Abstract
Background
Evidence regarding the relationship of n-3 fatty acids (FA) to type 2 diabetes (T2D) and metabolic syndrome components (MetS) is inconsistent.
Objective
To examine associations of adipose tissue n-3 FA with MetS.
Design
We studied 1611 participants without prior history of diabetes or heart disease who were participants in a population-based case-control study of diet and heart disease (The Costa Rica Heart Study). We calculated prevalence ratios (PR) and 95% confidence intervals (CI) for MetS by quartile of n-3 FA in adipose tissue derived mainly from plants [α-Linolenic acid (ALA)], fish [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)], or metabolism [docosapentaenoic acid (DPA), as well as the EPA:ALA ratio, a surrogate marker of delta-6 desaturase activity].
Results
N-3 FA levels in adipose tissue were associated with MetS prevalence in opposite directions. The PR (95% CI) for the highest compared to the lowest quartile adjusted for age, sex, BMI, residence, lifestyle, diet and other fatty acids were 0.60 (0.44, 0.81) for ALA, 1.43 (1.12, 1.82) for EPA, 1.63 (1.22, 2.18) for DPA, and 1.47 (1.14, 1.88) for EPA:ALA, all p for trend <0.05. Although these associations were no longer significant (except DPA) after adjustment for BMI, ALA and DPA were associated with lower glucose and higher triglyceride levels, p<0.05 (respectively).
Conclusions
These results suggest that ALA could exert a modest protective benefit, while EPA and DHA are not implicated in MetS. The positive associations for DPA and MetS could reflect higher delta-6 desaturase activity caused by increased adiposity.
Keywords: Adipose tissue, omega-3 fatty acids, metabolic syndrome
INTRODUCTION
Worldwide, an estimated 371 million individuals had type 2 diabetes (T2D) in 2012 and related health care expenditures totaled approximately 471.6 billion USD. 80% of diabetics live in low- and middle-income countries such as Costa Rica and Mexico (1). To stem this rising health and economic burden, metabolic syndrome is of particular interest due to the clustering of factors that elevate risk for T2D and cardiovascular disease. Metabolic syndrome components include abdominal obesity, dyslipidemia, elevated blood pressure, and impaired blood glucose levels (2).
In an effort to understand and delay metabolic syndrome onset, attention has been paid to essential n-3 polyunsaturated fatty acids, which have numerous other health benefits (3). Some studies found an inverse association between circulating marine-source n-3 fatty acids and metabolic syndrome (4, 5), while others found no association (6, 7). Results from a meta-analysis showed similar contradictory findings with T2D. Increased intake of fish and/or seafood is associated with lower risk of T2D in studies conducted in Asian countries but higher risk in studies conducted in North American and European countries (8). Additionally, circulating levels of EPA and DHA were not associated with T2D in American and European countries (8). These opposing results seem to arise, in part, from differences in geographic location, and/or methods to assess n-3 fatty acids.
Data on the association of plant-source n-3 fatty acids and metabolic syndrome are also inconsistent and insufficient. ALA levels in red blood cells or plasma phospholipids as biomarkers of intake have been positively associated with metabolic syndrome in some studies and inversely associated in others (4, 5). ALA intake and circulating ALA levels were inversely associated with T2D in a meta-analysis, although the results did not reach statistical significance. Thus, dietary the role of ALA in metabolic syndrome and T2D remains to be clarified (8).
It is possible that this relationship depends on fatty acid availability. Studies on the role of plant-source ALA in metabolic syndrome as a precursor of T2D are needed because the current consumption of fish in low and middle-income countries is insufficient to meet dietary recommendations (9). The purpose of this study was to examine associations of plant- and marine-source n-3 fatty acids in adipose tissue with metabolic syndrome. As a secondary aim, we evaluated the association of these n-3 fatty acids with metabolic syndrome components.
METHODS
Study Population
The study population includes 1,208 men and 403 women, out of 1,669 men and 605 women who participated as controls in a population-based case-control study of diet and heart disease in Costa Rica (The Costa Rica Heart Study) (10). Participants were not included if they had diabetes and/or did not have compete information on confounders and fatty acid levels (n = 461 men and 202 women).
The catchment area of the Costa Rica Heart Study consisted of 34 counties in Costa Rica’s Central Valley. Incident cases of myocardial infarction were matched by age (±5 years), sex and area of residence to population controls that were randomly identified with the aid of data from the National Census and Statistics Bureau of Costa Rica. Because of the comprehensive social services provided in Costa Rica, all persons living in the catchment area had access to medical care without regard to income. Therefore, control subjects came from the source population that gave rise to the cases and are not likely to have been having cardiovascular disease that was not diagnosed because of poor access to medical care (11). Participation was 88% among controls in The Costa Rica Heart Study. Participants provided informed consent by signing an “informed consent form” that described in detail the objective of the study and what their participation entailed. The conduct of the study was approved by the Institutional Review Boards at the Harvard School of Public Health and the University of Costa Rica.
There were 481 subjects who met the metabolic syndrome criteria (n=292 men and n=189 women) and 1,130 subjects who did not met these criteria. Metabolic syndrome was defined following Adult Treatment Panel (ATP) III guidelines (12). Subjects with metabolic syndrome had three or more of the following: cholesterol (<1.03 mmol/L or 40 mg/dL in men, <1.29 mmol/L or 50 mg/dL in women), high triglycerides (≥1.69 mmol/L or 150 mg/dL), elevated BP (>130/85 mm Hg), and impaired fasting glucose (≥5.6 mmol/L or 100 mg/dL).
Data Collection
Data on socio-demographic characteristics, smoking status, physical activity, medical history, and anthropometric measurements were collected at the subject’s home following standardized protocols (11). Fasting blood samples and adipose tissue biopsies were collected in the morning as described in detail previously (13).
Adipose tissue was chosen for measurement because previous studies have shown that the turnover of fatty acids in adipose tissue occurs over two years, allowing adipose tissue fatty acid concentrations to represent a long-term integrated measure of exposure, both from dietary intake and conversion (14, 15). Samples were collected from the buttock with a 16″ gauge needle and syringe and immediately immersed in ice and transported within three hours to the field station. The samples were diluted in 1.5 cc of hexane:isopropanol (3:2), sealed, and stored at −80°C until they were analyzed for fatty acid content at the Harvard School of Public Health.
