Abstract
Objective
Myofascial trigger points (MTrPs) are focal disruptions in skeletal muscle that can refer pain to the head and reproduce the pain patterns of tension-type headache (TTH). The present study applied massage focused on MTrPs of subjects with TTH in a placebo-controlled, clinical trial to assess efficacy on reducing headache pain.
Methods
Fifty-six subjects with TTH were randomized to receive 12 massage or placebo (detuned ultrasound) sessions over six weeks, or to wait-list. Trigger point release (TPR) massage focused on MTrPs in cervical musculature. Headache pain (frequency, intensity and duration) was recorded in a daily headache diary. Additional outcome measures included self-report of perceived clinical change in headache pain and pressure-pain threshold (PPT) at MTrPs in the upper trapezius and sub-occipital muscles.
Results
From diary recordings, group differences across time were detected in headache frequency (p=0.026), but not for intensity or duration. Post hoc analysis indicated headache frequency decreased from baseline for both massage (p<0.0003) and placebo (p=0.013), but no difference was detected between massage and placebo. Subject report of perceived clinical change was a greater reduction in headache pain for massage than placebo or wait-list groups (p=0.002). PPT improved in all muscles tested for massage only (all p's<0.002).
Discussion
Two findings from this study are apparent: 1) MTrPs are important components in the treatment of TTH, and 2) TTH, like other chronic conditions, is responsive to placebo. Clinical trials on headache that do not include a placebo group are at risk for overestimating the specific contribution from the active intervention.
Keywords: Episodic tension-type headache, chronic tension-type headache, complementary medicine, headache frequency, algometer
Introduction
Tension-type headache (TTH) is a widespread and major health concern. The one-year prevalence of episodic and chronic tension-type headache is 38% and 2-3%, respectively [1, 2]. Tension-type headache affects daily functioning, resulting in limitations in performance and participation [1]. Although TTH is the most prevalent headache disorder, resulting in greater societal burden than migraine, investigation into interventional avenues lags other headache categories such as migraine. [3-5] Others have stressed the need for development of treatment interventions for TTH with fewer side effects than currently recommended medications [6].
In recent years the myofascial trigger point (MTrP) has become a site of interest in the pathology of TTH. Patients with TTH exhibit increased presence and tenderness of MTrPs in pericranial muscles. There is evidence to suggest that the presence and pain sensitivity of MTrPs are sufficient to make distinctions between headache and non-headache populations [7-9]. MTrPs within skeletal muscle are characterized by a number of physical features including a palpable tender nodule within a taut muscular band, point tenderness at the nodule, characteristic patterns of referred pain and the presence of a local twitch response when stimulated [10]. To underscore the importance of the MTrP and its relationship to headache, one prominent and recurring theory is that progression from episodic to chronic forms of TTH is related to prolonged nociceptive input from peripheral myofascial tissues, which sensitize the central nervous system, thereby increasing its excitability. [11, 12] While pathogenesis of TTH remains unclear and is likely multifactorial, MTrPs have an intriguing connection to TTH in that active MTrPs can elicit referred pain phenomenon that reproduce patient pain complaints, thus providing a direct connection between peripheral tissue and the central pain of headache. [7, 12-18] In recent years, interventions directed at a MTrP have led to reduction in local pain, referred pain intensity, and the extent of referred pain fields originating from the MTrP in both headache and non-headache populations. [19, 20]
Treatment that addresses MTrPs has been emphasized as an interventional strategy for management of TTH. [21] To that end, several pilot studies with a focus on the MTrP have found positive effects in primary clinical measures of TTH. [22, 23] Preliminary research also indicates that interventions that address the MTrP as part of a more comprehensive treatment plan may be beneficial in the management of TTH [24-26]. Two recent non-controlled studies were designed to identify clinical characteristics of TTH patients likely to achieve short-term success with myofascial trigger point therapy. [27, 28] In the earlier study, four variables were identified for immediate short-term (headache duration < 8.5 hour/day, headache frequency < 5.5 days/week, bodily pain < 47, vitality < 47.5) and two variables stood out at the 1-month follow-up (headache frequency < 5.5 days/week and bodily pain < 47). The second study identified eight prognostic factors from the history and physical examination of TTH patients that link to treatment success of myofascial trigger point therapy for TTH. The eight variables were punctuated by six that are associated with myofascial trigger points or cervical musculature: the presence of MTrP's in the suboccipitals, left sternocleidomastoid, left superior oblique; referred pain area of right upper trapezius MTrP, total muscle tenderness score, and reduced cervical rotation to the left. Age less than 44.5 years and a score of 18.5 or less on the Neck Disability Index test constitute the remaining two prognostic factors. The authors report that patients expressing 5 or more variables exhibit an increased likelihood of treatment success with myofascial trigger point therapy. While techniques that reduce MTrP pain are a logical interventional target for TTH, no randomized, placebo-controlled trial has been conducted to determine the efficacy of an intervention focused solely on the MTrP for reducing headache pain. [29]
While many techniques exist that can impact MTrPs, massage therapy is particularly interesting due to its availability [30], relatively low cost [31], patient interest [32], informality, and treatment effectiveness. [19, 33] The aim of the present study was to determine the efficacy of trigger point release (TPR) massage for reducing tension-type headache pain in a randomized, placebo-controlled trial. Secondary aims were to determine the effects of multiple TPR massage sessions on MTrP tenderness and assess quality of life variables in those with TTH.
Methods
The present study conformed to International Headache Society (IHS) guidelines for the design of randomized clinical trials for headache. [34] Study procedures were approved by the Colorado Multiple Institutional Review Board (COMIRB) at the University of Colorado Denver. Informed consent was obtained from all subjects prior to study enrollment. The trial is registered at ClinicalTrials.gov, number NCT01244555.
Study population
Subjects were recruited from a large metropolitan setting near a University hospital. All subjects were screened for TTH by an anesthesiologist (JK) with specialty experience in headache. Participants were recruited and enrolled between September 2010 and May 2012.
For inclusion in the study, participants had to have two or more headaches per week meeting International Classification of Headache Disorders, 2nd edition (ICHD-2) criteria for TTH defined as head pain of bilateral location, pressing or tightening quality, mild to moderate intensity, and not aggravated by routine physical activity (e.g. walking or stair climbing). [35] Both of the following criteria also had to be met: no nausea or vomiting in association with the headache; and no more than one of the following two symptoms, photophobia or phonophobia. Participants between the ages of 18-59 years of age were recruited. Exclusion criteria included migraine (>1/month), headache originating from a secondary cause (e.g. cancer or injury), fibromyalgia, diabetes, major depression, neurological or cardiovascular disease, pregnancy, use of professional massage or ultrasound specifically for headache in the prior 6 months. Subjects taking prophylactic medication for headache were also excluded; abortive medication was permitted provided a self-reported use for no more than 75 % of headache episodes. All subjects were screened by a massage therapist for the presence of at least one active MTrP in the primary (bilateral) muscles addressed (upper trapezius, suboccipital muscles, sternocleidomastoid).
Design
The study was a randomized, placebo-controlled clinical trial conducted in three consecutive phases: baseline (4 weeks), treatment (6 weeks), and run-out (4 weeks). The treatment phase was divided into two three-week phases in order to detect any treatment plateau (Figure 1). Headache was recorded in a daily headache diary for the duration (14 weeks) of the study. Immediately prior to the first treatment session, subjects were block randomized (blocks of six) to receive massage, placebo, or wait-listed. Individuals randomized to massage or placebo were then scheduled for twice weekly sessions over six weeks for a maximum of 12 treatment sessions. Subjects randomized to the wait-list group did not attend intervening sessions.
