Abstract
Alzheimer’s disease (AD) is the most common cause of dementia, affecting more than 10% of people over the age of 65. Age is the greatest risk factor for AD, although a combination of genetic, lifestyle and environmental factors also contribute to disease development. Common features of AD are the formation of plaques composed of beta-amyloid peptides (Aβ) and neuronal death in brain regions involved in learning and memory. Although Aβ is neurotoxic, the primary mechanisms by which Aβ affects AD development remain uncertain and controversial. Mouse models overexpressing amyloid precursor protein and Aβ have revealed that Aβ has potent effects on neuroinflammation and cerebral blood flow that contribute to AD progression. Therefore, it is important to consider how endogenous signaling in the brain responds to Aβ and contributes to AD pathology. In recent years, Aβ has been shown to affect ATP release from brain and blood cells and alter the expression of G protein-coupled P2Y receptors that respond to ATP and other nucleotides. Accumulating evidence reveals a prominent role for P2Y receptors in AD pathology, including Aβ production and elimination, neuroinflammation, neuronal function and cerebral blood flow.
Keywords: Alzheimer’s disease, P2Y receptors, neuroinflammation, neurovascular regulation, ATP receptors, purinergic receptors
Alzheimer disease (AD) is a chronic neurodegenerative disorder characterized by progressive cognitive decline causing the most prevalent form of dementia in western society. The global incidence of AD is estimated to be around 24 million worldwide with a predicted doubling every 20 years until 2040 (Mayeux and Stern, 2012). The neuropathological lesions manifested in AD include senile amyloid plaques and neurofibrillary tangles, and are accompanied by reactive microgliosis, dystrophic neurites and loss of neurons and synapses (Serrano-Pozo et al., 2011). The main components of senile plaques are the neurotoxic beta-amyloid peptides (Aβ) that are derived from cleavage of amyloid precursor protein (APP) by β- and γ-secretase (Haass and Selkoe, 2007; Mayeux and Stern, 2012). Conversely, the neurofibrillary tangles are mainly constituted by intracellular accumulation of τ protein (Liu et al., 2005; Selkoe, 2001). Research efforts in the past two decades in animal models of AD have revealed that neuroinflammation plays a crucial role in AD progression. It is well known that Aβ induces microglia to produce nitric oxide (NO), reactive oxygen species (ROS), proinflammatory cytokines (e.g., tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-18) and prostaglandins (e.g., PGE2), which contribute to neuronal death (Akiyama et al., 2000). Furthermore, studies have also shown that cells involved in the central nervous system (CNS) immune response, such as dendritic cells, microglia, and astrocytes, are able to detect Aβ through Toll-like receptor (TLR) signaling. TLR2, in particular, plays a key role in triggering neuroinflammatory events that lead to the activation of signal-dependent transcription factors driving expression of downstream genes associated with the inflammatory pathway (Liu et al., 2005). In addition to a neuroinflammatory phenotype, AD is often preceded by malfunctions in the cerebrovascular system, including accumulation of Aβ in cerebral blood vessels (cerebral amyloid angiopathy) and decreased cerebral blood flow, which leads to hypoxia, breakdown of the blood-brain barrier and ultimately to brain atrophy and death (Bell et al., 2009; Iadecola, 2004; Kelleher and Soiza, 2013; Sagare et al., 2013; Zlokovic, 2008). Studies in mice and humans indicate that Aβ peptides cause forceful constriction of cerebral blood vessels (Niwa et al., 2001; Paris et al., 2003), suggesting that vascular factors play an early pathogenic role in AD.
Purinergic signaling, through activation of A1–3 adenosine receptors, P2X cation channel receptors for ATP and P2Y nucleotide receptors with subtype specificity for ATP, ADP, UTP, UDP and UDP-glucose (Table 1), has been implicated in neuroinflammation, vascular regulation and the progression of many neurodegenerative diseases, including AD (Burnstock, 2008; Burnstock and Ralevic, 2014; Erlinge and Burnstock, 2008; Rahman, 2009; Takenouchi et al., 2010; Weisman et al., 2012a). In the following sections, we discuss experimental evidence linking P2Y receptors to neuroinflammatory events, neuronal functions, the regulation of cerebral blood flow and the pathophysiology of AD.
Table 1.
Known agonists and antagonists of P2YRs
| P2YR subtypes | Endogenous agonists | Synthetic agonists | Synthetic antagonists |
|---|---|---|---|
| P2Y1R | ADP>ATP | 2MeSADP, 2MeSATP, ATPγS, MRS2365 | Suramin, RB2, MRS2179, MRS2279, MRS2365, MRS2500, A3P5PS, PPADS, BzATP |
| P2Y2R | ATP=UTP | ATPγS, UTPγS, BzATP | Suramin, RB2 |
| P2Y4R | UTP (human) ATP (rat, mouse) |
UTPγS | MRS2578, ATP (human), RB2 (rat), PPADS (weak), BzATP (rat) |
| P2Y6R | UDP | UDPγS | Suramin, RB2, PPADS |
| P2Y11R | ATP | BzATP, ADPγS | Suramin, RB2 |
| P2Y12R | ADP | 2MeSADP, ADPγS | Suramin, RB2, ARC69931, ARC66096, MRS2395 |
| P2Y13R | ADP | 2MeSADP, ADPγS | Suramin, RB2, PPADS, ARC69931, ARC66096, MRS2211 |
| P2Y14R | UDP-glucose |
Abbreviations: 2MeSADP, 2-methylthio-adenosine 5′-diphosphate; 2MeSATP, 2-methylthio-adenosine 5′-triphosphate; ATPγS, adenosine 5′-(γ-thio)triphosphate; RB2, reactive blue 2; PPADS, pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid; BzATP, 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate; UTPγS, uridine 5′-(γ-thio)triphosphate; UDPγS, uridine 5′-(γ-thio)diphosphate
CHANGES IN P2Y RECEPTOR EXPRESSION AND NUCLEOTIDE RELEASE IN AD
P2Y receptor expression in human and animal models of AD
Expression of all eight subtypes of P2YRs (P2Y1,2,4,6,11,12,13,14) has been documented in the brain (Weisman et al., 2012a). In AD patients and mouse models of AD, changes in expression patterns of P2Y1,2,4 receptors have been observed. Immunohistochemical localization of P2Y1R with neurofibrillary tangles, neuritic plaques and neuropil threads, which are associated with AD, has been reported in human AD brain samples (Moore et al., 2000b). Lai et al. assessed the expression of P2Y2,4,6 receptors in human AD brain samples by immunoblot analysis and found lower protein levels of P2Y2R compared to age-matched controls, while P2Y4R and P2Y6R levels were unaltered (Lai et al., 2008). However, lack of specificity for commercially available P2Y2R and P2Y6R antibodies has also been reported (Ben Yebdri et al., 2009; Yu and Hill, 2013). In the TgCRND8 mouse model of AD, which expresses the Swedish (K670N/M671L) and Indiana (V717F) mutations in human APP, P2Y2R mRNA expression is elevated in the brain at 10 weeks of age and reduced after 25 weeks of age compared to age-matched controls (Ajit et al., 2013). In an AD mouse model expressing the Swedish APP mutation and the γ-secretase mutation (PS1dE9), an increase in P2Y4R expression in cerebral cortical sections was observed in activated microglia compared to resting microglia (Li et al., 2013). This study also demonstrated that the microglial P2Y4R contributes to pinocytosis of soluble Aβ, suggesting that the murine P2Y4R is neuroprotective by stimulating microglia-mediated clearance of Aβ (Li et al., 2013).
Effects of Aβ on P2YR expression and nucleotide release
Aβ has been found to alter ATP release and/or P2YR expression in microglia and erythrocytes. In rat primary cortical astrocytes, oligomeric Aβ1–42 (oAβ1–42) induces the release of endogenous ATP (Peterson et al., 2010). In mouse primary microglial cells, fibrillar Aβ (fAβ1–42) and oAβ1–42 aggregates cause ATP release (Kim et al., 2012) and in microglial N13 cells, the Aβ25–35 peptide causes a dose-dependent release of ATP (Sanz et al., 2009). Aβ-induced release of ATP from microglial cells, as well as microglial activation, are dependent on expression of the ionotropic P2X7 receptor, which is thought to release ATP through the formation of a membrane pore (Sanz et al., 2009). Expression of the P2Y2R is upregulated in mouse primary microglia treated with fAβ1–42 or oAβ1–42 aggregates (Kim et al., 2012). In the vasculature, hypoxia causes the release ATP from erythrocytes into the blood stream (Bergfeld and Forrester, 1992) and a 24 h exposure of human erythrocytes to Aβ inhibits ATP release from deoxygenated cells (Misiti et al., 2008). P2Y receptors expressed in human vascular endothelium, including P2Y1,2,4,6R, trigger vasodilation through release of NO, endothelium-derived hyperpolarizing factor (EDHF) and prostacyclin (Burnstock and Ralevic, 2014), thus activation of these receptors may help to counteract the vasoconstrictive effects of Aβ.
ROLE OF P2Y RECEPTORS IN THE REGULATION OF AD SYMPTOMS AND BIOMARKERS
Effects of P2Y receptors on Aβ metabolism
APP production and cleavage
Although it remains unclear whether nucleotides and P2YRs affect the production and/or release of APP in neurons, ATP and UTP have been shown to increase the extracellular signal-regulated kinase (ERK)-dependent production and secretion of APP in primary rat cortical astrocytes (Tran, 2011), presumably through activation of the P2Y2R and P2Y4R. The non-selective P2YR antagonists, suramin and reactive blue 2 (RB2), inhibited expression of 130 kD APP to similar extents, but suramin was more effective at reducing 110 kD APP expression while RB2 was more effective at inhibiting APP release (Tran, 2011). Considering that suramin is a more potent antagonist of P2Y2R and RB2 is a more potent antagonist of P2Y4R, it was suggested that these two P2YRs may play different roles in the regulation of APP production and release.
Cleavage of APP by β- and γ-secretases produces neurotoxic peptides, Aβ1–40 and Aβ1–42, whereas cleavage of APP by α- and γ-secretases produces the non-amyloidogenic Aβ17–40/42 fragments (Roberts et al., 1994; Sisodia, 1992; Vassar et al., 2009). Competition between α-secretases (i.e., ADAM9/10/17) and β-secretase (i.e., BACE1) in APP processing has been documented (Deuss et al., 2008; Lichtenthaler, 2012; O’Brien and Wong, 2011; Skovronsky et al., 2000) and production of neurotoxic Aβ is reduced by promoting α-secretase activity (Caccamo et al., 2006; Kuhn et al., 2010). Activation of the P2Y2R enhances α-secretase-mediated APP processing in primary rat cortical neurons and human astrocytoma cells, which was dependent on two members of the ADAM family: ADAM 10 and ADAM17 (Camden et al., 2005; Kong et al., 2009; Leon-Otegui et al., 2011). Regulation of ADAM10/17 activity by the P2Y2R requires phosphatidylinositol 3-kinase (PI3K) and ERK1/2 activity, whereas the involvement of protein kinase C (PKC) varies with cell type (Camden et al., 2005; Kong et al., 2009).
Matrix metalloprotease activity and Aβ degradation
Matrix metalloprotease-9 (MMP-9) is a secreted protease that breaks down extracellular matrix in normal physiological conditions, but is expressed at higher levels in AD patients (Lorenzl et al., 2003) and co-localizes with Aβ plaques (Backstrom et al., 1996). Aβ induces the astrocytic production and activation of MMP-9 (Hernandez-Guillamon et al., 2009; Mizoguchi et al., 2009; Talamagas et al., 2007), which in turn degrades Aβ peptide (Backstrom et al., 1996) and fibrils in vitro (Yan et al., 2006), and compact plaques in situ (Yan et al., 2006). However, homozygous knockout of MMP-9 in mice or administration of GM6001, a broad-spectrum MMP inhibitor, was found to improve Aβ-induced memory loss, suggesting that MMP-9 activity promotes Aβ-induced cognitive impairment and neurotoxicity (Mizoguchi et al., 2009). Recently, Kinoshita et al. (2013) reported that MMP-9 production in rat primary astrocytes is increased upon treatment with apyrase, RB2 and pertussis toxin (PTX, a Gi-inhibitor). By pharmacological examination with P2Y agonists and antagonists, P2Y14R was identified to be associated with MMP-9 production and knockdown of P2Y14R led to a significant increase in MMP-9 expression. Treatment of astrocytes with apyrase, RB2 or PTX, also increased expression of cytokine-induced neutrophil chemoattractant-3, TNF-α, tissue inhibitory metalloproteinase (TIMP)-1 and monocyte chemoattrant protein-1 (MCP-1). While TIMP-1 is known to bind MMP-9 and inhibit its proteolytic activity (Hernandez-Guillamon et al., 2009), TNF-α induces MMP-9 release from astrocytes in vitro (Kinoshita et al., 2013). These findings suggest a protective role of the P2Y14R in AD via suppressing MMP-9 expression.
Effects of P2Y receptors on neuroinflammation
There is now a consensus that neuroinflammation is involved in AD, although its role remains ambiguous, whether as a primary cause of disease progression or a protective response (Rivest, 2009; Wyss-Coray and Rogers, 2012). While clinical trials of anti-inflammatory drugs are still inconclusive for AD treatment (Wyss-Coray and Rogers, 2012), modulation of inflammation has been effective in animal models of AD (Klegeris et al., 2007; McGeer et al., 2005; Rozemuller et al., 2005).
