Abstract
We compared whole transcriptome variation in six preadult stages and seven adult female ages in two populations of cactophilic Drosophila mojavensis reared on two host plants in order to understand how differences in gene expression influence standing life history variation. We used Singular Value Decomposition (SVD) to identify dominant trajectories of life cycle gene expression variation, performed pair-wise comparisons of stage and age differences in gene expression across the life cycle, identified when genes exhibited maximum levels of life cycle gene expression, and assessed population and host cactus effects on gene expression. Life cycle SVD analysis returned four significant components of transcriptional variation, revealing functional enrichment of genes responsible for growth, metabolic function, sensory perception, neural function, translation and aging. Host cactus effects on female gene expression revealed population and stage specific differences, including significant host plant effects on larval metabolism and development, as well as adult neurotransmitter binding and courtship behavior gene expression levels. In 3 - 6 day old virgin females, significant up-regulation of genes associated with meiosis and oogenesis was accompanied by down-regulation of genes associated with somatic maintenance, evidence for a life history tradeoff. The transcriptome of D. mojavensis reared in natural environments throughout its life cycle revealed core developmental transitions and genome wide influences on life history variation in natural populations.
Keywords: life history, development, gene expression, microarray, aging, Drosophila
Introduction
Understanding life history evolution requires knowledge of the forces shaping correlated suites of fitness characters in response to patterns of age-specific mortality (Hamilton 1966; Reznick 1982; Reznick et al. 2004; Roff 2002; Stearns 1977; Williams 1957). Therefore, it is necessary to integrate how life history traits are expressed across environments (Caswell 1983; Etges 1993; Gupta & Lewontin 1982; Scheiner 1993) and standing levels of genetic variation in fitness components (Gustafsson 1986; Istock et al. 1976; Price & Schluter 1991; Walsh & Blows 2009) with patterns of demographic and environmental variability (Caswell 2009; Orzack & Tuljapurkar 1989; Steiner & Tuljapurkar 2012; Tuljapurkar 1989). In order to predict life history patterns, we must also examine the genetic architecture of life history variation and the unfolding of organismal developmental programs over the life cycle (Levitis 2011). In particular, we need to understand the number and kind of genes responsible for life history differences, how coordinated groups of genes are expressed at different life cycle stages, and how environmental effects on genes influence internal and external buffering and genotype by environment (GxE) interactions (Arbeitman et al. 2002; Fiedler et al. 2010; Koutsos et al. 2007; Stolc et al. 2004).
Currently, large gaps remain in our understanding of how genomic expression throughout the life cycle is influenced by relevant ecological variables. In organisms where expression of genetic differences in life histories depends upon local ecological variation, examination of the sensitivity of gene expression, as well as gene expression-environment interactions, is necessary to evaluate the adaptive significance of life history variation in response to ecological variability. Environmental variation can maintain genetic polymorphism in populations, directly influence gene expression leading to GxE interactions (Gillespie & Turelli 1989), and be limited in its selective effects if alleles are neutral in some environments but not others (Anderson et al. 2013). Limits to plasticity of genome expression (Zhou et al. 2012) are of direct concern to organismal persistence in changing environments unless standing levels of genetic variability are high enough to allow short-term microevolutionary change. Although levels of genetic variation in components of fitness, as well as fitness itself, are sometimes low (Gustafsson 1986), it is essential to understand the nature of genome expression throughout the life history (Gibson 2008; Hodgins-Davis & Townsend 2009).
Here, we examine transcriptional profiles throughout the life cycle in Drosophila mojavensis, a cactophilic species endemic to the deserts of northwestern Mexico and southwestern USA, using whole transcriptome microarrays in order to document patterns and sensitivity of gene expression in populations characterized by genetically differentiated life history differences. We assessed transcriptional variation from embryogenesis to four week-old female adults to characterize the range of variation in gene expression and gene function in interrelated groups of genes. We focused on pre-adult stages and revealed expression shifts related to development, while analyses across female adult life stages revealed expression changes underlying maturation, senescence, and tradeoffs between reproduction and somatic maintenance in different environments.
Ecology and evolution of D. mojavensis
Throughout the arid lands of the New World, over half of the ca 100 species in the large D. repleta group use fermenting cactus tissues to carry out their life cycles (Filchak et al. 2005; Heed 1982; Oliveira et al. 2012; Wasserman 1992). Within the mulleri species complex, D. mojavensis and its two closest relatives, D. arizonae and D. navojoa, form a monophyletic group endemic to Mexico and the southwestern United States (Ruiz et al. 1990). Drosophila mojavensis became isolated in present-day peninsular Baja California from its closest relative, D. arizonae, on the mainland due to tectonic drift and changing sea levels (Gastil et al. 1975). Natural populations of D. mojavensis from the Sonoran and Mojave Deserts and adjacent arid lands use different host cacti across their range, i.e. pitaya agria cactus, Stenocereus gummosus, on the peninsula and organ pipe, S. thurberi, and sina cactus, S. alamosensis in mainland Mexico and Arizona (Etges et al. 1999; Heed & Mangan 1986). In the Mojave Desert in southern California and central Arizona, barrel cactus, Ferocactus cylindraceus, is a major host and populations of D. mojavensis on Santa Catalina Island near Los Angeles, California use Opuntia cactus. Southern California populations likely split from mainland Sonora-southern Arizona populations ca. 117–135 kya with little recurring gene flow (Smith et al. 2012).
Natural populations of D. mojavensis show considerable genetic variation in life histories, including host plant-influenced differences in adult mortality rates (Jaureguy & Etges 2007). Baja California populations express shorter egg to adult development times, higher viabilities, and smaller thorax sizes than mainland populations when reared on fermenting agria vs. organ pipe cactus in common garden experiments suggesting adaptation to these hosts in nature (Etges 1990; Etges et al. 2010; Etges & Heed 1987). Mainland Sonoran Desert D. mojavensis are characterized by larger body sizes (Etges 1992; Etges & Ahrens 2001), higher metabolic rates, more ovarioles (Heed, unpubl. data) and higher lifetime fecundities, but earlier ages at first reproduction than Baja populations (Etges & Klassen 1989). Genetic variation in development time and thorax size in both Baja and mainland populations, as well as significant GxE interactions when reared on different host plants, and positive across-host genetic correlations suggested ongoing life history evolution and evidence for ecological generalism (Etges 1993). Baja California and mainland populations also harbor significant genetic variation for adult longevity and average numbers of eggs laid per day, as well as a genetic tradeoff between early and late-life fecundity (Etges & Heed 1992). Together, these data suggest that as D. mojavensis colonized mainland Mexico and Arizona by switching host cacti, new life histories evolved in these derived populations (Etges 1993), with correlated shifts in reproductive isolation (Etges 1998; Etges et al. 2010).
We measured whole genome transcriptional responses of D. mojavensis from two populations exposed to fermenting tissues of two host cacti, i.e. agria, S. gummosus, and organ pipe cactus, S. thurberi, in pre-adult stages and adult of increasing age in order to reveal whole transcriptome responses to different host plants over the life cycle. We approached the analysis of our data with two distinct goals in mind. First, we assessed effects of stage/age independent of population and diet by generating a pooled dataset composed of mean expression levels for all genes (averaged across populations, diets, and biological replicates) at each stage/age. We used this averaged dataset to investigate highly conserved trajectories of gene expression across the D. mojavensis life cycle, independent of diet or population effects. To identify clusters of genes with similar age-trajectories of expression, we performed a singular value decomposition (SVD) of total genome expression (Alter 2006; Alter et al. 2000) on this pooled dataset. The SVD cluster analyses revealed continuous changes difficult to observe with simple pair-wise comparisons between stages and ages. We then considered as correlated gene clusters those sets of genes whose expression closely correlated with the dominant trajectories revealed in the SVD analysis. We also performed pair-wise comparisons, e.g., comparing expression at adjacent stages/ages, with the primary aim of mapping gene expression levels into functional domains as in previous studies (Kimet al. 2005; Koutsos et al. 2007; McCarroll et al. 2004; Pletcher et al. 2002; Remolina et al. 2012).
Second, we searched for evidence of divergence in gene expression patterns at each stage and age in our four population X cactus treatment groups. By teasing out expression differences into shifts due to population, host plant, and their interactions, we revealed gene expression/regulatory changes potentially responsible for their recent divergence in life histories.
Materials and Methods
Origin of Stocks
Populations of D. mojavensis were collected in nature by baiting over fermented bananas or by collecting adults emerged from cactus rots returned to the lab. A total of 465 adults were baited in Punta Prieta, Baja California in January 2008, and 1264 baited adults plus 9 adults that emerged from sina, S. alamosensis, rots were collected from Las Bocas, Sonora in March 2009. All flies were returned to the lab and each population was cultured on banana food (Brazner & Etges 1993) in 8 dr shell vials at room temperature until the experiments began in September 2009.
Preadult stage culture conditions
Thousands of adult flies from each population were introduced into separate population cages (12,720 cm3) for 7-10 days and allowed to choose mates. Population cages were maintained in an incubator programmed for a 14:10 LD photoperiod and 27:17 ° C. Flies were allowed to oviposit in cups containing fermenting agria or organ pipe cactus (see below). We used both cacti for egg oviposition because we were also interested in the effects of alternate cactus substrates on gene expression at all stages, including fertilized eggs. Thousands of eggs (~200 μg) were collected for six hours and briefly rinsed in deionized water to remove cactus media, snap frozen in liquid nitrogen, and stored at −80° C prior to RNA extraction. For larval and pupal stages, approximately 200 eggs were transferred to cups containing fermenting cactus media (see below) and allowed to develop to the stage of interest. Development times for the pre-adult stages were estimated from analysis of the duration of stage specific differences in larval mouth hook morphology and pupal periods (D. White and W. J. Etges, unpubl. results). A total of six pre-adult stages were used: fertilized embryos (6 hr), first instar larvae (48 hr), second instar larvae (144 hr), third instar larvae (240 hr), early pupae (288 hr), and late pupae (384 hr). Egg hatch is ca 24-25 hr under these conditions. In addition to age in hours, we verified each larval and pupal stage morphologically and discarded individuals that were early or advanced for each developmental stage. Each sample of larvae consisted of thousands of individuals for the first and second instars and hundreds of individuals for the third instar. For early and late pupae, 30 individuals were used in each sample.
Cactus media for rearing pre-adult stages was prepared with 400 g of cactus (either agria or organ pipe), 600 mL of deionized water and 4 g of agar. First, fresh cactus tissue was blended using 2/3 of the water, boiled, and then strained twice to remove large cactus fibers. This media was strained a third time using a fine mesh to remove excess fibers and the resulting liquid paste-like solution was added to the agar dissolved in boiling water. This media was then boiled for 10 min, autoclaved for 8 min and poured into food cups. After the medium cooled, it was inoculated with a pectolytic bacterium, Erwinia cacticida (Alcorn et al. 1991), and a mixture of seven cactophilic yeasts: Dipodascus starmeri, Candida sonorensis, C. valida, Starmera amethionina, Pichia cactophila, P. mexicana, and Sporopachydermia cereana. One mL of yeast and bacterial solution was injected into the cactus media every 48 hr to yield constant fermentation of the cacti. The final media was soft enough to separate the larvae (especially the first and second instars) from the cactus media.
Adult culture conditions
Flies were raised for one generation in population cages (described above), and eggs collected from these cages were reared to eclosion on banana food at moderate larval densities in half-pint bottles. Emerged adults were transferred to 8 dr shell vials in small same sex groups containing banana food until they were sexually mature (8-10 days). Approximately 400 adults (200 females and 200 males) from each population were introduced into separate oviposition chambers and allowed to mate and oviposit for 10 h each day. Eggs were collected from a 5.5 cm diameter petri dish containing agar-cactus media attached to each oviposition chamber, and washed in sterile deionized water, 70% ethanol, and again in deionized water. Eggs were counted into groups of 200, transferred to a 1 cm2 piece of sterilized filter paper, and placed in bottles containing 75 g of fermenting cactus tissue in the incubator described above. All unhatched eggs were counted to allow calculation of egg to adult viability. Eclosed adults from each replicate culture were counted daily, allowing determination of egg-to adult development time, separated by sex, and immediately transferred to vials containing fermenting cactus (see below) in same sex groups of 30 flies. All cultures were maintained in an incubator (described above).
