Abstract
RHO GTPases, members of the RAS superfamily of small GTPases, are adhesion and growth-factor activated molecular switches that play important roles in tumor development and progression. When activated, RHO-family GTPases such as RAC1, CDC42, and RHOA, transmit signals by recruiting a variety of effector proteins, including the protein kinases PAK, ACK, MLK, MRCK, and ROCK. Genetically-induced loss of RHO function impedes transformation by a number of oncogenic stimuli, leading to an interest in developing small molecule inhibitors that either target RHO GTPases directly, or that target their downstream protein kinase effectors. Although inhibitors of RHO GTPases and their downstream signaling kinases have not yet been widely adopted for clinical use, their potential value as cancer therapeutics continues to facilitate pharmaceutical research and development and is a promising therapeutic strategy.
Background
The RHO family proteins RAC1, CDC42 and RHOA are small GTP-binding proteins that act as molecular switches, shifting between an inactive, GDP-bound form and an active, GTP-bound form that define functions of RHO GTPases. This process is regulated by guanine nucleotide-exchange factors, GTPase-activating proteins, and guanine nucleotide-dissociation inhibitors (1). There are many signaling pathways that lead to RHO activation, including those initiated by physical stimuli (mechanical stress or cell-cell and cell-substrate adhesion) and chemical factors (growth factors and cytokines) (2). Upon activation, GTP-bound RHO-GTPases interact with a wide spectrum of effectors to regulate various cellular pathways, including cytoskeletal dynamics, motility, cytokinesis, cell growth, apoptosis, and transcriptional activity. The three best studied members of the RHO family – RAC1, CDC42, and RHOA – are essential for transformation by activated RAS (3, 4), and, in the case of RAC1 and RAC2, themselves can be oncogenic drivers in human malignancies (5, 6).
As with RAS, the RHO GTPases have proven difficult to target directly with small molecule inhibitors. There have been limited successes with molecules that disrupt the binding of guanine nucleotide exchange factors to RAC and CDC42 (7–10), as well as with molecules that disrupt GTPase membrane association (11). While efforts continue to develop direct small GTPase inhibitors, a promising and more conventional therapeutic approach has been to block the activities of RHO GTPase effectors. Among these effectors are several protein kinases that either are or might be amenable to small molecule inhibition. For example, RAC and CDC42 share two protein serine-threonine kinase effectors in common – PAK and MLK – and inhibitors for both these kinases have been developed. CDC42 also has distinct kinase effectors, such as MRCK and the tyrosine kinase ACK, and these kinases too might provide suitable drug targets in cancer. RHO-A has a distinct set of effector kinases, including the ROCK, CITRON, and PRK1, all of which regulate cellular processes that contribute to tumorigenesis, invasion, and metastasis (12).
p21 activated kinases (PAKs), the most extensively studied CDC42 and RAC effector proteins, consist of two subgroups containing three members each: group I (PAK1–3) and group II (PAK4–6). PAKs have been implicated in a number of cellular processes critical for oncogenic transformation, including cell proliferation, cell survival, adhesion and migration, and anchorage-independent growth (13). PAKs are overexpressed or/and hyperactivated in variety of human malignancies including bladder, melanoma, breast, prostate, colorectal, and ovarian carcinoma (13). Importantly, compelling genetic and pharmacologic evidence exists that shows that inhibiting group I PAKs can block transformation by oncogenic drivers such as ERBB2 (14), and K-RAS (15). Thus, this kinase is a well-validated anti-cancer target. Importantly, loss of Pak1 is well-tolerated in mice (16), implying that PAK1 inhibitors might not have unacceptable toxicities.
Another common effector of CDC42 and RAC, the mixed-lineage kinases (MLKs), are a family of serine/threonine kinases that translate signals from cell surface receptors to MAPKs. MLKs can function as MAP Kinase Kinase Kinases. Due to their ability to activate multiple MAPK pathways, MLKs mediate a variety of biological processes. For example, overexpression of MLK3 induces transformation and anchorage-independent growth of NIH-3T3 fibroblasts (17), MLK3 is required for proliferation/survival of various cancer cell lines including colon, ovarian (18) and breast cells (19), and for the migration/invasion of ovarian, triple negative/basal breast (20) and gastric carcinoma cells (21).
