization of beta-tubulin isotypes in differentiating neurites. The Journal of cell biology. 1989;109:663–673. doi: 10.1083/jcb.109.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang F, Purich DL, Southwick FS. Profilin promotes barbed-end actin filament assembly without lowering the critical concentration. The Journal of biological chemistry. 1999;274:36963–36972. doi: 10.1074/jbc.274.52.36963. [DOI] [PubMed] [Google Scholar]
- Katoh H, Aoki J, Yamaguchi Y, Kitano Y, Ichikawa A, Negishi M. Constitutively active Galpha12, Galpha13, and Galphaq induce Rho-dependent neurite retraction through different signaling pathways. The Journal of biological chemistry. 1998;273:28700–28707. doi: 10.1074/jbc.273.44.28700. [DOI] [PubMed] [Google Scholar]
- Ketschek A, Gallo G. Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia. J Neurosci. 2010;30:12185–12197. doi: 10.1523/JNEUROSCI.1740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek AR, Jones SL, Gallo G. Axon extension in the fast and slow lanes: substratum-dependent engagement of myosin II functions. Developmental neurobiology. 2007;67:1305–1320. doi: 10.1002/dneu.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Binder LI, Rosenbaum JL. The periodic association of MAP2 with brain microtubules in vitro. The Journal of cell biology. 1979;80:266–276. doi: 10.1083/jcb.80.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoops B, Octave JN. Alpha 1-tubulin mRNA level is increased during neurite outgrowth of NG 108-15 cells but not during neurite outgrowth inhibition by CNS myelin. Neuroreport. 1997;8:795–798. doi: 10.1097/00001756-199702100-00043. [DOI] [PubMed] [Google Scholar]
- Kollins KM, Hu J, Bridgman PC, Huang YQ, Gallo G. Myosin-II negatively regulates minor process extension and the temporal development of neuronal polarity. Developmental neurobiology. 2009;69:279–298. doi: 10.1002/dneu.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Molecular biology of the cell. 2008;19:1561–1574. doi: 10.1091/mbc.E07-09-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski AV, Rubinson DA, Dent EW, Edward van Veen J, Leslie JD, Zhang J, Mebane LM, Philippar U, Pinheiro EM, Burds AA, et al. Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron. 2007;56:441–455. doi: 10.1016/j.neuron.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Lee K, Gallop JL, Rambani K, Kirschner MW. Self-assembly of filopodia-like structures on supported lipid bilayers. Science. 2010;329:1341–1345. doi: 10.1126/science.1191710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC, Ressler AH. Inhibition of neurite initiation and growth by taxol. The Journal of cell biology. 1984;98:1355–1362. doi: 10.1083/jcb.98.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC, Wire JP. Three-dimensional organization of stable microtubules and the Golgi apparatus in the somata of developing chick sensory neurons. Journal of neurocytology. 1995;24:207–223. doi: 10.1007/BF01181535. [DOI] [PubMed] [Google Scholar]
- Li YH, Ghavampur S, Bondallaz P, Will L, Grenningloh G, Puschel AW. Rnd1 regulates axon extension by enhancing the microtubule destabilizing activity of SCG10. The Journal of biological chemistry. 2009;284:363–371. doi: 10.1074/jbc.A808126200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Szaro BG. Effects of intermediate filament disruption on the early development of the peripheral nervous system of Xenopus laevis. Developmental biology. 1996;179:197–211. doi: 10.1006/dbio.1996.0251. [DOI] [PubMed] [Google Scholar]
- Lindenbaum MH, Carbonetto S, Grosveld F, Flavell D, Mushynski WE. Transcriptional and post-transcriptional effects of nerve growth factor on expression of the three neurofilament subunits in PC-12 cells. The Journal of biological chemistry. 1988;263:5662–5667. [PubMed] [Google Scholar]
- Lu W, Fox P, Lakonishok M, Davidson MW, Gelfand VI. Initial neurite outgrowth in Drosophila neurons is driven by kinesin-powered microtubule sliding. Curr Biol. 2013;23:1018–1023. doi: 10.1016/j.cub.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart BW, Zhang W, Wayman GA, Kwon CH, Westbrook GL, Parada LF. Neurotrophin-dependent dendritic filopodial motility: a convergence on PI3K signaling. J Neurosci. 2008;28:7006–7012. doi: 10.1523/JNEUROSCI.0195-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J, Fok L, Gao H, Zhang X, Poo MM. Axon initiation and growth cone turning on bound protein gradients. J Neurosci. 