Figure 1.
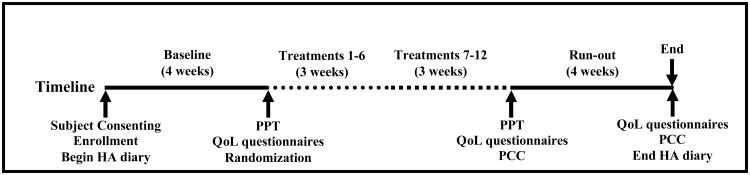
Study timeline for the 14 week clinical trial depicting the four week baseline, two 3-week treatment phases, the four week run-out, and time points for outcome measure assessments. Key: HA – headache; PPT – pressure-pain threshold; QoL – quality of life; PCC- perceived clinical change.
All subjects were informed, prior to consenting, that they could receive the alternate treatment (massage or ultrasound) program upon completion of the study free of charge. During the run-out phase, subjects received no intervention. No subject reported initiating a new treatment intervention during the 14 week study. All interventions and assessments were conducted at the Clinical and Translational Research Center (CTRC) at the University of Colorado Hospital.
Interventions
Each massage or placebo session was 45 minutes in duration, administered twice per week, and separated by at least 48 hours.
Massage
All of the massage therapists who delivered that intervention had state licensure in Colorado to practice massage, prior training and work experience in the identification of MTrPs, at least 3 years of professional practice experience, and regular use of the general massage techniques used in the protocol. Massage therapists underwent two 3-hour study protocol training sessions to ensure consistent application of the massage procedure. Each massage therapist was observed by the lead massage therapist (NB) on three unannounced occasions to confirm adherence to massage protocol administration. Only minimal deviations from protocol were observed and none detected after the first observation session. Six massage therapists participated in the study. Subjects were assigned a primary therapist for the study duration based on therapist and subject schedules. No therapist was assigned to more than five subjects.
Each massage session followed a standardized protocol lasting 45 minutes, consisting of 15 minutes of myofascial release to warm soft tissues of the back, shoulders, chest, and neck [36]; 20 minutes of trigger point release (TPR) applied bilaterally to address MTrPs in the upper trapezius, the sub-occipital muscle groups, and the sternocleidomastoids [22, 37]; the final 10 minutes consisted of post-isometric relaxation directed at right and left lateral cervical flexion, circular or cross-fiber friction on the masseter, temporalis, and occipital-frontalis muscles, as well as gentle effleurage and petrissage to the neck and shoulders [38, 39]. The upper trapezius, sub-occipital muscles, and the sternocleidomastoids were selected for TPR treatment due to the high frequency of MTrPs in these muscles that refer pain to the head and reproduce the subject headache pain complaint. [16, 21, 40]
MTrPs were identified using published criteria. [10] Briefly, muscles were palpated for a tender nodule along taut bands within the respective muscle groups. Force was progressively applied to the nodule with the patient instructed to verbally indicate whether they felt pain locally or in other areas (referred pain). For sites where the subject indicated referred pain, subjects were asked whether the pain was familiar (e.g. reproduced their typical headache). If multiple MTrPs were identified within a muscle, the MTrP that reproduced the subjects' typical headache (active MTrP) was addressed. If no active MTrP was identified the most tender site was treated. TPR was applied as follows: pincer grip of sufficient force to just elicit referred pain (or 6 on a 10-point scale) was applied to the identified site. Duration was until subject verbally reported dissipation of referred pain, the therapist detected a physical softening in the MTrP, or a maximum of 60 seconds had elapsed. Up to five compressions were performed at each site with a 10-second rest between compressions to allow blood reperfusion. TPR has been extensively used by our research team [22, 41] and others.[42-44]
Placebo
Proper placebo control for massage-based studies is inherently difficult since many sham treatments lack plausibility for the health issue or could inadvertently contribute a therapeutic benefit. A proper control intervention for massage must seem plausible for the ailment, account for contextual aspects of the intervention such as patient-therapist individual time, and incorporate a tactile interaction with the subject. Detuned ultrasound (US) meets all of these parameters as it: 1) is believable as an intervention for MTrPs [33, 45-48]; 2) controls for therapist-subject interaction; 3) involves tactile contact with the subject; and 4) provides no potentially effective treatment. Furthermore, patients are unable to distinguish an active from an inactive US treatment, thus ensuring that blinding was maintained. [49, 50]
Detuned US was administered by a nurse or nurse's aide who had received a one-hour training on ultrasound application from a certified ultrasound technician. Nurses were used due to the high plausibility for a nurse to administer this type of intervention in a medical setting, thus enhancing the believability of the placebo intervention. Detuned-US was administered using a Dynatron 150plus (Dynatronics Corporation, 7030 Park Centre Drive, Salt Lack City, UT 84121) with display settings at 1W/cm2 intensity, 1.0Mhz frequency, and 20% duty cycle. A non-functional soundhead (5cm2) provided by the manufacturer was used. A standardized protocol was followed to ensure consistency in its application. Subjects were seated in a massage chair with detuned-US administered sequentially to four regions of the upper back and neck to approximate location of the left and right upper trapezius and sub-occipital muscle groups. Water soluble coupling agent (ScripHessco Ultrasound Gel, Script ScripHessco 360 Veterans Parkway, Suite 115, Bolingbrook, IL 60440-4607) was applied to the skin surface and the soundhead moved in small overlapping circles at a rate of 2-3cm/s. Each region was treated for 10 minutes with the area cleaned of coupling agent before proceeding to the next region. Each US session lasted 45 minutes.
Eight nurses or nurse's aides participated in the study; each was observed by the certified ultrasound technician on one unannounced visit to assess US application and confirm treatment protocol compliance. No technical deviations were observed. Both nurses and subjects were blind to the non-functional nature of the ultrasound equipment.
Wait-list group
A wait-list comparator group was included to assess the natural course of TTH over time. Subjects randomized to this group did not receive any intervention, but completed all assessments in a time-matched manner. They maintained and returned their headache diaries similar to the massage and placebo groups.
All subjects and study personnel were blind to subject group assignment until immediately prior to the first treatment intervention (Figure 1) when a sealed envelope was opened that designated group assignment. Envelopes containing group allocation were prepared by departmental staff not affiliated with the study, but with guidance from the study statistician.
Outcome measures
Daily Headache Diary
Subjects maintained a paper daily headache diary for the duration of the study (14 weeks) with each phase representing three (treatment phase A and B) or four (baseline and run-out) week intervals. Diary recordings of three to four weeks have been shown to be reliable for headache measures in research investigations. [34, 51] Subjects recorded headache frequency, duration (hours and minutes), use of pain medication for headache, and headache pain intensity (100 mm visual analog scale (VAS) with anchors of “No headache pain” and “Maximum headache pain”). Diaries were returned by mail or directly to study personnel at two week intervals. Headache frequency was calculated as a weekly average for each subject during the time frame measured; headache duration and intensity are presented as the average value recorded per headache during the time frame measured; non-prescription medication use for headache was calculated as a weekly sum of the number of doses taken.
Perceived clinical change
Participant perceived change in headache pain was recorded on a 15 point scale ranging from -7 to +7. [56] Descriptors were provided with the numerical range where 0 was associated with “about the same;” ±1, “A tiny bit better/worse;” ± 2, “A little bit worse/better;” ± 3, “Somewhat worse/better;” ± 4, “Moderately worse/better;” ± 5, “Quite a bit worse/better;” ± 6, “A great deal worse/better;” ± 7, “A very great deal worse/better.” Subjects recorded perceived change in headache pain just prior to the final treatment (end of the treatment phase) and at the end of the four week run-out relative to how they felt at the start of treatment.