As described previously, total fatty acids (derived mostly from triglyceride) were extracted from adipose tissue with hexane:isopropanol (3:2) and esterified (16) and fatty acid methyl esters were separated on a 100m SP 2330 column on a HP 6980 gas chromatographer (13, 17). Qualitative analysis using peak retention and area percentage of 50 fatty acid peaks were identified using pure standards (NuCheck Prep, Elysium, MN). Fatty acids were expressed as a percentage of total fatty acid identified.
Plasma triglycerides were measured using enzymatic reagents (Boehringer-Mannheim Diagnostics, Indianapolis, IN) and capillary whole blood glucose was measured using an Accu-Check II Blood Glucose Monitor with Chemstrip bG Test Strips (Boehringer-Mannheim Diagnostics, Indianapolis, IN) immediately after sample collection.
Statistical Analysis
All statistical analyses were performed using the SAS 9.3 (SAS Institute Inc, Cary, NC). Differences in descriptive characteristics between subjects with and without metabolic syndrome were tested using t tests if normally distributed or Wilcoxon’s signed rank tests if not normally distributed for continuous variables. Chi-square tests were used for categorical variables.
The following adipose tissue n-3 PUFA were considered as main exposures: ALA, EPA, DPA, and DHA, along with the EPA:ALA ratio. The EPA:ALA ratio was computed by dividing the percent of total fatty acid EPA by percent of total fatty acid ALA. The EPA:ALA ratio provides a surrogate for delta-6 desaturase conversion activity, the rate-limiting step in EPA biosynthesis, which, in turn, is converted to docosapentaenoic acid (DPA, 22:5n-3), a fatty acid rarely found in foods (18, 19). Similarly, we calculated the ratio of ALA:LA to examine the proportion of ALA relative to the potential pro-lipogenic effects of LA (20, 21).
Each of these fatty acid exposures were categorized in quartiles for prevalence models. We evaluated whether the potential confounders age, sex, area of residence, physical activity (measured in metabolic equivalents, METS), current smoking status (yes/no), BMI (kg/m2), and alcohol intake (0 for non-drinkers, <1g/d and tertiles for those with ≥ 1g/d) were associated with our exposures of interest in subjects without metabolic syndrome. Given our previous findings (22), we also evaluated arachidonic acid as a potential confounder, but it did not change the results.
Prevalence ratios (PR) and 95% confidence intervals (CI) of metabolic syndrome according to quartiles of fatty acids in adipose tissue were calculated using SAS PROC GENMOD’s modified log-binomial regression (Poisson regression capability with robust variance) (23). The “Basic” model adjusted for matching factors only (sex, age, and area of residence), whereas the first “Adjusted” model also included adjustments for lifestyle (smoking status, alcohol intake, and physical activity) and dietary confounders (saturated fat and total calories), all other n-3 fatty acids (e.g. ALA, EPA, DHA, and/or DPA), linoleic acid (LA) 18:2n-6, and total trans (16:1 trans+ 18:1 trans + 18:2 trans) categorized in quartiles. The main source of ALA, trans fatty acids and LA in this population is soybean oil. The second “Adjusted” model additionally adjusted for BMI. Other potential confounders that were tested were sugar-sweetened beverage intake and the bean:rice ratio. These two confounders were previously associated with metabolic syndrome in this population and also serve as indicators of the carbohydrate quality of the diet (24, 25). However, these did not modify the results and thus were not included in analyses. Tests for trend were performed using the median of the quartiles as a continuous variable in the linear regression models. We also conducted sex-stratified analyses to examine whether observed associations of metabolic syndrome prevalence with the fatty of acids of interest were sex-dependent.
Predicted population means (least square means, LSMEANS) for each metabolic syndrome component were calculated using linear regression with empirical variances to account for the non-normal distributions of some variables (implemented in SAS PROC MIXED) (26).
RESULTS
Table 1 shows general characteristics of the study population. Metabolic syndrome prevalence was 30%. Subjects that fit the criteria for metabolic syndrome were less physically active and had higher BMI values and waist circumferences. Additionally, there were fewer females, smokers, and alcohol drinkers than among those without metabolic syndrome. With respect to polyunsaturated n-3 fatty acid levels, ALA as a percentage of adipose tissue was lower while the sum of EPA, DHA and DPA as a percentage of total fatty acids in adipose tissue was higher among those with metabolic syndrome than those without.
Table 1.
General Characteristics of the Study Population1
| Parameter | Metabolic Syndrome | |
|---|---|---|
| No (n = 1130) | Yes (n = 481) | |
| Age, years | 56±12 | 60±10 |
| Female, % | 19 | 39 |
| Rural residence, % | 24 | 26 |
| Monthly household income, US $ | 588±430 | 599±428 |
| Current smoker, % | 25 | 16 |
| Physical activity, daily METS2 | 37±17 | 34±13 |
| Body mass index, kg/m2 | 25±3 | 29±4 |
| Waist circumference, cm | 88±9 | 96±10 |
| History of hypertension, % | 14 | 53 |
| Systolic blood pressure, mm Hg | 131±21 | 146±22 |
| Diastolic blood pressure, mm Hg | 80±9 | 88±11 |
| Alcohol, % drinkers3 | 45 | 41 |
| Glucose, mg/dL | 74±16 | 83±27 |
| LDL cholesterol, mg/dl | 131±36 | 121±35 |
| HDL cholesterol, mg/dl | 42±9 | 38±7 |
| Total triglycerides, mg/dl | 201±128 | 240±98 |
| Metabolic syndrome components4 | ||
| High blood pressure, % | 23 | 81 |
| Abdominal obesity, % | 5 | 53 |
| Elevated triglycerides, % | 59 | 92 |
| Low HDL cholesterol, % | 52 | 88 |
| Elevated glucose, % | 2 | 16 |
| Adipose tissue fatty acids (% total, g/100g)5 | ||
| ALA | 0.68±0.22 | 0.62±0.19 |
| EPA | 0.04±0.02 | 0.05±0.02 |
| DPA | 0.17±0.05 | 0.19±0.05 |
| DHA | 0.14±0.05 | 0.15±0.05 |
| EPA + DPA + DHA | 0.35±0.10 | 0.39±0.11 |
| Total trans fatty acids | 2.74±0.75 | 2.61±0.67 |
Subjects with a history of diabetes were excluded to avoid reverse causation. Values are mean ± SD when appropriate.