2009;29:7450–7458. doi: 10.1523/JNEUROSCI.1121-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nature reviews. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Partin J. Evidence for mitochondrial control of neuronal polarity. Journal of neuroscience research. 1999;56:8–20. doi: 10.1002/(SICI)1097-4547(19990401)56:1<8::AID-JNR2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Matus A, Delhaye-Bouchaud N, Mariani J. Microtubule-associated protein 2 (MAP2) in Purkinje cell dendrites: evidence that factors other than binding to microtubules are involved in determining its cytoplasmic distribution. The Journal of comparative neurology. 1990;297:435–440. doi: 10.1002/cne.902970308. [DOI] [PubMed] [Google Scholar]
- Mellor H. The role of formins in filopodia formation. Biochimica et biophysica acta. 2010;1803:191–200. doi: 10.1016/j.bbamcr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Menna E, Fossati G, Scita G, Matteoli M. From filopodia to synapses: the role of actin-capping and anti-capping proteins. The European journal of neuroscience. 2011;34:1655–1662. doi: 10.1111/j.1460-9568.2011.07897.x. [DOI] [PubMed] [Google Scholar]
- Mizui T, Kojima N, Yamazaki H, Katayama M, Hanamura K, Shirao T. Drebrin E is involved in the regulation of axonal growth through actin-myosin interactions. Journal of neurochemistry. 2009;109:611–622. doi: 10.1111/j.1471-4159.2009.05993.x. [DOI] [PubMed] [Google Scholar]
- Mogilner A, Rubinstein B. The physics of filopodial protrusion. Biophysical journal. 2005;89:782–795. doi: 10.1529/biophysj.104.056515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Aoki K, Matsuda M. FRET imaging and in silico simulation: analysis of the signaling network of nerve growth factor-induced neuritogenesis. Brain cell biology. 2008;36:19–30. doi: 10.1007/s11068-008-9028-5. [DOI] [PubMed] [Google Scholar]
- Nusser N, Gosmanova E, Zheng Y, Tigyi G. Nerve growth factor signals through TrkA, phosphatidylinositol 3-kinase, and Rac1 to inactivate RhoA during the initiation of neuronal differentiation of PC12 cells. The Journal of biological chemistry. 2002;277:35840–35846. doi: 10.1074/jbc.M203617200. [DOI] [PubMed] [Google Scholar]
- Pantaloni D, Carlier MF. How profilin promotes actin filament assembly in the presence of thymosin beta 4. Cell. 1993;75:1007–1014. doi: 10.1016/0092-8674(93)90544-z. [DOI] [PubMed] [Google Scholar]
- Pedrotti B, Islam K. Microtubule associated protein 1B (MAP1B) promotes efficient tubulin polymerisation in vitro. FEBS letters. 1995;371:29–31. doi: 10.1016/0014-5793(95)00842-w. [DOI] [PubMed] [Google Scholar]
- Pinyol R, Haeckel A, Ritter A, Qualmann B, Kessels MM. Regulation of N-WASP and the Arp2/3 complex by Abp1 controls neuronal morphology. PloS one. 2007;2:e400. doi: 10.1371/journal.pone.0000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer BM. Microtubule-associated protein 1B, a growth-associated and phosphorylated scaffold protein. Brain research bulletin. 2007;71:541–558. doi: 10.1016/j.brainresbull.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Riederer BM, Pellier V, Antonsson B, Di Paolo G, Stimpson SA, Lutjens R, Catsicas S, Grenningloh G. Regulation of microtubule dynamics by the neuronal growth-associated protein SCG10. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:741–745. doi: 10.1073/pnas.94.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Galbraith RH, Gould KL. Cell cycle. Vol. 9. Georgetown, Tex: 2010. Setting the F-BAR: functions and regulation of the F-BAR protein family; pp. 4091–4097. [DOI] [PubMed] [Google Scholar]
- Roger B, Al-Bassam J, Dehmelt L, Milligan RA, Halpain S. MAP2c, but not tau, binds and bundles F-actin via its microtubule binding domain. Curr Biol. 2004;14:363–371. doi: 10.1016/j.cub.2004.01.058. [DOI] [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. Response of mitochondrial traffic to axon determination and differential branch growth. J Neurosci. 2003;23:8618–8624. doi: 10.1523/JNEUROSCI.23-24-08618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengsawang W, Mitok K, Viesselmann C, Pietila L, Lumbard DC, Corey SJ, Dent EW. The F-BAR protein CIP4 inhibits neurite formation by producing lamellipodial protrusions. Curr Biol. 2012;22:494–501. doi: 10.1016/j.cub.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengsawang W, Taylor KL, Lumbard DC, Mitok K, Price A, Pietila L, Gomez TM, Dent EW. CIP4 coordinates with phospholipids and actin-associated proteins to localize to the protruding edge and produce actin ribs and veils. Journal of cell science. 