Abstract
Therapeutics that induce cancer cell senescence can block cell proliferation and promote immune rejection. However, the risk of tumor relapse due to senescence escape may remain high due to the long lifespan of senescent cells that are not cleared. Here we show how combining a senescence-inducing inhibitor of the mitotic kinase Aurora A (AURKA) with an MDM2 antagonist activates p53 in senescent tumors harboring wildtype 53. In the model studied, this effect is accompanied proliferation arrest, mitochondrial depolarization, apoptosis and immune clearance of cancer cells by antitumor leukocytes in a manner reliant upon CCL5, CCL1 and CXCL9. The AURKA/MDM2 combination therapy shows adequate bioavailability and low toxicity to the host. Moreover, the prominent response of patient-derived melanoma tumors to co-administered MDM2 and AURKA inhibitors offers a sound rationale for clinical evaluation. Taken together, our work provides a preclinical proof-of-concept for a combination treatment which leverages both senescence and immune surveillance to therapeutic ends.
Introduction
Metastatic melanoma is a highly aggressive disease and one of the most challenging malignancies to treat. MAPK pathway targeting with inhibitors of BRAF (vemurafenib, dabrafenib) and/or MEK (trametinib) provide a therapeutic option for non-resectable melanoma tumors driven by oncogenic BRAF kinase (BRAFV600E) (1). However the majority of patients promptly develop resistance to these therapies (median progression-free survival is 6 months for dabrafenib alone and 9.4 months for dabrafeneb and trametinib combination, with a median overall survival of 23.8 months (1–5). Furthermore, about half of melanoma tumors are BRAFWT and thus are not eligible for BRAF targeted therapies. New therapeutic options are needed for patients with BRAFWT and BRAF inhibitor-resistant tumors.
Aurora kinase A (AURKA), an essential mitotic kinase indispensable for cell proliferation, is a promising therapeutic target in cancer. AURKA inhibitor MLN8237 (alisertib) is now being evaluated in several clinical trials for melanoma and other malignancies, though as a single agent the benefit has been somewhat limited (clinicaltrials.gov), (6). In our pre-clinical study MLN8237 treatment markedly slowed growth of >75% of patient-derived metastatic melanoma tumors independent of BRAF and NRAS mutational status. AURKA inhibition induced tumor senescence but not apoptosis (7). To improve treatment efficacy, we sought to identify a potential therapeutic partner for the AURKA inhibitor that is capable of activating death pathways in senescent melanoma cells.
The inactivation of the pro-apoptotic cell fate regulator p53 is considered indispensable for oncogenic transformation (8). In mouse melanoma models either genetic loss or down-regulation of p53 through p14/ARF inactivation or MDM4 overexpression cooperated with oncogenic BRAFV600E and NRAS in melanoma tumorigenesis (9–11). Overexpression of p53 due to Mdm4 inactivation, in turn, blocked RAS-driven melanoma progression (12). Thus restoring p53 function is a viable strategy for melanoma intervention especially since mutations and allelic loss of TP53 are relatively rare in those malignancies (16–19%) (13,14).
The CDKN2A locus (INK4a/ARF) is often compromised by deletions, promoter methylation or genetic mutations in both sporadic and hereditary melanoma (13,15,16). One of its products, p14/ARF, is a negative regulator of MDM2 ubiquitin ligase that controls p53 degradation (17). Loss of p14/ARF upon CDKN2A inactivation has been implicated in disabling p53 tumor suppressor activity in melanoma, as illustrated by the mutually exclusive pattern of CDKN2A and TP53 mutations (13,18). Therefore targeting MDM2 which is downstream of p14/ARF could restore compromised p53 activity in melanoma.
Apart from CDKN2A inactivation, overexpression of MDM4 has also been shown to contribute to p53 inactivation in a substantial proportion of human melanomas (11). It is plausible that co-targeting MDM4 with MDM2 may achieve robust p53 induction in these tumors. However, currently there are no specific inhibitors of MDM4 or dual MDM2/MDM4 inhibitors available for clinical testing. In contrast, compounds that specifically target p53 interaction with MDM2 show promising results in clinical trials (19). However, a recent study indicated that MDM2 antagonism alone may not be sufficient to restore p53-mediated tumor suppression in melanoma. Therapeutic efficacy of MDM2 inhibition was enhanced by co-targeting BRAF and iASPP, but complete abrogation of tumor growth was not achieved (20).
Induction of senescence has been found to be essential for the regression of established tumors upon genetic p53 reconstitution as it promotes immune-mediated tumor clearance (21). Hence, we tested whether combining p53-activating MDM2 antagonist with senescence-inducing AURKA inhibitor can benefit melanoma therapy.
Materials and Methods
Chemical reagents and cell culture
For in vitro studies, stock solutions of (−)-Nutlin-3 (30mM) and MLN8237 (20mM) were prepared in DMSO. The pan-caspase inhibitor Z-VAD-FMK was obtained from Molecular Probes (Eugene). Cisplatin was purchased from Sigma-Aldrich and a 10mM stock solution was prepared in DMSO. SK-Mel5, HS294T, SK-Mel28 human melanoma cells and B16F0 mouse melanoma cells were obtained from ATCC. Cells were cultured in DMEM/F12 media supplemented with 10% FBS, 100U/ml penicillin and 100ug/ml streptomycin.
Animal studies
Experiments were conducted in accordance with Vanderbilt University Animal Care and Use Committee (IACUC) guidelines (protocol M/10/034). To establish tumors 2x106 SK-Mel5 or 4x104 B16F0 cells were injected subcutaneously (SC) in both flanks of BALB/C nu/Foxn1 athymic nude mice or C57Bl/6 mice (Harlan-Sprague-Dawley), respectively. Generation of PDX was approved by the Institutional Review Board and has been described previously (22). Mutational status of TP53 was determined by direct exon sequencing with primers available from IARC TP53 Database (R16, November 2012). (−)-Nutlin-3 was synthesized as described previously (23) . MLN8237 was provided by Millennium Pharmaceuticals, Inc. At least 5 mice per treatment group were used. Drugs were given by oral gavage in 2% Klucel, 0.5% Tween 80 ((−)-Nutlin-3) and water (MLN8237). MLN8237 was administered once a day; (−)-Nutlin-3 was given twice daily in experiments with B16F0 melanoma tumors, PDXs of Patient 2 and Patient 3, and once a day on other experiments. Due to the aggressiveness of B16F0 tumors, treatment began when they were palpable. In all other experiments treatment began when tumors were 100mm3. Animal weight and tumor dimensions were measured 2–4 times weekly. Tumor volumes were calculated as 0.5 × length × width × width. In all animal experiments the endpoint was when tumors in any of the treatment groups exceeded 15 mm in diameter at which time mice in all treatment groups were sacrificed and tumors collected for downstream analyses, e.g. western blot, flow cytometry, qPCR, IF, IHC, H&E and SA-βGal staining .