Cactus media for rearing adults for RNA extraction was prepared by mixing cactus (agria or organ pipe), water, and agar homogenized in a blender in the following proportions: 953 g cactus, 486 ml deionized water, and 5 g agar. This mixture was autoclaved for 15 min, cooled, and inoculated with bacteria and yeasts (see above). This cactus media was prepared one week prior to use and kept in an incubator at 37 °C to maximize microbial fermentation. This media was then loaded into individual cup-like 2.2 cm diameter plastic barrel plugs (Alliance Express, Little Rock, Arkansas USA) that were pressed into one end of autoclaved 25 × 95 mm glass tubes. An additional inoculating loop containing a mixture of bacteria and seven cactophilic yeasts was added to the fermenting cactus tissue in each food cap to supplement nutrition. After adding 30 adult females or males to each tube, the other end of each tube was closed with a barrel plug that had been drilled with a 1.75 cm hole sealed with fine mesh to allow air circulation. Flies were fed atmospheric ethanol vapor by placing tubes in sealed desiccators containing 1 L of 4% ethanol (Etges 1989; Etges & Klassen 1989) from 8:00 AM to 6:00 PM in the incubator described above. For the remaining 14 hr each day, all tubes were removed from each desiccator and kept in the incubator to minimize condensation inside the tubes. Plugs containing fermenting cactus were replaced every four days.
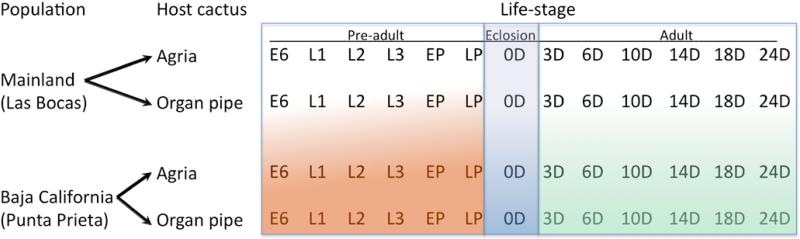
Adult females for RNA extraction were sampled at 8 time intervals: 0, 3, 6, 10, 14, 18, 24, and 28 days. Each adult sample consisted of 24 virgin females that were snap frozen in liquid nitrogen and stored at −80° C. Additional tubes of females and males sampled at each time interval were frozen at −20° C and used for cuticular hydrocarbon analysis (Etges & de Oliveira 2014). Overall, we planned 24 treatment combinations for pre-adult stages (2 populations × 2 cacti × 6 stages) and 32 combinations (2 populations × 2 cacti × 8 ages) for adult females (Fig. 1). Each combination was replicated four times for RNA extraction and microarray analysis; however, samples of 28 day old females were missing because few flies survived past 28 days in these conditions (Etges & Heed 1992; Jaureguy & Etges 2007), so we pooled them resulting in 7 ages sampled. A number of missing replicates resulted in 86 (pre-adult) and 86 (adult) samples (Suppl. Table 1).
Figure 1.
Experimental design for RNA sampling of the two populations of D. mojavensis reared on two host plants throughout the life cycle where L1 = first instar, L2 = second instar, L3 = third instar, EP = early pupae, LP = late pupae, 0 D = adult day of emergence, 3 D = 3 day old adults, etc. Day 24 adults were pooled with Day 28 adults because so few Day 28 adults were available due to mortality.
cDNA synthesis, hybridization and visualization
Total RNA was isolated from each sample using RNeasy mini-kits (Qiagen, Valencia, California USA) and stored at −80 °C until cDNA was prepared. Double-stranded cDNA was synthesized using Invitrogen Superscript Double-Stranded cDNA Synthesis kits, and cDNA concentrations were measured using a NanoDrop spectrophotometer (NanoDrop Technologies) to verify that all cDNA samples were ≥ 100ng/ul, A260/A280 ≥ 1.8, and A260/A230 ≥ 1.8. All cDNA samples were Cy3 labeled using a NimbleGen One Color DNA Labeling kit.
Our Roche NimbleGen microarray design contained a total of 14,528 unique transcripts based on the D. mojavensis genome (http://flybase.net/genomes/Drosophila_mojavensis/current/fasta/dmoj-all-transcript-r1.3.fasta.gz ; 4/14/2009) with nine probes per transcript for a total of 130,705 probes (each microarray in the 12-plex design included 135K probes; see Gene Expression Omnibus entry GSE43220 for details). Hybridizations were performed with a NimbleGen Hybridization System (Hybridization System 4, BioMicro Systems, Inc.) and spot intensity scanning was carried out with a GenePix 4000B scanner (Molecular Devices) and GenePix Pro software. All hybridization intensities were normalized using quantiles (Bolstad et al. 2003) with NimbleScan v2.5 software. Gene call files were generated using the Robust Multichip Average (RMA) algorithm as described by Irizarry et al. (2003).
Data Analysis
Whole-dataset analysis
We assessed time-series gene expression dynamics using Singular Value Decomposition (SVD) analysis (Alter et al. 2000; Alter et al. 2003). SVD is a linear transformation of expression data from genes × arrays space to a reduced “eigengenes” × “eigenarrays” space. In our case, the SVD took our 14528 gene × 13 stage/age data matrix and returned a 13 × 13 matrix where each row is an eigengene. Each of these eigengenes represents a consensus trajectory of gene expression, similar to a principal component, encompassing a proportion of the overall variation in gene expression over time. This application of SVD is closely analogous to its usual use in signal processing, with each eigengene representing a common trajectory of expression with a strong signal in the data. These eigengene profiles provide a way to cluster genes according to their correlation with these dominant trajectories of gene expression across the life cycle.
SVD analysis was performed on an averaged D. mojavensis dataset, consisting of mean within-life-stage gene expression values for all genes at each stage/age to evaluate overall gene expression variation changes. Preliminary analysis revealed a single eigengene representing steady-state expression that accounted for 99.6% of all variation in the data. The entropy of this dataset was also low (d = 0.012 <<< 1) suggesting that stage-specific changes in expression were relatively small deviations from lifetime mean expression (Alter et al. 2000). We therefore mean-centered the data by filtering out this eigengene (Alter et al. 2000), and all further analyses were undertaken on the resulting normalized dataset. After normalization, the stage-specific expression levels for each gene had values between −1 and 1, with positive relative expression levels indicating overexpression and negative expression indicating under-expression relative to the lifetime mean.
SVD analysis contains an inherent sign ambiguity, thus for each eigengene its complementary (i.e., equal and opposite relative expression level at each stage and age) trajectory is equally significant. While heuristic methods do exist to try to work around this ambiguity, we chose to exploit it by treating significant eigengenes as paired sets of correlated and anticorrelated gene expression trajectories. For each significant eigengene pair, we arbitrarily designated the “positive” eigengene to be the trajectory with positive relative expression in adult stages (Fig 2). The corresponding “negative” eigengene trajectory is a mirror image about zero of its complementary “positive” eigengene. Thus, genes significantly correlated with a “positive” eigengene will be significantly anticorrelated with the corresponding negative eigengene and vice versa.
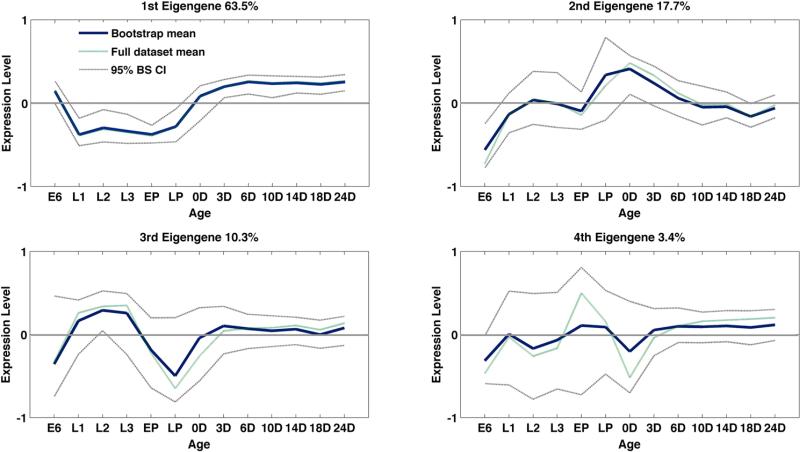
Figure 2.
The first four eigengenes plotted across the life cycle in D. mojavensis. The proportion of the total variation explained by each eigengene is listed. Plus/minus correlations with each eigengene are plotted for the overall data set means, bootstrap means, and 95 % bootstrap mean confidence intervals. The X axis represents the six preadult stages: embryo, larval, and pupal plus adults defined in Figure 1 and the text.
Serial resampling of the biological replicates was used to assess variation within stage/age samples and its impact on eigengenes revealed by SVD analysis. 10,000 resampled datasets were created by randomly selecting one biological replicate from the available samples at each life stage/age. These resampled datasets were subjected to SVD analysis just as with the averaged dataset, and were used to form 95% confidence bounds around the original eigengenes (Ghosh 2002). To determine which transcripts were contributing most to each eigengene pattern, genes were sorted by their correlation with the eigengene's trajectory over the life history (top 10%), and then these transcripts were sorted again by the magnitude of their projection onto the eigengene (Alter et al. 2000) to arrive at 5% or 726 predicted genes. For simplicity, we included the top 750 genes with the highest +/− rank in this sorting with respect to each eigengene at each stage and age for gene annotation and functional clustering.
Peaks and variance in gene expression
At each life stage and age, we calculated the mean and variance of expression for each transcript across populations and diets. We then determined when each gene was at its highest observed level of expression allowing us to characterize differences in maximum gene expression across the life cycle. We were also interested in the variability of gene expression across our replicate samples to determine whether gene expression may become less tightly controlled with age (cf. Pletcher et al. 2002). Thus, we plotted changes in genome-wide variances in gene expression characterized as the stage or age-specific variance of all predicted genes in their expression levels. Different numbers of individuals were sampled at different pre-adult stages that might affect genome-wide variance estimates, but only gene expression variance increases in second instar larvae and late pupae (384 hr) were observed (see Results).
Pair-wise stage and age comparisons
We also assessed a set of specific pair-wise comparisons using datasets pooled in a different way, e.g., comparing expression at two ages, or comparing expression under two environments with the primary aim of mapping gene expression levels into functional domains as in previous studies (Kim et al. 2005; Koutsos et al. 2007; Pletcher et al. 2002) and to search for shared components of gene co-expression underlying development and aging (McCarroll et al. 2004). We chose to analyze targeted pairwise interactions rather than use a traditional linear model approach, since a fully parameterized model of our data would involve 2 × 2 × 13 possible comparisons which, in the end, would have needed to be assessed with the same set of pairwise tests. A full linear model for all genes across the life cycle produced stage/age differences in gene expression that were > 99 % similar to our pair-wise comparisons (results not shown). We identified transcripts that significantly increased or decreased in expression between each pair of consecutive life stages using t-tests corrected for false discovery rate (FDR) P < 0.05 (Benjamini & Hochberg 1995) and that had absolute fold-changes > 1.5 (the absolute value of the ratio of normalized intensities between two samples). These comparisons helped to tease out individual gene expression changes potentially responsible for, or caused by, important age-stage transitions.