Metabolic Equivalent of Task (MET).
Drinkers were defined as those who reported any current alcohol consumption.
High blood pressure (systolic >130 or diastolic >85 mm Hg, or treatment of previously diagnosed hypertension using beta-blockers, diuretics, and other hypertensive medication); Abdominal obesity (waist circumference >102 cm for males and >88 cm for females); Elevated triglycerides (>150 mg/dL); low HDL cholesterol (<50 for females, <40 for males); elevated fasting glucose (> 100 mg/dL; subjects with previously diagnosed type 2 diabetes were excluded).
α-Linolenic acid (ALA, 18:3n-3); Eicosapentaenoic acid (EPA, 20:5n-3); Docosahexaenoic acid (DHA, 22:6n-3); docosapentaenoic acid (DPA, 22:5n-3); Eicosapentaenoic acid (EPA, 20:5n-3); Docosapentaenoic acid (22:5n-3; DPA); Docosahexaenoic acid (DHA, 22:6n-3).
Table 2 shows the age-adjusted distribution of potential confounders among subjects without metabolic syndrome for the first and fourth quartile of ALA and the sum of EPA, DPA, and DHA in adipose tissue. Quartiles of ALA were inversely correlated with smoking status, alcohol intake, female sex, and total calories, and directly correlated with LA and trans fatty acid levels in adipose tissue. A similar pattern was observed for quartiles of the sum of EPA, DPA, and DHA, except that correlations with LA and trans fatty acids in adipose tissue were inverse and correlations with female sex and alcohol intake were positive. Similar results for potential confounder relationships were obtained when EPA, DPA, and DHA were analyzed independently.
Table 2.
General characteristics and potential confounders by quartiles of ALA and EPA, DPA and DHA in adipose tissue of subjects without metabolic syndrome 1
| ALA | EPA + DPA + DHA | |||
|---|---|---|---|---|
| Quartile of adipose fatty acid | 1 | 4 | 1 | 4 |
| Mean of quartile (% total fatty acids) | (0.42) | (0.94) | (0.25) | (0.48) |
| Age, years | 56 | 56 | 56 | 56 |
| Female, % | 23% | 14% | 14% | 25% |
| Rural residence, % | 30% | 20% | 20% | 20% |
| Monthly household income, US $ | 491 | 546 | 545 | 594 |
| Current smoker, % | 33% | 24% | 29% | 24% |
| Physical activity, daily METS 2 | 37 | 39 | 37 | 36 |
| Body mass index, kg/m2 | 25 | 25 | 24 | 26 |
| Waist circumference, cm | 88 | 87 | 87 | 89 |
| History of hypertension, % | 13% | 15% | 12% | 19% |
| Systolic blood pressure, mm Hg | 132 | 132 | 131 | 13 |
| Diastolic blood pressure, mm Hg | 80 | 79 | 79 | 81 |
| Alcohol, % drinkers 3 | 48% | 39% | 38% | 51% |
| Total calories | 2560 | 2491 | 2508 | 2462 |
| Total saturated fat, % of calories | 12 | 9 | 11 | 10 |
| Glucose, mg/dL | 73 | 75 | 74 | 72 |
| LDL cholesterol, mg/dl | 128 | 132 | 129 | 131 |
| HDL cholesterol, mg/dl | 43 | 41 | 41 | 44 |
| Total triglycerides, mg/dl | 193 | 204 | 189 | 198 |
| Adipose tissue fatty acids (% total fatty acids) 4 | ||||
| ALA | 0.42 | 0.94 | 0.67 | 0.65 |
| EPA | 0.04 | 0.04 | 0.03 | 0.06 |
| DPA | 0.14 | 0.14 | 0.09 | 0.20 |
| DHA | 0.17 | 0.17 | 0.12 | 0.24 |
| EPA + DPA + DHA | 0.35 | 0.35 | 0.25 | 0.48 |
| Linoleic acid | 11.8 | 19.6 | 16.2 | 15.1 |
| Total trans fatty acids | 2.39 | 2.90 | 2.84 | 2.73 |
N = 1130; all values are adjusted for age - mean age reflects adjustment for age.
Metabolic Equivalent of Task (MET).
Drinkers were defined as those who reported any current alcohol consumption.
α-Linolenic acid (ALA, 18:3n-3); Eicosapentaenoic acid (EPA, 20:5n-3); Docosahexaenoic acid (DHA, 22:6n-3); docosapentaenoic acid (DPA, 22:5n-3); Eicosapentaenoic acid (EPA, 20:5n-3); Docosapentaenoic acid (22:5n-3; DPA); Docosahexaenoic acid (DHA, 22:6n-3).
Table 3 shows the PRs (95 % CI) for the associations between the fourth and first quartiles of each fatty acid of interest – ALA, EPA, DPA, DHA and the EPA:ALA and ALA:LA ratio in adipose tissue – and prevalence of metabolic syndrome. Higher ALA levels in adipose tissue were associated with lower prevalence of metabolic syndrome in the “Basic” model [0.69 (0.55, 0.86)]. Adjustment for confounders strengthened this result while further adjustment for BMI attenuated the association. Similar results were obtained when the ALA:LA ratio was used. By contrast, higher adipose tissue EPA levels were associated with greater prevalence of metabolic syndrome in the “Basic” model [1.62 (1.30, 2.03)], but this association was attenuated after multivariate adjustment and disappeared with adjustment for BMI [1.15 (0.92, 1.43)]. Like EPA, higher DPA levels in adipose tissue were associated with greater prevalence of metabolic syndrome in the “Basic” model but the trend for DPA remained statistically significant even after further adjustment for BMI. Higher DHA levels in adipose tissue were associated with higher metabolic syndrome prevalence in the “Basic” model [1.50 (1.20, 1.87)], but this association was no longer present after adjustment for confounders, particularly EPA and DPA levels in adipose tissue. A higher EPA:ALA ratio was associated with higher prevalence of metabolic syndrome in the “Basic” and multivariate (“Adjusted”) models [1.72 (1.38, 2.15)] and disappeared after adjustment for BMI.
Table 3.