2013;126:2411–2423. doi: 10.1242/jcs.117473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon WC, Adams MC, Waterman-Storer CM. Dual-wavelength fluorescent speckle microscopy reveals coupling of microtubule and actin movements in migrating cells. The Journal of cell biology. 2002;158:31–37. doi: 10.1083/jcb.200203022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Hayashi K, Shirao T, Ishikawa R, Kohama K. Inhibition by drebrin of the actin-bundling activity of brain fascin, a protein localized in filopodia of growth cones. Journal of neurochemistry. 1996;66:980–988. doi: 10.1046/j.1471-4159.1996.66030980.x. [DOI] [PubMed] [Google Scholar]
- Schaefer AW, Kabir N, Forscher P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. The Journal of cell biology. 2002;158:139–152. doi: 10.1083/jcb.200203038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TA, Obar RA. Diverse distribution and function of fibrous microtubule-associated proteins in the nervous system. International review of cytology. 1994;151:67–137. doi: 10.1016/s0074-7696(08)62631-5. [DOI] [PubMed] [Google Scholar]
- Schwamborn JC, Puschel AW. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nature neuroscience. 2004;7:923–929. doi: 10.1038/nn1295. [DOI] [PubMed] [Google Scholar]
- Sebok A, Nusser N, Debreceni B, Guo Z, Santos MF, Szeberenyi J, Tigyi G. Different roles for RhoA during neurite initiation, elongation, and regeneration in PC12 cells. Journal of neurochemistry. 1999;73:949–960. doi: 10.1046/j.1471-4159.1999.0730949.x. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Kuriyama R, Baas PW. Expression of a kinesin-related motor protein induces Sf9 cells to form dendrite-like processes with nonuniform microtubule polarity orientation. J Neurosci. 1996;16:4370–4375. doi: 10.1523/JNEUROSCI.16-14-04370.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Kuriyama R, Essner R, Baas PW. Expression of a minus-end-directed motor protein induces Sf9 cells to form axon-like processes with uniform microtubule polarity orientation. Journal of cell science. 1997;110(Pt 19):2373–2380. doi: 10.1242/jcs.110.19.2373. [DOI] [PubMed] [Google Scholar]
- Shea TB, Beermann ML. Neuronal intermediate filament protein alpha-internexin facilitates axonal neurite elongation in neuroblastoma cells. Cell motility and the cytoskeleton. 1999;43:322–333. doi: 10.1002/(SICI)1097-0169(1999)43:4<322::AID-CM5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Shelly M, Lim BK, Cancedda L, Heilshorn SC, Gao H, Poo MM. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327:547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- Skoble J, Auerbuch V, Goley ED, Welch MD, Portnoy DA. Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility. The Journal of cell biology. 2001;155:89–100. doi: 10.1083/jcb.200106061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL. The initiation of neurite outgrowth by sympathetic neurons grown in vitro does not depend on assembly of microtubules. The Journal of cell biology. 1994;127:1407–1418. doi: 10.1083/jcb.127.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G. Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell reports. 2013;5:1564–1575. doi: 10.1016/j.celrep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, Lanier LM. Arp2/3 is a negative regulator of growth cone translocation. Neuron. 2004;43:81–94. doi: 10.1016/j.neuron.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Sullivan KF. Structure and utilization of tubulin isotypes. Annual review of cell biology. 1988;4:687–716. doi: 10.1146/annurev.cb.04.110188.003351. [DOI] [PubMed] [Google Scholar]
- Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell reports. 2013;4:413–419. doi: 10.1016/j.celrep.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. The Journal of cell biology. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. The Journal of cell biology. 2003;160:409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaro BG, Grant P, Lee VM, Gainer H. Inhibition of axonal development after injection of neurofilament antibodies into a Xenopus laevis embryo. The Journal of comparative neurology. 1991;308:576–585. doi: 10.1002/cne.903080406. [DOI] [PubMed] [Google Scholar]
- Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N. Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. Journal of cell science. 1992;103(Pt 4):953–964. doi: 10.1242/jcs.103.4.953. [DOI] [PubMed] [Google Scholar]