(−)-Nutlin-3 and MLN8237 pharmacokinetics
Blood and tumor samples were harvested over a time course of 24 hours after drug administration (n=4 per time point). (−)-Nutlin-3 concentrations were assayed by validated high-performance liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) assay (24). Detailed explanation of MLN8237 analysis and sample preparation is given in supplemental experimental procedures. (−)-Nutlin-3 and MLN8237 concentration-time data were fitted by a two-compartment plasma model coupled to a perfusion-limited tumor compartment (Fig. S1) using a nonlinear mixed effects pharmacokinetic modeling approach implemented in NONMEM (25). Estimated pharmacokinetic parameters are shown in Table S1. The proportional residual error term was fixed to 10%, tumor volume was fixed to 0.339 cm3 (1.65 × 10−5 mL/kg), and tumor blood flow (Qt) was fixed to 5.8% of cardiac input (approximate blood flow to the skin) (26). The AUC0–24hr was estimated based on the individual simulated concentration-time curves using the trapezoidal method.
Western blotting, viability, apoptosis, flow cytometry, IF and SA-βGal analyses and antibodies are described in detail in supplemental experimental procedures.
Statistical analyses
Unless otherwise stated, ANOVA followed by Dunnett’s test for pair-wise comparisons was used. To compare mouse treatment groups the natural log of average tumor burden over time was analyzed using mixed models ANOVA for repeated measures with an AR(1) covariance structure for random effects. The log transformation was used to correct for heteroscedacity inherent in tumor growth models. The AR(1) covariance structure was uniformly selected across all xenograft models, based on the Akaike information criteria (AIC) measure. Quadratic and interaction effects were included as appropriate based on the likelihood ratio test. Group means and their differences were estimated and compared using linear contrasts. Group differences in tumor volume were considered statistically significant for p-values smaller than their Bonferroni correction that protects the per-experiment type I error rate <5%. The results of the statistical comparisons are specified on figures as * - p<0.05, ** - p<0.01, *** - p<0.001, ns - p>0.05.
Results
MDM2 antagonism limits lifespan of senescent melanoma cells
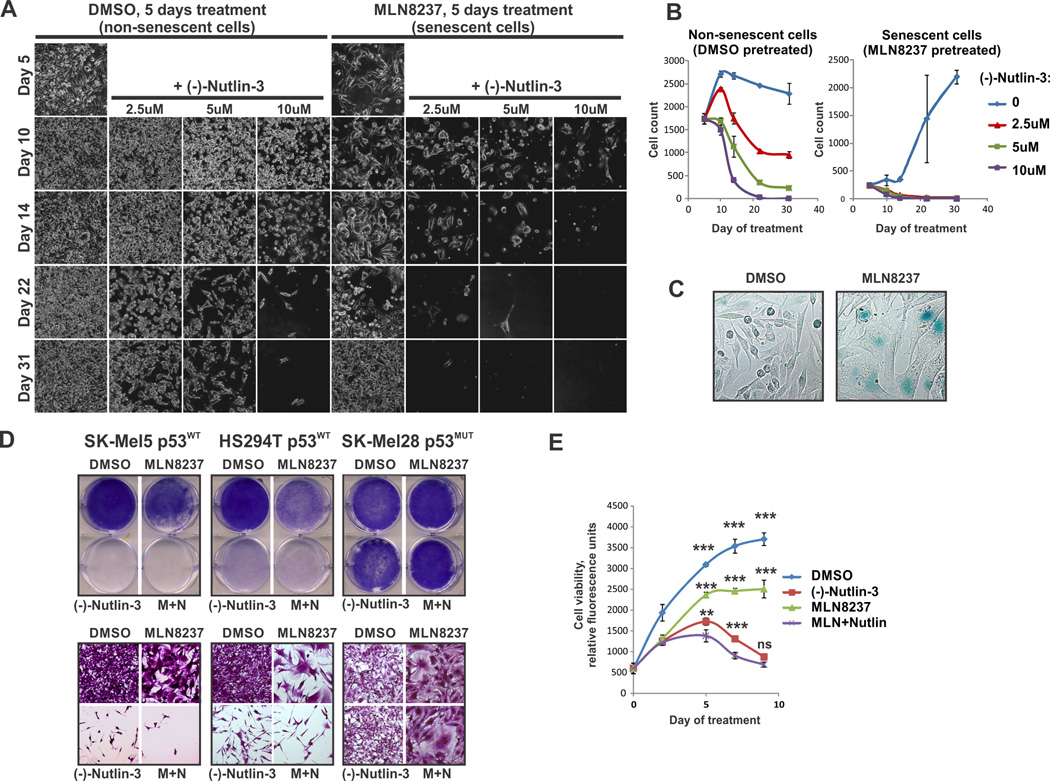
Senescent cells can acquire resistance to certain pro-apoptotic stimuli, including serum withdrawal, radiation, oxidative stress and treatment with DNA damaging drugs (27–30). Notably, the senescence-associated resistance to apoptosis has been linked to impaired activation of p53 (29,31). Therefore we hypothesized that activation of p53 in senescent cells may restore death pathway functionality. To initiate senescence the AURKA inhibitor MLN8237 was used (7,32). (−)-Nutlin-3 (Nutlin-3a) was used to induce expression of endogenous p53 by inhibiting MDM2-p53 interaction (33). We observed dose-dependent cytotoxicity of (−)-Nutlin-3 in both senescent (MLN8237 pre-treated) and non-senescent (vehicle pre-treated) cells (Fig. 1A, B). MLN8237 pre-treated cells remained static but viable after drug withdrawal. Notably, without p53 activation, some cells were able to escape senescence and regain their proliferative potential which resulted in culture outgrowth by day 31. In contrast, all (−)-Nutlin-3-treated senescent cells died before any of them could re-grow. The induction of senescence was confirmed using SA-β-Gal staining (Fig. 1C). This suggests that p53 activation prevents senescence escape by reducing lifespan of senescent cells. We also tested concurrent AURKA and MDM2 inhibition. Melanoma cells remained viable after a week of continuous exposure to MLN8237, suggesting a mostly cytostatic response (Fig. 1D, E). In contrast, p53 activation by (−)-Nutlin-3 administered alone or in combination with MLN8237, killed p53WT melanoma cells (Fig. 1E). These results show that the reduction in viable tumor cells with combined treatment is a result of both proliferative arrest caused by AURKA inhibition and cell death driven by MDM2 antagonism. Importantly, (−)-Nutlin-3 did not affect viability of cells expressing non-functional mutated p53 (Fig. 1D) or cells with p53 knockout (Fig. S2A), demonstrating the lack of p53-independent/off-target effects under these experimental conditions.
Figure 1.