Prevalence ratios and 95% confidence intervals for risk of metabolic syndrome by quartiles of fatty acids in adipose tissue
| Quartiles of Adipose Tissue ALA | |||||
| 1 | 2 | 3 | 4 | ||
| Median adipose | 0.44 | 0.56 | 0.69 | 0.90 | |
| PR (95% CI)1 | PR (95% CI) | PR (95% CI) | P for trend2 | ||
| Basic model3 | 1 (Reference) | 1.01 (0.84, 1.21) | 0.80 (0.66, 0.98) | 0.69 (0.55, 0.86) | 0.0002 |
| Adjusted model4 | 1 (Reference) | 0.88 (0.71, 1.08) | 0.64 (0.51, 0.81) | 0.60 (0.44, 0.81) | 0.0003 |
| Adjusted model5 | 1 (Reference) | 0.99 (0.82, 1.19) | 0.80 (0.64, 0.99) | 0.84 (0.63, 1.12) | 0.13 |
| Quartiles of Adipose Tissue EPA | |||||
| 1 | 2 | 3 | 4 | ||
| Median adipose | 0.02 | 0.04 | 0.05 | 0.07 | |
| PR (95% CI)1 | PR (95% CI) | PR (95% CI) | P for trend2 | ||
| Basic model3 | 1 (Reference) | 1.29 (1.02, 1.63) | 1.43 (1.14, 1.79) | 1.62 (1.30, 2.03) | <.0001 |
| Adjusted model4 | 1 (Reference) | 1.28 (1.01, 1.61) | 1.35 (1.07, 1.69) | 1.43 (1.12, 1.82) | 0.003 |
| Adjusted model5 | 1 (Reference) | 1.17 (0.94, 1.44) | 1.20 (0.98, 1.48) | 1.15 (0.92, 1.43) | 0.20 |
| Quartiles of Adipose Tissue DPA | |||||
| 1 | 2 | 3 | 4 | ||
| Median adipose | 0.12 | 0.16 | 0.19 | 0.24 | |
| PR (95% CI)1 | PR (95% CI) | PR (95% CI) | P for trend2 | ||
| Basic model3 | 1 (Reference) | 1.39 (1.08, 1.80) | 1.64 (1.29, 2.09) | 1.81 (1.42, 2.30) | <.0001 |
| Adjusted model4 | 1 (Reference) | 1.28 (0.98, 1.68) | 1.50 (1.14, 1.98) | 1.63 (1.22, 2.18) | 0.001 |
| Adjusted model5 | 1 (Reference) | 1.34 (1.06, 1.70) | 1.34 (1.05, 1.72) | 1.45 (1.11, 1.90) | 0.02 |
| Quartiles of Adipose Tissue DHA | |||||
| 1 | 2 | 3 | 4 | ||
| Median adipose | 0.09 | 0.12 | 0.15 | 0.20 | |
| PR (95% CI)1 | PR (95% CI) | PR (95% CI) | P for trend2 | ||
| Basic model3 | 1 (Reference) | 1.31 (1.04, 1.64) | 1.25 (0.99, 1.57) | 1.50 (1.20, 1.87) | 0.0009 |
| Adjusted model4 | 1 (Reference) | 1.07 (0.84, 1.36) | 0.94 (0.73, 1.21) | 0.98 (0.75, 1.28) | 0.67 |
| Adjusted model5 | 1 (Reference) | 1.02 (0.82, 1.27) | 0.87 (0.70, 1.09) | 0.95 (0.73, 1.22) | 0.52 |
| Quartiles of Adipose Tissue EPA:ALA | |||||
| 1 | 2 | 3 | 4 | ||
| Median adipose | 0.02 | 0.05 | 0.08 | 0.11 | |
| PR (95% CI)1 | PR (95% CI) | PR (95% CI) | P for trend2 | ||
| Basic model3 | 1 (Reference) | 1.16 (0.91, 1.48) | 1.44 (1.14, 1.80) | 1.72 (1.38, 2.15) | <.0001 |
| Adjusted model4 | 1 (Reference) | 1.11 (0.87, 1.41) | 1.27 (1.00, 1.61) | 1.47 (1.14, 1.88) | 0.002 |
| Adjusted model5 | 1 (Reference) | 1.09 (0.87, 1.35) | 1.12 (0.91, 1.39) | 1.12 (0.90, 1.39) | 0.32 |
| Quartiles of Adipose Tissue ALA:LA | |||||
| 1 | 2 | 3 | 4 | ||
| Median adipose | 0.03 | 0.04 | 0.04 | 0.05 | |
| PR (95% CI)1 | PR (95% CI) | PR (95% CI) | P for trend2 | ||
| Basic model3 | 1 (Reference) | 0.85 (0.71, 1.02) | 0.65 (0.53, 0.80) | 0.67 (0.53, 0.83) | <.0001 |
| Adjusted model4 | 1 (Reference) | 0.82 (0.69, 0.99) | 0.64 (0.52, 0.79) | 0.64 (0.51, 0.80) | <.0001 |
| Adjusted model5 | 1 (Reference) | 0.93 (0.78, 1.10) | 0.77 (0.63, 0.93) | 0.88 (0.72, 1.08) | 0.08 |
Prevalence ratios (PR) and 95% confidence intervals were estimated using log-binomial models. n = 1611.
Test for trend were performed using median of the quartile as a continuous variable in the linear regression models.
Adjusted for matching factors: age, sex, and area of residence.
Basic model above plus lifestyle (smoking, alcohol intake and physical activity), diet (saturated fat and total calories) and all other fatty acids (ALA, LA, EPA, DPA, DHA and total trans).
Adjusted model above plus BMI.
Table 4 shows predicted population means (least square means) for each component of metabolic syndrome with adjustments for matching, lifestyle and dietary factors, other fatty acids, and BMI. Higher ALA and the ALA:LA ratio levels in adipose tissue were associated with smaller waist circumference and lower fasting glucose levels. Conversely, EPA, DPA, and the EPA:ALA ratio were associated with larger waist circumference, DPA was associated with higher triglyceride levels. DHA showed no indication of a relationship with any component of metabolic syndrome. To assess if the association between ALA and DPA and plasma biomarkers was mediated by adiposity, we further adjusted for waist circumference. The fasting glucose levels for each quartile of adipose tissue ALA after further adjustment for waist circumference were 77, 78, 75, and 75 (p for trend = 0.05). The plasma triglyceride levels for each quartile of adipose tissue DPA were 211, 217, 230, and 232 (p for trend = 0.04).
Table 4.