Activated CDC42 kinase (ACK or TNK2) is a ubiquitously expressed non-receptor tyrosine kinase that binds to and is activated by CDC42 (22). ACK1 has been reported to regulate the receptor tyrosine kinase AXL, to promote activation of AKT, androgen receptor, and negatively regulate the tumor suppressor WWOX (23). Recent findings implicate ACK1 in carcinogenesis. Amplification or activating mutations of ACK1 have been identified in prostate, lung, ovarian, and pancreatic cancers, and ACK1 expression positively correlates with increased tumor invasiveness (24). Inhibition of ACK causes cell cycle arrest, sensitizes cells to ionizing radiation, and induces apoptosis (25). Recently, a micro-RNA (miRNA) miR-7 was identified as a negative regulator of ACK1 expression in human schwannoma (26). Overexpression of miR-7 inhibited NF2-null schwannoma cells growth both in culture and in the xenograft tumor models in vivo, suggesting that ACK1 inhibitors could be potential therapeutic molecules for targeting malignant schwannoma (26).
Myotonic dystrophy kinase-related CDC42-binding kinase (MRCK) acts as a downstream effector of CDC42, affecting actin/myosin reorganization by phosphorylating myosin-II light chain (Mlc2) and the myosin-binding subunit (MYPT1, also known as MBS) of myosin light-chain phosphatase (MLCP) (27). MRCKα was also found to phosphorylate LIM Kinases 1 and 2 (LIMK1 and LIMK2), resulting in increased phosphorylation and inactivation of the filamentous actin severing protein cofilin (28), which would contribute to actin-myosin contractility. In addition, MRCKα induced phosphorylation of moesin in vitro (29), which links integral membrane proteins to filamentous actin, suggesting that actin-myosin contractility may be further promoted by MRCK through enhanced coupling of the cytoskeleton to the membrane. Elevated MRCK expression has been found in various cancer cells, such as lymphoma, breast cancer, lung cancer, and pancreatic adenocarcinoma (30).
RHO-associated protein kinases (ROCK I and II), are key regulators of the actin cytoskeleton downstream of the small GTPase RHO. The main function of ROCK signaling is regulation of the cytoskeleton through the phosphorylation of downstream substrates, leading to increased actin filament stabilization and generation of actin-myosin contractility (31). Upon activation, ROCK promotes actin filament stabilization through LIMK-mediated phosphorylation and also through MLC phosphorylation, leading to increased actin filament bundling and myosin-driven contraction.
ROCK signaling is required for many cytoskeleton-dependent processes, including cell adhesion, motility, phagocytosis, and cell–cell and cell–matrix adhesion. As regulators of such cellular processes, ROCKs play critical roles in a range of human diseases, including cancer. ROCK activation is associated with cancer progression; its expression is elevated in several types of cancers, and ROCK inhibition has been shown to block ROCK tumor growth and reduced metastasis in vivo (32).
The LIMKs (LIMK1 and LIMK2) can be phosphorylated by PAKs, ROCK, and MRCK. The major LIMK substrate is the actin depolymerizing and severing protein cofilin (33). LIMK-dependent phosphorylation of cofilin leads to its inactivation and promotes stabilization of actin cytoskeleton (33). LIMKs also translocate from the cortical cytoplasm and focal adhesions, where they modulate cell morphology and motility, and to the centrosome and nucleus, where they regulate mitosis and cytokinesis (34). LIMKs are overexpressed and implicated in various cancers, such as breast, lung, skin, liver and prostate, and several tumor cell lines (35, 36). Down-regulation of LIMK1 activity reduces the invasiveness in several cancer cells, such as breast cancer and hepatoma cells (37).
Levels of LIMK1/2 are elevated in human vestibular schwannomas, and its inhibition arrests cells in early mitosis (38). Knockdown of either LIMK1 and LIMK2 has also been shown to decrease invasiveness, metastatsis, and cell-induced angiogenesis in pancreatic cancer cells in zebrafish xenografts; double knock down completely blocked invasion and formation of micrometastasis in vivo (39).
CLINICAL-TRANSLATIONAL ADVANCES
Despite the challenge presented by small GTPases, some small molecule inhibitors of RHO GTPases have shown promising results in in vitro and in vivo studies. For example, NSC23766, a first generation RAC-specific inhibitor, has been shown to inhibit RAC-GEF, TIAM1, and oncogenic RAS-induced transformation as well as to suppress prostate cancer cell proliferation and invasion (40, 41). Recently, more potent NSC23766 derivatives have been developed for possible clinical application (42). One of these, AZA1, a specific small molecule inhibitor of CDC42/RAC1 GTPase, was shown to suppress the growth prostate cancer in vivo and also improve survival in mice (43). Another related CDC42 inhibitor, AZA197, reduced colon cancer growth and prolonged survival to 50% in a preclinical mouse xenograft model (10). Finally, EHT 1864, a RAC1 inhibitor, and RHOsin, a RHOA specific inhibitor, was shown to inhibit proliferation and invasion of breast cancer cells, suggesting therapeutic potential of these inhibitors in breast cancer therapy (44, 45). Despite these promising results, currently there are no RHO GTPase inhibitors in clinical use. However, due to the potential value of these inhibitors as cancer therapeutics, there remains substantial interest in the development of RHO GTPase inhibitors for use in cancer patients.