Pressure-Pain Threshold (PPT)
PPT was assessed bilaterally in the upper trapezius and subobccipital muscles using pressure algometry (Wagner FPN 50 algometer, Wagner Instruments, Inc. Greenwich, CT). Briefly, muscles were palpated for MTrPs as described above. [10] When multiple MTrPs were identified, measurement was conducted at the site that generated referral pain reproducing the subjects' typical headache (e.g. active MTrP). If no active MTrP was identified, assessment was made at the most tender site identified. The algometer tip was placed over the site and force slowly applied until the subject verbally indicated that sensation changed from pressure to pain. The average of three assessments at the same site is reported; no change in PPT was detected across replicate measures (all p's>0.23 for each muscle group). A 20 second recovery was given between measurements. PPT was conducted immediately before randomization into treatment group and the final (12th) treatment visit, or, in the case of the wait-list group at a time- matched period six weeks after the first assessment. In all cases, the final PPT assessment was conducted at least 48 hours following any previous intervention session. Measurement of PPT in the SCM was not conducted due to the sensitive location and lack of a smooth, hard surface (e.g. bone) from which to apply constant pressure. Due to technical reasons it was not possible to blind the assessor to subject treatment group assignment.
Quality of life
The impact of headache on daily life was assessed by two validated questionnaires that capture different aspects of headache-associated disability. Questionnaires were administered at three time points: 1) the end of baseline phase (immediately prior to randomization); 2) the end of the treatment phase (six weeks later); 3) and at the end of run-out. Assessment at the end of treatment occurred just prior to the final treatment and at least 48 hours following any previous intervention.
The Headache Disability Inventory (HDI) is a principal measure of disability focusing on emotional and functional components of headache [52]. The HDI consists of 25 questions based on items derived empirically from case history responses of participants with headache. Responses are scored requiring a “yes,”2 points; “sometimes,” 1 point; or “no,” 0 points response. A total score is computed by summing all scores resulting in an individual HDI score ranging from 0 (no disability) to 100 (severe disability). A decrease in the total HDI score of ≥16 points is considered to be a clinically significant improvement. [53] The HDI has good internal consistency (0.89), robust short- and long-term retest reliability (0.83), and good construct validity [52, 53].
The Headache Impact Test (HIT-6) is a validated measure for assessing quality of life in patients with headache as a function of headache frequency.[34, 54] The HIT-6 consists of six items (pain, social functioning, role functioning, vitality, cognitive functioning, psychological distress) where respondents select one of five options: “never,” 6 points; “rarely,” 8 points; “sometimes,” 10 points; “very often,” 11 points; “always,” 13 points. Total score ranges from 36 to 78 points. A decrease of 2.3 to 8 points among patients with chronic daily headache reflects improvement in headache that is considered clinically significant. [55, 56] The HIT-6 has good internal consistency (Cronbach alpha 0.89), and sound test-retest reliability (0.80)[57].
Sample Size Calculation
An a priori power analysis was conducted assuming beta = 0.20, alpha = 0.05, and using effect size estimates from data obtained from published research for the primary headache indicator (headache frequency) in a preliminary study of massage intervention for tension-type headache. [22] Based on an effect size (Cohen's d) of approximately 0.84 for headache frequency enrollment of 20 subjects per group was anticipated to provide at least 80% power for headache frequency and allow for at least a modest amount of attrition (15%) during the study.
Statistical Analysis
All analyses were performed in SAS Version 9.2. All outcomes, with the exception of perceived clinical change, were modeled in a multilevel modeling framework in SAS Proc Mixed as linear mixed models. The models included a treatment main effect to determine an overall difference between groups and a time main effect to determine whether scores changed over time. The primary interest was in the treatment by time interaction to address the primary hypothesis that changes over time differed by group. If a significant treatment by time interaction was observed (e.g. p<0.05), simple effects tests were conducted to examine how scores changed over time within each of the three groups. The same modeling framework was followed for all outcomes, although there were four timepoints for headache diary outcomes (baseline, first half of treatment (phase A), second half of treatment (phase B), and end of run-out), two timepoints for PPT outcomes (baseline and end of treatment), and three timepoints for the HDI and HIT-6 (baseline, end of treatment, end of run-out). Several potential confounding variables were identified: age, gender, race/ethnicity, headache diagnosis (chronic or episodic), years with TTH, recent history of professional massage, activity level, depression, and anxiety. Each potential covariate was tested to determine whether it differed by condition or was related to the primary headache outcomes. None of these tests were statistically significant. Consistent with prior literature, the perceived clinical change score was broken down into four categories: 0 = No change, ±1 to ±3 = Small change; ±4 to ±5 = Moderate change; and ±6 to ±7 = Large change.[58] Fisher's exact test was used to test for differences in the proportion of those reporting no, small, moderate, or large change across the three conditions. ANOVA models were used to assess mean change in headache frequency from baseline to the end of treatment and run-out across the four change categories (collapsed across condition).
Results
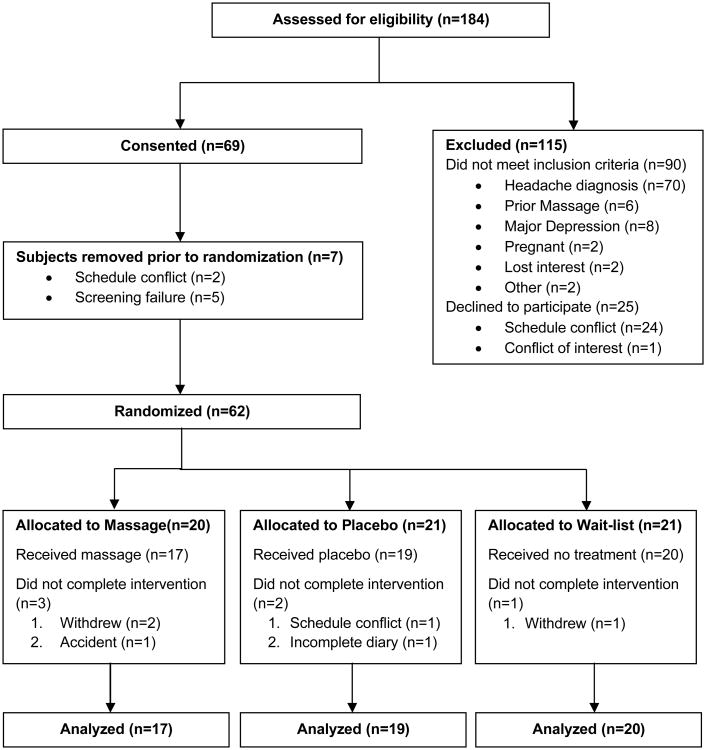
A total of 184 individuals were assessed for study eligibility; 69 met eligibility and were enrolled (Figure 2). Five subjects were removed from the study prior to randomization due to incorrect headache diagnosis based on headache diary recordings; two subjects withdrew, prior to randomization, due to conflict with scheduling. Sixty-two subjects were randomized to massage (20), placebo (21) or wait-list (21) groups. Six subjects (3 in massage, 2 in placebo, 1 in wait-list) subsequently withdrew or were lost to follow-up. To examine whether the data from those who completed the study could generalize to those who dropped, the completer group was compared to the dropped group on baseline demographic and headache variables. Of 25 tests conducted, the only significant difference between groups was on headache duration, where duration was significantly higher in the group who dropped due to a single outlier. Overall, this provides evidence that those who dropped were, on average, similar to those retained.