Multivariate adjusted means and 95% confidence intervals of metabolic syndrome components by quartiles of adipose tissue fatty acids1
| Quartiles of adipose ALA | |||||
| 1 | 2 | 3 | 4 | ||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P-trend2 | |
| Waist circumference, cm | 91 (90, 92) | 91 (90, 91) | 90 (90, 91) | 90 (89, 91) | 0.01 |
| Triglycerides, mg/dL | 231 (216, 247) | 229 (212, 246) | 216 (200, 231) | 215 (197, 233) | 0.13 |
| HDL cholesterol, mg/dL | 41 (40, 42) | 41 (40, 42) | 41 (40, 42) | 41 (39, 42) | 0.60 |
| Glucose, mg/dL | 78 (75, 81) | 78 (75, 81) | 75 (73, 77) | 75 (72, 77) | 0.03 |
| Diastolic blood pressure, mmHg | 82 (81, 84) | 81 (80, 82) | 80 (79, 81) | 81 (79, 82) | 0.19 |
| Systolic blood pressure, mmHg | 134 (131, 137) | 133 (131, 136) | 132 (130, 134) | 135 (132, 137) | 0.57 |
| Quartiles of adipose EPA | |||||
| 1 | 2 | 3 | 4 | ||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P-trend2 | |
| Waist circumference, cm | 90 (89, 91) | 90 (90, 91) | 91 (90, 91) | 91 (90, 91) | 0.04 |
| Triglycerides, mg/dL | 215 (201, 229) | 228 (212, 244) | 229 (214, 244) | 219 (202, 235) | 0.51 |
| HDL cholesterol, mg/dL | 41 (40, 42) | 41 (40, 42) | 40 (39, 41) | 41 (40, 43) | 0.85 |
| Glucose, mg/dL | 77 (74, 79) | 77 (74, 80) | 75 (73, 77) | 77 (75, 79) | 0.84 |
| Diastolic blood pressure, mmHg | 80 (79, 81) | 82 (81, 83) | 82 (80, 83) | 80 (79, 82) | 0.28 |
| Systolic blood pressure, mmHg | 132 (130, | 134 (132, 136) | 135 (133, 138) | 132 (129, 134) | 0.94 |
| Quartiles of adipose DPA | |||||
| 1 | 2 | 3 | 4 | ||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P-trend2 | |
| Waist circumference, cm | 90 (89, 90) | 91 (90, 91) | 90 (90, 91) | 91 (90, 92) | 0.02 |
| Triglycerides, mg/dL | 210 (194, 26) | 217 (203, 231) | 230 (214, 247) | 233 (215, 252) | 0.03 |
| HDL cholesterol, mg/dL | 41 (40, 42) | 41 (40, 42) | 41 (40, 42) | 41 (40, 42) | 0.92 |
| Glucose, mg/dL | 75 (73, 78) | 76 (74, 78) | 78 (75, 80) | 76 (73, 80) | 0.59 |
| Diastolic blood pressure, mmHg | 81 (79, 82) | 80 (79, 82) | 82 (80, 83) | 81 (80, 83) | 0.31 |
| Systolic blood pressure, mmHg | 134 (131, 136) | 133 (131, 136) | 133 (131, 136) | 133 (130, 135) | 0.59 |
| Quartiles of adipose DHA | |||||
| 1 | 2 | 3 | 4 | ||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P-trend2 | |
| Waist circumference, cm | 91 (90, 91) | 90 (90, 91) | 91 (90, 91) | 90 (89, 91) | 0.10 |
| Triglycerides, mg/dL | 225 (208, 243) | 227 (211, 243) | 224 (210, 239) | 214 (198, 231) | 0.31 |
| HDL cholesterol | 41 (40, 42) | 40 (39, 42) | 41 (40, 42) | 41 (40, 42) | 0.23 |
| Glucose, mg/dL | 76 (73, 78) | 77 (75, 79) | 77 (74, 79) | 76 (73, 79) | 0.87 |
| Diastolic blood pressure, mmHg | 81 (80, 82) | 81 (80, 83) | 80 (79, 82) | 81 (80, 82) | 0.84 |
| Systolic blood pressure, mmHg | 134 (131, 136) | 133 (131, 136) | 131 (129, 133) | 135 (132, 138) | 0.48 |
| Quartiles of adipose EPA:ALA ratio | |||||
| 1 | 2 | 3 | 4 | ||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P-trend2 | |
| Waist circumference, cm | 90 (90, 91) | 90 (89, 91) | 91 (90, 91) | 91 (90, 92) | 0.02 |
| Triglycerides, mg/dL | 212 (198, 227) | 216 (203, 229) | 243 (224, 262) | 220 (206, 234) | 0.17 |
| HDL cholesterol, mg/dL | 41 (40, 42) | 41 (39, 42) | 41 (40, 42) | 41 (40, 43) | 0.34 |
| Glucose, mg/dL | 76 (74, 79) | 77 (74, 80) | 76 (73, 78) | 77 (74, 79) | 0.96 |
| Diastolic blood pressure, mmHg | 80 (79, 81) | 82 (81, 83) | 81 (80, 82) | 81 (80, 82) | 0.24 |
| Systolic blood pressure, mmHg | 132 (129, 134) | 135 (133, 137) | 134 (131, 136) | 133 (130, 135) | 0.63 |
| Quartiles of adipose ALA:LA ratio | |||||
| 1 | 2 | 3 | 4 | ||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P-trend2 | |
| Waist circumference, cm | 91 (90, 91) | 91 (90, 91) | 91 (90, 91) | 90 (89, 90) | 0.03 |
| Triglycerides, mg/dL | 224 (2100, 238) | 217 (203, 231) | 214 (198, 230) | 236 (219, 253) | 0.23 |
| HDL cholesterol, mg/dL | 41 (40, 42) | 41 (40, 42) | 41 (40, 42) | 41 (40, 42) | 0.98 |
| Glucose, mg/dL | 78 (75, 81) | 77 (75, 80) | 75 (73, 77) | 75.01 (73, 77) | 0.04 |
| Diastolic blood pressure, mmHg | 82 (81, 83) | 81 (80, 82) | 80 (78, 81) | 81 (80, 82) | 0.37 |
| Systolic blood pressure, mmHg | 134 (132, 137) | 132 (130, 135) | 132 (130, 134) | 134 (132, 137) | 0.86 |
Models adjust for matching factors (age, sex, and area of residence); lifestyle (smoking, alcohol intake and physical activity); diet (saturated fat and total calories); all other fatty acids (ALA, LA, EPA, DHA, DPA and total trans) and BMI.