We also identified transcripts that significantly increased or decreased in expression between 3 day old (young adult) and > 18 day old (senescent) adults because many aging studies have focused on such pair-wise comparisions between ‘young’ and ‘old’ age classes (e.g. de Magalhães et al. 2009; Landis et al. 2004; Southworth et al. 2009). We pooled samples from ages 18+ days to increase sample sizes, since at older ages only enough flies remained to produce one or two replicate samples per treatment. We also assessed numbers of genes differentially expressed due to host cacti in preadult and adult stages by using a dataset with all preadult stages pooled together and all adult stages pooled together.
Host cactus and population effects
For each preadult stage and adults on day of eclosion, we assessed all gene expression differences due to cactus with t-tests with FDR P < 0.05 and absolute fold-changes > 1.5. The remaining adult data were assessed by ANOVA with cactus, population, and cactus by population interaction included with ages pooled (Etges 2014).
Ortholog search and functional annotation clustering
Submission of the 14,528 D. mojavensis transcripts to Flybase (Tweedie et al. 2009) produced 9117 D. melanogaster orthologs, i.e. only ~ 63 percent of predicted D. mojavensis genes could be functionally analyzed. Reciprocal BLAST searches with the other 10 available Drosophila genomes did not increase this number (results not shown). These 9117 orthologs were used in gene ontology analyses using DAVID Bioinformatics Resources 6.7 (Huang et al. 2009). Thus, for a given list of D. mojavensis transcripts of interest, we first determined the subset of those transcripts that had D. melanogaster orthologs, and used the corresponding D. melanogaster genes in our gene ontology analysis.
Gene annotation clusters were determined by DAVID's clustering algorithm with initial classification stringencies set to ‘Moderate’. We also used GO-Module (Yang et al. 2011) to reduce redundancy in numbers of annotated clusters when there were several overlapping functional clusters produced by DAVID. Further inspection of annotated gene function was enabled by identifying KEGG pathways (Kanehisa & Goto 2000).
Due to limited annotation of the D. mojavensis genome, our gene ontology analysis has two main potential sources of error. First, we could only include genes that have known D. melanogaster orthologs. Thus, the gene lists used in our analyses are missing ca 37 percent of the original transcripts of interest. The addition of this missing data could change the significance of the clusters reported here, and could also contain enriched clusters undetectable in our current dataset. Second, our enrichment analyses compared gene lists of interest with the list of 9117 orthologs as background, not with the entire D. melanogaster genome. An ‘enriched’ cluster of GO-terms, then, means that terms within that cluster were proportionately overrepresented in the subset of the original transcript list of interest that had known D. melanogaster orthologs, as compared to the total set of orthologs. Given these limitations, we interpret our gene ontology results with caution and focus primarily on broader trends. We performed the same annotation cluster analysis with the top 5% of genes corresponding to each eigengene, genes with maximal expression over the life cycle, and genes differing in expression between consecutive life cycle stages/ages.
Results
Singular Value Decomposition (SVD) analysis
SVD analysis revealed four eigengenes that explained 95% of the variation in the normalized dataset (Fig. 2). All four eigengenes showed life cycle shifts in gene co-expression associated with transitions from egg to larval stages and pupae to day of eclosion, with relatively little change from eclosion to adults of older ages (Fig. 2). We pooled replicates from population and cactus diet treatments because there were no significant differences observed in eigengene structure between these groups, as revealed by overlap in their 95% bootstrap confidence intervals at all life stages (results not shown). SVD analysis of overall life cycle variation in gene expression was thus insensitive to differences due to rearing substrates or population origin, likely in part because the number of replicates for each age-population-diet combination was limited to four.
The first eigengene accounted for 63.5 % of the overall variation in gene expression and so represented the largest correlated “structure” of life cycle gene expression in the normalized dataset. This trajectory was characterized by a negative relationship between pre-adult and adult gene expression patterns – transcripts that were down-regulated in pre-adult stages were up-regulated in adults and vice versa (Fig. 2). Interestingly, gene expression in 6 h embryos was concordant with expression in adult ages (Suppl. Table 2). This was expected in part because adult females contained developing eggs (cf. Graveley et al. 2011). Of the top 5% of all genes with the highest positive correlation with eigengene 1, just 38.7 percent were annotated, and were significantly enriched for general growth and metabolic function including protein synthesis, cell division, and secretory functions (Table 1).
Table 1.
Gene ontology terms identified with DAVID using the top 5% (750 genes) of all predicted D. mojavensis genes. Results for the top 4 significant eigengene clusters based on all life stages and ages are shown. Numbers in parentheses are the percentages of the top 750 genes with D. melanogaster orthologs.
| Eigengene 1 (63.5%) | Eigengene 2 (17.7%) | ||||||
|---|---|---|---|---|---|---|---|
| Positive correlation | Negative correlation | Positive correlation | Negative correlation | ||||
| 290 (38.7%)+ | 648 (86.4) | 574 (76.5) | 531 (70.8) | ||||
| GOTerm | enrich | GOTerm | enrich | GOTerm | enrich | GOTerm | enrich |
| protein transport, localization | 5.82**** | G-protein coupled receptor protein sensory perception, taste, chemical stimulus | 4.62**** | Membrane structure | 8.9**** | RNA, mRNA, tRNA processing, splicing, transport | 10.38**** |
| Vesicle transport, endocytosis, secretion | 3.35*** | Cell morphogenesis | 2.1** | Glycoproteins | 4.79**** | Zinc finger, metal ion binding | 6.05**** |
| Nucleotide binding | 2.74* | Ion transport, sodium channel activity | 1.96* | ATP transmembrane metabolism, ion transport | 4.32*** | Chromosomal structure | 4.24**** |
| Synaptic transmission | 2.06* | Transcriptional regulation | 1.64* | Plasma membrane | 3.2* | Transcription, protein synthesis | 4.1**** |
| Translation in mitochondrion | 1.99** | Peptidase activity | 1.25* | Larval behavior | 2.35* | Chromosome organization, modification | 3.86*** |
| Cell-cell signaling and neurological function | 2.33** | Protein folding | 3.21** | ||||
| Oxidative phosphorylation | 2.1*** | Ribonuclease activity | 2.92* | ||||
| g-protein coupled receptors | 1.54* | Epigenetic regulation of gene expression | 1.36* | ||||
| Detection of external stimulus | 1.38** | ||||||
| Eigengene 3 (10.3%) | Eigengene 4 (3.4%) | ||||||
|---|---|---|---|---|---|---|---|
| Positive correlation | Negative correlation | Positive correlation | Negative correlation | ||||
| 457 (60.9%) | 422 (56.3%) | 406 (54.1%) | 545 (72.7%) | ||||
| GOTerm | enrich | GOTerm | enrich | GOTerm | enrich | GOTerm | enrich |
| Peptidase activity | 3.78**** | Neuron differentiation & development, axonogenesis | 5.4**** | Ribosome | 6.27**** | HOX/homeobox, development protein, DNA binding, transcriptional regulation | 7.98**** |
| Endoplasmic reticulum | 2.88*** | Cell migration | 5.32**** | Structural constituent of ribosome | 2.81**** | Mitochondrion, mitochondrial ribosome | 5.13**** |
| Organ formation | 4.58* | Organ formation | 4.52**** | ||||
| Eye development | 4.22* | Regionalization, pattern specification | 4.24**** | ||||
| Instar larva or pupal morphogenesis, metamorphosis | 3.23* | Neuron development | 3.8*** | ||||
| Salivary gland development | 2.22*** | Transcription repressor activity, negative regulation of transcription | 3.67*** | ||||
| Head segmentation | 3.57** | ||||||
| Oxidative phosphorylation | 3.49** | ||||||
| Gut development | 3.14** | ||||||
| Respiratory system development | 2.31* | ||||||
| Wnt signaling | 2.16** | ||||||
Number (percentage of top 750) of genes annotated
FDR
P < 0.05
P < 0.01
P < 0.005
P < 0.0001
Of the genes with transcription levels negatively correlated with eigengene 1, 86.4 percent were annotated, and were enriched for protease activity, G-protein coupled receptor function, ion transport, sensory perception, and transcriptional regulation (Suppl. Table 2). These functional groups were expressed from first instar larvae to late pupae consistent with protein degradation, larval molting, tissue remodeling in pupation, and increased larval expression of sensory and gustatory genes (Vosshall & Stocker 2007). Many of these genes in this cluster were olfactory (Or) and gustatory receptor (Gr) orthologs that were up-regulated in first instar larvae (Table 2). Thus, the largest sources of life cycle gene co-expression variation for orthologs with inferred functions were due to increased expression of ribosomal-associated translation capacity in embryo and adult stages with correspondingly increased expression of gene clusters with protein degradation and sensory perception function in larval through pupal stages.
Table 2.
Changes in expression (all 1.5 X fold change, FDR P < 0.05) of sensory genes from 6 h embryos to first instar larvae (Egg-L1), second to third instar (L2-L3), and late pupae to day of eclosion (LP-0D) in two populations of D. mojavensis. All significant changes were identified in enriched functional clusters of genes differing in expression between consecutive life stages identified by DAVID (Huang et al., 2009). Direction of arrows indicates increased or decreased transcript abundance.