Figure 2.
Subject flow diagram illustrating subjects assessed for eligibility, randomization to groups (massage, placebo, and wait-list), and number analyzed in the study.
Table 1 depicts demographic information for the 56 subjects analyzed in the study, by treatment group. The three groups did not differ statistically in terms of any of the demographic variables examined. On average, subjects were 33.5 years old and the majority were female, Caucasian, employed full time, and had experienced TTH for an average of 8.9 years (SD = 6.9). Participants attended a mean of 11.6 (SD, 1.0) massage and 11.5 (SD, 0.84) placebo sessions of the planned 12 sessions (p=0.96).
Table 1. Subject demographic and headache information by treatment group.
| Demographic Variable | Massage (N=17) | Placebo (N=19) | Wait-list (N=20) |
|---|---|---|---|
| Mean ±SD or % | Mean ±SD or % | Mean ±SD or % | |
| Age | 32.1 ± 12.0 | 34.7 ± 11.1 | 33.4 ± 9.0 |
| % Female | 88.2% | 89.5% | 80.0% |
| % White | 88.2% | 79.0% | 85.0% |
| % Committed relationship / Married | 35.3% | 36.8% | 40.0% |
| % Employed Full Time | 64.7% | 68.4% | 80.0% |
| Headache | |||
| Chronic TTH | 64.7% | 52.6% | 45.0% |
| Episodic TTH | 35.3% | 47.4% | 55.0% |
| Years with TTH | 8.18 ± 6.6 | 9.34 ± 6.9 | 9.05 ± 7.5 |
| Treatment Sessions Attended | 11.6 ± 1.0 | 11.5 ± 0.84 | - |
Note. All comparisons between groups were non-significant (all ps > 0.44). TTH, Tension-type Headache
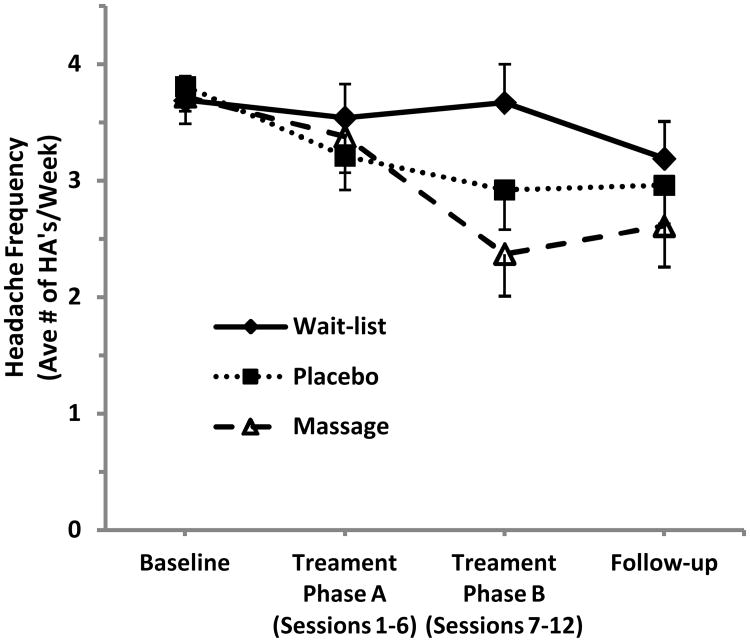
Table 2 depicts the results of the analyses examining treatment effects on changes in headache outcomes over time. Means ± standard error are presented for each timepoint, by treatment group. Treatment group differences were detected in changes in headache frequency over time (F (6, 52) = 2.65, p=0.026). Post-hoc analysis identified that headache frequency decreased for both massage (p=0.0003) and placebo (p=0.013) relative to their baseline but not for the wait-list group (p=0.098). No statistical difference was detected between the massage and placebo treatment groups (p=0.26). The pattern of means for headache frequency are also illustrated in Figure 3, and, as shown, the largest difference between conditions was during the second phase (phase B) of treatment (p=0.033). No significant treatment group differences were detected for headache duration, headache intensity, or medication use.
Table 2. Primary and secondary headache outcomes and medication use across the study time frame.
| Variable | Group | Baseline | Treatments 1-6 | Treatments 7-12 | Follow-up | Group*Time Interaction |
|---|---|---|---|---|---|---|
| Headache Frequency (headache/week) | Massage | 3.72±0.23 | 3.38±0.31 | 2.37±0.36 | 2.61±0.35 | F(6, 52) = 2.65, p = 0.026 |
| Placebo | 3.81±0.21 | 3.21±0.29 | 2.92±0.34 | 2.96±0.33 | ||
| Wait-list | 3.69±0.21 | 3.54±0.29 | 3.67±0.33 | 3.19±0.32 | ||
|
| ||||||
| Headache Duration (h) | Massage | 3.15±0.43 | 3.20±0.55 | 2.81±0.50 | 2.65±0.47 | F(6, 52) = 0.92, p = 0.49 |
| Placebo | 2.86±0.40 | 2.70±0.52 | 2.84±0.46 | 3.01±0.44 | ||
| Wait-list | 3.02±0.39 | 3.53±0.51 | 3.36±0.45 | 3.12±0.43 | ||
|
| ||||||
| Headache Intensity (100mm VAS) | Massage | 31.4±2.69 | 26.3±2.50 | 27.3±3.04 | 22.8±3.03 | F(6, 52) = 1.23, p = 0.30 |
| Placebo | 33.3±2.52 | 30.8±2.34 | 29.8±2.82 | 31.5±2.80 | ||
| Wait-list | 31.2±2.46 | 27.8±2.28 | 29.5±2.75 | 29.0±2.74 | ||
|
| ||||||
| Medication Use (doses per week) | Massage | 2.97±0.82 | 1.92±0.71 | 2.24±0.60 | 2.36±0.70 | F(6,52) = 1.12, p = 0.37 |
| Placebo | 1.75±0.76 | 1.55±0.66 | 1.02±0.56 | 1.05±0.66 | ||
| Wait-list | 3.13±0.74 | 3.06±0.65 | 2.96±0.54 | 2.47±0.64 | ||
Data are presented as mean ± standard error.
Figure 3.
Headache frequency means ± standard error over the four study phases (baseline, first and second halves of treatment, run-out) for the three study groups (massage, placebo, and wait-list). Values represent the average number of headache days per week during the respective time frame. Significant change from baseline was detected for massage (p=0.0003) and placebo (p=0.013), but not for wait-list (p=0.098).
Table 3 shows group effects on quality of life measures across time. Significant condition effects were observed on changes in HDI (F(4,52) = 3.26, p = 0.019) and HIT-6 (F(4,52) = 3.27, p = 0.018). Post-hoc tests showed a significant decrease in HDI scores in the intervention group (p = 0.0003) but not in the placebo (p = 0.06) or wait-list (p = 0.39) groups; a significant change in HIT-6 scores was detected over time in both the intervention (p = 0.0002) and placebo (p = 0.011) groups but not in the wait-list group (p = 0.52).