- Tang D, Goldberg DJ. Bundling of microtubules in the growth cone induced by laminin. Molecular and cellular neurosciences. 2000;15:303–313. doi: 10.1006/mcne.1999.0820. [DOI] [PubMed] [Google Scholar]
- Tao K, Matsuki N, Koyama R. AMP-activated protein kinase mediates activity-dependent axon branching by recruiting mitochondria to axon. Developmental neurobiology. 2013 doi: 10.1002/dneu.22149. [DOI] [PubMed] [Google Scholar]
- Teng J, Takei Y, Harada A, Nakata T, Chen J, Hirokawa N. Synergistic effects of MAP2 and MAP1B knockout in neuronal migration, dendritic outgrowth, and microtubule organization. The Journal of cell biology. 2001;155:65–76. doi: 10.1083/jcb.200106025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii T, Miyamoto Y, Nakamura K, Maeda M, Yamauchi J, Tanoue A. Arf6 guanine-nucleotide exchange factor, cytohesin-2, interacts with actinin-1 to regulate neurite extension. Cellular signalling. 2012;24:1872–1882. doi: 10.1016/j.cellsig.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Vandecandelaere A, Pedrotti B, Utton MA, Calvert RA, Bayley PM. Differences in the regulation of microtubule dynamics by microtubule-associated proteins MAP1B and MAP2. Cell motility and the cytoskeleton. 1996;35:134–146. doi: 10.1002/(SICI)1097-0169(1996)35:2<134::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Verheyen EM, Cooley L. Profilin mutations disrupt multiple actin-dependent processes during Drosophila development. Development. 1994;120:717–728. doi: 10.1242/dev.120.4.717. [DOI] [PubMed] [Google Scholar]
- Vignjevic D, Peloquin J, Borisy GG. In vitro assembly of filopodia-like bundles. Methods in enzymology. 2006;406:727–739. doi: 10.1016/S0076-6879(06)06057-5. [DOI] [PubMed] [Google Scholar]
- Villarroel-Campos D, Gastaldi L, Conde C, Caceres A, Gonzalez-Billault C. Rab-mediated trafficking role in neurite formation. Journal of neurochemistry. 2014 doi: 10.1111/jnc.12676. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Luan Q, Liu SL, Nolen BJ. Dip1 defines a class of Arp2/3 complex activators that function without preformed actin filaments. Curr Biol. 2013;23:1990–1998. doi: 10.1016/j.cub.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman JD, Bilancia CG, Peifer M, Kovar DR. Ena/VASP Enabled is a highly processive actin polymerase tailored to self-assemble parallel-bundled F-actin networks with Fascin. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:4121–4126. doi: 10.1073/pnas.1322093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Zhong G, Zhuang X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 2013;339:452–456. doi: 10.1126/science.1232251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Katoh H, Yasui H, Mori K, Negishi M. RhoA inhibits the nerve growth factor-induced Rac1 activation through Rho-associated kinase-dependent pathway. The Journal of biological chemistry. 2001;276:18977–18983. doi: 10.1074/jbc.M100254200. [DOI] [PubMed] [Google Scholar]
- Yamakita Y, Matsumura F, Yamashiro S. Fascin1 is dispensable for mouse development but is favorable for neonatal survival. Cell motility and the cytoskeleton. 2009;66:524–534. doi: 10.1002/cm.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Svitkina T. Filopodia initiation: focus on the Arp2/3 complex and formins. Cell adhesion & migration. 2011;5:402–408. doi: 10.4161/cam.5.5.16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y, Fujita S. Distribution of MAP1A, MAP1B, and MAP2A&B during layer formation in the optic tectum of developing chick embryos. Cell and tissue research. 2003;314:315–324. doi: 10.1007/s00441-003-0796-z. [DOI] [PubMed] [Google Scholar]
- Yu W, Ling C, Baas PW. Microtubule reconfiguration during axogenesis. Journal of neurocytology. 2001;30:861–875. doi: 10.1023/a:1020622530831. [DOI] [PubMed] [Google Scholar]
- Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments at a glance. Journal of cell science. 2012;125:3257–3263. doi: 10.1242/jcs.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Forscher P. Rac1 modulates stimulus-evoked Ca(2+) release in neuronal growth cones via parallel effects on microtubule/endoplasmic reticulum dynamics and reactive oxygen species production. Molecular biology of the cell. 2009;20:3700–3712. doi: 10.1091/mbc.E08-07-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, Poo MM. Calcium signaling in neuronal motility. Annual review of cell and developmental biology. 2007;23:375–404. doi: 10.1146/annurev.cellbio.23.090506.123221. [DOI] [PubMed] [Google Scholar]