As downstream effectors of RHO GTPases play important roles in regulating oncogenic processes, and are generally amenable to inhibition by small molecules, the best understood effectors - PAK, LIMK, ROCK, MRCK and ACK – have become attractive targets for anti-cancer drug development. For example, because mounting evidence suggests that PAKs act as oncogenes in number of human cancers, the search for specific PAK inhibitors has intensified. The best studied of these compounds, and the only compound to be used so far in a human clinical trial, is the pan-PAK inhibitor PF3758309 (46). Despite its potency and promising effects in preclinical models, the phase I trial of PF3758309 was discontinued due to undesirable pharmacological properties, primarily drug efflux. Another PAK inhibitor, the group I PAK-specific FRAX 597, was recently shown to reduce cancer cells proliferation in vitro and tumor progression in animal models. FRAX597 inhibits the proliferation of NF2-deficient schwannoma cells in culture and displayed potent anti-tumor activity in a K-RAS mouse model; tumor volume was reduced by 89% (15, 47). PAK inhibitors have also shown promise in a number of in vivo studies in melanoma, breast and prostate cancer, and in NF2-deficient schwannoma, indicating the potential benefit of clinical PAK pathway inhibition (13).
Initial functional analyses of MLKs suggested their role in promoting cell death in neuronal cells, and therefore, the inhibitor of MLKs, CEP-1347, ATP analogues that selectively inhibit the MLK family of kinases, was used in clinical trials for Parkinson's Disease, where it lacked efficacy in delaying disease progression (48). The fact that MLKs promote cell proliferation, survival and migration makes them potential targets for targeted cancer therapy. Recent studies have shown that CEP-1347 induces G2/M arrest, metaphase entry block, and apoptosis in three different ER+ breast cancer cell lines, with only minor effects on non-tumorigenic mammary epithelial cell lines (49). Whereas CEP-1347 displayed minimum toxicity in clinical trials, it has a potential in breast cancer therapeutics.
ACK1 activation and/or gene amplification occurs in multiple types of cancer. Recently, the ACK inhibitor AIM-100 was shown to suppress ACK1-mediated phosphorylation and to inhibit the growth of prostate cells by causing cell cycle arrest in G0/G1 phase (50). The ability of AIM-100 to inhibit autoactivated ACK1 (E346K mutant) further shows that it is effective in repressing both oncogene-induced and ligand-modulated ACK, indicating the potential therapeutic importance of this inhibitor (23).
Two independent screens identified Dasatinib as a potent inhibitor of ACK1 (Kd = 6 nM, IC50 1 nM) (51, 52). Dasatinib was originally identified as an inhibitor of the tyrosine protein kinases SRC and ABL (53), and is commercially available as an oral multi-family tyrosine kinase inhibitor for patients with chronic myelogenous leukemia and is in clinical trials for other malignancies. Interestingly, ACK1 failed to rescue H292 lung cancer cells from dasatinib-induced cell death, while other dasatinib target kinases such SRC, FYN, LYN, and LCK were able to do so (51). These results call into question the role of ACK1 as an oncogenic driver in these lung cancer cells.
Several inhibitors of MRCK have been described, including the natural compounds chelerythrine, cycloartane-3,24,25-triol and staurosporine. Chelerythrine, originally identified as a PKC inhibitor with in vitro IC50 of 660 nM (54), was subsequently shown to inhibit MRCKα kinase activity with an in vitro IC50 of 1.77 µM (55). Other off-target effects have also been found for chelerythrine, such as reactive oxygen species generation, DNA intercalation and inhibition of acetylcholinesterases, making this compound difficult for use for identifying MRCK functions (30). Another compound, the natural product cycloartane 3,24,25-triol, was discovered as a potential MRCKα inhibitor (56) due to its ability to compete an immobilized ligand for binding to the ATP-binding site. Cycloartane-3,24,25-triol reduced the viability of PC-3 and DU145 cell lines with IC50 values of 2.226 ± 0.28 µM and 1.67 ± 0.18 µM respectively (57). The non-selective kinase inhibitor staurosporine, the ROCK inhibitors, fasudil, H89 and Y27632, and the IκB Kinase 2 inhibitor TCPA-1 have also been shown to inhibit MRCKβ kinase domain activity in vitro (58). However, at this time there are no sufficiently selective MRCK inhibitors to use as adequate tools to study MRCK activity and functions. Despite this lack, given the differences in structure between the catalytic domains of MRCK and its close relative ROCK, it seems likely that such selective inhibitors could be identified.