Tests for trend were performed using median of the quartile as a continuous variable in the linear regression models.
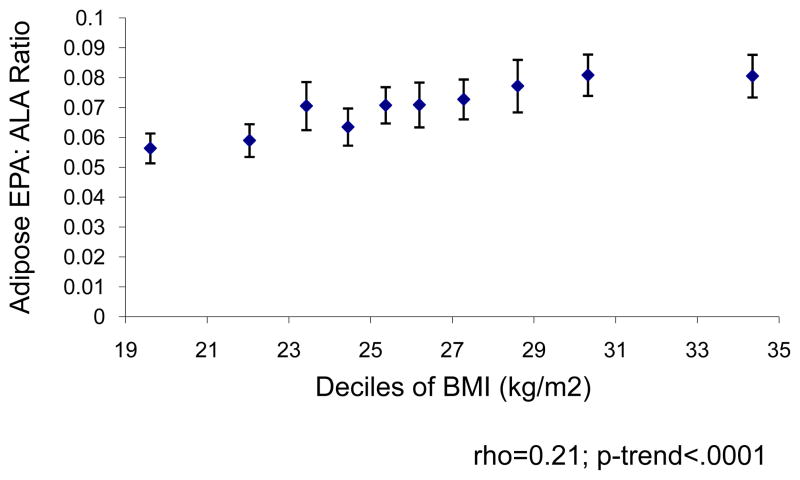
We evaluated the association between the EPA:ALA ratio and BMI to examine whether the positive association between EPA and DPA in adipose tissue and metabolic syndrome could be attributed to increased adiposity, rather than dietary intake. Figure 1 shows the Spearman correlation between the EPA:ALA ratio in adipose tissue and BMI. Increased BMI was positively associated with the EPA:ALA ratio although no further increases in EPA:ALA were observed with BMI greater than 29 kg/m2.
Figure 1. EPA: ALA ratio in adipose tissue by mean BMI within deciles.
Plot of EPA: ALA ratio in adipose tissue by mean BMI within deciles to illustrate the hypothesized upregulation of delta-6 desaturase in obesity. Delta-6 desaturase increases conversion of ALA to EPA and DPA.
In sensitivity analyses presented in Supplemental Tables 1–2, we considered the associations of fatty acid levels in adipose tissue separately in men and women and found similar results.
DISCUSSION
This cross-sectional study compared adipose tissue levels of n-3 fatty acids in samples from individuals with and without metabolic syndrome and found associations in opposite directions. We observed lower metabolic syndrome prevalence and lower fasting glucose levels among individuals with higher adipose tissue ALA. Adjusting for BMI attenuated this association and results did not reach statistical significance for metabolic syndrome, but remained significant for fasting glucose levels. This may still suggest a possible beneficial effect of ALA. In contrast, no evidence of a beneficial effect was found for the other fatty acids of interest (EPA, DHA, and DPA). In fact, metabolic syndrome prevalence and plasma triglyceride levels were higher among individuals with higher adipose tissue DPA, even after adjustment for BMI and waist circumference.
The associations observed in our study between adipose tissue n-3 fatty acids and prevalence of metabolic syndrome, after making adjustments for BMI, could highlight the different dietary and/or metabolic pathways at work that must be isolated for each fatty acid of interest. ALA cannot be endogenously synthesized, so its presence in human tissue is likely an indicator of dietary intake from plant sources. Numerous studies have found an inverse association between levels of ALA from diet and prevalence of metabolic syndrome and its components, as reviewed by Carpentier et al (27). A recent meta-analysis revealed that increased ALA intake was associated with lower T2D prevalence, although results did not reach statistical significance (8). A study of elderly Swedish men by Iggman et al. found inverse associations of insulin resistance measured through euglycaemic clamp and adipose tissue ALA and DHA but not EPA, and noted that most associations diminished or disappeared in lean individuals (28). These results are consistent with our findings. The potential benefit of plant-source n-3 fatty acids is of particular interest due to their greater ecologic sustainability and availability compared with marine sources (EPA, DHA). More research in this area is warranted (29).
Unlike ALA, the presence of EPA and DHA can reflect not just dietary intake but also metabolic activity. With respect to diet, our results for adipose tissue levels of EPA and DHA are in line with studies conducted in populations living in Europe and the United States that show no association between dietary intake or circulating levels of EPA+DHA and T2D (8). It is possible that these results are related to a similarity in genetic background as the Costa Rican population is predominantly of Southern European ancestry (30). Our results contrast findings from studies conducted in Asian populations, where higher dietary fish and/or seafood is associated with 11% lower risk of T2D (8). Additionally, results from studies among Chinese, Korean, or Inupiat Eskimo adult populations show that erythrocyte DHA levels (4) and dietary n-3 intake particularly from fish (7, 31) were associated with lower risk of metabolic syndrome and/or its components. Low fish intake in the Costa Rican population could also explain the lack of an association between DHA levels and metabolic syndrome in the current study (9, 10).
Our data also suggest that EPA and DHA might not be interchangeable biomarkers of dietary intake because they are metabolized differently in tissue. In our study, the association between adipose tissue levels of EPA, but not levels of DHA, and risk for metabolic syndrome was strongly influenced by adjustments for BMI. In support of this hypothesis, Sun et al. evaluated this relationship in plasma and red blood cells found higher correlations for DHA intake and its levels in plasma and red blood cells (erythrocyte r = 0.56 and plasma r = 0.48) than EPA intake (erythrocyte r = 0.38 and plasma r = 0.21) (32). Thus, EPA levels in adipose tissue may not simply be affected by dietary intake but also by higher delta-6 desaturase activity induced by adiposity (33, 34). Animal studies show that delta-6 desaturase expression is up regulated in obese (ob/ob) mice and Zucker fatty-rat models as compared to lean littermates (35, 36). A higher intake of marine fatty acids relative to plant-based fatty acids is another possible explanation for higher EPA:ALA ratios in adipose tissue; however, the more likely reason for a high EPA:ALA ratio in this population is higher delta-6 desaturase activity given the low consumption of fish and other seafood in the Costa Rican population (9, 10). Consistent with these and previous studies in humans, we found a high positive correlation between the EPA:ALA ratio in adipose tissue and adiposity.