Cytokine and chemokine production
In monocytes and microglia, Aβ induces secretion of proinflammatory cytokines, including IL-1β, IL-6 and TNF-α, as well as chemokines, including CC chemokines CCL2/macrophage inflammatory protein-1α/β (MIP-1α/β), MCP-1 and CXC chemokine IL-8 (Akiyama et al., 2000; Fiala et al., 1998; Walker et al., 1995). Among these cytokines, IL-1β is particularly important for regulating the expression of other proinflammatory cytokines (e.g., TNF-α, IL-6, and interferons) and chemokines (e.g., CXCL1 and CXCL2) (Rothwell and Luheshi, 2000; Shaftel et al., 2008). In vivo, over-expression of IL-1β in the hippocampus accelerates phagocyte recruitment and clearance of Aβ plaques in a mouse model of AD (Shaftel et al., 2007). In vitro, Aβ causes IL-1β release from microglia that is dependent on P2X7R expression (Sanz et al., 2009). Aβ treatment also increases expression of the P2Y2R in microglia (Kim et al., 2012) and neurons (Kong et al., 2009; Peterson et al., 2013), which is likely triggered by P2X7R-mediated IL-1β release (Chakfe et al., 2002; Choi et al., 2007; D’Alimonte et al., 2007; Mingam et al., 2008; Sanz et al., 2009; Suzuki et al., 2004; Takenouchi et al., 2011; Takenouchi et al., 2009).
P2Y1 receptors are important for regulating cytokine and chemokine production in a rat cerebral stroke model and, therefore, may also affect neuroinflammatory conditions occurring in AD. In the cerebral stroke model, administration of the P2Y1R specific agonist MRS2365 significantly increased the infarct volume (a measure of cerebral damage) after a 30-min occlusion of the middle cerebral artery (MCAO), whereas administration of the P2Y1R antagonists MRS2179, A3P5PS and MRS2279 significantly reduced infarct volume (Kuboyama et al., 2011). Furthermore, upregulation of cytokines and chemokines, including IL-6, TNF-α, CCL2/MCP-1 and interferon-inducible protein-10 (IP-10)/CXCL10, was inhibited after MCAO by MRS2179 application. The P2Y1R-dependent upregulation of cytokines and chemokines and cerebral infarct were suggested to act via the NF-κB signaling pathway (Kuboyama et al., 2011). Similarly, Chin et al. (2013) observed hippocampal neuroinflammation and microstructural alterations, as well as a long-lasting deficit in cognitive function, after a 90-min MCAO in wild-type rats and mice. However, MCAO in P2Y1R−/− mice caused defective sensory-motor function but cognition and hippocampal neuroinflammation and microstructure were unaltered. In addition, treatment with the P2Y1R-specific antagonist MRS2500 helped wild-type mice maintain cognitive function after MCAO (Chin et al., 2013).
Microglia recruitment
Microglial cells are CNS macrophages that differentiate from monocytes. As the primary immune effector cells in the CNS, activated microglia are thought to play a neuroprotective role in AD by migrating towards Aβ plaques (Bolmont et al., 2008; Frautschy et al., 1998; Meyer-Luehmann et al., 2008; Perlmutter et al., 1990; Zheng et al., 2010) and clearing neurotoxic Aβ by endocytosis (Bolmont et al., 2008; Kim et al., 2012; Shie et al., 2005) and degradation (Jiang et al., 2008). The importance of microglia in reducing Aβ levels has been confirmed not only in AD mouse models, but also in Aβ-immunized AD patients who showed decreased plaque load and increased microglial cell accumulation (Boche et al., 2010; Nicoll et al., 2003; Schenk et al., 1999). The population of microglia in the CNS can be amplified by recruiting blood-derived macrophages/monocytes (Hao et al., 2011; Simard et al., 2006). In an in vitro blood-brain barrier model, monocytes pretreated with Aβ1–42 on the “brain side” were recruited much more effectively from the “blood side” than untreated monocytes, a process thought to be related to Aβ1–42-induced secretion of chemokines (Fiala et al., 1998).
In an early in vitro study, extracellular ATP or ADP enhanced membrane ruffling and migration of cultured microglia (Honda et al., 2001). Later, local ATP was found to cause a rapid chemotactic response in microglia to local brain injury and ATP induced further release of ATP from surrounding astrocytes that was essential to the microglial response (Davalos et al., 2005). Although the P2 receptor subtype responsible for microglial migration to the injured site was not determined, application of the ATP-hydrolyzing enzyme apyrase or the P2YR inhibitors, RB2 and PPADS, showed efficient inhibition, whereas inhibition by suramin was incomplete (Davalos et al., 2005). ADP-induced membrane ruffling and chemokinesis of microglia is inhibited by the P2Y12/13R antagonist ARC69931 or by elevated intracellular cAMP levels, indicating P2Y12/13R-mediated microglia chemokinesis towards ADP (Nasu-Tada et al., 2005). Moreover, the β1 integrin is thought to be involved since it traffics to membrane ruffles after ADP stimulation (Nasu-Tada et al., 2005).
The importance of the P2Y12R in microglia polarization, migration and extension of processes toward nucleotides in vitro and in vivo was later supported by Haynes et al. (2006). They showed primary brain microglia from wild-type, but not the P2Y12R−/− mice, underwent membrane ruffling after exposure to ATP, ADP, or 2MeSADP. In living brains of P2Y12R−/− mice, the microglial processes extending toward the exogenous ATP resource or sites of focal laser ablation were significantly reduced in comparison to those in wild-type mice (Haynes et al., 2006). Impairment of microglial chemotaxis to ATPγS (an ATP homologue) in rat primary microglia after treatment with MRS2395, a selective antagonist of the P2Y12R, has also been reported (Li et al., 2013). These observations are consistent with an earlier report that P2Y12R-expressing microglia accumulate around damaged neurons in vivo (Sasaki et al., 2003). Interestingly, it was found that “resting” (albeit quite dynamic) microglia express more P2Y12R than activated microglia (Haynes et al., 2006), whereas the immunoreactivity of P2Y12R was not observed in macrophages in spleen or abdominal cavity (Haynes et al., 2006; Sasaki et al., 2003). Hence, the function of the P2Y12R might be to selectively motivate resting microglia for chemotaxis in the CNS.
Although microglial P2Y1R is undetectable, cytokines and chemokines released by P2Y1R-expressing astrocytes lead to macrophage infiltration and microglia activation in ischemic rat brain, which are abrogated by the P2Y1R antagonist MRS2179 (Kuboyama et al., 2011). The importance of P2Y1R in astrocytes challenged with extracellular ATP has also been implied by its involvement in ATP-induced ERK activation in response to stretch-induced injury (Neary et al., 2003).
P2Y6R was at first thought to mediate only the phagocytosis but not the chemotaxis or migration of microglia (Koizumi et al., 2007). However, Kim et al. (2011) found in primary cultured microglia, astrocytes and cortical slice cultures that UDP, a selective agonist of P2Y6R, induces expression of the chemokines CCL2 (MCP-1) and CCL3 (MIP-1α), which are important for monocyte infiltration into the injured brain. Two calcium-activated transcription factors, NFATc1 and c2, rather than NF-κB, were responsible for UDP-induced chemokine expression. Moreover, UDP-treated astrocytes recruited monocytes in an in vitro transmigration assay (Kim et al., 2011). Based on their regulation of microglia migration, P2Y1,6,12,13Rs in microglia may contribute to the pathophysiology of AD, although direct evidence is still lacking.
More evidence exists for the essential role of the P2Y2R in recruiting microglia to the brain during the development of AD. Although UTP was reported to be insufficient to induce ruffle formation in rat primary microglia (Haynes et al., 2006; Honda et al., 2001), it enhances migration of mouse primary microglia in vitro (Kim et al., 2012). In the TgCRND8 mouse model of AD, expression of CD11b, a marker for activated microglia, is elevated in hippocampal brain sections but reduced when P2Y2R expression is suppressed (Ajit et al., 2013).
Aβ uptake and degradation by microglia
Phagocytes, including microglia, use two types of clathrin-independent endocytosis: pinocytosis for uptake of liquids and phagocytosis for uptake of solids. Pinocytosis is further divided into two separate pathways: micropinocytosis and macropinocytosis, whose vesicle diameters and mechanisms differ. It has been experimentally demonstrated that microglia-mediated clearance of soluble Aβ is via macropinocytosis, both in vitro and in vivo (Mandrekar et al., 2009). The clearance of insoluble fAβ and fAβ-containing plaques is carried out by microglial phagocytosis, during which fAβ interacts with a cell surface receptor complex formed by the B-class scavenger receptor CD36, the α6β1 integrin and CD47/integrin associated protein (Bamberger et al., 2003; Koenigsknecht and Landreth, 2004).
The UDP-activated P2Y6R was found to stimulate phagocytosis in rat primary microglia where a CysLT receptor was involved (Koizumi et al., 2007). This P2Y6R-mediated microglial phagocytosis was confirmed by an in vivo phagocytosis assay in which fluorescently-labeled microspheres were injected into rats receiving kainic acid (KA), a brain injury model (Koizumi et al., 2007). Recently, time-lapse imaging visualized P2Y6R-mediated microglial cell membrane motility. Application of 100 μM UDP stimulated both immediate formation of lamellipodia-like structures and long-lasting active motility in the cell membranes (Uesugi et al., 2012). Moreover, UDP treatment induced formation of large vacuoles that had taken up extracellular fluorescently-labeled dextran, soluble Aβ1–42 or microspheres, which demonstrated that the P2Y6R mediates macropinocytosis and phagocytosis. These endocytic processes involved PKC-independent functions and protein kinase D (Uesugi et al., 2012).
In monocytes and macrophages, the P2Y2R is a critical sensor of nucleotides released by apoptotic cells, whose phagocytic clearance function is promoted (Elliott et al., 2009). Consistent with this, the phagocytosis and degradation of insoluble fAβ1–42 and oAβ1–42 aggregates by primary microglia was enhanced after UTP stimulation that activated the P2Y2R, as well as the αv integrin, Rac and Src pathways, neuroprotective responses (Kim et al., 2012). There is a similarity between the tyrosine kinase-Vav-Rac1 signaling cascade following fAβ-CD36 binding in microglia (Wilkinson et al., 2006) and the Vav2-Rac1 signaling cascade activated by association of the P2Y2R and αv integrin (Bagchi et al., 2005). Therefore, it was hypothesized that the P2Y2R and CD36 both interact with CD47 and its associated integrins to induce phagocytosis of insoluble Aβ by microglia (Kim et al., 2012).
Li et al. (2013) found that application of UTP, ATP or ATPγS, a non-hydrolyzable ATP homologue, efficiently stimulated pinocytosis in primary rat microglia. At first, both rat P2Y4R and P2Y2R were suspected to be involved, since unlike humans where ATP is an antagonist of the P2Y4R, in rats ATP is an agonist of P2Y4R with less potency than UTP (Kennedy et al., 2000). Using specific siRNAs, it was determined that the P2Y4R was responsible for pinocytosis. Such P2Y4R-mediated pinocytosis occurs spontaneously without exogenous nucleotide stimulation to internalize soluble Aβ1–42, but not an Aβ42-1 control (Li et al., 2013). In regards to the pharmacological differences between murine and human P2Y4Rs, it will be important to assess whether human P2Y4Rs mediate microglial pinocytosis of soluble Aβ.
Oxidative stress
Reactive oxygen species (ROS), mainly superoxide (O2−) and hydrogen peroxide (H2O2), are unavoidable by-products of cellular respiration. ROS react with molecules within cells, including DNA, and cause cell damage, i.e., oxidative stress. One of the major contributors of oxidative stress is the NADPH oxidase (NOX) complex that produces O2−.
Oxidative stress is a noted contributor to the pathogenesis of AD and has been recently reviewed (Mao and Reddy, 2011). Antioxidants and NOX-targeting therapeutics have shown positive effects in counteracting oxidative stress in vitro and in rodent models of AD (Simonyi et al., 2010). Primary cortical neurons exposed to Aβ1–42 had significant ROS production, which required NOX activity (Shelat et al., 2008). Primary hippocampal neurons treated with the general NOX inhibitor apocynin and soluble oAβ1–42, survived better than neurons treated with oAβ1–42 alone (Bruce-Keller et al., 2010). AD patients with mild cognitive impairment followed longitudinally had higher NOX expression and activity in the temporal gyri than controls, whereas preclinical or late-stage AD samples showed no difference (Bruce-Keller et al., 2010). Hence, the NOX-mediated redox pathway may be a pathogenic mechanism mainly in early AD within vulnerable regions of the brain, which may relate to previous clinical trials with antioxidants that failed to significantly retard AD progression (Pratico, 2008).
Microglia are known to express the NOX1, NOX2 and NOX4 subtypes, and treatment of mouse microglial BV2 cells with the P2Y2/4R specific agonist UTPγS increases expression of NOX1 and NOX2, but not NOX4 (Mead et al., 2012). UTPγS also increased O2− production, whereas the P2Y1R agonist MRS2365 had no effect. Interestingly, the UTPγS-induced production of O2− was inhibited by apocynin, but not by thioridazine (an inhibitor of the ferrocytochrome subunit of NOX) or wortmanin (an inhibitor of PI3K-mediated NOX2 regulation), whereas rottlerin (an inhibitor of PKC-mediated NOX1 regulation) slightly enhanced UTPγS-induced O2− production. Apocynin-sensitive O2− production in primary rat microglia was induced by BzATP (Mead et al., 2012), a stable ATP homologue and a potent agonist of the P2X7R, but also an agonist for the rat P2Y2R (Wildman et al., 2003).