ROCK
Due to its effects on cell motility, invasion, and angiogenesis (59), as well as effects on tumor stromal cells (60), ROCK has long been considered as a potential target in oncology. The ROCK inhibitors Y-27632 and Fasudil have been used in several cancer studies (32). Y27632 is effective in vitro, however, the existence of multiple off-targets effects makes it less than ideal for use in in vivo studies. Fasudil has been shown to inhibit tumor progression in vivo; moreover, this inhibitor has been tested extensively in humans for the treatment of cerebral vasospasm. The recently discovered ROCKI/II inhibitors, RKI-18 and RKI-1447 have been shown to prevent migration, invasion and anchorage-independent growth of human breast cancer cells (61, 62). Further, RKI-1447 also demonstrated antitumor activities in transgenic mouse model of breast cancer, reducing mammary tumor growth by 87% (61). However, despite the potential value of ROCK inhibitors for cancer treatment, to date such compounds have not found a therapeutic niche.
As members of the LIMK family are key regulators of the actin cytoskeleton and are involved in cell motility and invasion, LIMK is considered as a potential therapeutic target for metastatic disease. A small molecule inhibitor of LIMK, Pyr1 (apelin-13), has been shown to stabilize microtubules, inhibit cell motility, and induced complete survival increase survival in a mouse model of leukemia (63). Currently Pyr1 is in clinical trials for non-cancer indication, idiopathic pulmonary arterial hypertension.
T56-LIMKi, a novel LIMK inhibitor, has been shown to inhibit neurofibromin deficient cell growth, migration and colony formation. Further, this LIMK1/2 inhibitor was found to synergize with salirasib, a RAS inhibitor, to inhibit tumor cell growth and destabilization of actin cytoskeleton; together these findings suggest that this drug combination could be considered to treat neurofibromatosis-1 (64).
In another study, two effective LIMK inhibitors, damnacanthal and MO-26 (a pyrazolopyrimidine derivative), were identified. These compounds had previously been shown to inhibit LCK, a SRC family tyrosine kinase. However, in vitro kinase assays revealed that damnacanthal inhibited LIMK1 more effectively than LCK. Damnacanthal suppressed LIMK1-induced cofilin phosphorylation, and inhibited migration and invasion MDA-MB-231 cell line. Topical application of damnacanthal also suppressed hapten-induced migration of epidermal Langerhans cells in mouse ears (65). These inhibitors provide useful tools for investigating the cellular and physiological functions of LIMKs and have a great potential for the development of agents against LIMK-related diseases.
Summary
Given the increasing recognition of the importance of RHO GTPase-activated kinases in cancer, and the proven suitability of protein kinases as clinical drug targets, we can expect increasing efforts in development of cancer drugs that target these enzymes. As most of these kinases have multiple cellular functions, inhibitors of these enzymes may have potent anti-cancer effects, but may also be associated with limiting toxicities. However, the same conceptual issues are also true for other recent successful targeted agents, and thus, while they warrant caution, they should not dampen enthusiasm for the development of inhibitors of RHO-activated kinases.