| D. melanogaster ID | gene | Egg-L1 | L2-L3 | LP-0D | GO annotation |
|---|---|---|---|---|---|
| FBgn0000120 | Arr1 | ↑ ↑ | deactivation of rhodopsin mediated signaling | ||
| FBgn0000206 | boss | ↑ ↑ | R7 cell fate commitment; GO:0007465 compound eye development; GO:0048749 | ||
| FBgn0000313 | chp | ↑ ↑ | homophilic cell adhesion; GO:0007156 rhabdomere development; GO:0042052 | ||
| FBgn0066293 | CheB42b | ↑ ↑ | detection of pheromone; GO:0043695 | ||
| FBgn0040726 | dpr | ↑ ↑ | salt aversion; GO:0035199, sensory perception of salty taste | ||
| FBgn0004623 | Gbeta76C | ↑ ↑ | deactivation of rhodopsin mediated signaling; GO:0016059, G-protein coupled receptor protein signaling pathway; GO:0007186 | ||
| FBgn0028433 | Ggamma30A | ↑ ↑ | G-protein coupled receptor protein signaling pathway; GO:0007186, phototransduction; GO:0007602 | ||
| FBgn0004618 | gl | ↑ ↑ | response to red light; GO:0010114, entrainment of circadian clock by photoperiod | ||
| FBgn0045502 | Gr10a | ↑ ↑ | sensory perception of taste; GO:0050909 | ||
| FBgn0041250 | Gr21a | ↑ ↑ | sensory perception of taste; GO:0050909 | ||
| FBgn0041248 | Gr23a | ↑ ↑ | sensory perception of taste; GO:0050909 | ||
| FBgn0041247 | Gr28a | ↑ ↑ | ↓ ↓ | sensory perception of taste; GO:0050909 | |
| FBgn0045495 | Gr28b | ↑ ↑ | sensory perception of taste; GO:0050909 | ||
| FBgn0032416 | Gr33a | ↑ ↑ | ↓ ↓ | sensory perception of taste; GO:0050909 | |
| FBgn0041236 | Gr59d | ↑ ↑ | ↓ ↓ | sensory perception of taste; GO:0050909 | |
| FBgn0035468 | Gr63a | ↑ ↑ | ↓ ↓ | sensory perception of taste; GO:0050909, response to carbon dioxide; GO:0010037 | |
| FBgn0045479 | Gr64a | ↑ ↑ | ↓ ↓ | sensory perception of taste; GO:0050909, detection of glucose; GO:0051594 | |
| FBgn0045478 | Gr64b | ↑ ↑ | ↓ ↓ | sensory perception of taste; GO:0050909 | |
| FBgn0045477 | Gr64c | ↑ ↑ | ↓ ↓ | sensory perception of taste; GO:0050909 | |
| FBgn0045476 | Gr64e | ↑ ↑ | ↓ ↓ | sensory perception of taste; GO:0050909 | |
| FBgn0052255 | Gr64f | ↑ ↑ | ↓ ↓ | sensory perception of taste; GO:0050909 | |
| FBgn0035870 | Gr66a | ↓ ↓ | sensory perception of taste; GO:0050909 | ||
| FBgn0046885 | Gr98d | ↑ ↑ | ↓ ↓ | sensory perception of taste; GO:0050909, sensory perception of taste; GO:0050909 | |
| FBgn0011672 | Mvl | ↓ ↓ | sensory perception of taste; GO:0050909, taste receptor activity; GO:0008527 | ||
| FBgn0013972 | Gycalpha99B | ↑ ↑ | positive phototaxis; GO:0046956 rhodopsin mediated phototransduction; GO:0009586, guanylate cyclase complex, soluble; GO:0008074 | ||
| FBgn0004784 | inaC | ↑ ↑ | adaptation of rhodopsin mediated signaling; GO:0016062 phototransduction; GO:0007602 | ||
| FBgn0001263 | inaD | ↑ ↑ | deactivation of rhodopsin mediated signaling; GO:0016059, phototransduction; GO:0007602 | ||
| FBgn0053197 | mbl | ↑ ↑ | embryonic development; GO:0009790, muscle organ development; GO:0007517, compound eye photoreceptor cell differentiation; GO:0001751 | ||
| FBgn0036414 | nan | ↑ ↑ | calcium ion transport; GO:0006816, sensory perception of sound; GO:0007605 | ||
| FBgn0002936 | ninaA | ↑ ↑ | rhodopsin biosynthetic process; GO:0016063 | ||
| FBgn0002938 | ninaC | ↑ ↑ | cytoskeleton organization; GO:0007010, phototransduction, visible light; GO: adaptation of rhodopsin mediated signaling; GO:0016062 | ||
| FBgn0002940 | ninaE | ↑ ↑ | phototransduction; GO:0007602, photoreceptor cell morphogenesis; GO:0008594 | ||
| FBgn0037896 | ninaG | ↑ ↑ | retinoid metabolic process; GO:0001523 | ||
| FBgn0016047 | nompA | ↑ ↑ | dendrite morphogenesis; GO:0048813, detection of mechanical stimulus involved in sensory perception of sound; GO:0050910 | ||
| FBgn0016919 | nompB | ↑ ↑ | flagellum assembly; GO:0009296, sensory cilium assembly; GO:0035058 | ||
| FBgn0016920 | nompC | ↑ ↑ | calcium ion transport; GO:0006816, mechanosensory behavior; GO:0007638, sensory perception of sound; GO:0007605 | ||
| FBgn0031110 | Obp19b | ↑ ↑ | sensory perception of chemical stimulus; GO:0007606 | ||
| FBgn0033268 | Obp44a | ↑ ↑ | sensory perception of chemical stimulus; GO:0007606 | ||
| FBgn0033508 | Obp46a | ↑ ↑ | ↓ ↓ | sensory perception of chemical stimulus; GO:0007606 | |
| FBgn0033573 | Obp47a | ↑ ↑ | sensory perception of chemical stimulus; GO:0007606, odorant binding GO:0005549 | ||
| FBgn0050067 | Obp50a | ↑ ↑ | sensory perception of chemical stimulus; GO:0007606 | ||
| FBgn0033931 | Obp50e | ↑ ↑ | sensory perception of chemical stimulus; GO:0007606 | ||
| FBgn0034468 | Obp56a | ↑ ↑ | sensory perception of smell; GO:0007608, response to pheromone; GO:0019236 | ||
| FBgn0046879 | Obp56c | ↑ ↑ | ↓ ↓ | sensory perception of chemical stimulus; GO:0007606 | |
| FBgn0034471 | Obp56e | ↑ ↑ | sensory perception of chemical stimulus; GO:0007606 | ||
| FBgn0034474 | Obp56g | ↑ ↑ | sensory perception of chemical stimulus; GO:0007606 | ||
| FBgn0034475 | Obp56h | ↑ ↑ | sensory perception of smell; GO:0007608 response to pheromone; GO:0019236 | ||
| FBgn0034768 | Obp58b | ↑ ↑ | ↓ ↓ | sensory perception of chemical stimulus; GO:0007606 | |
| FBgn0034769 | Obp58c | ↑ ↑ | ↓ ↓ | sensory perception of chemical stimulus; GO:0007606 | |
| FBgn0034770 | Obp58d | ↑ ↑ | ↓ ↓ | sensory perception of chemical stimulus; GO:0007606 | |
| FBgn0046876 | Obp83ef | ↓ ↓ | sensory perception of chemical stimulus; GO:0007606 | ||
| FBgn0046875 | Obp83g | ↑ ↑ | ↓ ↓ | olfactory behavior; GO:0042048, response to pheromone; GO:0019236 | |
| FBgn0038859 | Obp93a | ↑ ↑ | ↓ ↓ | sensory perception of chemical stimulus; GO:0007606 | |
| FBgn0039685 | Obp99b | ↑ ↑ | ↑ ↑ | ↓ ↓ | autophagic cell death; GO:0048102, salivary gland cell autophagic cell death; GO:0035071, olfactory behavior; GO:0042048, response to pheromone; GO:0019236 |
| FBgn0039682 | Obp99c | ↑ ↑ | sensory perception of chemical stimulus; GO:0007606 | ||
| FBgn0026396 | Or22c | ↓ ↓ | sensory perception of smell; GO:0007608 | ||
| FBgn0026394 | Or24a | ↑ ↑ | sensory perception of smell; GO:0007608 | ||
| FBgn0032096 | Or30a | ↑ ↑ | ↓ ↓ | sensory perception of smell; GO:0007608 | |
| FBgn0026390 | Or33c | ↑ ↑ | ↓ ↓ | olfactory behavior; GO:0042048 | |
| FBgn0033041 | Or42a | ↑ ↑ | sensory perception of smell; GO:0007608 | ||
| FBgn0026389 | Or43a | ↑ ↑ | ↓ ↓ | sensory perception of smell; GO:0007608 | |
| FBgn0033422 | Or45b | ↑ ↑ | sensory perception of smell; GO:0007608 | ||
| FBgn0026388 | Or46a | ↑ ↑ | olfactory behavior; GO:0042048, sensory perception of smell; GO:0007608 | ||
| FBgn0033727 | Or49a | ↑ ↑ | ↓ ↓ | sensory perception of smell; GO:0007608 | |
| FBgn0028963 | Or49b | ↑ ↑ | ↓ ↓ | sensory perception of smell; GO:0007608 | |
| FBgn0034473 | Or56a | ↑ ↑ | ↓ ↓ | sensory perception of smell; GO:0007608 | |
| FBgn0026384 | Or59a | ↑ ↑ | ↓ ↓ | sensory perception of smell; GO:0007608 | |
| FBgn0035382 | Or63a | ↑ ↑ | ↓ ↓ | sensory perception of smell; GO:0007608 | |
| FBgn0036078 | Or67c | ↑ ↑ | ↓ ↓ | sensory perception of smell; GO:0007608 | |
| FBgn0036709 | Or74a | ↑ ↑ | ↓ ↓ | integral to membrane; GO:0016021, olfactory receptor activity; GO:0004984, odorant binding; GO:0005549 | |
| FBgn0037322 | Or83a | ↑ ↑ | ↓ ↓ | sensory perception of smell; GO:0007608 | |
| FBgn0037324 | Or83b | ↑ ↑ | ↓ ↓ | olfactory behavior; GO:0042048 sensory perception of smell; GO:0007608 response to pheromone; GO:0019236 | |
| FBgn0037576 | Or85a | ↑ ↑ | ↓ ↓ | sensory perception of smell; GO:0007608 | |
| FBgn0037594 | Or85d | ↑ ↑ | ↓ ↓ | sensory perception of smell; GO:0007608 | |
| FBgn0038798 | Or92a | ↑ ↑ | ↓ ↓ | sensory perception of smell; GO:0007608 | |
| FBgn0030204 | Or9a | ↑ ↑ | sensory perception of smell; GO:0007608 | ||
| FBgn0060296 | pain | ↑ ↑ | calcium ion transport; GO:0006816, sensory perception of pain; GO:0019233, response to mechanical stimulus; GO:0009612, feeding behavior | ||
| FBgn0011283 | Pbprp5 | ↑ ↑ | sensory perception of chemical stimulus; GO:0007606 | ||
| FBgn0065109 | ppk11 | ↑ ↑ | sodium ion transport; GO:0006814, liquid clearance, open tracheal system; GO:0035002, sensory perception of salty taste; GO:0050914 | ||
| FBgn0085373 | rdgA | ↑ ↑ | diacylglycerol kinase activity | ||
| FBgn0004366 | rdgC | ↑ ↑ | phototransduction; GO:0007602, photoreceptor cell maintenance; GO:0045494, visual perception; GO:0007601 | ||
| FBgn0003248 | Rh2 | ↑ ↑ | phototransduction; GO:0007602 | ||
| FBgn0003249 | Rh3 | ↑ ↑ | phototransduction, UV; GO:0007604 | ||
| FBgn0014019 | Rh5 | ↑ ↑ | phototransduction; GO:0007602 | ||
| FBgn0019940 | Rh6 | ↑ ↑ | phototransduction; GO:0007602 | ||
| FBgn0036260 | Rh7 | ↑ ↑ | G-protein coupled receptor protein signaling pathway; GO:0007186, phototransduction; GO:0007602 | ||
| FBgn0003380 | Sh | ↑ ↑ | flight behavior; GO:0007629, potassium ion transport; GO:0006813, courtship behavior; GO:0007619 | ||
| FBgn0086367 | t | ↑ ↑ | flight behavior; GO:0007629, cuticle pigmentation; GO:0048067, visual perception; GO:0007601, dopamine biosynthetic process; GO:0042416 | ||
| FBgn0014395 | tilB | ↑ ↑ | sensory perception of sound; GO:0007605, male courtship behavior, veined wing generated song production; GO:0045433 | ||
| FBgn0005614 | trpI | ↑ ↑ | calcium ion transport; GO:0006816, response to abiotic stimulus; GO:0009628, response to light stimulus; GO:0009416 | ||
| FBgn0004514 | TyrR | ↑ ↑ | sensory perception of smell; GO:0007608, octopamine/tyramine signaling pathway; GO:0007211 | ||
| FBgn0039482 | CG14258 | ↑ ↑ | pheromone/odorant binding GO:0005549 | ||
| FBgn0051345 | CG31345 | ↑ ↑ | detection of calcium ion; GO:0005513, phagocytosis, engulfment; GO:0006911 | ||
| FBgn0147028 (Dmoj) | Dmoj_GI24305 | ↑ ↑ | IPR004272: Odorant binding protein, IPR013053: Hormone binding, (no D. meianogaster ortholog) |
The second significant eigengene accounted for 17.7 % of the variation in lifetime gene expression. The ‘positive’ complement of this trajectory was characterized by down-regulated expression in egg and early pupa stages, with close to mean expression levels during larval stages, strongly up-regulated expression in late pupae and day of eclosion, then a slow monotonic decrease in expression with adult age (Fig. 2). The negative complement showed, conversely, up-regulated expression in egg and early pupal stages, mean expression levels in larval stages, down-regulation at late pupae and eclosion, and monotonic increases in expression with adult age. Thus, increased transcription in 6 h embryos and in aging, post-eclosion adults likely involved common gene clusters.