To protect cells from oxidative stress, heme oxygenase-1 (HO-1) and biliverdin reductase-A can be induced to neutralize ROS. In the hippocampus of samples from postmortem AD patients, HO-1 protein levels and serine phosphorylation are significantly higher than in the age-matched controls (Barone et al., 2012). Potential roles for nucleotides and P2YRs in protection from oxidative stress in AD were suggested by Espada et al. (2010), who found that HO-1 was upregulated in primary cerebellar granule neurons (CGN) from rats and mice after treatment with ADP or its stable analogue 2MeSADP. Among the three 2MeSADP-reactive P2YR subtypes, P2Y12R was barely expressed in CGN and it was determined that the P2Y13R and not the P2Y1R mediates ADP/2MeSADP-induced HO-1 expression, using the selective antagonists of P2Y13R and P2Y1R, MRS2179 and MRS2211, respectively. It also was shown that activation of the transcription factor Nrf2 is critical to 2MeSADP-evoked protection of CGN from H2O2-induced death (Espada et al., 2010). In contrast, Fujita et al. (2009) found that ATP and 2MeSADP had no impact on the cell death of primary rat hippocampal neurons exposed to H2O2. However, when these neurons were co-cultured with primary astrocytes, the H2O2-induced neuronal death was greatly decreased after ATP or 2MeSADP pretreatment. Using P2Y1R-specific siRNA, it was shown that this neuroprotection against oxidative stress was conferred by activation of astrocytic P2Y1R and release of IL-6 in response to extracellular ATP (Fujita et al., 2009).
Effects of P2Y receptors on neuronal function
P2Y receptors in neurons have been shown to mediate neuroprotective responses through their regulation of axonal growth, neurite extension and synaptic transmission. All of the P2Y receptor subtypes are expressed in neurons or oligodendrocytes under a variety of conditions (Amadio et al., 2007; del Puerto et al., 2012; Heine et al., 2007; Koles et al., 2011; Moore et al., 2000a; Weisman et al., 2012b). Although relatively few studies have examined the roles of these P2Y receptors in the context of AD, studies have determined functional responses in neurons mediated by each P2Y receptor subtype that are thought to be protective in the face of inflammation or other pathological insults in the brain. In addition, a wide range of agonists and antagonists that are relatively selective for specific P2Y receptor subtypes have been developed (Weisman et al., 2012b) that may ultimately prove useful in the treatment of AD and other neurodegenerative diseases.
Expression of P2Y receptors in neurons
The P2Y1R for ADP is widely expressed in the brains of mammals, including in the cerebral cortex and hippocampus (Koles et al., 2011; Moore et al., 2000a; Moran-Jimenez and Matute, 2000; Simon et al., 1997), regions predominantly impacted in AD (Lee et al., 2010; Obulesu et al., 2011; Wyss-Coray and Rogers, 2012; Zilka et al., 2006). The P2Y1R has been shown to be expressed in glutamatergic and glycinergic neurons (Jimenez et al., 2011; Rodrigues et al., 2005), oligodendrocytes (Agresti et al., 2005), neuroprogenitor cells (Mishra et al., 2006), dorsal root ganglia and horn neurons (Kobayashi et al., 2006; Ruan and Burnstock, 2003; Sanada et al., 2002). In human brain from AD patients, P2Y1Rs in neurons co-localize with tau tangles and Aβ plaques (Moore et al., 2000b).
Low expression levels of the P2Y2R for ATP and UTP have been demonstrated in neurons of the cerebral cortex and hippocampus (Moore et al., 2001; Verkhratsky et al., 2009). However, P2Y2R expression has been shown to be significantly increased in cortical neurons upon treatment with the proinflammatory cytokine IL-1β, whose levels are elevated in AD patients and animal models of AD, as compared to normal controls (Cacabelos et al., 1994; Lee et al., 2010). Interestingly, the P2Y2R is also upregulated in mammalian cells and tissues under conditions associated with inflammation or injury (Kong et al., 2009; Koshiba et al., 1997; Schrader et al., 2005; Seye et al., 2002; Shen et al., 2004; Turner et al., 1997), including in the spinal cord (Rodriguez-Zayas et al., 2010) and brain (Franke et al., 2004). These findings suggest that P2Y2R upregulation in neurons in response to cell damage plays a potential role in neuroprotection.
Recent studies have shown that P2Y4R and P2Y6R can function as homo- or heterodimers in neurons (D’Ambrosi et al., 2007). Both P2Y4R and P2Y6R mRNA are detected in rat hippocampal pyramidal neurons (Rodrigues et al., 2005), whereas P2Y6R mRNA is expressed in mouse superior cervical ganglion (Calvert et al., 2004) and rat dorsal-root ganglion neurons (Ruan and Burnstock, 2003; Sanada et al., 2002). The P2Y4 and P2Y6 receptors (D’Ambrosi et al., 2007; Koles et al., 2011) are expressed in other neuronal cell types of the CNS (Moore et al., 2001; Verkhratsky et al., 2009).
Expression of the P2Y11R for ATP has been described in rat hippocampal pyramidal neurons, cerebellar Purkinje cells (Rodrigues et al., 2005; Volonte et al., 2006) and other neuronal cell types (Moore et al., 2001; Verkhratsky et al., 2009). P2Y11R activation has been shown to inhibit apoptosis induced by pathogens or inflammation, suggesting a role in neuroprotection (Vaughan et al., 2007). Other studies have shown that the P2Y12R, known to regulate platelet aggregation (Dorsam and Kunapuli, 2004; Jantzen et al., 1999; Wallentin, 2009; Xu et al., 2002), is expressed in neurons (Jimenez et al., 2011; Moore et al., 2001; Rodrigues et al., 2005; Verkhratsky et al., 2009) and oligodendrocytes (Amadio et al., 2006).
P2Y13Rs for ADP are expressed in a variety of neuronal cell types (Jimenez et al., 2011; Moore et al., 2001; Verkhratsky et al., 2009), where they have been postulated to play a role in cell survival (Weisman et al., 2012b). P2Y14Rs for UDP-glucose have been detected in neurons (Moore et al., 2001; Verkhratsky et al., 2009), and sensory neurons have been shown to express P2Y13 and P2Y14, as well as P2Y1 receptors (Malin and Molliver, 2010).
Neuroprotective responses mediated by P2Y receptors
Cellular functions coupled to the activation of various P2YR subtypes in neurons appear to be largely neuroprotective, suggesting that these receptors could serve as effective pharmacological targets to impede the progression of AD. However, these pathways have not been investigated as extensively as the neuroprotective functions of P2YRs in glial cells of the brain (see above). Nonetheless, the activation of specific P2YR subtypes in neurons has been shown to stimulate neurite extension/axonal elongation and non-amyloidogenic processing of APP and promote cell survival, as described below.
The coordinate regulation of axonal growth by the P2Y1 and P2Y13 receptors has been demonstrated in rat hippocampal neurons, where axonal elongation is stimulated by the P2Y1R through Gq-dependent activation of PI3K and axonal elongation is inhibited by the P2Y13R via Gi-dependent activation of adenylate cyclase, pathways that could be targeted to counteract the neurodegenerative effects of neurofibrillary tangles (del Puerto et al., 2012). Studies with rat primary cortical neurons indicate that neurite extension is stimulated by P2Y2R-mediated interactions with αvβ3/5 integrin leading to activation of a pathway involving G12-dependent Rho activation and the phosphorylation of the actin-depolymerizing protein cofilin (Peterson et al., 2013), which is known to promote neurite extension (Aizawa et al., 2001; Endo et al., 2007; Endo et al., 2003; Figge et al., 2012; Meberg and Bamburg, 2000; Meberg et al., 1998). Activation of the P2Y2R in the presence of nerve growth factor has been shown to enhance tyrosine kinase A-dependent neuronal differentiation and acceleration of neurite formation in PC12 cells and dorsal root ganglion neurons (Arthur et al., 2005). Therefore, the ability of P2Y2Rs to be upregulated by the cytokine IL-1β, whose levels are elevated in the AD brain, strongly suggests that acute inflammation serves to stimulate P2Y2R-mediated neuroprotection (i.e., enhanced axonal elongation/neurite outgrowth) in response to extracellular nucleotide release from activated glia or apoptotic cells in the inflammatory environment of early AD.
Activation of the P2Y2R in primary cortical neurons and neuroblastoma cells increases the α-secretase-dependent degradation of APP to generate the soluble APPα peptide rather than neurotoxic Aβ1–42 peptide, a response that can be prevented by pretreatment with inhibitors of the metalloproteases ADAM10/17, i.e., the TNF-α converting enzyme (Camden et al., 2005; Kong et al., 2009). It has been shown that neuropathology in AD (e.g., synapse loss) is correlated with a decrease in P2Y2R expression in the parietal cortex of human postmortem AD brain samples (Lai et al., 2008), as compared to controls, which is similar to the enhanced neuroinflammatory and neurodegenerative phenotype seen in the TgCRND8 mouse model of AD upon knockout of the P2Y2R (see below). The P2Y2R also protects against trauma-induced death of astrocytic cells (Burgos et al., 2007), through P2Y2R-mediated phosphorylation of the pro-apoptotic factor Bad and reduction in the bax/bcl-2 mRNA expression ratio (Chorna et al., 2004), which are known to be involved in cell survival mechanisms. Thus, loss of protective functions regulated by the P2Y2R in brain neurons and glial cells in humans and mice appears to promote the progression of the AD phenotype and suggests that activation of P2Y2Rs may serve to delay neurodegeneration caused by inflammation in the AD brain.
Other potential neuroprotective responses mediated by P2YR activation in neurons include the enhancement of Na+- and Cl−-dependent glycine transport in the synaptic cleft by P2Y1, P2Y12 and P2Y13 receptor activation (Jimenez et al., 2011) and resensitization of ionotropic P2X2 receptors (Chen et al., 2010) and vanilloid type 1 channels (TRPV1) in sensory neurons by P2Y2R activation (Wang et al., 2010). P2Y11R activation in glutamatergic neurons also can delay cell apoptosis by a cAMP-dependent mechanism (Vaughan et al., 2007), whereas P2Y13R activation inhibits glycogen synthase kinase-3 phosphorylation and enhances the PI3K/Akt-dependent nuclear translocation of β-catenin leading to the elevated expression of cell survival genes (Ortega et al., 2008). Activation of the P2Y13R also has been shown to induce the expression of Nrf2 and cytoprotective heme oxygenase-1 to prevent oxidative stress-induced death in neurons (Espada et al., 2010). As recently reviewed (Koles et al., 2011), activation of P2YRs can increase dopamine (Heine et al., 2007; Krugel et al., 1999; Krugel et al., 2001) and glutamate release (Price et al., 2003), but also can inhibit neurotransmitter release (De Lorenzo et al., 2006) in the cerebral cortex(Bennett and Boarder, 2000; Cunha et al., 1994; Luthardt et al., 2003; Rodrigues et al., 2005; von Kugelgen et al., 1994) and hippocampus (Csolle et al., 2008; Koch et al., 1997; Mendoza-Fernandez et al., 2000). This wide diversity in the regulatory effects on neurotransmission caused by activation of individual P2Y receptor subtypes requires further investigation, particularly in the context of neurodegenerative diseases, such as AD.
Although P2YRs, including the P2Y1 and P2Y12 receptors, are expressed in oligodendrocytes (Agresti et al., 2005; Amadio et al., 2006), little is known about the functional role of these P2Y receptors in this important neuroprotective cell type that regulates axonal myelination and other functions (Liu et al., 2013). Since the AD phenotype has been associated with oligodendrocyte dysfunction (Higuchi et al., 2005; Kohama et al., 2012; Liu et al., 2013; Rowe et al., 2007), the investigation of P2YR functions in these cells may elucidate novel pharmacological targets to enhance neuroregenerative pathways mediated by oligodendrocytes. P2Y12R expression in oligodendrocytes has been found to decrease with demyelination in cortical grey matter lesions of post-mortem multiple sclerosis brain samples (Amadio et al., 2010) and decreases in P2Y2R expression have been reported in human AD brain (Lai et al., 2008). Thus, the loss of P2YR receptor functions appears to correlate with neuronal dysfunction associated with neurodegenerative diseases and more studies are needed to determine whether approaches that promote upregulation and sustained activation of specific P2YR subtypes in neurons will lead to a slower onset of neurological deficits in AD.