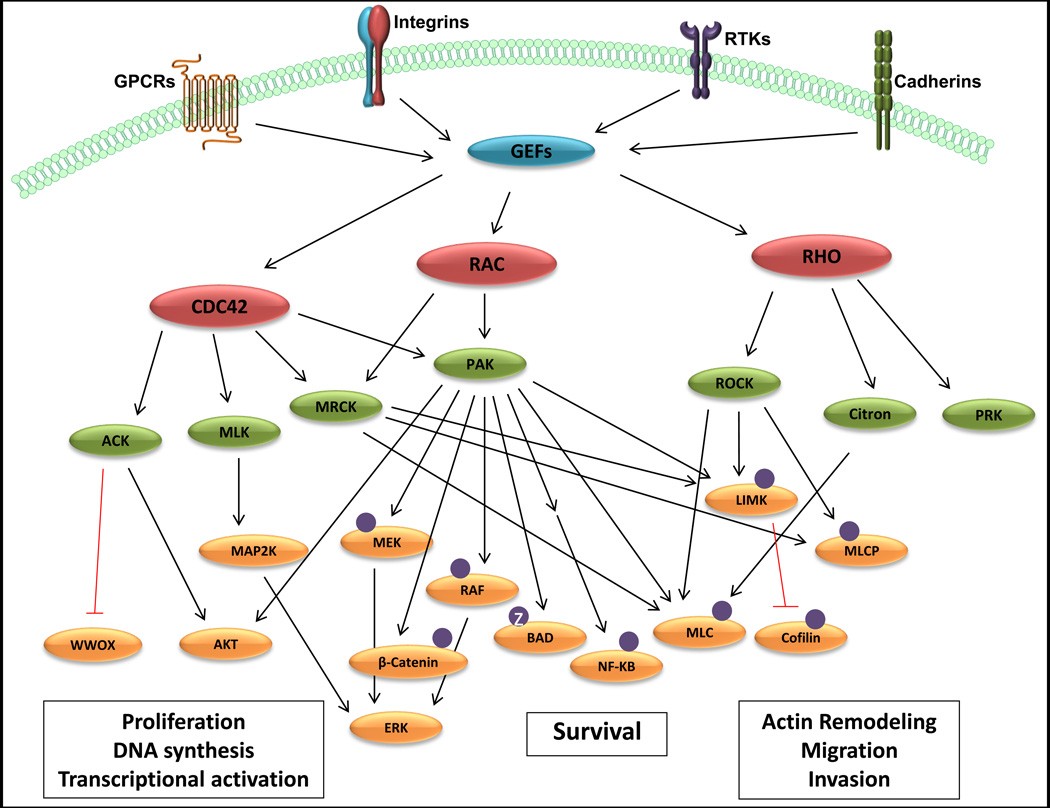
Figure 1. Signaling from RHO-family GTPases.
The three major RHO-family GTPases, CDC42, RAC, and RHO, are activated by guanine-nucleotide exchange factors (GEFs), which are in turn regulated by signals originating at the cell surface. Upon activation, RHO proteins recruit and activate a number of cytoplasmic protein kinases, indicated in green. These protein kinases in turn regulate the activities of a number of additional effector proteins, including those that modulate cell proliferation, survival, and migration. For detailed description of signaling pathways, see main text.
Acknowledgments
Financial Support: This work was supported by grants from the NIH (R01 CA148805 and R01 CA098830), DOD (NF130108), and Children’s Tumor Foundation to JC, and an appropriation from the state of Pennsylvania to the Fox Chase Cancer Center (P30 CA006927).
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest
References
- 1.Walker K, Olson MF. Targeting Ras and Rho GTPases as opportunities for cancer therapeutics. Curr Opin Genet Dev. 2005;15:62–68. doi: 10.1016/j.gde.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Buchsbaum RJ. Rho activation at a glance. J Cell Sci. 2007;120:1149–1152. doi: 10.1242/jcs.03428. [DOI] [PubMed] [Google Scholar]
- 3.Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu RG, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol. 1997;17:3449–3458. doi: 10.1128/mcb.17.6.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis MJ, Ha BH, Holman EC, Halaban R, Schlessinger J, Boggon TJ. RAC1P29S is a spontaneously activating cancer-associated GTPase. Proc Natl Acad Sci U S A. 2013;110:912–917. doi: 10.1073/pnas.1220895110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawazu M, Ueno T, Kontani K, Ogita Y, Ando M, Fukumura K, et al. Transforming mutations of RAC guanosine triphosphatases in human cancers. Proc Natl Acad Sci U S A. 2013;110:3029–3034. doi: 10.1073/pnas.1216141110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onesto C, Shutes A, Picard V, Schweighoffer F, Der CJ. Characterization of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. Methods Enzymol. 2008;439:111–129. doi: 10.1016/S0076-6879(07)00409-0. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferri N, Corsini A, Bottino P, Clerici F, Contini A. Virtual screening approach for the identification of new Rac1 inhibitors. J Med Chem. 2009;52:4087–4090. doi: 10.1021/jm8015987. [DOI] [PubMed] [Google Scholar]
- 10.Zins K, Gunawardhana S, Lucas T, Abraham D, Aharinejad S. Targeting Cdc42 with the small molecule drug AZA197 suppresses primary colon cancer growth and prolongs survival in a preclinical mouse xenograft model by downregulation of PAK1 activity. J Transl Med. 2013;11:295. doi: 10.1186/1479-5876-11-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelish HE, Peterson JR, Salvarezza SB, Rodriguez-Boulan E, Chen JL, Stamnes M, et al. Secramine inhibits Cdc42-dependent functions in cells and Cdc42 activation in vitro. Nat Chem Biol. 2006;2:39–46. doi: 10.1038/nchembio751. [DOI] [PubMed] [Google Scholar]
- 12.Teramoto H, Malek RL, Behbahani B, Castellone MD, Lee NH, Gutkind JS. Identification of H-Ras, RhoA, Rac1 and Cdc42 responsive genes. Oncogene. 2003;22:2689–2697. doi: 10.1038/sj.onc.1206364. [DOI] [PubMed] [Google Scholar]