Inhibition of MDM2 limits survival of senescent melanoma cells. (A) Microphotographs of live SK-Mel5 melanoma cells pre-treated with 1 µM MLN8237 or DMSO followed by treatment with (−)-Nutlin-3 as indicated. (B) Quantification of experiments shown in A. Average number of cells per microphotograph ± SD is shown. (C) Senescence-associated β-Galactosidase (SA-β-Gal) staining of SK-Mel5 cells treated with 1 µM MLN8237 or DMSO for 5 days. (D) Crystal violet staining shows cell density on macro- (top panels) and microscopic (bottom panels) levels. Cells were treated with (−)-Nutlin-3 (10µM), MLN8237 (1µM), combination of both (M+N), or vehicle for 7 days. (E) Time-dependent changes in viability of SK-Mel5 cells treated as in A using the MultitoxFluor kit. Experiments were performed twice in triplicate; average viability ± SEM of a representative experiment is shown. Two-way ANOVA with Bonferroni post-test was used for data analysis.
p53 activation induces caspase-independent cell death in senescent melanoma cells
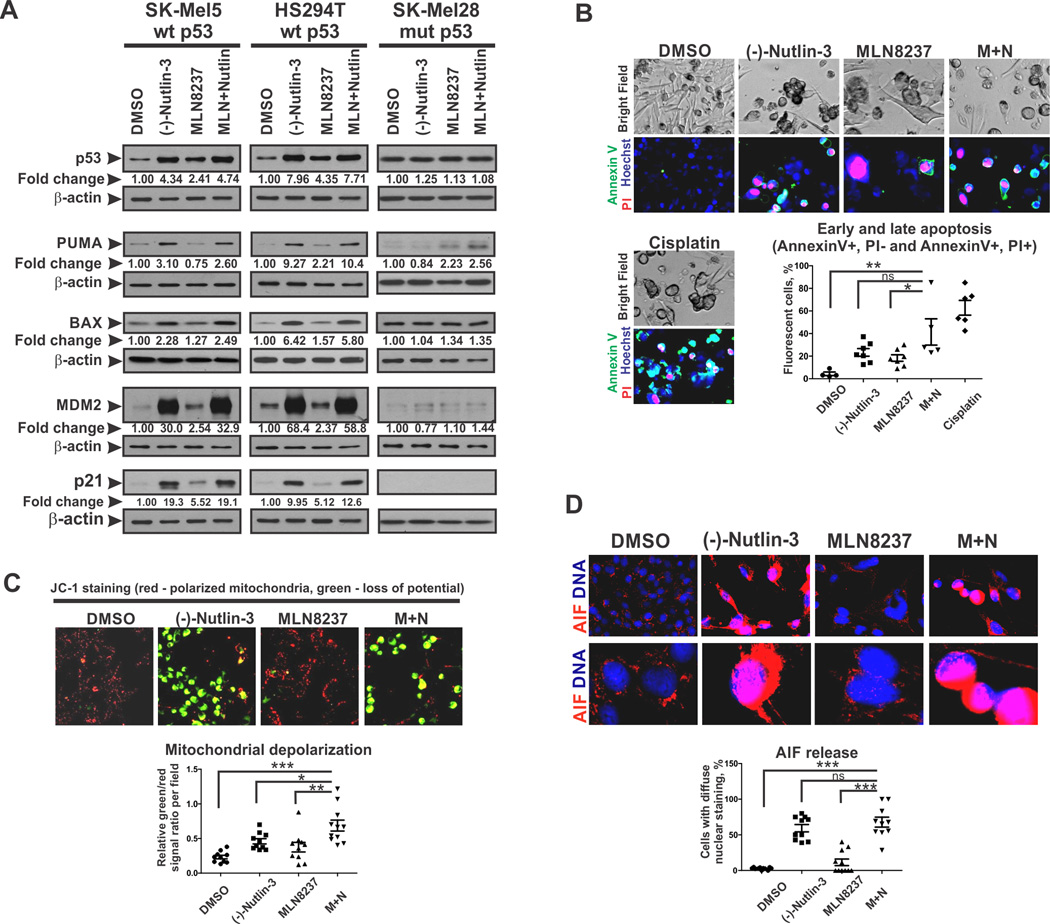
Since we have previously characterized the mechanisms by which AURKA inhibition induced melanoma senescence (7), we focused here on consequences of AURKA inhibition combined with MDM2 antagonism. As expected, (−)-Nutlin-3 induced accumulation of p53 and its transcriptional targets p21, PUMA, BAX and MDM2 in p53WT cells (SK-Mel5 and Hs294T) (Fig. 2A, S2B). Of note, MDM2 accumulation does not cause p53 degradation in the context of (−)-Nutlin-3 treatment because this drug interferes with the binding of MDM2 to p53. Consistent with high specificity of this drug, the level and activity of p53 was unaffected by (−)-Nutlin-3 in p53MUT SK-Mel28 cells (Fig. 2A). Interestingly, treatment with the AURKA inhibitor induced p53 accumulation in p53WT-expressing melanoma cells, although it was less effective compared to (−)-Nutlin-3.
Figure 2.
MDM2 antagonism induces mitochondrial-mediated apoptosis in senescent melanoma cells. (A) Western Blot analysis of p53 levels and expression of p53 target genes after 5 days of treatment with (−)-Nutlin-3 (10µM), MLN8237 (1µM), combination of both, or vehicle. Numbers are average β-actin-normalized densitometry values from 3 independent experiments. (B) Analysis of apoptosis using Annexin V-FITC and PI staining. SK-Mel5 cells were treated for 5 days as in A. Bottom panel shows the percentages of apoptotic cells per random microscope field. (C) Mitochondrial membrane potential in cells treated as described in A was analyzed using JC-1 dual emission dye. Fluorescence of cells in red (polarized mitochondria) and green (loss of mitochondrial membrane potential) channels were evaluated. Graph shows relative green to red signal ratios in random fields. (D) IF staining of AIF in SK-Mel5 cells treated as described in A. Bottom panel shows the percentages of cells with nuclear AIF staining in random fields. Kruskal-Wallis test with Dunn’s multiple comparison was applied. Experiments were performed at least 3 times with consistent results. Images were quantified with ImageJ software.
p53 stabilization by (−)-Nutlin-3 induced apoptosis in p53WT melanoma cells (Fig. 2B). p53 activates expression of key pro-apoptotic mitochondrial proteins, including PUMA, NOXA and BAX, that promote mitochondrial membrane depolarization, leading to release of apoptotic effectors(34). Indeed, we found that p53 activation by (−)-Nutlin-3 alone and in combination with MLN8237 increased expression of PUMA and BAX (Fig. 2A) which was accompanied by the decrease in mitochondrial membrane potential (Fig. 2C). Interestingly, despite the strong evidence of apoptosis, cleavage of caspase 3 was not detected in cells treated with (−)-Nutlin-3 (Fig. S2C). In addition, treatment with pan-caspase inhibitor did not interfere with cytotoxic response to p53 activation (Fig. S2D). These data argue for a caspase-independent mechanism of (−)-Nutlin-3-induced apoptosis. Consistently, we found that treatment of melanoma cells with (−)-Nutlin-3 induced activation, mitochondrial release and nuclear translocation of Apoptosis Inducing Factor (AIF) (Fig. 2D), which is a known effector of caspase-independent apoptosis (35). Collectively these results demonstrate that prolonged activation of p53 in melanoma cells initiates caspase-independent programmed cell death driven by mitochondrial depolarization and AIF activation.