Neurovascular dysregulation in AD
Evidence over the last decade suggests that vascular dysfunction in the brain plays a central role in the development of AD and is the subject of several reviews (Bell and Zlokovic, 2009; Iadecola, 2004; Kelleher and Soiza, 2013; Sagare et al., 2013; Zlokovic, 2008). Breakdown of the blood-brain barrier (BBB) is observed more frequently in AD patients than age-matched controls without AD (Claudio, 1996; Matsumoto et al., 2007) and brain-imaging techniques have revealed that there is typically a reduction in cerebral blood flow (CBF) during the development of AD (Alsop et al., 2000; Ruitenberg et al., 2005; Schuff et al., 2009; Warkentin et al., 2004). In transgenic mice overexpressing the Swedish mutation of APP, behavioral deficits and memory loss occur at 6 months of age and amyloid plaques at 9–12 months (Hsiao et al., 1996; Kawarabayashi et al., 2001; Westerman et al., 2002). However, reductions in CBF precede these events and are observed as early as 2 months of age and include disruption of cerebrovascular autoregulation and attenuation of increases in CBF caused by endothelium-dependent vasodilators (Niwa et al., 2002; Niwa et al., 2000b). These cerebrovascular effects are greater in transgenic mice that express higher brain levels of Aβ and occur in the absence of cognitive impairment or amyloid plaques (Niwa et al., 2002; Niwa et al., 2000b). Furthermore, cerebrovascular dysregulation can be reproduced in normal mice by topical application of Aβ1–40 to the neocortex (Niwa et al., 2000a). In isolated cerebral arteries from rodents and humans, Aβ causes blood vessel constriction that can be reversed by treatment with free radical scavengers, such as superoxide dismutase (SOD1) (Niwa et al., 2001; Paris et al., 2003). SOD1 treatment also reverses cerebrovascular dysfunction in mice overexpressing mutant APP (Iadecola et al., 1999), suggesting that Aβ causes vasoconstriction through production of free radicals. These observations support the hypothesis that soluble Aβ is a crucial factor in cerebrovascular dysfunction in AD that occurs early in disease progression, well before deposition of Aβ in amyloid plaques.
Hypoxia is a direct consequence of reduced CBF and is a common component of many AD risk factors, including stroke, atherosclerosis, hypertension and diabetes mellitus. In vivo studies show that exposure of mice overexpressing mutant APP to hypoxia greatly accelerates the appearance of AD symptoms and biomarkers, including memory loss and increased β-amyloid plaque deposition and brain levels of Aβ and BACE1, the secretase that is critical for the generation of Aβ (Sun et al., 2006). Furthermore, in vitro experiments show that hypoxia increases the level of hypoxia-inducible factor-1α (HIF-1α) and BACE1 in SH-SY5Y neuroblastoma cells and that the BACE1 promoter contains a hypoxia response element that binds and is activated by HIF-1α (Sun et al., 2006). Therefore, it is thought that reduced CBF and the resulting hypoxia facilitates AD pathogenesis by increasing the production of HIF-1α and HIF-1α-inducible proteins, including BACE1 (Zhang et al., 2007), VEGF (a vascular endothelial growth factor that increases vascular permeability and breakdown of the BBB) (Yeh et al., 2007), endothelin-1 (a potent vasoconstrictor) (Hisada et al., 2012), RAGE (a receptor for advanced glycation end products that transports Aβ from the bloodstream to underlying tissues) (Pichiule et al., 2007), serum response factor and myocardin (transcription factors that suppress expression of LRP1, a major transporter of Aβ from vascular cells in the brain to the bloodstream) (Bell et al., 2009; Chow et al., 2007) and PERK (an endoplasmic reticulum kinase that deactivates translation initiation factor eIF2α and represses global protein synthesis that is necessary for synaptic plasticity and memory function) (Costa-Mattioli et al., 2009; Ma et al., 2013).
Cerebrovascular effects of nucleotides and P2Y receptors
Purinergic signaling regulates vascular blood flow throughout the body and brain and has been extensively reviewed (Burnstock and Ralevic, 2014; Erlinge and Burnstock, 2008). Long ago, ATP release from active brain slices was detected (Pull and McIlwain, 1972) and shown to stimulate oxygen uptake and increase CBF (Forrester, 1978). In general, regulation of cerebrovascular tone by nucleotides (i.e., vasoconstriction versus vasodilation) depends on the nucleotide source. When ATP is released as a neurotransmitter or cotransmitter with noradrenaline from perivascular sympathetic nerves it causes vasoconstriction by activating P2X1 and P2Y2,4,6 receptors on vascular smooth muscle cells (Burnstock and Knight, 2004; Burnstock and Ralevic, 2014). When ATP and UTP are released into the vascular lumen (e.g., from endothelial cells in response to changes in blood flow induced by exercise (Farias et al., 2005; Hashimoto et al., 1999) or hypoxia (Bodin and Burnstock, 1995), from erythrocytes in response to hypoxia (Bergfeld and Forrester, 1992) or from activated platelets and immune cells (Beigi et al., 1999; Eltzschig et al., 2006; Piccini et al., 2008)) they cause vasodilation by activating P2Y1,2,4,6 receptors on endothelial cells (Burnstock and Ralevic, 2014). Studies in various vasculature systems, including the cerebral vasculature, have shown that intraluminal application of ATP or UTP (equipotent agonists of the P2Y2R) induces endothelium-dependent dilation of blood vessels and a decrease in blood pressure, which have been attributed to activation of the endothelial P2Y2R (Dietrich et al., 2009; Guns et al., 2006; Kennedy and Burnstock, 1985; Miyagi et al., 1996; Ralevic and Burnstock, 1996; Rieg et al., 2007; Rieg et al., 2011; Terada et al., 1976). P2Y1,2,4,6 receptors, which are expressed in human and rodent vascular endothelium (Guns et al., 2005; Wihlborg et al., 2003), increase blood flow through the release of NO, prostaglandins and EDHF (Buvinic et al., 2002; Guns et al., 2006; Lustig et al., 1992; Wihlborg et al., 2003).
The effects of nucleotides on cerebrovascular tone vary, however, depending on the species, size and age of blood vessel being studied. For example, in isolated human pial arteries, 10−7 to 10−5 M UTP and UDP cause transient, endothelium-dependent dilation and endothelium-independent constriction at higher concentrations (Hardebo et al., 1987), suggesting that P2Y2,4,6 receptors are involved. In human cerebral arteries denuded of endothelium, activation of the P2Y6 receptor causes vasoconstriction (Malmsjo et al., 2003). ATP- and ADP-induced dilation of arteries isolated from monkeys was endothelium-dependent in temporal arteries, but largely endothelium-independent in cerebral arteries (Toda et al., 1991). In baboon and cat cerebral arterioles, perivascular and intracarotid application of ATP increases CBF at concentrations ranging from 10−11 to 4×10−8 M (Forrester et al., 1979). In bovine middle cerebral arterial strips, UTP causes constriction when the endothelium is removed, but causes dilation via NO when the endothelium is present (Miyagi et al., 1996). ADP-induced dilation of rabbit small cerebral arteries was endothelium-dependent (Brayden, 1991). In isolated rat cerebral arterioles, microapplication of ATP causes transient constriction via smooth muscle P2X1 receptors and sustained dilation that is primarily due to endothelial P2Y receptor activation (Dietrich et al., 2009). In aging rat cerebral arteries, downregulation of P2X1 and upregulation of P2Y1 and P2Y2 receptor mRNA levels are observed in smooth muscle cells, whereas downregulation of P2Y1 and P2Y2 receptor mRNA levels are observed in endothelial cells (Miao et al., 2001), suggesting that age affects cerebrovascular responses to nucleotides. Although cerebral capillary studies are not available, capillary blood flow in the subventricular region of the mouse brain in response to UTP has been investigated. Using laser Doppler flowmetry, Lacar et al. (2012) showed that intraventricular UTP injection transiently decreases blood flow. Furthermore, they demonstrate that UTP application to acute brain slices increases calcium levels in pericytes leading to capillary constriction (Lacar et al., 2012).
A recent ex vivo study by Dietrich et al. (2010) in rat penetrating cerebral arterioles, the vessels most responsible for controlling cerebrovascular resistance, looked at the vasoconstrictive/vasodilatory effects of extraluminally applied ATP and soluble Aβ. They found that extraluminal application of ATP causes transient vasoconstriction and sustained vasodilation. Furthermore, they demonstrate that extraluminal application of Aβ causes significant vasoconstriction through a ROS-dependent mechanism and that co-application of ATP and Aβ enhances the transient ATP-induced vasoconstriction and diminishes the sustained ATP-induced vasodilation (Dietrich et al., 2010). This suggests that endothelial P2YRs play an important role in counteracting cerebral vasoconstriction and reduced CBF caused by Aβ as it begins to accumulate during the early stages of AD.
In vivo effects of the loss of P2Y2 receptor expression in an AD mouse model
Previous studies with post-mortem human AD brain tissue show decreased expression of P2Y2Rs, as compared to non-AD controls (Lai et al., 2008). Interestingly, recent studies show initial upregulation of the P2Y2R during early stage disease pathology (10–25 weeks) in TgCRND8 mice, a well-characterized AD mouse model. However, the expression of P2Y2R decreases by 25–48 weeks of age with disease progression (Ajit et al., 2013), suggesting that P2Y2R-mediated neuroprotection is available early in AD, but is lost at later stages of the disease.
Mortality
The heterozygous deletion of the P2Y2R in the TgCRND8 mouse model of AD leads to accelerated pathology and premature death (10–12 weeks) compared to TgCRND8 mice expressing the full complement of P2Y2R. Furthermore, homozygous deletion of the P2Y2R in TgCRND8 mice leads to a sharp decline in the survival rate with death occurring at 4–5 weeks of age (Ajit et al., 2013). Other studies indicate that the cellular distribution of the P2Y1R is altered in human AD brains, where the P2Y1R is localized to neurofibrillary tangles, neuropil threads and neuritic plaques (Moore et al., 2000b). However, the functional consequences of P2Y1R redistribution in AD require further investigation. Nonetheless, these observations indicate a potential beneficial role for P2YRs in the prevention of AD.
Aβ accumulation
Chronic inflammation contributes to neurodegeneration in AD (Akiyama et al., 2000; Griffin, 2006; Ho et al., 2005), however, acute inflammation is important for tissue repair and may limit brain damage (Monsonego and Weiner, 2003), in part by promoting the clearance of Aβ. Accumulation of Aβ is a characteristic feature of AD and current therapeutic strategies are targeting mechanisms that enhance the clearance of Aβ. Previous studies have shown that P2Y2R upregulation and activation enhances fibrillar Aβ1–42 uptake by microglial cells through P2Y2R interaction with αv integrins that results in activation of Rac1, a signaling protein known to regulate phagocytosis (Kim et al., 2012). These results were substantiated by heterozygous deletion of the P2Y2R in TgCRND8 mice, which accelerates accumulation of soluble Aβ in the brain as compared to age-matched TgCRND8 mice with a full complement of P2Y2R. These studies support a role for the P2Y2R in the phagocytosis and degradation of Aβ in the brain, particularly since heterozygous deletion of the P2Y2R in TgCRND8 mice did not alter levels of APP or the APP processing enzymes ADAM10, ADAM17 or BACE1. Therefore, it seems likely that increased Aβ accumulation upon P2Y2R deletion is related to the inefficient clearance of Aβ.
Microglial cell recruitment
Under neuroinflammatory conditions associated with AD, resident microglia become activated, proliferate and migrate towards and surround Aβ plaques (Bolmont et al., 2008), and blood-derived macrophages infiltrate the brain (Hao et al., 2011; Simard et al., 2006). These activated microglia are thought to play a neuroprotective role through the phagocytosis and degradation of neurotoxic forms of Aβ (Jiang et al., 2008). The crucial role of microglia in controlling Aβ levels has been demonstrated in AD mouse models and AD patients where Aβ immunization has been shown to decrease plaque load and increase microglial cell accumulation (Boche et al., 2010; Nicoll et al., 2003; Schenk et al., 1999).
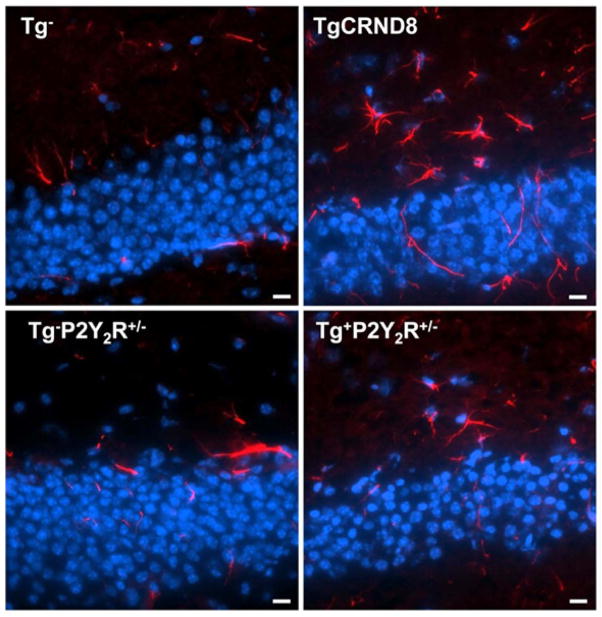
Upregulation and activation of P2Y2Rs in mouse primary microglial cells treated with oligomeric Aβ increased migration of microglial cells towards Aβ and enhanced Aβ uptake and degradation (Kim et al., 2012), a neuroprotective mechanism that could slow disease progression in vivo. Accordingly, heterozygous deletion of P2Y2Rs in TgCRND8 mice decreased the number of CD11b+ and CD45+ (Figure 1) microglial cells surrounding Aβ plaques in the brain, suggesting that a reduction in P2Y2R expression leads to inefficient microglial cell accumulation and activation that would be expected to have negative effects on nerve function (Kim et al., 2012).
Figure 1. Heterozygous knockout of the P2Y2R attenuates CD45+ microglial cell accumulation in the brains of TgCRND8 mice.