- 13.Radu M, Semenova G, Kosoff R, Chernoff J. PAK signalling during the development and progression of cancer. Nat Rev Cancer. 2014;14:13–25. doi: 10.1038/nrc3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arias-Romero LE, Villamar-Cruz O, Huang M, Hoeflich KP, Chernoff J. Pak1 kinase links ErbB2 to beta-catenin in transformation of breast epithelial cells. Cancer Res. 2013;73:3671–3682. doi: 10.1158/0008-5472.CAN-12-4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow HY, Jubb AM, Koch JN, Jaffer ZM, Stepanova D, Campbell DA, et al. p21-Activated kinase 1 is required for efficient tumor formation and progression in a Ras-mediated skin cancer model. Cancer Res. 2012;72:5966–5975. doi: 10.1158/0008-5472.CAN-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen JD, Jaffer ZM, Park SJ, Burgin S, Hofmann C, Sells MA, et al. p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood. 2009;113:2695–2705. doi: 10.1182/blood-2008-06-160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartkamp J, Troppmair J, Rapp UR. The JNK/SAPK activator mixed lineage kinase 3 (MLK3) transforms NIH 3T3 cells in a MEK-dependent fashion. Cancer Res. 1999;59:2195–2202. [PubMed] [Google Scholar]
- 18.Chadee DN, Kyriakis JM. MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat Cell Biol. 2004;6:770–776. doi: 10.1038/ncb1152. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Miller EM, Gallo KA. MLK3 is critical for breast cancer cell migration and promotes a malignant phenotype in mammary epithelial cells. Oncogene. 2010;29:4399–4411. doi: 10.1038/onc.2010.198. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Gallo KA. MLK3 regulates paxillin phosphorylation in chemokine-mediated breast cancer cell migration and invasion to drive metastasis. Cancer Res. 2012;72:4130–4140. doi: 10.1158/0008-5472.CAN-12-0655. [DOI] [PubMed] [Google Scholar]
- 21.Mishra P, Senthivinayagam S, Rangasamy V, Sondarva G, Rana B. Mixed lineage kinase-3/JNK1 axis promotes migration of human gastric cancer cells following gastrin stimulation. Mol Endocrinol. 2010;24:598–607. doi: 10.1210/me.2009-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prieto-Echagüe V, Miller WT. Regulation of Ack-Family Nonreceptor Tyrosine Kinases. Journal of Signal Transduction. 2011;2011:9. doi: 10.1155/2011/742372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahajan K, Mahajan NP. Shepherding AKT and androgen receptor by Ack1 tyrosine kinase. J Cell Physiol. 2010;224:327–333. doi: 10.1002/jcp.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Horst EH, Degenhardt YY, Strelow A, Slavin A, Chinn L, Orf J, et al. Metastatic properties and genomic amplification of the tyrosine kinase gene ACK1. Proc Natl Acad Sci U S A. 2005;102:15901–15906. doi: 10.1073/pnas.0508014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prieto-Echague V, Miller WT. Regulation of ack-family nonreceptor tyrosine kinases. J Signal Transduct. 2011;2011:742372. doi: 10.1155/2011/742372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saydam O, Senol O, Wurdinger T, Mizrak A, Ozdener GB, Stemmer-Rachamimov AO, et al. miRNA-7 attenuation in Schwannoma tumors stimulates growth by upregulating three oncogenic signaling pathways. Cancer Res. 2011;71:852–861. doi: 10.1158/0008-5472.CAN-10-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan I, Yong J, Dong JM, Lim L, Leung T. A tripartite complex containing MRCK modulates lamellar actomyosin retrograde flow. Cell. 2008;135:123–136. doi: 10.1016/j.cell.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Sumi T, Matsumoto K, Shibuya A, Nakamura T. Activation of LIM kinases by myotonic dystrophy kinase-related Cdc42-binding kinase alpha. J Biol Chem. 2001;276:23092–23096. doi: 10.1074/jbc.C100196200. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura N, Oshiro N, Fukata Y, Amano M, Fukata M, Kuroda S, et al. Phosphorylation of ERM proteins at filopodia induced by Cdc42. Genes Cells. 2000;5:571–581. doi: 10.1046/j.1365-2443.2000.00348.x. [DOI] [PubMed] [Google Scholar]
- 30.Unbekandt M, Olson MF. The actin-myosin regulatory MRCK kinases: regulation, biological functions and associations with human cancer. J Mol Med (Berl) 2014;92:217–225. doi: 10.1007/s00109-014-1133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rath N, Olson MF. Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012;13:900–908. doi: 10.1038/embor.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manetti F. LIM kinases are attractive targets with many macromolecular partners and only a few small molecule regulators. Med Res Rev. 2012;32:968–998. doi: 10.1002/med.20230. [DOI] [PubMed] [Google Scholar]