Further evidence that DHA levels in adipose tissue might be a better marker of dietary intake was found in examining the association between DPA and metabolic syndrome. Since DPA from diet is negligible (37, 38), its presence likely arises in part from the upregulation of delta-6 desaturase, which increases conversion of ALA to EPA and DPA. The association between DPA levels in adipose tissue and risk of metabolic syndrome remained significant even after adjusting for BMI and waist circumference. Thus, it is possible that higher DPA levels reflect low fish intake as dietary DHA decreases accumulation of DPA in plasma by accelerating its clearance (39). However, residual confounding by adiposity likely remains. Our study found a positive association of DPA levels in adipose tissue and metabolic syndrome, which is important to be aware of as a precursor to T2D. However, there is no direct evidence from prospective studies that DPA is associated with T2D. Rather, DPA was associated with lower risk in studies that did not find an association for EPA or DHA (40–43).
This study provides needed data on diet and metabolic dysregulation that increase the risk of T2D in low-income countries such as Costa Rica, particularly because findings from Asian countries differ from those in the United States and Europe (8). Citizens of lower-income countries make up most of the world’s population (9) and in most of these regions, low availability of marine sources of n-3 fatty acids make it difficult for individuals to meet the WHO’s recommendation of one to two servings of fish per week (9). Plant-source n-3 fatty acids may be able to replace marine-source n-3, but data are lacking. Improving our understanding of ALA and its metabolites is of particular relevance as global obesity prevalence increases.
One limitation of the present study is cross-sectional design: while we excluded identified diabetics to avoid reverse causation due to changes in diet after diagnosis, no causal relationships or temporality can be established. Reverse causation, particularly by BMI despite adjustment for it in the final model. BMI adjustment may have resulted in variance inflation due to collinearity because of its strong correlation with waist circumference, a criterion for the metabolic syndrome. Participants in this study are only representative of the source population within matching strata (age, sex, and area of residence), which limits the generalizability of the results. (11) In addition, this study cannot identify the mechanism by which ALA may relate to metabolic syndrome.
In sum, this study suggests that ALA could play a role in metabolic syndrome, while long-chain fatty acids from fish and/or seafood (EPA and DHA) are not implicated. Our study suggests that the association between adipose tissue DPA levels and metabolic syndrome could be, in part, the effect of adiposity on delta-6-desaturase activity.
Supplementary Material
Acknowledgments
Funding: Supported by the National Institutes of Health HL49086 and HL60692.
Abbreviations
- T2D
Type 2 diabetes
- CI
Confidence interval
- PUFA
Polyunsaturated fatty acids
- ALA
α-Linolenic acid
- EPA
Eicosapentaenoic acid
- DHA
Docosahexaenoic acid
- DPA
Docosapentaenoic acid
- BMI
Body Mass Index
- PR
Prevalence ratio
Footnotes
Reprints will not be available from the author.
The authors have no conflicts of interest to declare.
Supplementary information is available at EJCN’s website.
References
- 1.International Diabetes Foundation. IDF Diabetes Atlas. (6) [cited 2012 December 6, 2012]. 6th Edition:[Available from: http://www.idf.org/diabetesatlas.
- 2.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, et al. American Heart A. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Balk E, Chung M, Lichtenstein A, Chew P, Kupelnick B, Lawrence A, et al. Effects of omega-3 fatty acids on cardiovascular risk factors and intermediate markers of cardiovascular disease. Evid Rep Technol Assess (Summ) 2004;(93):1–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang G, Sun Q, Hu FB, Ye X, Yu Z, Zong G, et al. Erythrocyte n-3 fatty acids and metabolic syndrome in middle-aged and older Chinese. J Clin Endocrinol Metab. 2012;97(6):E973–7. doi: 10.1210/jc.2011-2997. [DOI] [PubMed] [Google Scholar]
- 5.Huang T, Bhulaidok S, Cai Z, Xu T, Xu F, Wahlqvist ML, et al. Plasma phospholipids n-3 polyunsaturated fatty acid is associated with metabolic syndrome. Mol Nutr Food Res. 2010;54(11):1628–35. doi: 10.1002/mnfr.201000025. [DOI] [PubMed] [Google Scholar]
- 6.Lai YH, Petrone AB, Pankow JS, Arnett DK, North KE, Ellison RC, et al. Association of dietary omega-3 fatty acids with prevalence of metabolic syndrome: The National Heart, Lung, and Blood Institute Family Heart Study. Clinical nutrition (Edinburgh, Scotland) 2013 doi: 10.1016/j.clnu.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baik I, Abbott RD, Curb JD, Shin C. Intake of fish and n-3 fatty acids and future risk of metabolic syndrome. J Am Diet Assoc. 2010;110(7):1018–26. doi: 10.1016/j.jada.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Wu JH, Micha R, Imamura F, Pan A, Biggs ML, Ajaz O, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012;107 (Suppl 2):S214–27. doi: 10.1017/S0007114512001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrova S, Dimitrov P, Willett WC, Campos H. The global availability of n-3 fatty acids. Public Health Nutr. 2011;14(7):1157–64. doi: 10.1017/S1368980010003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campos H, Baylin A, Willett WC. Alpha-linolenic acid and risk of nonfatal acute myocardial infarction. Circulation. 2008;118(4):339–45. doi: 10.1161/CIRCULATIONAHA.107.762419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabagambe EK, Baylin A, Ascherio A, Campos H. The type of oil used for cooking is associated with the risk of nonfatal acute myocardial infarction in costa rica. The Journal of nutrition. 2005;135(11):2674–9. doi: 10.1093/jn/135.11.2674. [DOI] [PubMed] [Google Scholar]
- 12.Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 13.Baylin A, Kabagambe EK, Siles X, Campos H. Adipose tissue biomarkers of fatty acid intake. The American journal of clinical nutrition. 2002;76(4):750–7. doi: 10.1093/ajcn/76.4.750. [DOI] [PubMed] [Google Scholar]
- 14.Dayton S, Hashimoto S, Dixon W, Pearce ML. Composition of lipids in human serum and adipose tissue during prolonged feeding of a diet high in unsaturated fat. J Lipid Res. 1966;7(1):103–11. [PubMed] [Google Scholar]