Microglial cell activation in the brains of 10-week-old TgCRND8 mice, and a non-transgenic littermate control (Tg−), TgCRND8 mice heterozygous for P2Y2R (Tg+P2Y2R+/−) and non-transgenic littermate control mice heterozygous for P2Y2R (Tg−P2Y2R+/−) were investigated by subjecting hippocampal brain sections to immunofluorescence analysis using anti-CD45 antibody (red) and Hoescht nuclear stain (blue). Results indicate a decrease in microglial cell accumulation in TgCRND8 mouse brain with deletion of the P2Y2R. Images are representative of results from 3 independent experiments and scale bar = 10 μm.
Neurological symptoms
Abnormal reflexes in limb-flexion and paw-clasping are non-specific markers of neurological deficits that have been used to evaluate neurodegeneration in AD and Huntington’s disease (Komatsu et al., 2006; Mangiarini et al., 1996). Impaired posture and gait have been previously reported even in early stages of AD (Pettersson et al., 2002).
P2Y2R deletion in TgCRND8 mice resulted in abnormal limb-flexion and paw-clasping at 10–12 weeks of age (Ajit et al., 2013). However, these symptoms were absent at this age in TgCRND8 mice with a full complement of P2Y2R, suggesting a neuroprotective role for the P2Y2R in AD. Additionally, heterozygous deletion of the P2Y2R in TgCRND8 mice results in loss of motor coordination. Gait analysis using a Noldus CatWalk system indicates that P2Y2R deletion in TgCRND8 mice reduces swing speed (i.e., the speed of the paw while not in contact with the walkway) and stride length (i.e., the distance between successive paw placements), as compared to age-matched TgCRND8 mice. Additionally, the regularity index, a measure of interpaw coordination defined as the number of normal step sequence patterns relative to the number of paw placements, was significantly decreased with heterozygous deletion of the P2Y2R in TgCRND8 mice. These results strongly support the hypothesis that the P2Y2R plays a neuroprotective function in the TgCRND8 mouse model of AD and encourage studies to assess the effects of the deletion of other P2YR subtypes in animal models of neurodegenerative diseases, including AD.
CONCLUSIONS
G protein-coupled P2Y nucleotide receptors play a role in neuroinflammation, cerebral blood flow and the progression of many neurodegenerative diseases, including AD, as described in this review. All eight known P2Y receptor subtypes are expressed in the brain where they regulate a variety of neuronal, neuroinflammatory and neurovascular functions, including non-amyloidogenic APP processing, the production of cytokines and chemokines, the migration of microglia, microglial cell-mediated endocytosis and degradation of neurotoxic Aβ, responses to oxidative stress, axonal outgrowth and neurite extension in neurons, the regulation of neurotransmission, and the endothelium-dependent dilation of cerebral blood vessels. Aβ can increase the release of nucleotides from cells and stimulate the upregulation of P2Y receptors. Overall, the collective results from numerous studies suggest that P2YRs can regulate neuroprotective effects, particularly under neuroinflammatory conditions. Therefore, there is considerable interest in targeting P2YRs to prevent the progression of neurodegenerative diseases, such as AD. Interestingly, deletion of the P2Y2R in a mouse model of AD accelerates mortality, enhances neurological deficits and Aβ accumulation in the brain and decreases the migration of microglial cells to Aβ plaques (pathways coupled to P2Y2R activation in the brain are shown in Figure 2). Similarly, reduced P2Y2R expression has been reported in post-mortem brain samples from AD patients, as compared to normal controls, suggesting that loss of P2Y2R expression in humans correlates with the AD phenotype. These findings encourage further research to evaluate the contribution of each P2Y receptor subtype to AD progression as a means to develop novel therapeutic approaches to delay the onset and retard pathological manifestations of this debilitating disease that is anticipated to impact 50 million people worldwide within the next few decades.
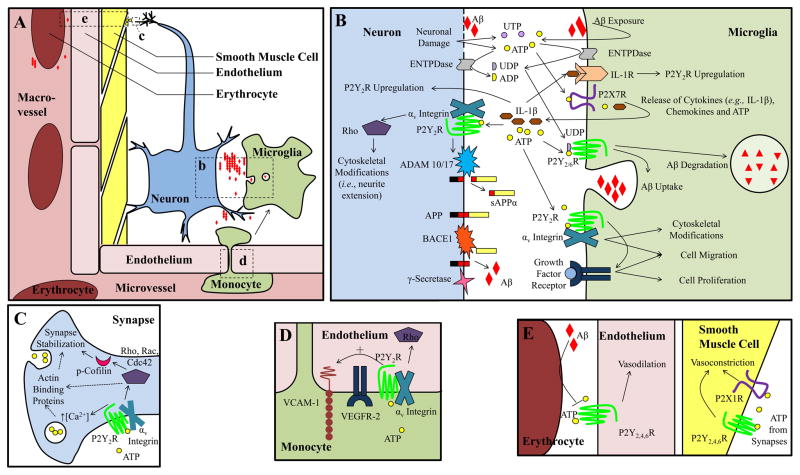
Figure 2. P2Y receptor function in AD.
A. Overview of cell types involved in neuroinflammation and the neurovascular unit. Areas b–e in panel A are magnified in panels B–E. Although intimately involved, neuron-associated astrocytes and oligodendrocytes and microvessel-associated pericytes are not shown for simplicity. Aβ alters cellular release of nucleotides, including increased ATP release from microglia and decreased ATP release from hypoxic erythrocytes. In perivascular neurons, ATP is released from synaptic vesicles and causes vasoconstriction by activating P2X1 and P2Y2,4,6 receptors on vascular smooth muscle cells. P2Y1,2,4,6 receptors in vascular endothelial cells promote vasodilation by responding to ATP in the blood stream. The endothelial P2Y2R facilitates monocyte adhesion to the vascular wall, through VEGFR-2-dependent upregulation of vascular cell adhesion molecule-1 (VCAM-1) as well as monocyte extravasation. Activation of neuronal P2Y1,2 receptors promote neurite extension and stabilization, whereas P2Y13R inhibits neurite extension. Neuronal P2Y2R facilitates non-amyloidogenic processing of APP through ADAM10/17-dependent production of soluble APPα (sAPPα). P2Y1,12,13 receptors enhance glycine transport in the synaptic cleft and P2Y11R activation in glutamatergic neurons delays apoptosis. P2YRs increase dopamine and glutamate release, but can also inhibit neurotransmitter release in the cerebral cortex and hippocampus. In microglia, P2Y1,2,6,12,13 receptor activation increases microglial cell migration and P2Y2,6 receptors promote Aβ uptake and degradation. In astrocytes, P2Y1,6 receptors stimulate the production of proinflammatory cytokines and chemokines, P2Y2,4 receptors increase the production and secretion of APP and P2Y14R increases expression of MMP9, which degrades Aβ.
Acknowledgments
Funding
This work was supported by research grants from the National Institute of Health (NIH grants AG018357 and HL088228) and the BrightFocus Foundation for Alzheimer’s disease research (grant A2013171S).
Abbreviations used
- Aβ
beta-amyloid peptide
- AD
Alzheimer’s disease
- ADAM
a disintegrin and metalloprotease
- APP
amyloid precursor protein
- BACE1
β-secretase
- BBB
blood-brain barrier
- CBF
cerebral blood flow
- CNS
central nervous system
- EDHF
endothelium-derived hyperpolarizing factor
- ERK
extracellular signal-regulated kinase
- fAβ
fibrillar Aβ
- HIF-1α
hypoxia-inducible factor-1α
- HO-1
heme oxygenase-1
- IL
interleukin
- MCAO
middle cerebral artery occlusion
- MCP-1
monocyte chemoattrant protein-1
- MMP-9
matrix metalloprotease-9
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- NOX
NADPH oxidase
- oAβ
oligomeric Aβ
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PTX
pertussis toxin
- ROS
reactive oxygen species
- SOD1
superoxide dismutase
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of interest
The authors have declared no conflict of interest.
References
- Agresti C, Meomartini ME, Amadio S, Ambrosini E, Volonte C, Aloisi F, Visentin S. ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res Brain Res Rev. 2005;48:157–165. doi: 10.1016/j.brainresrev.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4:367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Ajit D, Woods LT, Camden JM, Thebeau CN, El-Sayed FG, Greeson GW, Erb L, Petris MJ, Miller DC, Sun GY, Weisman GA. Loss of P2Y Nucleotide Receptors Enhances Early Pathology in the TgCRND8 Mouse Model of Alzheimer’s Disease. Mol Neurobiol. 2013;49:1031–1042. doi: 10.1007/s12035-013-8577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol. 2000;47:93–100. [PubMed] [Google Scholar]
- Amadio S, Montilli C, Magliozzi R, Bernardi G, Reynolds R, Volonte C. P2Y12 receptor protein in cortical gray matter lesions in multiple sclerosis. Cereb Cortex. 2010;20:1263–1273. doi: 10.1093/cercor/bhp193. [DOI] [PubMed] [Google Scholar]
- Amadio S, Montilli C, Picconi B, Calabresi P, Volonte C. Mapping P2X and P2Y receptor proteins in striatum and substantia nigra: An immunohistological study. Purinergic Signal. 2007;3:389–398. doi: 10.1007/s11302-007-9069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadio S, Tramini G, Martorana A, Viscomi MT, Sancesario G, Bernardi G, Volonte C. Oligodendrocytes express P2Y12 metabotropic receptor in adult rat brain. Neurosci. 2006;141:1171–1180. doi: 10.1016/j.neuroscience.2006.05.058. [DOI] [PubMed] [Google Scholar]
- Arthur DB, Akassoglou K, Insel PA. P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci USA. 2005;102:19138–19143. doi: 10.1073/pnas.0505913102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom JR, Lim GP, Cullen MJ, Tokes ZA. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1–40) J Neurosci. 1996;16:7910–7919. doi: 10.1523/JNEUROSCI.16-24-07910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi S, Liao Z, Gonzalez FA, Chorna NE, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor interacts with alphav integrins to activate Go and induce cell migration. J Biol Chem. 2005;280:39050–39057. doi: 10.1074/jbc.M504819200. [DOI] [PubMed] [Google Scholar]
- Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone E, Di Domenico F, Sultana R, Coccia R, Mancuso C, Perluigi M, Butterfield DA. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic Biol Med. 2012;52:2292–2301. doi: 10.1016/j.freeradbiomed.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigi R, Kobatake E, Aizawa M, Dubyak GR. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol. 1999;276:C267–278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, Streb JW, Guo H, Rubio A, Van Nostrand W, et al. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Yebdri F, Kukulski F, Tremblay A, Sevigny J. Concomitant activation of P2Y(2) and P2Y(6) receptors on monocytes is required for TLR1/2-induced neutrophil migration by regulating IL-8 secretion. Euro J Immunol. 2009;39:2885–2894. doi: 10.1002/eji.200939347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GC, Boarder MR. The effect of nucleotides and adenosine on stimulus-evoked glutamate release from rat brain cortical slices. Br J Pharmacol. 2000;131:617–623. doi: 10.1038/sj.bjp.0703598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- Boche D, Denham N, Holmes C, Nicoll JA. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer’s disease pathogenesis. Acta Neuropathol. 2010;120:369–384. doi: 10.1007/s00401-010-0719-5. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Synergistic effect of acute hypoxia on flow-induced release of ATP from cultured endothelial cells. Experientia. 1995;51:256–259. doi: 10.1007/BF01931108. [DOI] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden JE. Hyperpolarization and relaxation of resistance arteries in response to adenosine diphosphate. Distribution and mechanism of action. Circ Res. 1991;69:1415–1420. doi: 10.1161/01.res.69.5.1415. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Gupta S, Parrino TE, Knight AG, Ebenezer PJ, Weidner AM, LeVine H, 3rd, Keller JN, Markesbery WR. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010;12:1371–1382. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos M, Neary JT, Gonzalez FA. P2Y2 nucleotide receptors inhibit trauma-induced death of astrocytic cells. J Neurochem. 2007;103:1785–1800. doi: 10.1111/j.1471-4159.2007.04872.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacological Reviews. 2014;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- Buvinic S, Briones R, Huidobro-Toro JP. P2Y(1) and P2Y(2) receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol. 2002;136:847–856. doi: 10.1038/sj.bjp.0704789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacabelos R, Alvarez XA, Fernandez-Novoa L, Franco A, Mangues R, Pellicer A, Nishimura T. Brain interleukin-1 beta in Alzheimer’s disease and vascular dementia. Methods Find Exp Clin Pharmacol. 1994;16:141–151. [PubMed] [Google Scholar]
- Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Calvert JA, Atterbury-Thomas AE, Leon C, Forsythe ID, Gachet C, Evans RJ. Evidence for P2Y1, P2Y2, P2Y6 and atypical UTP-sensitive receptors coupled to rises in intracellular calcium in mouse cultured superior cervical ganglion neurons and glia. Br J Pharmacol. 2004;143:525–532. doi: 10.1038/sj.bjp.0705959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camden JM, Schrader AM, Camden RE, Gonzalez FA, Erb L, Seye CI, Weisman GA. P2Y2 nucleotide receptors enhance alpha-secretase-dependent amyloid precursor protein processing. J Biol Chem. 2005;280:18696–18702. doi: 10.1074/jbc.M500219200. [DOI] [PubMed] [Google Scholar]