Table 3. Quality of life outcomes from baseline to end if treatment and follow-up.
| Variable | Group | Baseline | End of Treatment | Follow-up | Group*Time Interaction |
|---|---|---|---|---|---|
| HDI | Massage | 26.5±3.51 | 19.6±3.38 | 17.8±3.11 | F(4,52) = 3.26 p = 0.019 |
| Placebo | 29.1±3.30 | 24.3±3.18 | 26.0±2.92 | ||
| Wait-list | 21.6±3.23 | 22.6±3.11 | 20.4±2.86 | ||
|
| |||||
| HIT-6 | Massage | 56.3±1.57 | 51.1±1.54 | 50.2±1.57 | F(4,52) = 3.27 p = 0.018 |
| Placebo | 54.7±1.48 | 50.5±1.45 | 52.3±1.47 | ||
| Wait-list | 53.2±1.45 | 53.4±1.41 | 52.1±1.44 | ||
Data are presented as mean ± standard error. HDI, Headache Disability Index; HIT-6, Headache Impact Test.
Perceived clinical change in headache pain is presented in Table 4. In the first set of analyses, the proportion of subjects reporting changes in headache pain across the three conditions was assessed. The results of the Fisher's exact test comparing reported change across the three conditions was significant both at the end of treatment and at the end of run-out. At post-treatment, the vast majority of those in the massage condition reported moderate or large improvement (84.7%), compared with 50% in the placebo group and 0% in the wait-list group (p<0.001). In contrast, 82.4% of those in the wait-list condition reported no change. These patterns were maintained at the end of run-out where 64.3% of those in the massage group reported moderate or large improvement compared to 36.8% in the placebo condition and 6.25% in the wait-list control condition (p=0.002).
Table 4. Percent reporting perceived clinical change in headache frequency at the end of treatment and follow-up.
| Time | Group | Small negative change | No change | Small positive change | Moderate positive change | Large positive change | Test Statistic |
|---|---|---|---|---|---|---|---|
| End of Treatment | Massage | 7.7% | 0 | 7.7% | 46.2% | 38.5% | p < 0.0001 a |
| Placebo | 5.6% | 16.7% | 27.8% | 33.3% | 16.7% | ||
| Wait-list | 5.9% | 82.4% | 11.7% | 0 | 0 | ||
| Change in Weekly HA Frequency | * | 0.14±0.22 | -0.43±0.25 | -1.01±0.40 | -2.10±0.52 | p = 0.0003 b | |
|
| |||||||
| End of Follow-up | Massage | 7.1% | 7.1% | 21.4% | 28.6% | 35.7% | p = 0.002 a |
| Placebo | 0 | 42.1% | 21.0% | 21.0% | 15.8% | ||
| Wait-list | 0 | 81.2% | 12.5% | 6.2% | 0 | ||
| Change in Weekly HA Frequency | * | -0.31±0.19 | -0.80±0.43 | -1.10±0.51 | -1.88±0.41 | p = 0.017 b | |
Based on Fisher's exact test of 3×4 table comparing percentage of subjects within each of the reported change categories across the three conditions
Based on univariate ANOVA examining mean headache frequency change score across the four change categories
Insufficient number of subjects for calculation
Table 4 also shows the mean change in headache pain that corresponds to change category membership. There were significant differences in the change in headache pain across the four categories at both assessment timepoints. A reduction of approximately 2 headaches (1.88-2.11) per week corresponded to a subject reported “large” perceived change.
Changes in MTrP PPT from start to end of the six week treatment phase are shown in Table 5. There was a significant time by treatment interaction for all four sites tested (F values ranged from 4.49 to 7.91, p values ranged from <0.001 to 0.015). Post hoc analyses showed that scores significantly improved in the massage group (p values ranged from <0.001 to 0.002 across outcomes), but did not change in the placebo and waitlist groups (all p's > 0.17).
Table 5. Myofascial trigger point pressure-pain threshold across the treatment phase.
| Muscle | Group | Week 1 Mean ± se | Week 6 Mean ± se | Group*Time Interaction |
|---|---|---|---|---|
| Upper Trapezius, Left | Massage | 1.89±0.16 | 2.44±0.17 | F(2,59) = 4.49, p = 0.015 |
| Placebo | 1.83±0.15 | 1.82±0.17 | ||
| Wait-list | 2.00±0.15 | 1.91±0.17 | ||
|
| ||||
| Upper Trapezius, Right | Massage | 1.83±0.16 | 2.46±0.20 | F(2,59) = 4.98, p = 0.010 |
| Placebo | 2.00±0.15 | 1.98±0.20 | ||
| Wait-list | 1.97±0.15 | 1.94±0.19 | ||
|
| ||||
| Suboccipital, Left | Massage | 1.61±0.12 | 2.14±0.13 | F(2,59) = 7.16, p = 0.002 |
| Placebo | 1.57±0.11 | 1.73±0.13 | ||
| Wait-list | 1.51±0.11 | 1.45±0.12 | ||
|
| ||||
| Suboccipital, Right | Massage | 1.57±0.13 | 2.26±0.15 | F(2,59) = 7.91, p < 0.001 |
| Placebo | 1.60±0.12 | 1.70±0.15 | ||
| Wait-list | 1.52±0.12 | 1.52±0.14 | ||
Discussion
The present clinical trial found that six-week programs of massage therapy, with an emphasis on MTrPs in key cervical musculature, or placebo (detuned ultrasound) were both efficacious for reducing headache frequency in a mixed population of episodic or chronic TTH. No statistical difference between massage and placebo was found for headache frequency as recorded on headache diary, although self-report of perceived clinical change detected greater improvement for those who received massage at both immediately post-intervention and end of run-out timepoints. Group or time changes to secondary headache measures (intensity and duration) were not impacted by massage or placebo, nor was medication use changed as measured by number of weekly doses consumed for headache pain.
Statistical change for secondary non-headache, quality of life measures was noted in both the HDI and HIT-6 questionnaires, with post-hoc statistical separation between massage and placebo groups detected for the HDI, but not the HIT-6. Only the change from baseline in the HIT-6 achieved the threshold to be considered clinically significant. [55] Finally, assessment of pressure-pain threshold (PPT) at MTrPs of the upper trapezius and sub-occipital muscles increased for subjects who received massage, but did not change for placebo or wait-list groups. This measure was conducted at least 48 hours after any treatment indicating a sustained effect in PPT for those who received massage.
The treatment phase of the present study was divided into two halves (phases A & B) to help identify a possible treatment plateau. For the massage intervention, a small reduction in headache frequency was detected from baseline to the first half (0.34 HA/week) of intervention; greater reduction in headache frequency (1.01 HA/week) was noted during the second half of the treatment phase. Aggravation of MTrPs can lead to increased local pain. [59] With direct pressure from TPR-massage at MTrPs coupled with uncertainty of optimal treatment pressure, it is possible that multiple sessions are needed to effect meaningful change or that gains from early sessions are counter-balanced by losses from aggravation of MTrPs. The effect of placebo intervention on headache frequency was the reverse, i.e. greater reduction in headache frequency occurred during the first three weeks (0.6 HA/week) with an additional gain of about half that during the second half of the treatment phase (0.29 HA/week). It is not clear if additional massage or placebo sessions beyond the 12 would provide additional benefit although 75% of those receiving massage and 53% in the placebo group reported a belief that additional sessions would be beneficial.