- 15.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38(10):2012–22. [PubMed] [Google Scholar]
- 16.Lillington JM, Trafford DJ, Makin HL. A rapid and simple method for the esterification of fatty acids and steroid carboxylic acids prior to gas-liquid chromatography. Clin Chim Acta. 1981;111(1):91–8. doi: 10.1016/0009-8981(81)90425-3. [DOI] [PubMed] [Google Scholar]
- 17.Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L, Tocco P, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. 2005;162(4):373–81. doi: 10.1093/aje/kwi213. [DOI] [PubMed] [Google Scholar]
- 18.Sprecher H, Luthria DL, Mohammed BS, Baykousheva SP. Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J Lipid Res. 1995;36(12):2471–7. [PubMed] [Google Scholar]
- 19.Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 2000;1486(2–3):219–31. doi: 10.1016/s1388-1981(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 20.Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri JM, Guesnet P. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Progress in lipid research. 2006;45(3):203–36. doi: 10.1016/j.plipres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Goyens PL, Mensink RP. The dietary alpha-linolenic acid to linoleic acid ratio does not affect the serum lipoprotein profile in humans. The Journal of nutrition. 2005;135(12):2799–804. doi: 10.1093/jn/135.12.2799. [DOI] [PubMed] [Google Scholar]
- 22.Williams ES, Baylin A, Campos H. Adipose tissue arachidonic acid and the metabolic syndrome in Costa Rican adults. Clinical nutrition (Edinburgh, Scotland) 2007;26(4):474–82. doi: 10.1016/j.clnu.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 24.Mattei J, Malik V, Hu FB, Campos H. Substituting homemade fruit juice for sugar-sweetened beverages is associated with lower odds of metabolic syndrome among Hispanic adults. The Journal of nutrition. 2012;142(6):1081–7. doi: 10.3945/jn.111.149344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattei J, Hu FB, Campos H. A higher ratio of beans to white rice is associated with lower cardiometabolic risk factors in Costa Rican adults. The American journal of clinical nutrition. 2011;94(3):869–76. doi: 10.3945/ajcn.111.013219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White H. A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica (pre-1986) 1980;48(4):817. [Google Scholar]
- 27.Carpentier YA, Portois L, Malaisse WJ. n-3 fatty acids and the metabolic syndrome. The American journal of clinical nutrition. 2006;83(6 Suppl):1499S–504S. doi: 10.1093/ajcn/83.6.1499S. [DOI] [PubMed] [Google Scholar]
- 28.Iggman D, Arnlov J, Vessby B, Cederholm T, Sjogren P, Riserus U. Adipose tissue fatty acids and insulin sensitivity in elderly men. Diabetologia. 2010;53(5):850–7. doi: 10.1007/s00125-010-1669-0. [DOI] [PubMed] [Google Scholar]
- 29.Brunner EJ, Jones PJ, Friel S, Bartley M. Fish, human health and marine ecosystem health: policies in collision. Int J Epidemiol. 2009;38(1):93–100. doi: 10.1093/ije/dyn157. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Narvaez EA, Bare L, Arellano A, Catanese J, Campos H. West African and Amerindian ancestry and risk of myocardial infarction and metabolic syndrome in the Central Valley population of Costa Rica. Hum Genet. 2010;127(6):629–38. doi: 10.1007/s00439-010-0803-x. [DOI] [PubMed] [Google Scholar]
- 31.Ebbesson SO, Tejero ME, Nobmann ED, Lopez-Alvarenga JC, Ebbesson L, Romenesko T, et al. Fatty acid consumption and metabolic syndrome components: the GOCADAN study. J Cardiometab Syndr. 2007;2(4):244–9. doi: 10.1111/j.1559-4564.2007.07393.x. [DOI] [PubMed] [Google Scholar]
- 32.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. The American journal of clinical nutrition. 2007;86(1):74–81. doi: 10.1093/ajcn/86.1.74. [DOI] [PubMed] [Google Scholar]
- 33.Warensjo E, Rosell M, Hellenius ML, Vessby B, De Faire U, Riserus U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis. 2009;8:37. doi: 10.1186/1476-511X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warensjo E, Sundstrom J, Lind L, Vessby B. Factor analysis of fatty acids in serum lipids as a measure of dietary fat quality in relation to the metabolic syndrome in men. The American journal of clinical nutrition. 2006;84(2):442–8. doi: 10.1093/ajcn/84.1.442. [DOI] [PubMed] [Google Scholar]
- 35.Fevre C, Bellenger S, Pierre AS, Minville M, Bellenger J, Gresti J, et al. The metabolic cascade leading to eicosanoid precursors--desaturases, elongases, and phospholipases A2--is altered in Zucker fatty rats. Biochim Biophys Acta. 2011;1811(6):409–17. doi: 10.1016/j.bbalip.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Botolin D, Xu J, Christian B, Mitchell E, Jayaprakasam B, et al. Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J Lipid Res. 2006;47(9):2028–41. doi: 10.1194/jlr.M600177-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sargent JR. Fish oils and human diet. Br J Nutr. 1997;78 (Suppl 1):S5–13. doi: 10.1079/bjn19970131. [DOI] [PubMed] [Google Scholar]
- 38.Baylin A, Siles X, Donovan-Palmer A, Fernandez X, Campos H. Fatty acid composition of Costa Rican foods including trans fatty acid content. Journal of Food Composition and Analysis. 2007;20(3–4):182–92. [Google Scholar]
- 39.Emken EA, Adlof RO, Duval SM, Nelson GJ. Effect of dietary docosahexaenoic acid on desaturation and uptake in vivo of isotope-labeled oleic, linoleic, and linolenic acids by male subjects. Lipids. 1999;34(8):785–91. doi: 10.1007/s11745-999-0424-2. [DOI] [PubMed] [Google Scholar]
- 40.Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, Wareham NJ, et al. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. The American journal of clinical nutrition. 2010;92(5):1214–22. doi: 10.3945/ajcn.2010.29182. [DOI] [PubMed] [Google Scholar]
- 41.Hodge AM, English DR, O’Dea K, Sinclair AJ, Makrides M, Gibson RA, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. The American journal of clinical nutrition. 2007;86(1):189–97. doi: 10.1093/ajcn/86.1.189. [DOI] [PubMed] [Google Scholar]
- 42.Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, et al. Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2008;18(7):503–10. doi: 10.1016/j.numecd.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Kroger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Doring F, et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. The American journal of clinical nutrition. 2011;93(1):127–42. doi: 10.3945/ajcn.110.005447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.