The largest positive loadings on this eigengene occurred from late pupae to eclosion and in young adults. Of the top 5% positively correlated genes, 574 genes were annotated and enriched for functional clusters involved with plasma membrane structure and ion transport, glycoprotein metabolism, neural development and function, sensory perception and oxidative phosphorylation (Table 1). This enrichment is consistent with expression of developmental genes in late pupae, as well as peak neural and metabolic function in young adults with decreases in neural and metabolic function with increasing adult age. Negative associations with eigengene 2 included RNA processing and transport, transcriptional regulation, protein folding, chromosomal organization, and epigenetic control of gene regulation (Suppl. Table 3). This enrichment pattern is consistent with protein synthesis in egg and pupal stages, and interestingly, again in late adult life. Thus, eigengene 2 included a significant component of lifetime gene co-expression associated with embryonic gene clusters and the pupa-eclosion transition that then shifted with adult age. This suggested that eigengene 2 structure was driven by post-eclosion shifts in gene cluster transcription associated with slowing of protein metabolism, reduction in neural function, detoxification activity, and chromatin silencing associated with aging, including Sirt6, a known determinant of adult lifespan (Kusama et al. 2006). Eigengene 2 is therefore an excellent genelet to pursue in order to understand expression of aging genes.
While the third and fourth significant eigengenes accounted for far smaller proportions of the total variation in our data, eigengene 3 was associated with contrasting larval and pupal patterns of gene expression and an overall lack of deviation from mean gene expression levels after eclosion (Fig. 2). The “positive” trajectory of this eigengene had peak expression in larval stages, with strong down-regulation in egg and pupal stages. Transcripts correlated with this trajectory were enriched for peptidase activity and endoplasmic reticulum function. The increased expression of these genes in larvae, with decreasing expression in pupal stages, is consistent with decreases in metabolic rates from early to late pupal stages (Lebo et al. 2009; Merkey et al. 2011)
The negative trajectory of eigengene 3 was characterized by peak expression in egg and pupal stages, with down-regulated expression in larvae. Transcripts with correlated with this trajectory were enriched for brain and organ development, and metamorphosis consistent with up-regulation of developmental processes in the embryo and pupae (Table 1).
The “positive” trajectory of the fourth eigengene was characterized by down-regulated expression in 6 hr embryos, weaker down-regulation in larval stages, peak expression in pupal stages followed by strong down-regulation of expression at eclosion and slowly increasing expression at adult ages (Fig. 2). Transcripts correlated with this trajectory were enriched for ribosomal function, consistent with the tissue remodeling during pupal stages.
Transcriptional variation correlated with the negative trajectory of this eigengene was associated with pattern formation and larval development, and enriched for Hox genes, organ system formation, segmentation and neuron development genes, as well as wnt signaling (Table 1). Enrichment for developmental genes is consistent with expression patterns in the embryonic stage, and likely has little to do with the eigengene's expected peak expression in young adults. wnt signaling was also associated with embryogenesis, and this enrichment is likely driven by overexpression of wnt associated genes in 6 hr embryos. All wnt signaling homologs showed peak expression early in embryogenesis, but some, e.g. boca, WntD, pangolin, and wingless (Suppl. Table 2), also showed increased expression in adults consistent with the positive expression in young adults for eigengene 4 (Fig. 2) similar to modENCODE expression levels in D. melanogaster (Tweedie et al. 2009).
Peak expression and transitions in gene expression levels over the life cycle
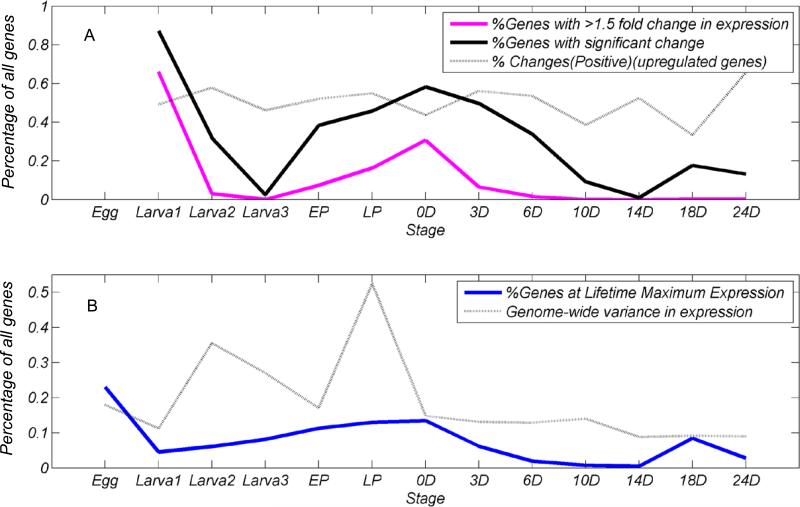
Both maximum expression data (percent of all genes at maximum lifetime expression levels, Fig. 3, Suppl. Table 4) and expression change data (percent of all genes with significant (FDR P < 0.05 and >1.5 X fold changes, Suppl. Table 5) between successive life stages/ages showed the same three distinct peaks over the life cycle of D. mojavensis (Fig. 4). There was a clear burst of genome-wide levels of expression in 6 h embryos that declined throughout larval stages, an increase in pupae to day of eclosion, and an almost monotonic decline until adults were 14 days old (Table 3; Fig. 4). A slight late-life peak in gene expression levels was apparent from 14 to 18 and 24 days, a peak also seen for genes at their maximum lifetime expression levels (Fig. 4).
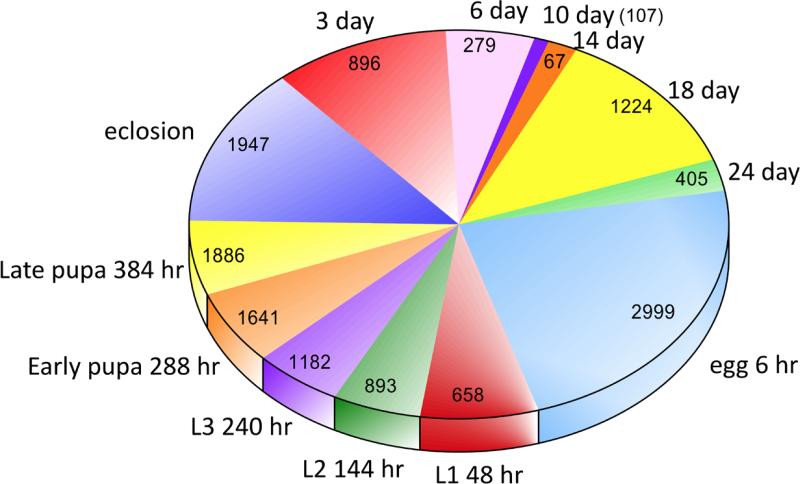
Figure 3.
Pie chart showing the numbers of genes at their maximum lifetime expression levels at each stage and age in this study. Stages and ages are defined in Figure 1 and in the text.
Figure 4.
A. Plots of the changes in gene expression compared with previous stages/ages for the proportion of all 14,528 genes with 1.5 × fold changes, significant changes (P < 0.05), percent of all genes that were upregulated from the previous stage/age. B. Plot showing the percentage of all genes at maximum lifetime expression levels and changes in the variance in gene expression for all genes at each stage/age.
Table 3.
Functional annotation clustering of genes with maximum lifetime expression levels in two populations of D. mojavensis using DAVID. Numbers of transcripts having their maximum expression at each life stage are indicated with the number of D. melanogaster orthologs (in parentheses) used for GO clustering. FDR P values associated with top GOterms are indicated. L1 = first instar, L2 = second instar, L3 = third instar, EP = early pupae, LP = late pupae, 0 D = adult day of emergence, 3 D = 3 day old adults, etc. See text for details.
| Life stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Embryo 6 h n = 2999 (2363) | L1 n = 658 (432) | L2 n = 893 (345) | L3 n = 1182 (451) | EP n = 1641 (602) | |||||
| GOTerm | enrich score | GOTerm | enrich score | GOTerm | enrich score | GOTerm | enrich score | GOTerm | enrich score |
| 1. RNA splicing, processing |
22.6**** | 1. peritrophic membrane structure, chitin binding |
4.4**** | 1. G-protein coupled peptide receptor activity |
4.2**** | 1. G-protein coupled receptor, sensory perception |
12.3**** | 1. cuticle structure | 4.2**** |
| 2. nuclear lumen, nucleoplasm |
19.6**** | 2. signal peptide | 3.1**** | 2. HOX , DNA binding | 3.0**** | 2. detection of chemical stimulus |
6.5**** | 2. salivary gland histolysis |
3.6*** |
| 3. DNA binding, transcription regulation |
19.3**** | 3. cell adhesion | 2.6* | 3. membrane receptor | 2.7**** | 3. sodium channel activity |
5.6**** | 3. chitin binding | 2.7** |
| 4. Chromosome | 16.1**** | 4. proton transport ATPase |
2.6**** | 4. carbohydrate binding |
2.1** | 4. peptide receptor activity |
2.5* | 4. neuron development |
2.6* |
| 5. Down regulation of gene expression |
13.5**** | 5. transmembrane function |
2.6** | 5. cuticle structure | 1.7* | 5. ligand-gated channel activity |
2.5**** | 5. Serine protease | 1.8** |
| 6. Mitosis, cell cycle | 12.5**** | 6. ribosome structure | 2.5**** | 6. serine-type peptidase activity |
1.7* | 6. chitin binding | 2.3** | 6. dynein complex | 1.7 |
| 7. Embryonic morphogenesis |
10.5**** | 7. procollagen- proline dioxygenase activity |
2.0** | 7. cell adhesion | 1.7* | ||||
| 8. Chromatin organization/ modification |
8.8**** | 8. neurotransmitter, gated channel activity |
1.9** | ||||||
| 9. Up regulation of gene expression |
8.1**** | ||||||||
| 10. Oogenesis | 7.9**** | ||||||||
| 11. Transcription factor binding |
7.9**** | ||||||||
| 12. DNA replication | 6.9**** | ||||||||
| 13. DNA repair | 6.2**** | ||||||||
| 14. Neuron differentiation, morphogensis |
5.8**** | ||||||||
| 15. Ribosome biogenesis |
5.5**** | ||||||||
| 16. Nucleocytoplasmic transport |
5.3**** | ||||||||
| 17. RNA helicase activity |
4.9**** | ||||||||
| 18. DNA packaging, nucleosome assembly |
4.8**** | ||||||||
| 19. Meiosis | 4.8**** | ||||||||
| 20. cell migration, motion |
4.7**** | ||||||||
| 21. compound eye development |
4.7**** | ||||||||
| 22. Nucleotidyl- transferase activity |
4.5**** | ||||||||
| 23. Stem cell maintenance |
4.4**** | ||||||||
| 24. Imaginal disc development |
4.1**** | ||||||||
| 25. Gene silencing |
3.5**** | ||||||||
| 26. Embryonic segmentation |
3.4**** | ||||||||
| Life stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| LP n = 1886 (818) | 0 day n = 1947 (1562) | 3 day n = 896 (613) | 6 day n = 279 (213) | 10 day n = 107 (79) | |||||
| GOTerm | enrich score | GOTerm | enrich score | GOTerm | enrich score | GOTerm | enrich score | GOTerm | enrich score |
| 1. cuticle structure | 3.1*** | 1. cellular respiration |
20.6**** | 1. fatty acid biosynthesis |
5.8**** | 1. cofactor binding | 2.5**** | 1. ribosome, translation |
2.8**** |
| 2. microtubule cytoskeleton |
2.6**** | 2. transit peptide: mitochondrion |
6.6**** | 2. iron ion binding | 5.0**** | 2. vitamin binding | 2.3**** | ||
| 3. ion channel activity | 2.2*** | 3. TCA cycle | 6.2**** | 3. cofactor binding, acyl-CoA DH activity |
4.3**** | 3. steroid metabolism |
2.3**** | ||
| 4. muscle development |
1.8**** | 4. photo- transduction, light detection |
5.5**** | 4. hexose metabolism |
4.0**** | 4. Golgi vesicle membrane |
1.9*** | ||
| 5. membrane transport |
1.8** | 5. ion transport | 4.9** | 5. transaminase activity |
3.8*** | 5. membrane, endocytosis |
1.8* | ||
| 6. mitochondrial membrane |
1.5 | 6. ATP metabolic process |
4.6**** | 6. glycosidase, lysosome |
3.8**** | 6. oogenesis | 1.8 | ||
| 7. neuropeptide binding |
1.5* | 7. mitochondrial electron transport |
4.4**** | 7. signal peptide secretion |
3.1*** | ||||
| 8. adult locomotory behavior |
4.3**** | 8. carboxylic acid catabolism |
3.0*** | ||||||
| 9. membrane transport |
4.2**** | 9. glutathione transferase activity, P450 |
2.9*** | ||||||
| 10. proteasome accessory complex |
3.7**** | 10. galactose metabolism |
2.9** | ||||||
| 11. glycolysis, alcohol metabolism |
3.7**** | 11. unsaturated FA synthesis |
2.9*** | ||||||
| 12. synaptic transmission |
3.3**** | 12. oxidoreductase activity, AA metabolism |
2.3*** | ||||||
| 13. glycogen biosynthetic process |
2.6*** | ||||||||
| 14. ion transport | 2.5**** | ||||||||
| 15. axon projection | 2.2** | ||||||||
| 14 day n = 67 (50) | 18 day n = 1224 (997) | 24 day n = 405 (316) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GOTerm | enrich score | GOTerm | enrich score | GOTerm | enrich score | ||||
| 1. ribosome, translation |
1.2 | 1. DNA repair | 7.8**** | 1. peroxisomal membrane |
3.0**** | ||||
| 2. oogenesis | 1.1 | 2. zinc finger, ion binding |
7.1**** | 2. translation, ribosome |
3.0**** | ||||
| 3. ATP binding | 4.3**** | 3. protein transport |
2.1* | ||||||
| 4. chaperonin, protein folding |
3.6*** | ||||||||
| 5. mitosis | 2.5** | ||||||||
| 6. Golgi apparatus | 2.2*** | ||||||||
| 7. phosphoinositide binding |
2.1*** | ||||||||
| 8. apoptosis regulation | 2.1* | ||||||||
| 9. protein transport | 2.1** | ||||||||
| 10. DNA helicase activity | 2.0** | ||||||||
| 11. meiosis | 2.0* | ||||||||
| 12. recombination | 1.8 | ||||||||
| 13. deoxyribonuclease activity |
1.7* | ||||||||
| 14. oocyte development | 1.7** | ||||||||
FDR
P < 0.05
P < 0.01
P < 0.005
P < 0.0001
Genome-wide variance in expression levels peaked at second larval instar and late pupal stages and remained relatively unchanged over adulthood (Fig. 4). Since sexes were pooled until day of eclosion, and only female adults were analyzed here, we could not separate variation due to sex-specific expression in preadult stages from other causes as a contributing factor to these variance increases. However, sex-specific differences in expression, particularly in pupae, were likely greater in germline than somatic tissues (Lebo et al. 2009).