MDM2 and AURKA antagonists work in concert to activate p53 and block melanoma tumor growth in vivo
Xenograft of human melanoma cells was used to evaluate responses to (−)-Nutlin-3 and/or MLN8237 in vivo. While single agent treatments only partially inhibited tumor progression, combined (−)-Nutlin-3 and MLN8237 treatment fully blocked tumor growth (Fig. 3A). As expected, MLN8237 inhibited AURKA activity, shown by loss of AURKA auto-phosphorylation in tumor lysates (Fig. 3B). This resulted in inhibition of cancer cell proliferation and induction of senescence, based upon the decrease of proliferative markers phosphorylated histone H3 and Ki-67 and the increase in SA-β-Gal activity (Fig. 3B, C). Consistent with our in vitro findings (Fig. 2A), MLN8237 treatment caused moderate induction of p53 (Fig. 3B). The lower of the 2 bands detected by the human p53-specific antibody may represent an alternative p53 isoform (36). Interestingly, while in cultured cells p53 levels were greatly elevated by MDM2 inhibitor, in vivo p53 activation by (−)-Nutlin-3 was modest, resulting in limited induction of p53-target genes p21, BAX, and apoptosis, based on AIF staining (Fig. 3B, C). To gain better understanding of these findings, we evaluated tumor cell expression of p53. Only 11% of cells in the (−)-Nutlin-3-treated tumors had elevated p53 (compared to 3–6% in untreated control tumors) (Fig. S3). This is much lower than the results obtained in vitro where 100% of cells showed strong p53 induction (Fig. S2B). This suggests that systemic administration of (−)-Nutlin-3 may not target all tumor cells equally, as opposed to in vitro cell culture conditions. In this case cells unaffected by (−)-Nutlin-3-induced apoptosis would have proliferative advantage and therefore addition of the cytostatic AURKA antagonist may promote (−)-Nutlin-3 efficacy. Indeed, we found that p53 activation and expression of p53 targets p21 and BAX was much more robust after combined MLN8237 and (−)-Nutlin-3 treatment in comparison with single agent therapies (Fig. 3B). Up to 50% of tumor cells showed strong p53 staining in the combination treatment group (Fig. S3). Similar to p53 levels, about 50% of tumor cells showed induction of the apoptosis marker AIF after combined MLN8237 and (−)-Nutlin-3 treatment, compared to no more than 15% after treatment with (−)-Nutlin-3 alone (Fig. 3C). Accumulation of the pro-apoptotic molecule BIMEL in the tumor lysates of the MLN8237 and (−)-Nutlin-3-treated mice confirmed the induction of apoptosis (Fig. 3B). We also detected down-regulation of proliferation markers phospho-H3 and Ki67 after combined MDM2 and AURKA inhibition consistent with higher p21 levels, indicating enhanced anti-proliferative effect (Fig. 3B, C). These results show that MDM2 antagonism and AURKA inhibition cooperate to activate p53, induce cell death and inhibit cell proliferation in vivo.
Figure 3.
Co-targeting AURKA and MDM2 blocks melanoma growth in vivo. (A) Mice bearing SK-Mel5 xenografts received daily treatments with (−)-Nutlin-3 (200mg/kg), MLN8237 (30mg/kg), combination of both or vehicle control. Tumor volume changes over time are shown. (B) Western Blot analysis of tumors (T) described in A. Expression was normalized to β-actin and average fold changes ± SD in different treatment groups over the placebo group were plotted (right panels). (C) Representative results of SA-βGal, Ki-67 and AIF staining in tumors described in A. Percentages of AIF and Ki-67-positive cells were quantified using ImageJ software; SA-βGal staining was scored by pathologist in a blind fashion. Five random fields of 2–3 tumors were evaluated in each treatment group and average values per tumor ± SD are shown. V- vehicle, N- (−)-Nutlin-3, M- MLN8237, MN- combined MLN8237 and (−)-Nutlin-3 treatment.
AURKA and MDM2 antagonism promotes tumor immune infiltration
Immune cells have been shown to target senescent tumor cells overexpressing p53 (21). To assess the effect of AURKA and MDM2 inhibitors on the anti-tumor immune response we employed an immunocompetent melanoma model where B16F0 tumors are grown in syngeneic C57Bl/6 mice. In vitro B16F0 cells showed decreased viability and induction of apoptosis in response to treatment with either drug, but the cytotoxic effect was most prominent with the combined treatment (Fig. S4A, B). In vivo MLN8237 alone partially inhibited tumor growth while (−)-Nutlin-3 showed no significant anti-tumor activity. Strikingly, combined (−)-Nutlin-3 and MLN8237 treatment elicited remarkable responses, completely blocking growth of aggressive B16 tumors (Fig. 4A, B). Drug responses in (−)-Nutlin-3 and MLN8237-treated tumors were associated with high p53 levels and increased expression of p53 transcriptional targets, such as pro-apoptotic molecules BAX and PUMA and cell cycle arrest mediator p21 (Fig. 4C,D).
Figure 4.
Co-targeting AURKA and MDM2 promotes immune infiltration of melanoma tumors. (A) Growth of B16F0 tumors C57Bl6 mice treated with (−)-Nutlin-3 (200mg/kg), MLN8237 (30mg/kg), combination of both, or vehicle. (B) Photographs of excised tumors described in A. (C) Real time PCR analysis of p53 transcriptional targets p21, BAX and PUMA in tumors described in A. (D) IF staining of p53 in tumors described in A. (E) IF staining of leukocytes in tumors described in A using CD45 antibody. Quantitative analysis of staining in D and E was performed using ImageJ software; 5 random fields of 3 individual tumors were evaluated in each treatment group. Average values per tumor were used for statistical comparison. (F) Flow cytometric analysis of indicated lymphoid and myeloid cells in tumors described in A. (G) H&E staining of tumors described in A. Arrows indicate necrotic areas. The percent of necrotic area in whole tumor sections was determined by a pathologist in a blind manner (right panel). (H) Drug response in immune deficient mice. Experiments were performed as described in A, except NSG mice were used. (I) Necrotic area in tumors described in H was quantified based on H&E staining by pathologist in a blind manner.