The origin of pain in TTH remains unknown, although both peripheral and central factors likely play a role in its pathophysiology. Episodic (ETTH) and chronic (CTTH) tension-type headache populations report increased presence of MTrPs in pericranial musculature [7, 13, 15, 40] that, when stimulated, precisely reproduce the typical headache complaint. It was hypothesized that massage of MTrPs in the cervical musculature would result in a reduction in headache pain. Our study provides some evidence that increase in MTrP PPT is a mechanism underlying the positive effect of intervention on headache frequency. Supplementary mediational analysis showed that massage intervention predicted change in MTrP PPT from beginning to end of the treatment phase (p < .001) and that the change in PPT predicted a decrease in the average number of headaches from the baseline to the follow-up period (p < 0.001). These analyses show that MTrP PPT change may be one mechanism by which reduction in headache occurs. However, since headache frequency also decreased in the placebo condition, even though the placebo treatment did not impact trigger point tenderness, a separate mechanism for headache reduction is likely. Since the placebo treatment contained all aspects of the massage treatment except an active intervention, it is probable that some of the observed gains in the massage group are related to contextual factors of the intervention.
A qualitatively altered pain perception and generalized hyperalgesia lead current thought that headache pain in CTTH is the result of chronic central sensitization, suggesting differing pain generation mechanisms between episodic and chronic TTH while questioning the role of MTrPs once CTTH has been established. [11, 60] Active MTrPs in TTH patients could be a source of frequent noxious stimuli to the CNS that assists in transforming ETTH to CTTH. Our study, suggests that a reduction in pericranial myofascial nociception (e.g. increased PPT) remains an important component for reducing headache frequency in both frequent episodic or chronic TTH.
Identification of individuals with TTH likely to respond to MTrP-based therapies is important for treatment efficacy. In our study, individuals presented with at least one active MTrP, but also had multiple other criteria described by Fernandez-de-la Penas as relevant to TTH for responsiveness to MTrP therapy. [27, 28] Subjects in the present study met the criteria for age, headache frequency and duration, as well as presence of MTrPs in cervical muscles, which are proposed characteristics correlated with greater likelihood for successful outcome from MTrP therapy. Our findings, in a clinical trial setting, give support to the clinical prediction rule for identification of subjects responsive to MTrP therapies.
The massage protocol used in the study was highly structured, with muscles most commonly associated with pain referral to the head (upper trapezius, sub-occipitals, and sternocleidomastoid) addressed for MTrPs during each visit. While this consistency enhanced reproducibility of procedure, it may have limited treatment efficacy as pericranial muscles (e.g. temporalis, superior oblique, masseter, levator scapula) also generate referred pain from MTrPs and may contribute to TTH [40, 61]. In the present study, some subjects had few active MTrPs in the muscles addressed, which could have resulted in limited improvement if MTrPs in other muscles were active in contributing to headache. Freedom for the therapist to palpate for, and address, MTrPs in a subject's most problematic muscles, in a pragmatic study design, would be helpful in identifying the treatment potential of TPR-massage for TTH.
Several limitations in our study should be noted. The study was powered to identify whether a TPR-focused massage could reduce headache pain relative to a wait-list population rather than compared to placebo intervention. While no statistical difference was observed from diary recordings between TPR-massage and placebo for headache frequency, self-report did indicate a difference in overall headache pain. Further research is needed to establish if a difference between placebo and TPR-massage exists. We screened for subjects presenting with TTH and excluded those with >1 migraine per month. The selection criteria resulted in a population largely free of non-TTH headache, which suggests the results are not generalizable to individuals with other headache types. Furthermore, while individuals with either frequent episodic and chronic TTH were recruited, the individuals who responded and met enrollment criteria were predominantly CTTH or experienced ETTH with 8 or more headaches per month; suggesting that the study findings are most relevant to those with greater headache frequency.
To our knowledge, this study marks for the first time, a placebo effect for a TTH interventional study. Inclusion of a placebo intervention for headache studies has been limited, especially for studies of TTH. [62] It is an important consideration because investigations involving placebo interventions can have profound implications [63]. Blinding of both subjects and those who administer a physical intervention is extremely difficult, but of critical importance. Interventional studies for TTH involving medications have found placebo medications may be as effective as an active medication, highlighting the need for placebo-controlled trials in body-oriented therapies, as well [64]. For studies involving physical therapies, placebo in the form of medication fails to address potentially important contextual aspects of an intervention such as patient-therapist individual time as well physical interactions. Furthermore, a placebo treatment that focuses on a non-affected area (e.g. foot massage) would likely raise doubts among subjects regarding its potential value and possibly leading to subject drop-out. Blinding of the technician who administers treatment is also important to study integrity since subjects may be influenced by practitioner behavior [65]. We addressed this last concern by keeping social interaction between subject and practitioner to a minimum and keeping the ultrasound technicians blind to the non-functional nature of the device.
In recent years, MTrPs have received increased attention for their role in TTH. While massage that focused on MTrPs and placebo both reduce tension headache frequency, their respective mechanisms of action for headache reduction may be different. Our findings suggest that reduction in MTrP PPT may be an important treatment avenue for addressing TTH pain although contextual factors associated with intervention also contribute to improvement in headache frequency.
Acknowledgments
The authors would like to thank Heather Gunnerson, Darci Gau, Lynne Jordan, Michelle Stevens-Hogue, Jennifer Leete, Ann Mathews, Jonathan Hebert, Crystal Escartega, Sharon Jordan, and the nursing and support staff at the Clinical and Translational Research Center at University of Colorado Hospital for their assistance on this study.
Source Funding: This study was supported by NIH/NCCAM Grant Number R21 AT004469 and by NIH/NCATS Colorado CTSI Grant Number UL1 TR000154. Contents are the authors' sole responsibility and do not necessarily represent official NIH views.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Crystal SC, Robbins MS. Epidemiology of tension-type headache. Curr Pain Headache Rep. 2010;14:449–54. doi: 10.1007/s11916-010-0146-2. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz BS, Stewart WF, Simon D, Lipton RB. Epidemiology of tension-type headache. JAMA. 1998;279:381–3. doi: 10.1001/jama.279.5.381. [DOI] [PubMed] [Google Scholar]