Almost 21% of all predicted genes were at their maximum transcription levels in 6 h embryos (n = 2999, Fig. 3), and were significantly enriched for 26 different functional gene clusters (Table 3). The second transcriptional peak in late pupae to day of emergence involved 1886 and 1947 genes, respectively, and a third peak in 18 day old females revealed 1224 genes at maximum lifetime expression (Fig. 3). This lifetime pattern was quite similar to that of eigengene 1 (Fig. 2). Numbers of functionally annotated clusters identified in DAVID (Huang et al. 2009) in each life stage were strongly correlated with numbers of D. mojavensis genes with D. melanogaster orthologs (Pearson r = 0.95, t = 10.09, P < 0.0001). Here, the average proportion ± 1 SD of annotated genes was 0.64 ± 0.18, with a range from 0.37 in early pupae to 0.80 on the day of eclosion (Table 3).
Annotation clustering of genes with maximal lifetime expression levels uncovered the largest number of functional terms in the 6 h embryo stage (Table 3), in part because 79% of these early developmental genes were annotated. A diverse set of gene clusters involved with development, segmentation, nucleic acid metabolism, oogenesis, cellular metabolism, negative and positive regulation of biosynthesis and transcription, mitosis, morphogenesis, and imaginal disc development were significantly enriched. That meiotic gene expression was enriched in embryos has been previously observed (Mukai et al. 2006), and was due to genes associated with meiotic chromosome segregation, microtubule binding, and cell cycle dynamics (Table 3).
The transition from 6 h embryo to first instar revealed a precipitous decline in the numbers of genes with maximal expression, the proportion of genes with significant changes in expression from the embryo stage (Fig. 3, 4), and numbers of enriched gene clusters of diverse function (Table 3). The most enriched gene cluster in the first instar stage was associated with formation of the peritrophic membrane, a lining of a specialized extracellular matrix in the gut, indicating significantly increased expression of genes associated with feeding and digestion. Other enriched clusters included those annotated for ribosome assembly, increased metabolism, and development (Table 3). Genes that increased in expression from embryo to first instar stages were significantly enriched for functional clusters with membrane, chitin, cuticle and a number of other metabolic pathways and sensory systems associated with larval development (Suppl. Table 6A). This transition was also characterized by significant decreases in expression of many of the embryonic gene clusters with maximal gene expression (Table 3). Thus, the embryo to larval transition involved the largest down-regulation of genome-wide expression across the life cycle in D. mojavensis.
Maximum expression of second and third instar larval genes was enriched for similar functional clusters associated with growth and development (Table 3). Membrane receptor function, HOX gene regulation, and cuticle formation gene clusters were at maximum expression levels in second instar larvae accompanied by significantly increased transcription of cellular respiration, energy production, and fatty acid metabolism genes as in first instar larvae (Suppl. Table 6A), while expression of cell division and DNA repair genes significantly declined. Third instar larvae showed enrichment for genes at maximum expression for sensory perception of chemical stimuli and increased membrane receptor activity (Table 3) with decreased expression of metabolic pathways including energy, sugar, amino acid, lipid, and P450 metabolism (Suppl. Table 6A). Decreased transcription of endoplasmic reticulum genes and increased expression of cuticle structure, fat body associated ADH, and odorant binding genes was consistent with the continued trajectory of increasing larval growth and size prior to pupation.
From first instar to late pupae, the numbers of genes at maximum expression increased (Fig. 3) while the fractions of annotated genes at maximum expression levels decreased from 0.66 (432/658) in first instars to as low as 0.37 (602/1641) in early pupae (Table 3) suggesting increases in expression of lineage–specific D. mojavensis genes, i.e. those with no D. melanogaster orthologues, during late preadult development. The third instar to early pupa transition revealed a drastic reduction in metabolic rates where mitochondrial, oxidative phosphorylation, citric acid cycle, and sugar, lipid and amino acid metabolism genes showed significant decreases in expression (Suppl. Table 6A). DAVID also identified a gene cluster with 54 annotated D. melanogaster orthologs enriched for spermatogenesis that was significantly up-regulated from L3 to EP (Suppl. Tables 6A, 7) consistent with the known timing of testis development in D. melanogaster (Cooper 1950).
Significantly increased transcription of gene clusters enriched for mitochondrial and aerobic respiration function, the TCA cycle, and glycolysis (Suppl. Table 6A) was consistent with increases in metabolic rates in late pupae (Merkey et al. 2011). Increased expression of flight muscle genes (DeSimone et al. 1995; Fernandes et al. 1991) and associated mitochondrial genes, as well as gene clusters enriched for glycolysis, were accompanied by significant decreases in transcripts associated with DNA replication and RNA processing, DNA repair, sensory perception and steroid synthesis.
Almost 2000 genes were at maximum expression levels on the day of eclosion that accounted for 15 significantly enriched gene clusters, second only to the diversity of genes expressed in 6 h embryos across the entire life cycle (Fig. 3, Table 3). Highly significant GOterms included cellular respiration, mitochondrial and TCA cycle function, vision, adult movement, and other metabolic functions (Table 3). Eighty percent of genes showing increased expression from late pupa to day of emergence were annotated, and were functionally enriched for a number of metabolic functions including membrane transport, ATP binding, protein transport and catabolism, mitochondrial function and biogenesis, growth, and others (Suppl. Table 6A). Up-regulation of fatty acid metabolism was also apparent in KEGG pathway analysis (Kanehisa & Goto 2000). The transition from late pupae to emergence was characterized by decreases in cytoskeleton formation, ion transport, peptidase activity, and KEGG pathways involving carbohydrate and glutathione metabolism, and oxidative phosphorylation. An annotated cluster of 21 taste and olfactory receptor genes showed significant decreases in expression from late pupa to eclosion (Suppl. Table 6A). This was a subset of the 94 sensory, taste, and olfactory orthologs that were significantly up-regulated from egg to first instar (Table 2). Thus, the pupa-adult transition involved a large decrease in expression of sensory genes that were up-regulated in early larval stages.
From eclosion into adulthood, far fewer genes were expressed at maximum lifetime levels, except in day 18 adults, and there was a corresponding decrease in the numbers of genes showing significant decreases/increase in expression between sampling points (Fig. 3, Suppl. Table 6). In three day old adults, genes at maximum lifetime expression were functionally enriched for diverse metabolic functions including fatty acid metabolism, iron ion binding, sugar metabolism, carboxylic acid catabolism, and P450 activity (Table 3), and there were significant increases in gene expression for DNA replication, cell division, ribosome manufacture, egg production, and DNA repair (Suppl. Table 6A). In six day-old adults, fewer genes were at maximal expression (Fig. 3) and these were enriched for vitamin and cofactor binding, steroid hormone manufacture, and oogenesis. Three to six days is approximately the age at first reproduction for D. mojavensis females depending on temperature and nutrition (Etges & Klassen 1989; Markow 1982). Also seen in the transition from three to six day old adults were 621 genes associated with oogenesis, meiosis, cell division, DNA repair, and down-regulation of metabolism, as well as decreased expression of cuticle formation, immune response, melanin metabolism, sugar transport, and muscle development genes (Suppl. Table 6A) suggesting decreasing gene expression associated with somatic maintenance with the onset of female reproduction.
From the 6 to 10 day and 10 to 14 day intervals, there were continuing decreases in expression for cuticle gene expression, immune response, melanin metabolism, and muscle formation, and few significant increases in gene expression. Sixty-seven genes were at maximal expression levels at day 14 (Fig. 3) that were enriched for translation and oogenesis (Table 3). A larger number of genes, 1224, were at maximal lifetime expression at day 18 that were enriched for genes associated with aging including DNA repair, protein chaperones, DNAase activity, and apoptosis regulation, as well as control of gene regulation and oocyte development. Significant decreases in expression from 14 to 18 days involved gene clusters enriched for signal peptides, lipid synthesis, microsome-associated iron binding, immune response, and a number of other cellular catalytic functions (Suppl. Table 6A). Many of these same gene clusters were then up-regulated from 18 to 24 days (Fig. 2), including antimicrobial peptides, immune response, lipid metabolism, and microsome-associated iron binding, as well as amino acid metabolism, glutathione metabolism (KEGG), and P450 activity suggesting further regulatory changes associated with aging, increased oxidative stress, and immune response to microbes (Table 3, Suppl. Table 6A). This “late life” transition in gene expression from 14 to 18 to 24 days was also observed in both eigengenes 2 and 3 (Fig. 2).
Assessing differential expression between ‘young’ and ‘old’ adults (3-6 day vs. 18+ day) revealed changes consistent with other studies of aging (see de Magalhães et al. 2009 for a review). In ‘old’ samples, there was increased gene expression in DNA repair, DNA replication, stress response, mitosis and meiosis, and decreased expression of electron transport chain, muscle development, signaling and transport, hormone binding and locomotor genes (Suppl. Table 6B). However, as seen above, this simple young-old comparison missed the non-monotonic trajectories of expression through adulthood, particularly the ‘late life’ transitions observed between ages 14 and 18, and 18 and 24 (Fig. 2).