Consistent with limited anti-tumor activity, p53 was not strongly induced upon treatment with (−)-Nutlin-3 (Fig. 4D). While the resistance of B16 tumors to MDM2 inhibitor (−)-Nutlin-3 has been previously reported, the mechanism has not been determined (20). We found that microvessel density was decreased in tumors treated with (−)-Nutlin-3 alone (Fig. S5). This is consistent with the study showing that (−)-Nutlin-3 limits vessel formation in vivo by inhibiting migration, proliferation and survival of endothelial cells (37). Inadequate blood supply, in turn, has been shown to cause uneven distribution of anti-cancer drugs within tumor and cause drug resistance (38–40). Therefore cells in poorly vascularized areas can be protected from exposure to drug resulting in a low overall level of p53 activation. The anti-angiogenic effect of (−)-Nutlin-3 was not evident in combination with MLN8237 (Fig. S5). This may be due to anti-proliferative activity of MLN8237 which reduces the need for neo-angiogenesis.
We have previously reported that targeting AURKA induces infiltration of immune cells into the tumor (7). Here we found that combined MDM2 and AURKA inhibition further promoted tumor infiltration by host immune cells (Fig. 4E). Both lymphoid and myeloid cells accumulated in (−)-Nutlin-3 and MLN8237-treated tumors. The numbers of NK cells, macrophages, and antigen-presenting dendritic cells increased most prominently (Fig. 4F). These changes were present only at tumor sites and were not seen in either bone marrow or spleen (Fig. S6A, B). In fact, the numbers of dendritic cells and macrophages were decreased in the spleens of animals treated with the (−)-Nutlin-3 and MLN8237 combination, which may be a result of redistribution of these cell types to the tumor site (Fig. S6A). Figures S6C and S6D show the gating strategy for this experiment.
Aggressively growing B16 tumors usually contain large areas of necrotic tissue (Fig. 4G). We noted a striking absence of necrosis in MLN8237 and MLN8237 and (−)-Nutlin-3-treated tumors indicating tumor clearance. Absence of tumor necrosis after treatment was unlikely a result of growth inhibition, since some of the tumors in mice treated with single agent MLN8237 were comparable by size to vehicle-treated tumors (Fig. 4B), yet they exhibited a virtually absence of necrosis. In order to determine the significance of tumor-infiltrating immune cells in tumor clearance and overall drug response, we compared efficacy of MDM2 and AURKA inhibition against B16F0 melanoma tumors that were grown either in immunocompetent C57Bl6 mice or in severely immunodeficient NOD/SCID interleukin-2 receptor gamma chain null (NSG) mice that lack T, B, and NK cells, have functionally-defective macrophages, dendritic cells and compromised cytokine signaling. Therapeutic efficacy was greatly reduced in immunocompromised mice as compared to immunocompetent animals (compare Fig. 4H and Fig. 4A). The induction of the apoptotic marker BIMEL was comparable in immunocompetent and immunodeficient models (Fig. S4C). In contrast, clearance of necrotic areas in senescent tumors treated with MLN8237 or MLN8237 and (−)-Nutlin-3 combination was abrogated in the absence of functional immune system (Fig. 4I). This suggests that immune cells recruited into the tumors in response to combined MDM2 and AURKA antagonism facilitate tumor clearance rather than direct melanoma cell killing. This conclusion is also supported by the data from the T cell-deficient mouse model where the response to senescence-inducing therapy with the AURKA inhibitor, as well as to combined AURKA and MDM2 antagonists treatment, was associated with accumulation of phagocytic myeloid cells, e.g. macrophages and dendritic cells within the tumors (Fig. S7).
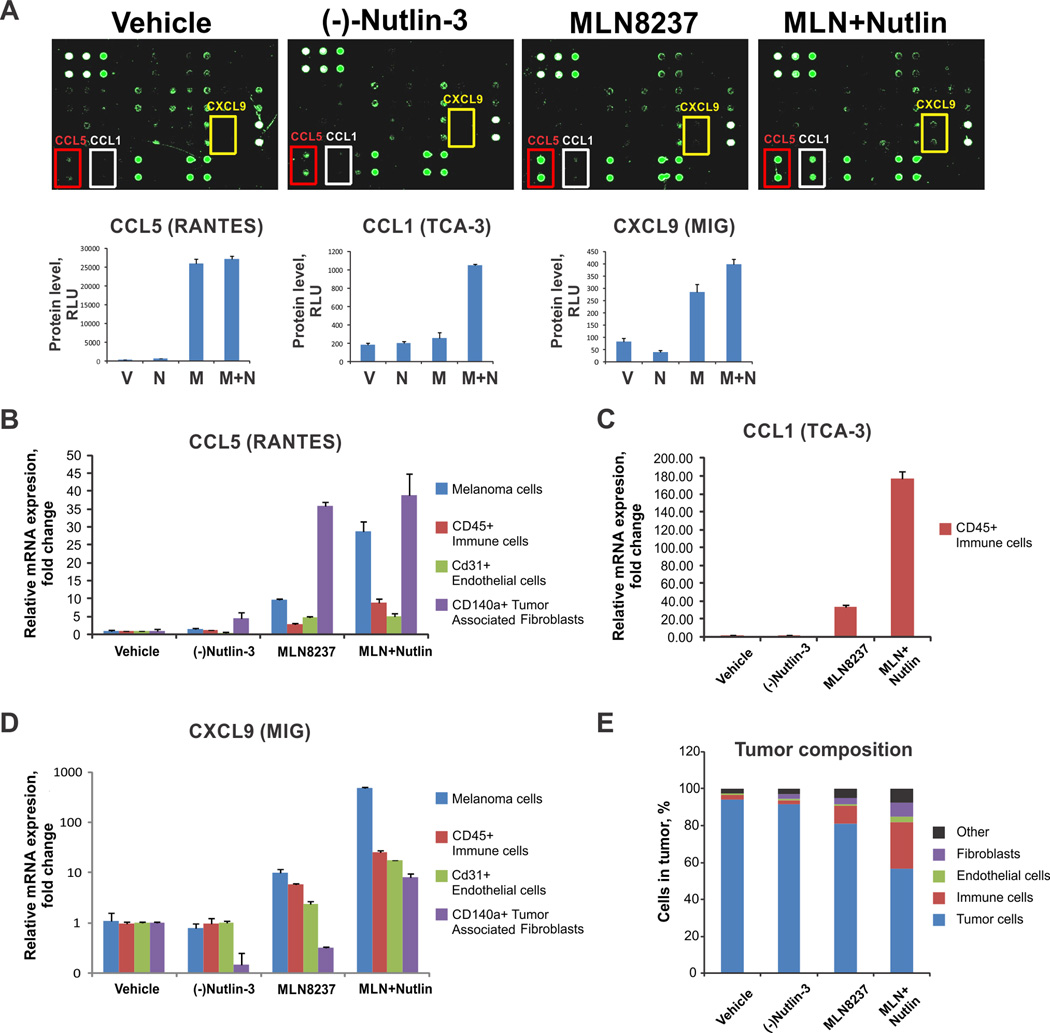
Senescent cells attract immune cells through induction of senescence associated secretory phenotype (SASP), characterized by increased secretion of pro-inflammatory cytokines and chemokines. We investigated which cytokines in particular orchestrated immune infiltration of tumors treated with MDM2 and AURKA inhibitors. A prominent increase of the CCL5 chemokine was detected in response to AURKA inhibitor treatment as well as an induction of CCL1 and CXCL9 in tumors treated with AURKA and MDM2 antagonists combined (Fig. 5A). CCL5 attracts monocytes, macrophages, dendritic and NK cells; CCL1 recruits monocytes, dendritic and T cells; and CXCL9 targets T and NK cells (41). These functional characteristics are consistent with the profiles of tumor-infiltrating leukocytes in MLN8237 and (−)-Nutlin-3-treated cells (Fig. 4F).