- Chakfe Y, Seguin R, Antel JP, Morissette C, Malo D, Henderson D, Seguela P. ADP and AMP induce interleukin-1beta release from microglial cells through activation of ATPprimed P2X7 receptor channels. J Neurosci. 2002;22:3061–3069. doi: 10.1523/JNEUROSCI.22-08-03061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Molliver DC, Gebhart GF. The P2Y2 receptor sensitizes mouse bladder sensory neurons and facilitates purinergic currents. J Neurosci. 2010;30:2365–2372. doi: 10.1523/JNEUROSCI.5462-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin Y, Kishi M, Sekino M, Nakajo F, Abe Y, Terazono Y, Hiroyuki O, Kato F, Koizumi S, Gachet C, Hisatsune T. Involvement of glial P2Y(1) receptors in cognitive deficit after focal cerebral stroke in a rodent model. J Neuroinflammation. 2013;10:95. doi: 10.1186/1742-2094-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27:4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorna NE, Santiago-Perez LI, Erb L, Seye CI, Neary JT, Sun GY, Weisman GA, Gonzalez FA. P2Y receptors activate neuroprotective mechanisms in astrocytic cells. J Neuroschem. 2004;91:119–132. doi: 10.1111/j.1471-4159.2004.02699.x. [DOI] [PubMed] [Google Scholar]
- Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van Nostrand W, Miano JM, Zlokovic BV. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci USA. 2007;104:823–828. doi: 10.1073/pnas.0608251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio L. Ultrastructural features of the blood-brain barrier in biopsy tissue from Alzheimer’s disease patients. Acta Neuropathol. 1996;91:6–14. doi: 10.1007/s004010050386. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csolle C, Heinrich A, Kittel A, Sperlagh B. P2Y receptor mediated inhibitory modulation of noradrenaline release in response to electrical field stimulation and ischemic conditions in superfused rat hippocampus slices. J Neurochem. 2008;106:347–360. doi: 10.1111/j.1471-4159.2008.05391.x. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Ribeiro JA, Sebastiao AM. Purinergic modulation of the evoked release of [3H]acetylcholine from the hippocampus and cerebral cortex of the rat: role of the ectonucleotidases. Eur J Neurosci. 1994;6:33–42. doi: 10.1111/j.1460-9568.1994.tb00245.x. [DOI] [PubMed] [Google Scholar]
- D’Alimonte I, Ciccarelli R, Di Iorio P, Nargi E, Buccella S, Giuliani P, Rathbone MP, Jiang S, Caciagli F, Ballerini P. Activation of P2X(7) receptors stimulates the expression of P2Y(2) receptor mRNA in astrocytes cultured from rat brain. Int J Immunopathol Pharmacol. 2007;20:301–316. doi: 10.1177/039463200702000210. [DOI] [PubMed] [Google Scholar]
- D’Ambrosi N, Iafrate M, Saba E, Rosa P, Volonte C. Comparative analysis of P2Y4 and P2Y6 receptor architecture in native and transfected neuronal systems. Biochim Biophys Acta. 2007;1768:1592–1599. doi: 10.1016/j.bbamem.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- De Lorenzo S, Veggetti M, Muchnik S, Losavio A. Presynaptic inhibition of spontaneous acetylcholine release mediated by P2Y receptors at the mouse neuromuscular junction. Neuroscience. 2006;142:71–85. doi: 10.1016/j.neuroscience.2006.05.062. [DOI] [PubMed] [Google Scholar]
- del Puerto A, Diaz-Hernandez JI, Tapia M, Gomez-Villafuertes R, Benitez MJ, Zhang J, Miras-Portugal MT, Wandosell F, Diaz-Hernandez M, Garrido JJ. Adenylate cyclase 5 coordinates the action of ADP, P2Y1, P2Y13 and ATP-gated P2X7 receptors on axonal elongation. J Cell Sci. 2012;125:176–188. doi: 10.1242/jcs.091736. [DOI] [PubMed] [Google Scholar]
- Deuss M, Reiss K, Hartmann D. Part-time alpha-secretases: the functional biology of ADAM 9, 10 and 17. Curr Alzheimer Res. 2008;5:187–201. doi: 10.2174/156720508783954686. [DOI] [PubMed] [Google Scholar]
- Dietrich HH, Horiuchi T, Xiang C, Hongo K, Falck JR, Dacey RG., Jr Mechanism of ATP-induced local and conducted vasomotor responses in isolated rat cerebral penetrating arterioles. J Vascular Res. 2009;46:253–264. doi: 10.1159/000167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich HH, Xiang C, Han BH, Zipfel GJ, Holtzman DM. Soluble amyloid-beta, effect on cerebral arteriolar regulation and vascular cells. Mol Neurodegener. 2010;5:15. doi: 10.1186/1750-1326-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004;113:340–345. doi: 10.1172/JCI20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- Endo M, Ohashi K, Mizuno K. LIM kinase and slingshot are critical for neurite extension. J Biol Chem. 2007;282:13692–13702. doi: 10.1074/jbc.M610873200. [DOI] [PubMed] [Google Scholar]
- Endo M, Ohashi K, Sasaki Y, Goshima Y, Niwa R, Uemura T, Mizuno K. Control of growth cone motility and morphology by LIM kinase and Slingshot via phosphorylation and dephosphorylation of cofilin. J Neurosci. 2003;23:2527–2537. doi: 10.1523/JNEUROSCI.23-07-02527.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espada S, Ortega F, Molina-Jijon E, Rojo AI, Perez-Sen R, Pedraza-Chaverri J, Miras-Portugal MT, Cuadrado A. The purinergic P2Y(13) receptor activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Free Radic Biol Med. 2010;49:416–426. doi: 10.1016/j.freeradbiomed.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Farias M, 3rd, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol. 2005;288:H1586–1590. doi: 10.1152/ajpheart.00983.2004. [DOI] [PubMed] [Google Scholar]
- Fiala M, Zhang L, Gan X, Sherry B, Taub D, Graves MC, Hama S, Way D, Weinand M, Witte M, et al. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood--brain barrier model. Mol Med. 1998;4:480–489. [PMC free article] [PubMed] [Google Scholar]
- Figge C, Loers G, Schachner M, Tilling T. Neurite outgrowth triggered by the cell adhesion molecule L1 requires activation and inactivation of the cytoskeletal protein cofilin. Mol Cell Neurosci. 2012;49:196–204. doi: 10.1016/j.mcn.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Forrester T. Extracellular nucleotides in exercise: possible effect on brain metabolism. J Physiol (Paris) 1978;74:477–483. [PubMed] [Google Scholar]
- Forrester T, Harper AM, MacKenzie ET, Thomson EM. Effect of adenosine triphosphate and some derivatives on cerebral blood flow and metabolism. J Physiol. 1979;296:343–355. doi: 10.1113/jphysiol.1979.sp013009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Krugel U, Grosche J, Heine C, Hartig W, Allgaier C, Illes P. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2004;127:431–441. doi: 10.1016/j.neuroscience.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Tozaki-Saitoh H, Inoue K. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia. 2009;57:244–257. doi: 10.1002/glia.20749. [DOI] [PubMed] [Google Scholar]
- Griffin WS. Inflammation and neurodegenerative diseases. Am J Clin Nutr. 2006;83:470S–474S. doi: 10.1093/ajcn/83.2.470S. [DOI] [PubMed] [Google Scholar]
- Guns PJ, Korda A, Crauwels HM, Van Assche T, Robaye B, Boeynaems JM, Bult H. Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol. 2005;146:288–295. doi: 10.1038/sj.bjp.0706326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guns PJ, Van Assche T, Fransen P, Robaye B, Boeynaems JM, Bult H. Endothelium-dependent relaxation evoked by ATP and UTP in the aorta of P2Y2-deficient mice. Br J Pharmacol. 2006;147:569–574. doi: 10.1038/sj.bjp.0706642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hao W, Liu Y, Liu S, Walter S, Grimm MO, Kiliaan AJ, Penke B, Hartmann T, Rube CE, Menger MD, Fassbender K. Myeloid differentiation factor 88-deficient bone marrow cells improve Alzheimer’s disease-related symptoms and pathology. Brain. 2011;134:278–292. doi: 10.1093/brain/awq325. [DOI] [PubMed] [Google Scholar]
- Hardebo JE, Kahrstrom J, Owman C, Salford LG. Endothelium-dependent relaxation by uridine tri- and diphosphate in isolated human pial vessels. Blood Vessels. 1987;24:150–155. doi: 10.1159/000158690. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Shinozuka K, Tanabe Y, Gamoh S, Hara T, Hossain MS, Kwon YM, Kunitomo M, Masumura S. Hypotension induced by exercise is associated with enhanced release of adenyl purines from aged rat artery. Am J Physiol. 1999;276:H970–975. doi: 10.1152/ajpheart.1999.276.3.H970. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Heine C, Wegner A, Grosche J, Allgaier C, Illes P, Franke H. P2 receptor expression in the dopaminergic system of the rat brain during development. Neuroscience. 2007;149:165–181. doi: 10.1016/j.neuroscience.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Hernandez-Guillamon M, Delgado P, Ortega L, Pares M, Rosell A, Garcia-Bonilla L, Fernandez-Cadenas I, Borrell-Pages M, Boada M, Montaner J. Neuronal TIMP-1 release accompanies astrocytic MMP-9 secretion and enhances astrocyte proliferation induced by beta-amyloid 25–35 fragment. J Neurosci Res. 2009;87:2115–2125. doi: 10.1002/jnr.22034. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Zhang B, Forman MS, Yoshiyama Y, Trojanowski JQ, Lee VM. Axonal degeneration induced by targeted expression of mutant human tau in oligodendrocytes of transgenic mice that model glial tauopathies. J Neurosci. 2005;25:9434–9443. doi: 10.1523/JNEUROSCI.2691-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada T, Ayaori M, Ohrui N, Nakashima H, Nakaya K, Uto-Kondo H, Yakushiji E, Takiguchi S, Terao Y, Miyamoto Y, et al. Statin inhibits hypoxia-induced endothelin-1 via accelerated degradation of HIF-1alpha in vascular smooth muscle cells. Cardiovasc Res. 2012;95:251–259. doi: 10.1093/cvr/cvs110. [DOI] [PubMed] [Google Scholar]
- Ho GJ, Drego R, Hakimian E, Masliah E. Mechanisms of cell signaling and inflammation in Alzheimer’s disease. Curr Drug Targets Inflamm Allergy. 2005;4:247–256. doi: 10.2174/1568010053586237. [DOI] [PubMed] [Google Scholar]
- Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- Jantzen HM, Gousset L, Bhaskar V, Vincent D, Tai A, Reynolds EE, Conley PB. Evidence for two distinct G-protein-coupled ADP receptors mediating platelet activation. Thromb Haemost. 1999;81:111–117. [PubMed] [Google Scholar]
- Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez E, Zafra F, Perez-Sen R, Delicado EG, Miras-Portugal MT, Aragon C, Lopez-Corcuera B. P2Y purinergic regulation of the glycine neurotransmitter transporters. J Biol Chem. 2011;286:10712–10724. doi: 10.1074/jbc.M110.167056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, Soiza RL. Evidence of endothelial dysfunction in the development of Alzheimer’s disease: Is Alzheimer’s a vascular disorder? Am J Cardiovasc Dis. 2013;3:197–226. [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Burnstock G. ATP produces vasodilation via P1 purinoceptors and vasoconstriction via P2 purinoceptors in the isolated rabbit central ear artery. Blood Vessels. 1985;22:145–155. doi: 10.1159/000158592. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Qi AD, Herold CL, Harden TK, Nicholas RA. ATP, an agonist at the rat P2Y(4) receptor, is an antagonist at the human P2Y(4) receptor. Mol Pharmacol. 2000;57:926–931. [PubMed] [Google Scholar]
- Kim B, Jeong HK, Kim JH, Lee SY, Jou I, Joe EH. Uridine 5′-diphosphate induces chemokine expression in microglia and astrocytes through activation of the P2Y6 receptor. J Immunol. 2011;186:3701–3709. doi: 10.4049/jimmunol.1000212. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Ajit D, Peterson TS, Wang Y, Camden JM, Gibson Wood W, Sun GY, Erb L, Petris M, Weisman GA. Nucleotides released from Abeta(1–42) -treated microglial cells increase cell migration and Abeta(1–42) uptake through P2Y(2) receptor activation. J Neurochem. 2012;121:228–238. doi: 10.1111/j.1471-4159.2012.07700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Nasu-Tada K, Fujishita K, Sato K, Koizumi S. Secretion of matrix metalloproteinase-9 from astrocytes by inhibition of tonic P2Y14-receptor-mediated signal(s) Cell Mol Neurobiol. 2013;33:47–58. doi: 10.1007/s10571-012-9869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, McGeer EG, McGeer PL. Therapeutic approaches to inflammation in neurodegenerative disease. Curr Opin Neurol. 2007;20:351–357. doi: 10.1097/WCO.0b013e3280adc943. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498:443–454. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- Koch H, von Kugelgen I, Starke K. P2-receptor-mediated inhibition of noradrenaline release in the rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:707–715. doi: 10.1007/pl00005003. [DOI] [PubMed] [Google Scholar]
- Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci. 2004;24:9838–9846. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama SG, Rosene DL, Sherman LS. Age-related changes in human and non-human primate white matter: from myelination disturbances to cognitive decline. Age. 2012;34:1093–1110. doi: 10.1007/s11357-011-9357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koles L, Leichsenring A, Rubini P, Illes P. P2 receptor signaling in neurons and glial cells of the central nervous system. Adv Pharmacol. 2011;61:441–493. doi: 10.1016/B978-0-12-385526-8.00014-X. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kong Q, Peterson TS, Baker O, Stanley E, Camden J, Seye CI, Erb L, Simonyi A, Wood WG, Sun GY, Weisman GA. Interleukin-1beta enhances nucleotide-induced and alpha-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y(2) receptor. J Neurochem. 2009;109:1300–1310. doi: 10.1111/j.1471-4159.2009.06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba M, Apasov S, Sverdlov V, Chen P, Erb L, Turner JT, Weisman GA, Sitkovsky MV. Transient up-regulation of P2Y2 nucleotide receptor mRNA expression is an immediate early gene response in activated thymocytes. Proc Natl Acad Sci USA. 1997;94:831–836. doi: 10.1073/pnas.94.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugel U, Kittner H, Illes P. Adenosine 5′-triphosphate-induced dopamine release in the rat nucleus accumbens in vivo. Neurosci Lett. 1999;265:49–52. doi: 10.1016/s0304-3940(99)00206-2. [DOI] [PubMed] [Google Scholar]