Host cactus effects on gene expression across the life cycle
Both host cactus and population effects influenced preadult stage-specific patterns of gene expression (fold change > 1.5 X and FDR P < 0.05). From the embryo stage to eclosion, there were significant differences between these two populations in the timing of differentially expressed genes due to host cactus (Table 4). Variation in egg to adult development time and viability in this experiment (Suppl. Tables 8, 9; Suppl. Fig. 1) was consistent with previous studies, so transcriptional variation here should help to identify causes of cactus-influenced shorter development times and higher viabilities of Baja populations vs. those on the mainland (Etges 1990; Etges et al. 2010). Cactus rearing substrates caused expression levels to differ in first and second instar stages in the mainland population, but in the Baja California population most transcriptional differences due to cactus occurred in early and late pupal stages (Table 5; Suppl. Table 10). There were no differences in the numbers of genes showing significant up/down transcription differences due to agria or organ pipe substrates (paired t = 0.66, P > 0.05), and the average proportion of predicted D. mojavensis genes with D. melanogaster orthologs that were influenced by rearing substrates for these two populations ranged from 55 to 64 percent.
Table 4.
Total numbers of genes differentially expressed (fold change > 1.5 X and FDR < 0.05) due to cactus rearing substrates in preadult stages, from egg to eclosion, in two populations of D. mojavensis reared on two cactus hosts. Contrasts indicate numbers of genes overexpressed due to organ pipe (OP) vs. agria (AG) cactus, OP>AG, and vice versa. Life stages are eggs (6 hr), L1 - first instar larvae (48 hr), L2 - second instar larvae (144 hr), L3 - third instar larvae (240 hr), EP - early pupae (288 hr), LP - late pupae (384 hr), and 0D – adults at day of eclosion.
| Life stage | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | Egg | L1 | L2 | L3 | EP | LP | 0D | |||||||
| Las Bocas * | OP>AG | AG>OP | OP>AG | AG>OP | OP>AG | AG>OP | OP>AG | AG>OP | OP>AG | AG>OP | OP>AG | AG>OP | OP>AG | AG>OP |
| D. mojavensis genes | - | - | 570 | 631 | 61 | 15 | - | 3 | 3 | 2 | - | - | 1 | - |
| D. melanogaster orthos | - | - | 222 | 529 | 53 | 9 | - | 3 | 1 | 2 | - | - | 0 | - |
| Proportion annotated | - | - | 0.389 | 0.838 | 0.869 | 0.6 | - | 1 | 0.33 | 1 | - | - | 0 | - |
| Average % annotated | 0.637 | |||||||||||||
| Population | Egg | L1 | L2 | L3 | EP | LP | 0D | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Punta Prieta # | OP>AG | AG>OP | OP>AG | AG>OP | OP>AG | AG>OP | OP>AG | AG>OP | OP>AG | AG>OP | OP>AG | AG>OP | OP>AG | AG>OP |
| D. mojavensis genes | 4 | 4 | 1 | 4 | - | - | - | - | 15 | 21 | 213 | 132 | - | 4 |
| D. melanogaster orthos | 0 | 1 | 0 | 4 | - | - | - | - | 2 | 14 | 137 | 59 | - | 3 |
| Proportion annotated | 0 | 0.25 | 0 | 1 | - | - | - | - | 0.13 | 0.67 | 0.643 | 0.447 | - | 0.75 |
| Average % annotated | 0.553 | |||||||||||||
mainland Sonora
Baja California
Table 5.
Gene ontology results for predicted genes that were differentially expressed (>1.5 fold difference, FDR P < 0.05) from the egg stage to day of eclosion in two populations of D. mojavensis reared on agria and organ pipe cacti. Life stages are defined in Table 3 and individual genes and their D. melanogaster ortholog names are listed when only several genes differed in expression and no functional clustering was observed in DAVID (Huang et al., 2009).
| Population | ||||||||
|---|---|---|---|---|---|---|---|---|
| Las Bocas (mainland) | Punta Prieta (Baja California) | |||||||
| OP>AG | AG>OP | OP>AG | AG>OP | |||||
| GOTerm (gene) | enrich score | GOTerm (gene) | enrich score | GOTerm (gene) | enrich score | GOTerm (gene) | enrich score | |
| Egg | - | - | - | - | - | - | GI22080 - Cep78 protein | - |
| L1 | 1. cuticle, chitin structure | 5.9**** | 1. transport | 5.9**** | - | - | GI24491 – Rheb | - |
| 2. cell metabolism | 4.3**** | GI24142 - Rab-protein 7 – GTPase mediated signal transduction | ||||||
| 2. Odorant binding, olfactory reception | 2.1*** | 3. protein transport | 3.5**** | |||||
| 4. Oxphos process/ ion transport | 3.4**** | |||||||
| 5. mitotic spindle/ribosome protein | 3.3**** | GI10537 - alternative testis transcripts open reading frame A | ||||||
| 6. cell morphogenesis | 3.0*** | |||||||
| 7. RNA binding | 3.0** | |||||||
| 8. larval development | 2.9** | GI17029 - split ends - post-embryonic morphogenesis | ||||||
| 9. actin filament processes | 2.9** | |||||||
| 10. protein synthesis | 2.5** | |||||||
| 11. RNA splicing | 2.5* | |||||||
| 12. axon development | 2.2** | |||||||
| 13.mitochondria transport peptide | 2.1** | |||||||
| 14. germ cell develop. | 1.8** | |||||||
| L2 | 1. cytoskeleton, ribosomal protein | 1.1 | 1. metal ion binding | 0.5 | - | - | - | - |
| L3 | - | - | GI16482 - ribosomal protein L40 | - | - | - | - | - |
| GI12755 – Rh50 (membrane transport) | ||||||||
| GI11050 – yu (ptn and RNA binding | ||||||||
| EP | GI11311 - GNBP2 (Gram-negative bacteria binding protein 2) | GI18191 – fbp (fructose-1,6-bisphosphatase) | - | GI24313 - Cpr30F (pupal chitin structure) | 1. ubl conjugation pathway, proteolysis | 2.0 * | ||
| GI15376 - mesoderm development | GI19286 | |||||||
| GI24782+ | ||||||||
| GI12221+ | ||||||||
| LP | - | - | - | - | 1. mitochondrial membrane | 1.6 | 1. cuticle structure | 1.2 |
| 0D | GI23541+ | - | - | - | - | - | GI16633 – heat shock ptn/metal ion binding | - |
| GI24251 – nucleotide binding | ||||||||
| GI16531 - Dpy-30 (adenylate kinase) | ||||||||
| GI19042 | ||||||||
False Discovery rate P values
P < 0.05
P < 0.01
P < 0.001
P < 0.0001
gene of unknown function
While just eight genes in 6 h embryos showed significant expression differences due to cactus (Table 5), just GI22080 was annotated, a Cep78 homolog, a centrosomal protein (The-UniProt-Consortium 2011) that showed increased transcription due to agria cactus (Table 5). A larger number of orthologs in first (n = 1201) and second (n = 76) instar larvae were differentially expressed in this mainland population than in the Baja population due to cactus substrates. Organ pipe cactus caused increased transcription of cuticle and odorant binding-related orthologs in first instar larvae, and moderate increases in expression in genes associated with cytoskeleton and ribosome function in second star larvae compared to agria-reared larvae. Agria cactus caused greater expression of a broad range of significantly enriched genes in first instar larvae associated with growth and development including protein transport, cell division, and ion transport than organ pipe cactus (Table 5). Just four genes of diverse function in Baja California first instar larvae were significantly over-expressed due to agria cactus, and few third instar genes showed any effect of cactus on expression levels. Of the 381 early and late pupal genes showing expression differences due to cactus, significant enrichment for ubiquitin conjugation function (proteolysis) genes was observed, as well as genes responsible for cuticle structure and mitochondrial membrane function. Thus, agria cactus caused increased expression of a broader spectrum of genes in different parts of the preadult life cycle than organ pipe cactus, particularly those associated with early larval development and metabolism, but the overall number of genes with significantly different levels of expression influenced by cactus was small.
For adults, samples were pooled across ages and variation in expression levels was assessed with a mixed model ANOVA with population, cactus, and population × cactus effects where cactus was a fixed effect. Organ pipe cactus caused increased expression of genes enriched for neurotransmitter binding, circadian rhythm, and courtship and mating behavioral functions. Mainland females showed significantly increased expression of genes enriched for fatty acid metabolism genes and iron binding functions, such as P450 genes associated with xenobiotic detoxification. Baja females had higher overall expression of genes associated with transcription than mainland females (Etges 2014). Overall, these patterns of differential gene expression in populations of D. mojavensis were influenced by cactus rearing substrates in both preadult and adult stages, where different cactus substrates influenced expression of a greater number of orthologs in preadult stages than in adults.
Discussion
The holometabolous life cycle of D. mojavensis is marked by major transitions in the expression of functional clusters of genes similar to those in D. melanogaster (Arbeitman et al. 2002; Graveley et al. 2011; Tennessen & Thummel 2011). SVD captured a portrait of gene expression throughout preadult development and adult aging in D. mojavensis expressed in environments designed to simulate natural conditions, yet these eigengenes were not overly sensitive to population or host cactus differences. This suggests that population origin and cactus substrates influenced expression for relatively small numbers of genes in relation to the major developmental transitions in gene expression, i.e. the life cycle transcriptome of D. mojavensis is relatively well buffered from differences in its host plants and has yet to become strongly geographically differentiated.
Population and cactus differences have previously been shown to influence both egg to adult development time and average longevity in adults in D. mojavensis (Etges 1990; Jaureguy & Etges 2007). Our sampling from egg to eclosion was stage-based, not age-based, and so would not reveal expression differences related to rate of development. Our sampling of adults was necessarily destructive, so it was not possible to infer whether gene expression levels of adults at different ages were related to their ultimate lifespans.
However, our experimental design included just four replicate samples at each age/stage with often surprisingly high within-age variance, so perhaps some influences of these environmental factors may be distinguishable with increased replication. The top four eigengenes revealed three major transitions, i.e., from embryos to larvae, larvae to pupae, and pupae to adults (Fig. 2). Thus, the latent patterns of biological organization and function revealed in eigengene analysis (Alter 2006; Ponnapalli et al. 2011) of the variation in lifetime gene expression occurred between, rather than within life stage types, and were uncovered through functional gene ontology clustering.
All four eigengenes were significantly influenced by variation in ribosome function and protein production (Table 1) suggesting that life cycle SVD analyses, when used to compare patterns of life gene expression in this and other organisms, may reveal fundamental insights into the general processes of development and senescence. Eigengene 1 encompassed most of overall transcriptional variation (63.5 %) due to increased expression of larval and pupal endopeptidases, embryonic and adult protein production and transport, as well as sensory perception (Fig. 2, Tables 1, 3, Suppl. Table 6). While biased due to the lack of annotation for ca. a third of predicted genes, life cycle shifts in protein metabolism and tissue remodeling were not surprisingly major sources of variation in lifetime gene expression.