Figure 5.
MDM2 and AURKA antagonism promotes tumor immune infiltration. (A) Cytokine levels in tumor lysates from B16F0 tumor-bearing immunocompetent mice were detected using cytokine array (Ray Bio). Cytokines differentially expressed between the treatment groups are indicated. The bottom panels show quantification of the results using manufacturer’s software. (B, C, D) Levels of CCL5, CCL1 and CXCL9 in different cell types within the B16F0 tumors were analyzed using qRT-PCR. (E) Cells were sorted by FACS.
To investigate what cell types within the tumor microenvironment (TME) were producing these chemokines, we FACS sorted immune cells, endothelial cells, fibroblasts, and melanoma cells in the tumor based on the surface markers CD45, CD31, CD140a and analyzed their expression of CCL1, CCL5, and CXCL9. CCL5 levels were increased in both melanoma cells and fibroblasts upon MLN8237 or MLN8237 and (−)-Nutlin-3 treatments (Fig. 5B). However, because fibroblasts represented a relatively minor cell population within the TME, melanoma cells were the main source of CCL5 in the tumor (Fig. 5E). Similarly, melanoma cells were the source of CXCL9 upon AURKA and MDM2 co-targeting, while the tumor-infiltrating immune cells produced CCL1 (Fig. 5C, D).
(−)-Nutlin-3 and MLN8237 drug combination exhibits good bioavailability and low toxicity
For pharmacokinetics analysis, matched plasma and tumor tissue samples from 56 B16F0 melanoma-bearing C57Bl6 mice treated with MLN8237 (30 mg/kg) and/or (−)-Nutlin-3 (200 mg/kg) were evaluated and modeled together using a population pharmacokinetic approach (Table S1). The population model-predicted plasma and tissue concentration-time plots for (−)-Nutlin-3 (Fig. 6A and 6B) and MLN8237 (Fig. 6C and 6D) indicate adequate model fitting of the observed data. The hybrid physiologically-based pharmacokinetic models for (−)-Nutlin-3 and MLN8237 are shown in Fig. S5.
Figure 6.
(−)-Nutlin-3 and MLN8237 pharmacokinetics and safety. (A–D) Population model-predicted (−) and observed (o) plasma and tumor concentrations of (−)-Nutlin-3 (A and B, respectively) and MLN8237 (C and D, respectively). Median (−)-Nutlin-3 and MLN8237 plasma and tumor exposure after indicated treatments. Single agent and combination treatment groups were compared using Mann-Whitney test (E–H). Body weight and serum levels of liver toxicity markers AST and ALT in tumor-free C57Bl6 mice that received daily treatments with (−)-Nutlin-3 (200mg/kg), MLN8237 (30mg/kg), combination of both or vehicle control (I–K).
The median (−)-Nutlin-3 AUC0–24hr estimate was 292.7 µg/mL×hr for plasma and 455.6 µg/mL×hr for tumor tissue. The overall median (range) MLN8237 area under the concentration-time curve from time 0 to 24 hrs (AUC0–24hr) estimate was 42.7 µg/mL×hr (14.0 – 119) for plasma and 37.4 µg/g×hr (4.59 – 147) for tumor tissue. The median (−)-Nutlin-3 plasma AUC0–24hr decreased 34% (p=0.0519) in mice that received (−)-Nutlin-3 and MLN8237 compared to the (−)-Nutlin-3 single agent arm (Fig. 6E). Likewise, Fig. 6F shows 55% reduction in the median (−)-Nutlin-3 tissue AUC0–24hr (p<0.05) for mice receiving concomitant MLN8237. The plasma and tumor exposure were similar comparing mice that received MLN8237 alone or combined with (−)-Nutlin-3 (Fig. 6G and 6H). These results suggest a potential interaction between (−)-Nutlin-3 and MLN8237 that reduces (−)-Nutlin-3 tumor tissue exposure. The clinical relevance of this interaction remains to be determined and further studies will be required to optimize the dosing and scheduling of these drugs as they are integrated into clinical practice. Nevertheless, the efficacy studies demonstrate excellent anti-tumor effect of combined (−)-Nutlin-3 and MLN8237 treatment despite the slight short term reduction in tumor tissue levels of (−)-Nutlin-3 when combined with MLN8237.
To assess safety/potential toxicity of this drug combination we monitored weight of laboratory animals receiving the therapy and analyzed the levels of liver toxicity markers ALT and AST in serum of treated mice. No weight loss or signs of liver toxicity were detected (Fig. 6 I–K).
Combined MDM2 and AURKA antagonism blocks growth of BRAFWT and BRAFV600E patient tumors
To evaluate anti-melanoma activity MDM2 and AURKA targeting in clinically relevant setting, 5 patient melanoma tumors were transplanted into nude mice creating patient-derived xenograft (PDX) model. Multiple studies demonstrated that PDX tumors retain the histology, gene alteration and expression profiles as well as drug response rates of their source tumors (reviewed in (42)) which makes them an excellent model for preclinical evaluation of new therapeutics. Our PDX set included tumors with unknown driver mutations (BRAFWT, c-KitWT and NRASWT; patients 2 and 4) and tumors with mutation in BRAF (BRAFV600E patients 1, 3 and 5). Patient 5 received treatment with BRAF inhibitor vemurafenib and progressed after an initial response. All tumors were p53WT based on direct sequencing of all TP53 coding exons. Combined (−)-Nutlin-3 and MLN8237 treatment was significantly more effective than vehicle or single agent treatment in all experiments and fully blocked the growth of 4 out of 5 tested patient tumors (Fig. 7A). Strong induction of p53, its targets p21,PUMA, and the mitochondrial apoptosis effector BIMEL was observed in tumor lysates of (−)-Nutlin-3 and MLN8237-treated mice (Fig. 7B). These data suggest that combined MDM2 and AURKA targeted therapy may benefit patients with BRAFWT and BRAF inhibitor-resistant tumors.