- Krugel U, Kittner H, Illes P. Mechanisms of adenosine 5′-triphosphate-induced dopamine release in the rat nucleus accumbens in vivo. Synapse. 2001;39:222–232. doi: 10.1002/1098-2396(20010301)39:3<222::AID-SYN1003>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Kuboyama K, Harada H, Tozaki-Saitoh H, Tsuda M, Ushijima K, Inoue K. Astrocytic P2Y(1) receptor is involved in the regulation of cytokine/chemokine transcription and cerebral damage in a rat model of cerebral ischemia. J Cereb Blood Flow Metab. 2011;31:1930–1941. doi: 10.1038/jcbfm.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn PH, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, Rossner S, Lichtenthaler SF. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010;29:3020–3032. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacar B, Herman P, Platel JC, Kubera C, Hyder F, Bordey A. Neural progenitor cells regulate capillary blood flow in the postnatal subventricular zone. J Neurosci. 2012;32:16435–16448. doi: 10.1523/JNEUROSCI.1457-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MK, Tan MG, Kirvell S, Hobbs C, Lee J, Esiri MM, Chen CP, Francis PT. Selective loss of P2Y2 nucleotide receptor immunoreactivity is associated with Alzheimer’s disease neuropathology. J Neural Transm. 2008a;115:1165–1172. doi: 10.1007/s00702-008-0067-y. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Han SB, Nam SY, Oh KW, Hong JT. Inflammation and Alzheimer’s disease. Arch Pharm Res. 2010;33:1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- Leon-Otegui M, Gomez-Villafuertes R, Diaz-Hernandez JI, Diaz-Hernandez M, Miras-Portugal MT, Gualix J. Opposite effects of P2X7 and P2Y2 nucleotide receptors on alphasecretase-dependent APP processing in Neuro-2a cells. FEBS Lett. 2011;585:2255–2262. doi: 10.1016/j.febslet.2011.05.048. [DOI] [PubMed] [Google Scholar]
- Li HQ, Chen C, Dou Y, Wu HJ, Liu YJ, Lou HF, Zhang JM, Li XM, Wang H, Duan S. P2Y4 receptor-mediated pinocytosis contributes to amyloid beta-induced self-uptake by microglia. Mol Cell Biol. 2013;33:4282–4293. doi: 10.1128/MCB.00544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler SF. Alpha-secretase cleavage of the amyloid precursor protein: proteolysis regulated by signaling pathways and protein trafficking. Curr Alzheimer Res. 2012;9:165–177. doi: 10.2174/156720512799361655. [DOI] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Rossie S, Gong CX. Dephosphorylation of tau by protein phosphatase 5: impairment in Alzheimer’s disease. J Biol Chem. 2005;280:1790–1796. doi: 10.1074/jbc.M410775200. [DOI] [PubMed] [Google Scholar]
- Liu XB, Shen Y, Plane JM, Deng W. Vulnerability of premyelinating oligodendrocytes to white-matter damage in neonatal brain injury. Neurosci Bull. 2013;29:229–238. doi: 10.1007/s12264-013-1311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzl S, Albers DS, Relkin N, Ngyuen T, Hilgenberg SL, Chirichigno J, Cudkowicz ME, Beal MF. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem Int. 2003;43:191–196. doi: 10.1016/s0197-0186(03)00004-4. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Erb L, Landis DM, Hicks-Taylor CS, Zhang X, Sportiello MG, Weisman GA. Mechanisms by which extracellular ATP and UTP stimulate the release of prostacyclin from bovine pulmonary artery endothelial cells. Biochim Biophys Acta. 1992;1134:61–72. doi: 10.1016/0167-4889(92)90028-a. [DOI] [PubMed] [Google Scholar]
- Luthardt J, Borvendeg SJ, Sperlagh B, Poelchen W, Wirkner K, Illes P. P2Y(1) receptor activation inhibits NMDA receptor-channels in layer V pyramidal neurons of the rat prefrontal and parietal cortex. Neurochem Int. 2003;42:161–172. doi: 10.1016/s0197-0186(02)00069-4. [DOI] [PubMed] [Google Scholar]
- Ma T, Trinh MA, Wexler AJ, Bourbon C, Gatti E, Pierre P, Cavener DR, Klann E. Suppression of eIF2alpha kinases alleviates Alzheimer’s disease-related plasticity and memory deficits. Nat Neurosci. 2013;16:1299–1305. doi: 10.1038/nn.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Molliver DC. Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Molecular Pain. 2010;6:21. doi: 10.1186/1744-8069-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmsjo M, Hou M, Pendergast W, Erlinge D, Edvinsson L. Potent P2Y6 receptor mediated contractions in human cerebral arteries. BMC Pharmacol. 2003;3:4. doi: 10.1186/1471-2210-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Mao P, Reddy PH. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: implications for early intervention and therapeutics. Biochim Biophys Acta. 2011;1812:1359–1370. doi: 10.1016/j.bbadis.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Yanase D, Noguchi-Shinohara M, Ono K, Yoshita M, Yamada M. Blood-brain barrier permeability correlates with medial temporal lobe atrophy but not with amyloid-beta protein transport across the blood-brain barrier in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;23:241–245. doi: 10.1159/000100019. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006239. doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, Klegeris A, McGeer PL. Inflammation, the complement system and the diseases of aging. Neurobiol Aging. 2005;26(Suppl 1):94–97. doi: 10.1016/j.neurobiolaging.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Mead EL, Mosley A, Eaton S, Dobson L, Heales SJ, Pocock JM. Microglial neurotransmitter receptors trigger superoxide production in microglia; consequences for microglial-neuronal interactions. J Neurochem. 2012;121:287–301. doi: 10.1111/j.1471-4159.2012.07659.x. [DOI] [PubMed] [Google Scholar]
- Meberg PJ, Bamburg JR. Increase in neurite outgrowth mediated by overexpression of actin depolymerizing factor. J Neurosci. 2000;20:2459–2469. doi: 10.1523/JNEUROSCI.20-07-02459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meberg PJ, Ono S, Minamide LS, Takahashi M, Bamburg JR. Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil Cytoskeleton. 1998;39:172–190. doi: 10.1002/(SICI)1097-0169(1998)39:2<172::AID-CM8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Mendoza-Fernandez V, Andrew RD, Barajas-Lopez C. ATP inhibits glutamate synaptic release by acting at P2Y receptors in pyramidal neurons of hippocampal slices. J Pharmacol Exp Ther. 2000;293:172–179. [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao LY, Tang JP, Esposito DP, Zhang JH. Age-related changes in P2 receptor mRNA of rat cerebral arteries. Exp Gerontol. 2001;37:67–79. doi: 10.1016/s0531-5565(01)00159-0. [DOI] [PubMed] [Google Scholar]
- Mingam R, De Smedt V, Amedee T, Bluthe RM, Kelley KW, Dantzer R, Laye S. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 beta in the murine brain. Brain Behav Immun. 2008;22:234–244. doi: 10.1016/j.bbi.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Braun N, Shukla V, Fullgrabe M, Schomerus C, Korf HW, Gachet C, Ikehara Y, Sevigny J, Robson SC, Zimmermann H. Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development. 2006;133:675–684. doi: 10.1242/dev.02233. [DOI] [PubMed] [Google Scholar]
- Misiti F, Orsini F, Clementi ME, Masala D, Tellone E, Galtieri A, Giardina B. Amyloid peptide inhibits ATP release from human erythrocytes. Biochem Cell Biol. 2008;86:501–508. doi: 10.1139/O08-139. [DOI] [PubMed] [Google Scholar]
- Miyagi Y, Kobayashi S, Nishimura J, Fukui M, Kanaide H. Dual regulation of cerebrovascular tone by UTP: P2U receptor-mediated contraction and endothelium-dependent relaxation. Br J Pharmacol. 1996;118:847–856. doi: 10.1111/j.1476-5381.1996.tb15477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi H, Takuma K, Fukuzaki E, Ibi D, Someya E, Akazawa KH, Alkam T, Tsunekawa H, Mouri A, Noda Y, et al. Matrix metalloprotease-9 inhibition improves amyloid beta-mediated cognitive impairment and neurotoxicity in mice. J Pharmacol Exp Ther. 2009;331:14–22. doi: 10.1124/jpet.109.154724. [DOI] [PubMed] [Google Scholar]
- Monsonego A, Weiner HL. Immunotherapeutic approaches to Alzheimer’s disease. Science. 2003;302:834–838. doi: 10.1126/science.1088469. [DOI] [PubMed] [Google Scholar]
- Moore D, Chambers J, Waldvogel H, Faull R, Emson P. Regional and cellular distribution of the P2Y(1) purinergic receptor in the human brain: striking neuronal localisation. J Comp Neurol. 2000a;421:374–384. doi: 10.1002/(sici)1096-9861(20000605)421:3<374::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Moore D, Iritani S, Chambers J, Emson P. Immunohistochemical localization of the P2Y1 purinergic receptor in Alzheimer’s disease. Neuroreport. 2000b;11:3799–3803. doi: 10.1097/00001756-200011270-00041. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Chambers JK, Wahlin JP, Tan KB, Moore GB, Jenkins O, Emson PC, Murdock PR. Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription-polymerase chain reaction study. Biochim Biophys Acta. 2001;1521:107–119. doi: 10.1016/s0167-4781(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Moran-Jimenez MJ, Matute C. Immunohistochemical localization of the P2Y(1) purinergic receptor in neurons and glial cells of the central nervous system. Brain Res Mol Brain Res. 2000;78:50–58. doi: 10.1016/s0169-328x(00)00067-x. [DOI] [PubMed] [Google Scholar]
- Nasu-Tada K, Koizumi S, Inoue K. Involvement of beta1 integrin in microglial chemotaxis and proliferation on fibronectin: different regulations by ADP through PKA. Glia. 2005;52:98–107. doi: 10.1002/glia.20224. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci. 2003;23:2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Niwa K, Carlson GA, Iadecola C. Exogenous A beta1–40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000a;20:1659–1668. doi: 10.1097/00004647-200012000-00005. [DOI] [PubMed] [Google Scholar]
- Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Ashe KH, Carlson GA, Iadecola C. Abeta 1–40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci USA. 2000b;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, Porter VA, Kazama K, Cornfield D, Carlson GA, Iadecola C. A beta-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol Heart Circ Physiol. 2001;281:H2417–2424. doi: 10.1152/ajpheart.2001.281.6.H2417. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002;283:H315–323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Ann Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obulesu M, Venu R, Somashekhar R. Tau mediated neurodegeneration: an insight into Alzheimer’s disease pathology. Neurochem Res. 2011;36:1329–1335. doi: 10.1007/s11064-011-0475-5. [DOI] [PubMed] [Google Scholar]
- Ortega F, Perez-Sen R, Miras-Portugal MT. Gi-coupled P2Y-ADP receptor mediates GSK-3 phosphorylation and beta-catenin nuclear translocation in granule neurons. J Neurochem. 2008;104:62–73. doi: 10.1111/j.1471-4159.2007.05021.x. [DOI] [PubMed] [Google Scholar]
- Paris D, Humphrey J, Quadros A, Patel N, Crescentini R, Crawford F, Mullan M. Vasoactive effects of A beta in isolated human cerebrovessels and in a transgenic mouse model of Alzheimer’s disease: role of inflammation. Neurol Res. 2003;25:642–651. doi: 10.1179/016164103101201940. [DOI] [PubMed] [Google Scholar]
- Perlmutter LS, Barron E, Chui HC. Morphologic association between microglia and senile plaque amyloid in Alzheimer’s disease. Neurosci Lett. 1990;119:32–36. doi: 10.1016/0304-3940(90)90748-x. [DOI] [PubMed] [Google Scholar]
- Peterson TS, Camden JM, Wang Y, Seye CI, Wood WG, Sun GY, Erb L, Petris MJ, Weisman GA. P2Y2 nucleotide receptor-mediated responses in brain cells. Mol Neurobiol. 2010;41:356–366. doi: 10.1007/s12035-010-8115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TS, Thebeau CN, Ajit D, Camden JM, Woods LT, Wood WG, Petris MJ, Sun GY, Erb L, Weisman GA. Up-regulation and activation of the P2Y(2) nucleotide receptor mediate neurite extension in IL-1beta-treated mouse primary cortical neurons. J Neurochem. 2013;125:885–896. doi: 10.1111/jnc.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson AF, Engardt M, Wahlund LO. Activity level and balance in subjects with mild Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;13:213–216. doi: 10.1159/000057699. [DOI] [PubMed] [Google Scholar]
- Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichiule P, Chavez JC, Schmidt AM, Vannucci SJ. Hypoxia-inducible factor-1 mediates neuronal expression of the receptor for advanced glycation end products following hypoxia/ischemia. J Biol Chem. 2007;282:36330–36340. doi: 10.1074/jbc.M706407200. [DOI] [PubMed] [Google Scholar]