- 3.Jensen R. Diagnosis, epidemiology, and impact of tension-type headache. Curr Pain Headache Rep. 2003;7:455–9. doi: 10.1007/s11916-003-0061-x. [DOI] [PubMed] [Google Scholar]
- 4.Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, Steiner T, Zwart JA. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 5.Stovner LJ, Andree C. Impact of headache in Europe: a review for the Eurolight project. J Headache Pain. 2008;9:139–46. doi: 10.1007/s10194-008-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyngberg AC, Rasmussen BK, Jorgensen T, Jensen R. Has the prevalence of migraine and tension-type headache changed over a 12-year period? A Danish population survey. European Journal of Epidemiology. 2005;20:243–9. doi: 10.1007/s10654-004-6519-2. [DOI] [PubMed] [Google Scholar]
- 7.Couppe C, Torelli P, Fuglsang-Frederiksen A, Andersen KV, Jensen R. Myofascial trigger points are very prevalent in patients with chronic tension-type headache: a double-blinded controlled study. Clin J Pain. 2007;23:23–7. doi: 10.1097/01.ajp.0000210946.34676.7d. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-de-las-Penas C, Alonso-Blanco C, Cuadrado ML, Gerwin RD, Pareja JA. Trigger points in the suboccipital muscles and forward head posture in tension-type headache. Headache. 2006;46:454–60. doi: 10.1111/j.1526-4610.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-de-Las-Penas C, Simons D, Cuadrado ML, Pareja J. The role of myofascial trigger points in musculoskeletal pain syndromes of the head and neck. Curr Pain Headache Rep. 2007;11:365–72. doi: 10.1007/s11916-007-0219-z. [DOI] [PubMed] [Google Scholar]
- 10.Simons DG, Travell JG, Simons LS. Travell & Simons' myofascial pain and dysfunction: the trigger point manual. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 11.Bendtsen L, Jensen R, Olesen J. Qualitatively altered nociception in chronic myofascial pain. Pain. 1996;65:259–64. doi: 10.1016/0304-3959(95)00239-1. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-de-las-Penas C, Cuadrado ML, Arendt-Nielsen L, Simons DG, Pareja JA. Myofascial trigger points and sensitization: an updated pain model for tension-type headache. Cephalalgia. 2007;27:383–93. doi: 10.1111/j.1468-2982.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- 13.Alonso-Blanco C, Fernandez-de-las-Penas C, Fernandez-Mayoralas DM, de-la-Llave-Rincon AI, Pareja JA, Svensson P. Prevalence and anatomical localization of muscle referred pain from active trigger points in head and neck musculature in adults and children with chronic tension-type headache. Pain Med. 2011;12:1453–63. doi: 10.1111/j.1526-4637.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- 14.Borg-Stein J. Cervical myofascial pain and headache. Curr Pain Headache Rep. 2002;6:324–30. doi: 10.1007/s11916-002-0055-0. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez de las Penas C. New evidence for trigger point involvement in tension-type headaches. Journal of Musculoskeletal Pain. 2010;18:354–360. [Google Scholar]
- 16.Fernandez-de-Las-Penas C, Alonso-Blanco C, Cuadrado ML, Gerwin RD, Pareja JA. Myofascial trigger points and their relationship to headache clinical parameters in chronic tension-type headache. Headache. 2006;46:1264–72. doi: 10.1111/j.1526-4610.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-de-Las-Penas C, Alonso-Blanco C, Cuadrado ML, Pareja JA. Myofascial trigger points in the suboccipital muscles in episodic tension-type headache. Man Ther. 2006;11:225–30. doi: 10.1016/j.math.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-de-Las-Penas C, Ge HY, Arendt-Nielsen L, Cuadrado ML, Pareja JA. Referred pain from trapezius muscle trigger points shares similar characteristics with chronic tension type headache. Eur J Pain. 2007;11:475–82. doi: 10.1016/j.ejpain.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez de las Penas C, Alonso-Blanco C, Fernandez-Carnero J, Miangolarra JC. The immediate effect of ischemic compression technique and transverse friction massage on tenderness of active and latent myofascial trigger points: a pilot study. J Bodyw Mov Ther. 2006;10:3–9. [Google Scholar]
- 20.Toro-Velasco C, Arroyo-Morales M, Fernandez-de-Las-Penas C, Cleland JA, Barrero-Hernandez FJ. Short-term effects of manual therapy on heart rate variability, mood state, and pressure pain sensitivity in patients with chronic tension-type headache: a pilot study. J Manipulative Physiol Ther. 2009;32:527–35. doi: 10.1016/j.jmpt.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Alonso-Blanco C, de-la-Llave-Rincon AI, Fernandez-de-las-Penas C. Muscle trigger point therapy in tension-type headache. Expert Rev Neurother. 2012;12:315–22. doi: 10.1586/ern.11.138. [DOI] [PubMed] [Google Scholar]
- 22.Moraska A, Chandler C. Changes in clinical parameters in patients with tension-type headache following massage therapy: a pilot study. J Man Manip Ther. 2008;16:106–12. doi: 10.1179/106698108790818468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Stulpnagel C, Reilich P, Straube A, Schafer J, Blaschek A, Lee SH, Muller-Felber W, Henschel V, Mansmann U, Heinen F. Myofascial trigger points in children with tension-type headache: a new diagnostic and therapeutic option. Journal of Child Neurology. 2009;24:406–9. doi: 10.1177/0883073808324540. [DOI] [PubMed] [Google Scholar]
- 24.Soderberg E, Carlsson J, Stener-Victorin E. Chronic tension-type headache treated with acupuncture, physical training and relaxation training. Between-group differences. Cephalalgia. 2006;26:1320–9. doi: 10.1111/j.1468-2982.2006.01209.x. [DOI] [PubMed] [Google Scholar]
- 25.Torelli P, Jensen R, Olesen J. Physiotherapy for tension-type headache: a controlled study. Cephalalgia. 2004;24:29–36. doi: 10.1111/j.1468-2982.2004.00633.x. [DOI] [PubMed] [Google Scholar]
- 26.van Ettekoven H, Lucas C. Efficacy of physiotherapy including a craniocervical training programme for tension-type headache; a randomized clinical trial. Cephalalgia. 2006;26:983–91. doi: 10.1111/j.1468-2982.2006.01163.x. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-de-las-Penas C, Cleland JA, Cuadrado ML, Pareja JA. Predictor variables for identifying patients with chronic tension-type headache who are likely to achieve short-term success with muscle trigger point therapy. Cephalalgia. 2008;28:264–75. doi: 10.1111/j.1468-2982.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-de-las-Penas C, Cleland JA, Palomeque-del-Cerro L, Caminero AB, Guillem-Mesado A, Jimenez-Garcia R. Development of a clinical prediction rule for identifying women with tension-type headache who are likely to achieve short-term success with joint mobilization and muscle trigger point therapy. Headache. 2011;51:246–61. doi: 10.1111/j.1526-4610.2010.01789.x. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-de-las-Penas C, Schoenen J. Chronic tension-type headache: what is new? Curr Opin Neurol. 2009;22:254–61. doi: 10.1097/WCO.0b013e32832973ce. [DOI] [PubMed] [Google Scholar]
- 30.American Massage Therapy Association. 2012 Massage Therapy Industry Fact Sheet. [Accessed Dec. 10, 2013]; [Web Page] Available at http://www.amtamassage.org/articles/2/PressRelease/detail/2545.