Figure 7.
Co-targeting AURKA and MDM2 blocks growth of PDX. (A) Mice bearing human melanoma implants (Patients 1–5) received daily treatments with (−)-Nutlin-3 (200mg/kg), MLN8237 (30mg/kg), combination of both, or vehicle control. (B) Western blot analysis of the indicated tumors shown in A.
Collectively our results demonstrate that combined MDM2 and AURKA antagonism halts melanoma growth by inducing growth arrest and senescence, limiting lifespan of senescent cells, and enhancing tumor immune infiltration and clearance.
Discussion
We hypothesized that p53 restorative therapy can synergize with concurrent senescence-promoting therapy. Tumor senescence and immune-mediated tumor clearance has been linked with response to genetic p53 restoration (21,43). However, we found that pharmacological p53 inducer (−)-Nutlin-3 elicited cytotoxic rather than senescence response in melanoma tumors. Therefore we combined (−)-Nutlin-3 with the AURKA antagonist MLN8237, which is capable of triggering melanoma cell senescence in vivo (7). While senescence induction was found to be critical for the therapeutic efficacy of this drug we cannot fully exclude the possibility that anti-melanoma effect of AURKA inhibition extends beyond senescence. A daily oral regimen with the combination of MDM2 and AURKA inhibitors blocked the growth of patient derived p53WT melanoma tumors independent of BRAF mutational status or resistance to BRAF inhibitor. These data suggest that p53-restoring-mitotic kinase-targeted therapy could benefit melanoma patients who are not eligible for BRAF and MEK inhibitors. Since both p53-activating drugs and mitotic kinase inhibitors have already been tried on human subjects, prompt translation of combination therapy to the clinic is highly feasible.
While (−)-Nutlin-3 alone was a modest inducer of p53 in vivo, addition of AURKA inhibitor promoted p53 activation in tumors. This is in agreement with studies showing that AURKA phosphorylates p53 on serine 315 and 215, which leads to MDM2-dependent p53 ubiquitination and degradation with inhibition of p53 transcriptional activity (44,45). Consistent with previous findings in hepatocellular carcinoma and sarcoma (21,43), p53 activation failed to initiate classical caspase-driven apoptosis in melanoma. Nevertheless, we noted a delayed caspase-independent cytotoxic response that may have been previously overlooked. Prolonged p53 activation in melanoma cells resulted in mitochondrial dysfunction and mitochondrial-nuclear translocation of AIF, a known mediator of caspase-independent cell death (35).
Accumulating evidence suggests that the TME can be an important positive or negative determinant of tumor response to therapy. For example, stromal cells have been implicated in the protection of tumor cells from MEK and BRAF inhibitor-induced toxicity in melanoma (46). Other studies showed that treatment with BRAF and MEK inhibitors restores function to compromised dendritic cell and T cells, thus establishing a tumor-suppressing TME (47,48). We found that non-malignant cells of TME can modulate the efficacy of p53-restorative therapy. Senescence induced by AURKA inhibition promoted accumulation of immune cells in melanoma tumors. These findings are in agreement with the critical role of immune cells in targeted elimination of senescent pre-malignant cells, as well as clearance of senescent tumor cells (49). As we reported previously, AURKA inhibition in melanoma cells initiates SASP driven by NF-κB activity (7). Interestingly, activation of p53 in senescent AURKA-deficient tumors further promoted recruitment of macrophages, dendritic cells, and NK cells by co-stimulating the expression of CCL5, CCL1 and CXCL9 chemokines in malignant and non-malignant cells of TME. These results are in concert with a recent finding that p53 can cooperate with NF-κB in SASP induction (50). Compromised anti-tumor activity of AURKA and MDM2 antagonism in severely immunodeficient animals indicated that immune-mediated tumor clearance plays a critical role in response to this therapy.
Our study demonstrates that the potent therapeutic benefit of MDM2 and AURKA co-targeting is derived from a direct effect on melanoma cells combined with stimulation of host’s anti-tumor defenses. Activation of p53 synergized with AURKA antagonism to inhibit growth and survival of melanoma cells and promote immune-mediated tumor clearance. These findings provide a strong rationale to further develop p53-reactivating therapies in combination with senescence-inducing drugs, for instance, mitotic kinase inhibitors, for treatment of p53WT metastatic melanoma.
Supplementary Material
Acknowledgements
This work was supported by grants from the Department of Veterans Affairs (5101BX000196-04), NIH (CA116021, CA116021-S1, CA90625, 5T32CA119925-03, 1F32CA171895-01, GM084333 and CA68485), and a Senior Research Career Scientist Award to AR.