- 34.Scott RW, Olson MF. LIM kinases: function, regulation and association with human disease. J Mol Med (Berl) 2007;85:555–568. doi: 10.1007/s00109-007-0165-6. [DOI] [PubMed] [Google Scholar]
- 35.Bagheri-Yarmand R, Mazumdar A, Sahin AA, Kumar R. LIM kinase 1 increases tumor metastasis of human breast cancer cells via regulation of the urokinase-type plasminogen activator system. Int J Cancer. 2006;118:2703–2710. doi: 10.1002/ijc.21650. [DOI] [PubMed] [Google Scholar]
- 36.Horita Y, Ohashi K, Mukai M, Inoue M, Mizuno K. Suppression of the invasive capacity of rat ascites hepatoma cells by knockdown of Slingshot or LIM kinase. J Biol Chem. 2008;283:6013–6021. doi: 10.1074/jbc.M706538200. [DOI] [PubMed] [Google Scholar]
- 37.Li R, Doherty J, Antonipillai J, Chen S, Devlin M, Visser K, et al. LIM kinase inhibition reduces breast cancer growth and invasiveness but systemic inhibition does not reduce metastasis in mice. Clinical & Experimental Metastasis. 2013;30:483–495. doi: 10.1007/s10585-012-9553-6. [DOI] [PubMed] [Google Scholar]
- 38.Petrilli A, Copik A, Posadas M, Chang LS, Welling DB, Giovannini M, et al. LIM domain kinases as potential therapeutic targets for neurofibromatosis type 2. Oncogene. 2014;33:3571–3582. doi: 10.1038/onc.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlecken D, Bagowski C. LIMK1 and LIMK2 Are Important for Metastatic Behavior and Tumor Cell-Induced Angiogenesis of Pancreatic Cancer Cells. Zebrafish. 2009;6:433–439. doi: 10.1089/zeb.2009.0602. [DOI] [PubMed] [Google Scholar]
- 40.Nassar N, Cancelas J, Zheng J, Williams DA, Zheng Y. Structure-function based design of small molecule inhibitors targeting Rho family GTPases. Curr Top Med Chem. 2006;6:1109–1116. doi: 10.2174/156802606777812095. [DOI] [PubMed] [Google Scholar]
- 41.Akbar H, Cancelas J, Williams DA, Zheng J, Zheng Y. Rational design and applications of a Rac GTPase-specific small molecule inhibitor. Methods Enzymol. 2006;406:554–565. doi: 10.1016/S0076-6879(06)06043-5. [DOI] [PubMed] [Google Scholar]
- 42.Montalvo-Ortiz BL, Castillo-Pichardo L, Hernandez E, Humphries-Bickley T, De la Mota-Peynado A, Cubano LA, et al. Characterization of EHop-016, novel small molecule inhibitor of Rac GTPase. J Biol Chem. 2012;287:13228–13238. doi: 10.1074/jbc.M111.334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zins K, Lucas T, Reichl P, Abraham D, Aharinejad S. A Rac1/Cdc42 GTPase-specific small molecule inhibitor suppresses growth of primary human prostate cancer xenografts and prolongs survival in mice. PLoS One. 2013;8:e74924. doi: 10.1371/journal.pone.0074924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenblatt AE, Garcia MI, Lyons L, Xie Y, Maiorino C, Desire L, et al. Inhibition of the Rho GTPase, Rac1, decreases estrogen receptor levels and is a novel therapeutic strategy in breast cancer. Endocr Relat Cancer. 2011;18:207–219. doi: 10.1677/ERC-10-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shang X, Marchioni F, Sipes N, Evelyn CR, Jerabek-Willemsen M, Duhr S, et al. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem Biol. 2012;19:699–710. doi: 10.1016/j.chembiol.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci U S A. 2010;107:9446–9451. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Licciulli S, Maksimoska J, Zhou C, Troutman S, Kota S, Liu Q, et al. FRAX597, a small molecule inhibitor of the p21-activated kinases, inhibits tumorigenesis of neurofibromatosis type 2 (NF2)-associated Schwannomas. J Biol Chem. 2013;288:29105–29114. doi: 10.1074/jbc.M113.510933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007;69:1480–1490. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Gallo KA, Conrad SE. Targeting mixed lineage kinases in ER-positive breast cancer cells leads to G2/M cell cycle arrest and apoptosis. Oncotarget. 2013;4:1158–1171. doi: 10.18632/oncotarget.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahajan K, Challa S, Coppola D, Lawrence H, Luo Y, Gevariya H, et al. Effect of Ack1 tyrosine kinase inhibitor on ligand-independent androgen receptor activity. Prostate. 2010;70:1274–1285. doi: 10.1002/pros.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Rix U, Fang B, Bai Y, Edwards A, Colinge J, et al. A chemical and phosphoproteomic characterization of dasatinib action in lung cancer. Nat Chem Biol. 2010;6:291–299. doi: 10.1038/nchembio.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phatak SS, Zhang S. A novel multi-modal drug repurposing approach for identification of potent ACK1 inhibitors. Pac Symp Biocomput. 2013:29–40. [PMC free article] [PubMed] [Google Scholar]
- 53.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 54.Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- 55.Tan I, Lai J, Yong J, Li SF, Leung T. Chelerythrine perturbs lamellar actomyosin filaments by selective inhibition of myotonic dystrophy kinase-related Cdc42-binding kinase. FEBS Lett. 2011;585:1260–1268. doi: 10.1016/j.febslet.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 56.Lowe HI, Watson CT, Badal S, Toyang NJ, Bryant J. Cycloartane-3,24,25-triol inhibits MRCKalpha kinase and demonstrates promising anti prostate cancer activity in vitro. Cancer Cell Int. 2012;12:46. doi: 10.1186/1475-2867-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 58.Podolin PL, Callahan JF, Bolognese BJ, Li YH, Carlson K, Davis TG, et al. Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IkappaB Kinase 2, TPCA-1 (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide), occurs via reduction of proinflammatory cytokines and antigen-induced T cell Proliferation. J Pharmacol Exp Ther. 2005;312:373–381. doi: 10.1124/jpet.104.074484. [DOI] [PubMed] [Google Scholar]
- 59.Amin E, Dubey BN, Zhang SC, Gremer L, Dvorsky R, Moll JM, et al. Rho-kinase: regulation, (dys)function, and inhibition. Biol Chem. 2013;394:1399–1410. doi: 10.1515/hsz-2013-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanz-Moreno V, Gaggioli C, Yeo M, Albrengues J, Wallberg F, Viros A, et al. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell. 2011;20:229–245. doi: 10.1016/j.ccr.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 61.Patel RA, Forinash KD, Pireddu R, Sun Y, Sun N, Martin MP, et al. RKI-1447 is a potent inhibitor of the Rho-associated ROCK kinases with anti-invasive and antitumor activities in breast cancer. Cancer Res. 2012;72:5025–5034. doi: 10.1158/0008-5472.CAN-12-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel RA, Liu Y, Wang B, Li R, Sebti SM. Identification of novel ROCK inhibitors with anti-migratory and anti-invasive activities. Oncogene. 2014;33:550–555. doi: 10.1038/onc.2012.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prudent R, Vassal-Stermann E, Nguyen C-H, Pillet C, Martinez A, Prunier C, et al. Pharmacological Inhibition of LIM Kinase Stabilizes Microtubules and Inhibits Neoplastic Growth. Cancer Research. 2012;72:4429–4439. doi: 10.1158/0008-5472.CAN-11-3342. [DOI] [PubMed] [Google Scholar]
- 64.Mashiach-Farkash E, Rak R, Elad-Sfadia G, Haklai R, Carmeli S, Kloog Y, et al. Computer-based identification of a novel LIMK1/2 inhibitor that synergizes with salirasib to destabilize the actin cytoskeleton. Oncotarget. 2012;3:629–639. doi: 10.18632/oncotarget.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohashi K, Sampei K, Nakagawa M, Uchiumi N, Amanuma T, Aiba S, et al. Damnacanthal, an effective inhibitor of LIM-kinase, inhibits cell migration and invasion. Mol Biol Cell. 2014;25:828–840. doi: 10.1091/mbc.E13-09-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]