- Pratico D. Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Pharmacol Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Price GD, Robertson SJ, Edwards FA. Long-term potentiation of glutamatergic synaptic transmission induced by activation of presynaptic P2Y receptors in the rat medial habenula nucleus. Eur J Neurosci. 2003;17:844–850. doi: 10.1046/j.1460-9568.2003.02501.x. [DOI] [PubMed] [Google Scholar]
- Pull I, McIlwain H. Adenine derivatives as neurohumoral agents in the brain. The quantities liberated on excitation of superfused cerebral tissues. Biochem J. 1972;130:975–981. doi: 10.1042/bj1300975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A. The role of adenosine in Alzheimer’s disease. Curr Neuropharmacol. 2009;7:207–216. doi: 10.2174/157015909789152119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Relative contribution of P2U- and P2Y-purinoceptors to endothelium-dependent vasodilatation in the golden hamster isolated mesenteric arterial bed. Br J Pharmacol. 1996;117:1797–1802. doi: 10.1111/j.1476-5381.1996.tb15357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J. 2007;21:3717–3726. doi: 10.1096/fj.07-8807com. [DOI] [PubMed] [Google Scholar]
- Rieg T, Gerasimova M, Boyer JL, Insel PA, Vallon V. P2Y(2) receptor activation decreases blood pressure and increases renal Na(+) excretion. Am J Physiol Regul Integr Comp Physiol. 2011;301:R510–518. doi: 10.1152/ajpregu.00148.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Ripellino JA, Ingalls KM, Robakis NK, Felsenstein KM. Nonamyloidogenic cleavage of the beta-amyloid precursor protein by an integral membrane metalloendopeptidase. J Biol Chem. 1994;269:3111–3116. [PubMed] [Google Scholar]
- Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci. 2005;25:6286–6295. doi: 10.1523/JNEUROSCI.0628-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Zayas AE, Torrado AI, Miranda JD. P2Y2 receptor expression is altered in rats after spinal cord injury. Int J Dev Neurosci. 2010;28:413–421. doi: 10.1016/j.ijdevneu.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Landfield PW. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007;27:3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozemuller AJ, van Gool WA, Eikelenboom P. The neuroinflammatory response in plaques and amyloid angiopathy in Alzheimer’s disease: therapeutic implications. Curr Drug Targets CNS Neurol Disord. 2005;4:223–233. doi: 10.2174/1568007054038229. [DOI] [PubMed] [Google Scholar]
- Ruan HZ, Burnstock G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem Cell Biol. 2003;120:415–426. doi: 10.1007/s00418-003-0579-3. [DOI] [PubMed] [Google Scholar]
- Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zlokovic BV. Neurovascular defects and faulty amyloid-beta vascular clearance in Alzheimer’s disease. J Alzheimers Dis. 2013;33(Suppl 1):S87–100. doi: 10.3233/JAD-2012-129037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada M, Yasuda H, Omatsu-Kanbe M, Sango K, Isono T, Matsuura H, Kikkawa R. Increase in intracellular Ca(2+) and calcitonin gene-related peptide release through metabotropic P2Y receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;111:413–422. doi: 10.1016/s0306-4522(02)00005-2. [DOI] [PubMed] [Google Scholar]
- Sanz JM, Chiozzi P, Ferrari D, Colaianna M, Idzko M, Falzoni S, Fellin R, Trabace L, Di Virgilio F. Activation of microglia by amyloid {beta} requires P2X7 receptor expression. J Immunol. 2009;182:4378–4385. doi: 10.4049/jimmunol.0803612. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Hoshi M, Akazawa C, Nakamura Y, Tsuzuki H, Inoue K, Kohsaka S. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44:242–250. doi: 10.1002/glia.10293. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schrader AM, Camden JM, Weisman GA. P2Y2 nucleotide receptor up-regulation in submandibular gland cells from the NOD. B10 mouse model of Sjogren’s syndrome. Arch Oral Biol. 2005;50:533–540. doi: 10.1016/j.archoralbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Schuff N, Matsumoto S, Kmiecik J, Studholme C, Du A, Ezekiel F, Miller BL, Kramer JH, Jagust WJ, Chui HC, Weiner MW. Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement. 2009;5:454–462. doi: 10.1016/j.jalz.2009.04.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seye CI, Kong Q, Erb L, Garrad RC, Krugh B, Wang M, Turner JT, Sturek M, Gonzalez FA, Weisman GA. Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106:2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Griffin WS, O’Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- Shen J, Seye CI, Wang M, Weisman GA, Wilden PA, Sturek M. Cloning, upregulation, and mitogenic role of porcine P2Y2 receptor in coronary artery smooth muscle cells. Mol Pharmacol. 2004;66:1265–1274. doi: 10.1124/mol.104.002642. [DOI] [PubMed] [Google Scholar]
- Shie FS, Breyer RM, Montine TJ. Microglia lacking E Prostanoid Receptor subtype 2 have enhanced Abeta phagocytosis yet lack Abeta-activated neurotoxicity. Am J Pathol. 2005;166:1163–1172. doi: 10.1016/s0002-9440(10)62336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Simon J, Webb TE, Barnard EA. Distribution of [35S]dATP alpha S binding sites in the adult rat neuraxis. Neuropharmacology. 1997;36:1243–1251. doi: 10.1016/s0028-3908(97)00124-x. [DOI] [PubMed] [Google Scholar]
- Simonyi A, He Y, Sheng W, Sun AY, Wood WG, Weisman GA, Sun GY. Targeting NADPH oxidase and phospholipases A2 in Alzheimer’s disease. Mol Neurobiol. 2010;41:73–86. doi: 10.1007/s12035-010-8107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia SS. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci USA. 1992;89:6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovronsky DM, Moore DB, Milla ME, Doms RW, Lee VM. Protein kinase Cdependent alpha-secretase competes with beta-secretase for cleavage of amyloid-beta precursor protein in the trans-golgi network. J Biol Chem. 2000;275:2568–2575. doi: 10.1074/jbc.275.4.2568. [DOI] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci USA. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Iwamaru Y, Sugama S, Tsukimoto M, Fujita M, Sekigawa A, Sekiyama K, Sato M, Kojima S, Conti B, et al. The activation of P2X7 receptor induces cathepsin D-dependent production of a 20-kDa form of IL-1beta under acidic extracellular pH in LPS-primed microglial cells. J Neurochem. 2011;117:712–723. doi: 10.1111/j.1471-4159.2011.07240.x. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Sekiyama K, Sekigawa A, Fujita M, Waragai M, Sugama S, Iwamaru Y, Kitani H, Hashimoto M. P2X7 receptor signaling pathway as a therapeutic target for neurodegenerative diseases. Arch Immunol Ther Exp (Warsz) 2010;58:91–96. doi: 10.1007/s00005-010-0069-y. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP-Induced release and processing of IL-1beta in microglial cells. Crit Rev Immunol. 2009;29:335–345. doi: 10.1615/critrevimmunol.v29.i4.40. [DOI] [PubMed] [Google Scholar]
- Talamagas AA, Efthimiopoulos S, Tsilibary EC, Figueiredo-Pereira ME, Tzinia AK. Abeta(1–40)-induced secretion of matrix metalloproteinase-9 results in sAPPalpha release by association with cell surface APP. Neurobiol Dis. 2007;28:304–315. doi: 10.1016/j.nbd.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Terada H, Kajiura T, Kyoi K, Utsumi S, Hori H. Effects of ATP on cerebral circulation--morphological observation of cerebral circulation and cerebral vasculature. No To Shinkei. 1976;28:151–156. [PubMed] [Google Scholar]
- Toda N, Kawakami M, Yamazaki M, Okamura T. Comparison of endothelium-dependent responses of monkey cerebral and temporal arteries. Br J Pharmacol. 1991;102:805–810. doi: 10.1111/j.1476-5381.1991.tb12256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran MD. P2 receptor stimulation induces amyloid precursor protein production and secretion in rat cortical astrocytes. Neurosci Lett. 2011;492:155–159. doi: 10.1016/j.neulet.2011.01.078. [DOI] [PubMed] [Google Scholar]
- Turner JT, Weisman GA, Camden JM. Upregulation of P2Y2 nucleotide receptors in rat salivary gland cells during short-term culture. Am J Physiol. 1997;273:C1100–1107. doi: 10.1152/ajpcell.1997.273.3.C1100. [DOI] [PubMed] [Google Scholar]
- Uesugi A, Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Robaye B, Boeynaems JM, Inoue K. Involvement of protein kinase D in uridine diphosphate-induced microglial macropinocytosis and phagocytosis. Glia. 2012;60:1094–1105. doi: 10.1002/glia.22337. [DOI] [PubMed] [Google Scholar]
- Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer’s disease: regulation, cell biology, function, and therapeutic potential. J Neurosci. 2009;29:12787–12794. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan KR, Stokes L, Prince LR, Marriott HM, Meis S, Kassack MU, Bingle CD, Sabroe I, Surprenant A, Whyte MK. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J Immunol. 2007;179:8544–8553. doi: 10.4049/jimmunol.179.12.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Mol Neurobiol. 2009;39:190–208. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- Volonte C, Amadio S, D’Ambrosi N, Colpi M, Burnstock G. P2 receptor web: complexity and fine-tuning. Pharmacol Ther. 2006;112:264–280. doi: 10.1016/j.pharmthera.2005.04.012. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I, Spath L, Starke K. Evidence for P2-purinoceptor-mediated inhibition of noradrenaline release in rat brain cortex. Br J Pharmacol. 1994;113:815–822. doi: 10.1111/j.1476-5381.1994.tb17066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Kim SU, McGeer PL. Complement and cytokine gene expression in cultured microglial derived from postmortem human brains. J Neurosci Res. 1995;40:478–493. doi: 10.1002/jnr.490400407. [DOI] [PubMed] [Google Scholar]
- Wallentin L. P2Y(12) inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. Eur Heart J. 2009;30:1964–1977. doi: 10.1093/eurheartj/ehp296. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang DH, Galligan JJ. P2Y2 receptors mediate ATP-induced resensitization of TRPV1 expressed by kidney projecting sensory neurons. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1634–1641. doi: 10.1152/ajpregu.00235.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkentin S, Ohlsson M, Wollmer P, Edenbrandt L, Minthon L. Regional cerebral blood flow in Alzheimer’s disease: classification and analysis of heterogeneity. Dement Geriatr Cogn Disord. 2004;17:207–214. doi: 10.1159/000076358. [DOI] [PubMed] [Google Scholar]
- Weisman GA, Camden JM, Peterson TS, Ajit D, Woods LT, Erb L. P2 receptors for extracellular nucleotides in the central nervous system: role of P2X7 and P2Y(2) receptor interactions in neuroinflammation. Mol Neurobiol. 2012a;46:96–113. doi: 10.1007/s12035-012-8263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman GA, Woods LT, Erb L, Seye CI. P2Y receptors in the mammalian nervous system: pharmacology, ligands and therapeutic potential. CNS Neurol Disord Drug Targets. 2012b;11:722–738. doi: 10.2174/187152712803581047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wihlborg AK, Malmsjo M, Eyjolfsson A, Gustafsson R, Jacobson K, Erlinge D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br J Pharmacol. 2003;138:1451–1458. doi: 10.1038/sj.bjp.0705186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, Unwin RJ, King BF. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol. 2003;140:1177–1186. doi: 10.1038/sj.bjp.0705544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Stephens A, Kirschenheuter G, Greslin AF, Cheng X, Sennelo J, Cattaneo M, Zighetti ML, Chen A, Kim SA, et al. Acyclic analogues of adenosine bisphosphates as P2Y receptor antagonists: phosphate substitution leads to multiple pathways of inhibition of platelet aggregation. J Med Chem. 2002;45:5694–5709. doi: 10.1021/jm020173u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, Xiao Q, Hsu FF, Turk JW, Xu J, et al. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J Biol Chem. 2006;281:24566–24574. doi: 10.1074/jbc.M602440200. [DOI] [PubMed] [Google Scholar]
- Yeh WL, Lu DY, Lin CJ, Liou HC, Fu WM. Inhibition of hypoxia-induced increase of blood-brain barrier permeability by YC-1 through the antagonism of HIF-1alpha accumulation and VEGF expression. Mol Pharmacol. 2007;72:440–449. doi: 10.1124/mol.107.036418. [DOI] [PubMed] [Google Scholar]
- Yu W, Hill WG. Lack of specificity shown by P2Y6 receptor antibodies. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:885–891. doi: 10.1007/s00210-013-0894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao FF, Xu H, Zhang YW. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J Biol Chem. 2007;282:10873–10880. doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]
- Zheng Z, White C, Lee J, Peterson TS, Bush AI, Sun GY, Weisman GA, Petris MJ. Altered microglial copper homeostasis in a mouse model of Alzheimer’s disease. J Neurochem. 2010;114:1630–1638. doi: 10.1111/j.1471-4159.2010.06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilka N, Ferencik M, Hulin I. Neuroinflammation in Alzheimer’s disease: protector or promoter? Bratisl Lek Listy. 2006;107:374–383. [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]