Footnotes
Authors declare no conflict of interest
References
- 1.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzies AM, Long GV. Dabrafenib and trametinib, alone and in combination for BRAF-mutant metastatic melanoma. Clin Cancer Res. 2014;20(8):2035–2043. doi: 10.1158/1078-0432.CCR-13-2054. [DOI] [PubMed] [Google Scholar]
- 6.Matulonis UA, Sharma S, Ghamande S, Gordon MS, Del Prete SA, Ray-Coquard I, et al. Phase II study of MLN8237 (alisertib), an investigational Aurora A kinase inhibitor, in patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Gynecologic oncology. 2012;127(1):63–69. doi: 10.1016/j.ygyno.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Hawkins OE, Su Y, Vilgelm AE, Sobolik T, Thu YM, et al. Targeting aurora kinases limits tumour growth through DNA damage-mediated senescence and blockade of NF-kappaB impairs this drug-induced senescence. EMBO Mol Med. 2013;5(1):149–166. doi: 10.1002/emmm.201201378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Junttila MR, Evan GI. p53--a Jack of all trades but master of none. Nat Rev Cancer. 2009;9(11):821–829. doi: 10.1038/nrc2728. [DOI] [PubMed] [Google Scholar]
- 9.Goel VK, Ibrahim N, Jiang G, Singhal M, Fee S, Flotte T, et al. Melanocytic nevus-like hyperplasia and melanoma in transgenic BRAFV600E mice. Oncogene. 2009;28(23):2289–2298. doi: 10.1038/onc.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bardeesy N, Bastian BC, Hezel A, Pinkel D, DePinho RA, Chin L. Dual inactivation of RB and p53 pathways in RAS-induced melanomas. Mol Cell Biol. 2001;21(6):2144–2153. doi: 10.1128/MCB.21.6.2144-2153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gembarska A, Luciani F, Fedele C, Russell EA, Dewaele M, Villar S, et al. MDM4 is a key therapeutic target in cutaneous melanoma. Nature medicine. 2012 doi: 10.1038/nm.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terzian T, Torchia EC, Dai D, Robinson SE, Murao K, Stiegmann RA, et al. p53 prevents progression of nevi to melanoma predominantly through cell cycle regulation. Pigment cell & melanoma research. 2010;23(6):781–794. doi: 10.1111/j.1755-148X.2010.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein AM, Chan M, Harland M, Hayward NK, Demenais F, Bishop DT, et al. Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J Med Genet. 2007;44(2):99–106. doi: 10.1136/jmg.2006.043802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedberg DE, Rigas SH, Russak J, Gai W, Kaplow M, Osman I, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100(11):784–795. doi: 10.1093/jnci/djn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubbutat MH, Ludwig RL, Ashcroft M, Vousden KH. Regulation of Mdm2-directed degradation by the C terminus of p53. Mol Cell Biol. 1998;18(10):5690–5698. doi: 10.1128/mcb.18.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445(7130):851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 19.Carry JC, Garcia-Echeverria C. Inhibitors of the p53/hdm2 protein-protein interaction-path to the clinic. Bioorganic & medicinal chemistry letters. 2013;23(9):2480–2485. doi: 10.1016/j.bmcl.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Lu M, Breyssens H, Salter V, Zhong S, Hu Y, Baer C, et al. Restoring p53 function in human melanoma cells by inhibiting MDM2 and cyclin B1/CDK1-phosphorylated nuclear iASPP. Cancer cell. 2013;23(5):618–633. doi: 10.1016/j.ccr.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445(7128):656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su Y, Vilgelm AE, Kelley MC, Hawkins OE, Liu Y, Boyd KL, et al. RAF265 inhibits the growth of advanced human melanoma tumors. Clin Cancer Res. 2012;18(8):2184–2198. doi: 10.1158/1078-0432.CCR-11-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis TA, Vilgelm AE, Richmond A, Johnston JN. Preparation of (−)-nutlin-3 using enantioselective organocatalysis at decagram scale. J Org Chem. 2013;78(21):10605–10616. doi: 10.1021/jo401321a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai F, Zhu F, Tagen M, Miller L, Owens TS, Mallari J, et al. Determination of nutlin-3a in murine plasma by liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) J Pharm Biomed Anal. 2010;51(4):915–920. doi: 10.1016/j.jpba.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer RJ, Guzy S, Ng C. A survey of population analysis methods and software for complex pharmacokinetic and pharmacodynamic models with examples. The AAPS journal. 2007;9(1):E60–E83. doi: 10.1208/aapsj0901007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13(4):407–484. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- 27.Wang E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer research. 1995;55(11):2284–2292. [PubMed] [Google Scholar]
- 28.Yeo EJ, Hwang YC, Kang CM, Choy HE, Park SC. Reduction of UV-induced cell death in the human senescent fibroblasts. Molecules and cells. 2000;10(4):415–422. [PubMed] [Google Scholar]
- 29.Seluanov A, Gorbunova V, Falcovitz A, Sigal A, Milyavsky M, Zurer I, et al. Change of the death pathway in senescent human fibroblasts in response to DNA damage is caused by an inability to stabilize p53. Mol Cell Biol. 2001;21(5):1552–1564. doi: 10.1128/MCB.21.5.1552-1564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders YY, Liu H, Zhang X, Hecker L, Bernard K, Desai L, et al. Histone modifications in senescence-associated resistance to apoptosis by oxidative stress. Redox biology. 2013;1(1):8–16. doi: 10.1016/j.redox.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uberti D, Carsana T, Bernardi E, Rodella L, Grigolato P, Lanni C, et al. Selective impairment of p53-mediated cell death in fibroblasts from sporadic Alzheimer's disease patients. Journal of cell science. 2002;115(Pt 15):3131–3138. doi: 10.1242/jcs.115.15.3131. [DOI] [PubMed] [Google Scholar]
- 32.Gorgun G, Calabrese E, Hideshima T, Ecsedy J, Perrone G, Mani M, et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010;115(25):5202–5213. doi: 10.1182/blood-2009-12-259523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 34.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2(8):594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 35.Cande C, Vahsen N, Garrido C, Kroemer G. Apoptosis-inducing factor (AIF): caspase-independent after all. Cell death and differentiation. 2004;11(6):591–595. doi: 10.1038/sj.cdd.4401400. [DOI] [PubMed] [Google Scholar]
- 36.Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP, et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19(18):2122–2137. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Secchiero P, Corallini F, Gonelli A, Dell'Eva R, Vitale M, Capitani S, et al. Antiangiogenic activity of the MDM2 antagonist nutlin-3. Circulation research. 2007;100(1):61–69. doi: 10.1161/01.RES.0000253975.76198.ff. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharya A, Toth K, Mazurchuk R, Spernyak JA, Slocum HK, Pendyala L, et al. Lack of microvessels in well-differentiated regions of human head and neck squamous cell carcinoma A253 associated with functional magnetic resonance imaging detectable hypoxia, limited drug delivery, and resistance to irinotecan therapy. Clin Cancer Res. 2004;10(23):8005–8017. doi: 10.1158/1078-0432.CCR-04-1306. [DOI] [PubMed] [Google Scholar]
- 39.Primeau AJ, Rendon A, Hedley D, Lilge L, Tannock IF. The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res. 2005;11(24 Pt 1):8782–8788. doi: 10.1158/1078-0432.CCR-05-1664. [DOI] [PubMed] [Google Scholar]
- 40.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 41.Bonecchi R, Galliera E, Borroni EM, Corsi MM, Locati M, Mantovani A. Chemokines and chemokine receptors: an overview. Frontiers in bioscience. 2009;14:540–551. doi: 10.2741/3261. [DOI] [PubMed] [Google Scholar]
- 42.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9(6):338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445(7128):661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 44.Katayama H, Sasai K, Kawai H, Yuan ZM, Bondaruk J, Suzuki F, et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nature genetics. 2004;36(1):55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 45.Liu Q, Kaneko S, Yang L, Feldman RI, Nicosia SV, Chen J, et al. Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. J Biol Chem. 2004;279(50):52175–52182. doi: 10.1074/jbc.M406802200. [DOI] [PubMed] [Google Scholar]
- 46.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487(7408):500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ott PA, Henry T, Baranda SJ, Frleta D, Manches O, Bogunovic D, et al. Inhibition of both BRAF and MEK in BRAF(V600E) mutant melanoma restores compromised dendritic cell (DC) function while having differential direct effects on DC properties. Cancer Immunol Immunother. 2013;62(4):811–822. doi: 10.1007/s00262-012-1389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19(5):1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serrano M. Cancer: final act of senescence. Nature. 2011;479(7374):481–482. doi: 10.1038/479481a. [DOI] [PubMed] [Google Scholar]
- 50.Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153(2):449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.