- 31.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1–23. [PubMed] [Google Scholar]
- 32.Rossi P, Di Lorenzo G, Faroni J, Malpezzi MG, Cesarino F, Nappi G. Use of complementary and alternative medicine by patients with chronic tension-type headache: results of a headache clinic survey. Headache. 2006;46:622–31. doi: 10.1111/j.1526-4610.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 33.Aguilera FJ, Martin DP, Masanet RA, Botella AC, Soler LB, Morell FB. Immediate effect of ultrasound and ischemic compression techniques for the treatment of trapezius latent myofascial trigger points in healthy subjects: a randomized controlled study. J Manipulative Physiol Ther. 2009;32:515–20. doi: 10.1016/j.jmpt.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Andrasik F, Lipchik GL, McCrory DC, Wittrock DA. Outcome measurement in behavioral headache research: headache parameters and psychosocial outcomes. Headache. 2005;45:429–37. doi: 10.1111/j.1526-4610.2005.05094.x. [DOI] [PubMed] [Google Scholar]
- 35.The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 36.Chaitow L, DeLany J. Clinical applications of neuromuscular technique. Edinburgh; New York: Churchill Livingstone; 2000. [Google Scholar]
- 37.Hou CR, Tsai LC, Cheng KF, Chung KC, Hong CZ. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger-point sensitivity. Archives of Physical Medicine and Rehabilitation. 2002;83:1406–14. doi: 10.1053/apmr.2002.34834. [DOI] [PubMed] [Google Scholar]
- 38.Hendrickson T. Massage for orthopedic conditions. Philadelphia, Pa: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 39.Lewit K, Simons DG. Myofascial pain: relief by post-isometric relaxation. Archives of Physical Medicine and Rehabilitation. 1984;65:452–6. [PubMed] [Google Scholar]
- 40.Fernandez-de-Las-Penas C, Ge HY, Alonso-Blanco C, Gonzalez-Iglesias J, Arendt-Nielsen L. Referred pain areas of active myofascial trigger points in head, neck, and shoulder muscles, in chronic tension type headache. J Bodyw Mov Ther. 2010;14:391–6. doi: 10.1016/j.jbmt.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Moraska AF, Hickner RC, Kohrt WM, Brewer A. Changes in blood flow and cellular metabolism at a myofascial trigger point with trigger point release (ischemic compression): a proof-of-principle pilot study. Archives of Physical Medicine and Rehabilitation. 2013;94:196–200. doi: 10.1016/j.apmr.2012.08.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fryer G, Hodgson L. The effect of manual pressure release on myofascial trigger points in the upper trapezius muscle. J Bodyw Mov Ther. 2005;9:248–255. [Google Scholar]
- 43.Hains G, Descarreaux M, Lamy AM, Hains F. A randomized controlled (intervention) trial of ischemic compression therapy for chronic carpal tunnel syndrome. J Can Chiropr Assoc. 2010;54:155–63. [PMC free article] [PubMed] [Google Scholar]
- 44.Montanez-Aguilera FJ, Valtuena-Gimeno N, Pecos-Martin D, Arnau-Masanet R, Barrios-Pitarque C, Bosch-Morell F. Changes in a patient with neck pain after application of ischemic compression as a trigger point therapy. J Back Musculoskelet Rehabil. 2010;23:101–4. doi: 10.3233/BMR-2010-0255. [DOI] [PubMed] [Google Scholar]
- 45.Draper DO, Mahaffey C, Kaiser D, Eggett D, Jarmin J. Thermal ultrasound decreases tissue stiffness of trigger points in upper trapezius muscles. Physiother Theory Pract. 2010;26:167–72. doi: 10.3109/09593980903423079. [DOI] [PubMed] [Google Scholar]
- 46.Majlesi J, Unalan H. High-power pain threshold ultrasound technique in the treatment of active myofascial trigger points: a randomized, double-blind, case-control study. Archives of Physical Medicine and Rehabilitation. 2004;85:833–6. doi: 10.1016/j.apmr.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 47.Srbely JZ, Dickey JP, Lowerison M, Edwards AM, Nolet PS, Wong LL. Stimulation of myofascial trigger points with ultrasound induces segmental antinociceptive effects: a randomized controlled study. Pain. 2008;139:260–6. doi: 10.1016/j.pain.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Unalan H, Majlesi J, Aydin FY, Palamar D. Comparison of high-power pain threshold ultrasound therapy with local injection in the treatment of active myofascial trigger points of the upper trapezius muscle. Archives of Physical Medicine and Rehabilitation. 2011;92:657–62. doi: 10.1016/j.apmr.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 49.Ebenbichler GR, Resch KL, Nicolakis P, Wiesinger GF, Uhl F, Ghanem AH, Fialka V. Ultrasound treatment for treating the carpal tunnel syndrome: randomised “sham” controlled trial. BMJ. 1998;316:731–5. doi: 10.1136/bmj.316.7133.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson VJ, Baker KG. A review of therapeutic ultrasound: effectiveness studies. Physical Therapy. 2001;81:1339–50. [PubMed] [Google Scholar]
- 51.Blanchard EB, Hillhouse J, Appelbaum KA, Jaccard J. What is an adequate length of baseline in research and clinical practice with chronic headache? Biofeedback and Self Regulation. 1987;12:323–9. doi: 10.1007/BF00998723. [DOI] [PubMed] [Google Scholar]
- 52.Jacobson GP, Ramadan NM, Aggarwal SK, Newman CW. The Henry Ford Hospital Headache Disability Inventory (HDI) Neurology. 1994;44:837–42. doi: 10.1212/wnl.44.5.837. [DOI] [PubMed] [Google Scholar]
- 53.Jacobson GP, Ramadan NM, Norris L, Newman CW. Headache disability inventory (HDI): short-term test-retest reliability and spouse perceptions. Headache. 1995;35:534–9. doi: 10.1111/j.1526-4610.1995.hed3509534.x. [DOI] [PubMed] [Google Scholar]
- 54.Sauro KM, Rose MS, Becker WJ, Christie SN, Giammarco R, Mackie GF, Eloff AG, Gawel MJ. HIT-6 and MIDAS as measures of headache disability in a headache referral population. Headache. 2010;50:383–95. doi: 10.1111/j.1526-4610.2009.01544.x. [DOI] [PubMed] [Google Scholar]
- 55.Coeytaux RR, Kaufman JS, Chao R, Mann JD, Devellis RF. Four methods of estimating the minimal important difference score were compared to establish a clinically significant change in Headache Impact Test. Journal of Clinical Epidemiology. 2006;59:374–80. doi: 10.1016/j.jclinepi.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 56.Castien RF, Blankenstein AH, Windt DA, Dekker J. Minimal clinically important change on the Headache Impact Test-6 questionnaire in patients with chronic tension-type headache. Cephalalgia. 2012;32:710–4. doi: 10.1177/0333102412449933. [DOI] [PubMed] [Google Scholar]
- 57.Kosinski M, Bayliss MS, Bjorner JB, Ware JE, Jr, Garber WH, Batenhorst A, Cady R, Dahlof CG, Dowson A, Tepper S. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12:963–74. doi: 10.1023/a:1026119331193. [DOI] [PubMed] [Google Scholar]
- 58.Jaeschke R, Singer J, Guyatt GH. Measurement of health status Ascertaining the minimal clinically important difference Controlled. Clinical Trials. 1989;10:407–15. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 59.Hoyle JA, Marras WS, Sheedy JE, Hart DE. Effects of postural and visual stressors on myofascial trigger point development and motor unit rotation during computer work. Journal of Electromyography and Kinesiology. 2011;21:41–8. doi: 10.1016/j.jelekin.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Ashina S, Bendtsen L, Ashina M, Magerl W, Jensen R. Generalized hyperalgesia in patients with chronic tension-type headache. Cephalalgia. 2006;26:940–8. doi: 10.1111/j.1468-2982.2006.01150.x. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez-de-Las-Penas C, Ge HY, Arendt-Nielsen L, Cuadrado ML, Pareja JA. The local and referred pain from myofascial trigger points in the temporalis muscle contributes to pain profile in chronic tension-type headache. Clin J Pain. 2007;23:786–92. doi: 10.1097/AJP.0b013e318153496a. [DOI] [PubMed] [Google Scholar]
- 62.Autret A, Valade D, Debiais S. Placebo and other psychological interactions in headache treatment. J Headache Pain. 2012;13:191–8. doi: 10.1007/s10194-012-0422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krogsboll LT, Hrobjartsson A, Gotzsche PC. Spontaneous improvement in randomised clinical trials: meta-analysis of three-armed trials comparing no treatment, placebo and active intervention. BMC Med Res Methodol. 2009;9:1. doi: 10.1186/1471-2288-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bendtsen L, Buchgreitz L, Ashina S, Jensen R. Combination of low-dose mirtazapine and ibuprofen for prophylaxis of chronic tension-type headache. Eur J Neurol. 2007;14:187–93. doi: 10.1111/j.1468-1331.2006.01607.x. [DOI] [PubMed] [Google Scholar]
- 65.Kelley JM, Lembo AJ, Ablon JS, Villanueva JJ, Conboy LA, Levy R, Marci CD, Kerr CE, Kirsch I, Jacobson EE, Riess H, Kaptchuk TJ. Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosomatic Medicine. 2009;71:789–97. doi: 10.1097/PSY.0b013e3181acee12. [DOI] [PMC free article] [PubMed] [Google Scholar]