In addition, a large number of gustatory, odorant binding, olfactory receptor, ion transport, and photoreceptor gene orthologs that increased in expression from embryo to first instar larvae, and then were down-regulated in adults were also highly correlated with eigengene 1 and revealed in comparisons of consecutive life cycle stages (Table 2). While adult sensory perception has been intensively studied because of its roles in chemical, host plant attraction/repulsion, and adult mating behavior (Amrein 2004; Carlson 1996; Olsson et al. 2006; Stokl et al. 2010; Thistle et al. 2012), sensory perception in preadult stages has been less well studied, but is known to be a determinant of successful larval feeding behavior, growth, and attainment of body critical mass prior to pupation (Beadle et al. 1938; Tennessen & Thummel 2011). The functional consequences of sensory genes across the life cycle in Drosophila species have been documented (Cobb 1999; c.f. de Belle et al. 1989; Gerber & Stocker 2007; Kent et al. 2009; Matsuo et al. 2007), but rarely in flies reared under natural conditions. In larvae, foraging behaviors are facilitated by chemical perception (Fishilevich et al. 2005) and thus resource acquisition during larval development. While D. mojavensis larval behavior in the wild has not been well studied (but see Fogleman et al. 1981), our results suggest that further study of the expression and evolution of these sensory gene families may help to unravel sensory behavior variation in nature and how it is related to resource exploitation, i.e. the cactus-influenced preadult life history differences between Baja California and mainland populations (Etges 1990, 1993; Etges et al. 2010). These patterns were far more subtle in the stage-specific GO clustering analyses (Table 1, 2) exemplifying the utility of SVD, and also emerged in analyses of host cactus effects (Table 4, 5).
Most emphasis on understanding host cactus preferences and subsequent larval and adult performance in desert Drosophila has been on production of and attraction to cactus fermentation by-products (Etges & Klassen 1989; Fanara et al. 1999; Newby & Etges 1998; Starmer 1982; Starmer et al. 1977) xenobiotic metabolism of cactus secondary compounds (Fogleman et al. 1998; Fogleman & Heed 1989; Matzkin 2008), and host cactus resource availability (Etges 1990; Etges et al. 2010; Heed & Mangan 1986). For D. mojavensis, use of agria and organ pipe cacti in the Sonoran Desert is due largely to its tolerance of medium sized fatty acids (C6 - C18, but most are C10 - C12 (Fogleman & Kircher 1986)), sterol diols, and high levels of triterpene glycosides. It can also tolerate the isoquinoline alkaloids present in the rarely used alternate hosts saguaro, Carnegiea gigantea, and cardón, Pachycereus pringlei, cacti (Fogleman & Danielson 2001), but secondary compounds of other alternate hosts, e.g. California barrel cactus, Ferocactus cylindraceus, sina cactus, S. alamosensis, cochal cactus, Myrtillocactus cochal in Baja California, and Opuntia species on Santa Catalina Island have not been as intensively studied. While differences between agria and organ pipe cacti on overall gene expression were sometimes small, there were significant preadult stage-specific differences in gene expression between populations (Table 5) and population and cactus effects on adult gene expression (Etges 2014). There was little evidence of cactus-induced differences in expression of detoxification genes in preadult stages (Table 5), but there was significantly greater enrichment of P-450 genes in adult mainland females reared on organ pipe cactus. Thus, larvae were less sensitive to differences in cactus secondary compounds than adults, perhaps helping to explain genetic evidence for host plant generalism in larval performance in D. mojavensis (Etges 1993).
Other ecological aspects of cactus rots influencing larval growth and development involving sensory perception include selective foraging and predator/parasite avoidance. Larval D. mojavensis prefer particular yeast species over others in naturally occurring rots, so larval olfactory and gustatory receptors are likely to be directly involved with foraging preferences (Fogleman et al. 1981). In addition to bacteria and yeasts, cactus rots comprise a complex fermenting environment of degraded cactus tissues, secondary compounds, volatiles, and other invertebrates as rots progress from early bacterial fermentation, but interactions between these organisms and drosophilids have only been partially assessed (Escalante & Benado 1990; Kiontkeet al. 2011; Mangan 1979; Polak 1998). Thus, understanding patterns of gustatory, odorant binding, olfactory receptor, and photoreceptor gene expression throughout the life cycle in D. mojavensis may contribute to our general understanding of patterns of resource use, life history variation, and host plant adaptation in natural populations of Drosophila.
Expression of life histories in contrasting environments
Central to a general understanding of life history evolution are the consequences of lifetime differences in environmental variability on survivorship and reproduction, and uncovering the environment-dependent expression of genetic variation underlying these components of fitness. Genetic differences in life histories between Baja California and mainland populations of D. mojavensis are host plant dependent, and thought to be influenced by differences in resource predictability at different stages of the life cycle (Etges 1990, 1993; Etges et al. 1999; Heed 1978, 1981). Cactus substrate-influenced development time differences between populations (Suppl. Fig. 1, Suppl. Table 8) were accompanied by larval, stage-specific differences in gene expression (Table 4, 5). Consistent with the increased development times of organ pipe-reared Las Bocas (mainland) flies and a Population × Cactus interaction, organ pipe-reared first instar larvae were enriched for 14 down-regulated gene clusters associated with larval development and metabolism. A handful of annotated orthologs were also down-regulated in the Punta Prieta, Baja California population due to organ pipe cactus, including GI17029, a D. melanogaster ortholog of split ends, involved in nucleic acid binding and postembryonic development (Table 5). Thus, decreased expression of developmental genes due to organ pipe cactus and increased expression of larval cuticle and olfactory reception genes in first instar larvae (Table 4, 5) suggests longer mainland development times result in part from transcriptional events early in larval development. Just a few gene clusters were functionally enriched for proteolysis associated with metamorphosis including the ubl conjugation pathway, and cuticle structure, where organ pipe cactus again caused reduced transcription levels in Baja flies. Several of these functional clusters including genes responsible for nucleic acid binding, cuticle proteins, and larval growth and metabolism were correlated with a tradeoff between larval mass and survival in D. melanogaster (Bochdanovits & de Jong 2004) suggesting there may be a shared genetic basis for preadult growth rates in these species.
In adults, co-expression of genes associated with aging and age-specific reproduction was revealed by different eigengenes, patterns of maximum lifetime gene expression (Table 3) and in pairwise comparisons between adjacent ages (Suppl. Table 6). From a positive eigengene 2 peak at eclosion through 18 to 24 days (Fig. 2), there was a monotonic shift from eclosion onwards reflecting shifts in neural functioning, cellular maintenance, metabolic rates, and P450 activity through adulthood (Suppl. Table 2). Also at day 18, there were 14 significantly enriched gene clusters based on genes at maximum lifetime expression levels (Table 3), most that were associated with aging related traits, patterns strikingly similar to those in D. melanogaster and Caenorhabditis elegans (McCarroll et al. 2004). After 18 days, there was significantly increased expression of five gene clusters enriched for antimicrobial peptides, immune response, lipid metabolism, membrane function, and further P450 activity (Suppl. Table 6) similar to replicate lines of D. melanogaster selected for late life reproduction (Remolina et al. 2012). The noticeable late life shift in eigengene 2 and 3 expression at 18 days was due in part to increased expression of genes responsible for DNA repair, protein chaperones, signal transduction, ATP production, apoptosis, and others. Thus, D. mojavensis females at ca three weeks of age reared on fermenting cactus exhibited transcriptional shifts associated with physiological signs of increased cellular maintenance and protection from microbes and harmful chemical compounds.
A classic life history tradeoff between somatic maintenance and reproduction was evident in decreases in gene expression associated with somatic maintenance with the onset of female reproduction. At the onset of sexual maturity at three to six days (Supple. Table 6A), increased expression of 621 genes were functionally enriched for reproduction and DNA repair, and showed decreased expression of cuticle formation, immune response, melanin metabolism, sugar transport, and muscle development genes (Supple. Table 6A). However, there was little evidence for down-regulation of genes associated with egg production as in D. melanogaster where decreases in transcript abundance of chorion formation genes with increasing age has been observed (Pletcher et al. 2002). Likely explanations for this are; 1) D. mojavensis females cultured on fermenting cactus and ethanol vapor rarely live more than 30 days and so may not reach reproductive senescence vs. the 60 + day survivorship of D. melanogaster cultured on artificial media, 2) our adult female D. mojavensis were unmated, so it is unlikely that we would expect to observe realistic lifetime shifts in expression of gene clusters associated with mating and egg production because female longevity, fecundity, and metabolism are significantly influenced by mating and remating (Etges & Heed 1992; Markow et al. 1990).
Conclusions
Comparative life cycle studies of genomic expression in different organisms are imperative for characterizing the genetic architecture and ontogeny of gene expression responsible for the life history variation we seek to understand. Only then can we evaluate the expression of genomic elements responsible for fitness tradeoffs and senescence in relation to phenotypic variation in life histories. Despite the limitations of genome annotation for most non-model species, SVD analysis successfully resolved many of the major developmental and adult shifts in the expression of correlated groups of genes from embryogenesis through senescence in this model insect. Ideally, future whole genome expression SVD studies should involve direct comparisons of the same life stages and ages under controlled environmental conditions. Although few whole genome studies assessing such life cycle variation have been performed under natural conditions for comparison, the transcriptome of D. mojavensis reared on two of its major host cacti throughout its life cycle has revealed similar core developmental transitions to those in D. melanogaster. However, there remains a significant fraction of the genome that is still unknown due to limited gene annotation, much that is necessary for understanding subtle expression differences due to population or host plants (Table 5). This will limit future comparative studies whether microarrays or other transcriptome methods are used.
Supplementary Material
Acknowledgements
We thank A. J. Marlon and G. Almeida for excellent technical assistance, C. Ross for statistical help, and D. Reznick and three reviewers for helpful comments on the manuscript. Funding was provided by National Science Foundation grant EF-0723930 to A. G. Gibbs and W. J. Etges, a grant from the Center on the Economics and Demography of Aging (CEDA) – University of California, Berkeley to S. Tuljapurkar and W. J. Etges, and National Institute of Aging grant R24AG039345 to S. Tuljapurkar. The UNLV Genomics Core Facility was supported by grants from the National Center for Research Resources (5P20RR016464-11) and the National Institute of General Medical Sciences (8P20GM103440-11).
Footnotes
Data archiving
MIAME compliant microarray data have been deposited at Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) with accession number GSE54510.
Literature Cited
- Alcorn SM, Orum TV, Steigerwalt AG, et al. Taxonomy and pathogenicity of Erwinia cacticida sp. nov. International Journal of Systematic Bacteriology. 1991;41:197–212. doi: 10.1099/00207713-41-2-197. [DOI] [PubMed] [Google Scholar]
- Alter O. Discovery of principles of nature from mathematical modeling of DNA microarray data. Proceedings of the National Academy of Sciences. 2006;103:16063–16064. doi: 10.1073/pnas.0607650103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter O, Brown PO, Botstein D. Singular value decomposition for genome-wide expression data processing and modeling. Proceedings of the National Academy of Sciences (USA) 2000;97:10101–10106. doi: 10.1073/pnas.97.18.10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter O, Brown PO, Botstein D. Generalized singular value decomposition for comparative analysis of genome-scale expression data sets of two different organisms. Proceedings of the National Academy of Sciences. 2003;100:3351–3356. doi: 10.1073/pnas.0530258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein H. Pheromone perception and behavior in Drosophila. Current Opinion in Neurobiology. 2004;14:435–442. doi: 10.1016/j.conb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Anderson JT, Lee C-R, Rushworth CA, Colautti RI, Mitchell-Olds T. Genetic trade-offs and conditional neutrality contribute to local adaptation. Molecular Ecology. 2013;22:699–708. doi: 10.1111/j.1365-294X.2012.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeitman MN, Furlong EEM, Imam F, et al. Gene expression during the life cycle of Drosophila melanogaster. Science. 2002;297:2270–2275. doi: 10.1126/science.1072152. [DOI] [PubMed] [Google Scholar]
- Beadle GW, Tatum EL, Clancy CW. Food level in relation to rate of development and eye pigmentation in Drosophila melanogaster. Biological Bulletin. 1938;75:447–462. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B. 1995;57:289–300. [Google Scholar]
- Bochdanovits Z, de Jong G. Antagonistic pleiotropy for life-history traits at the gene expression level. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2004;271:S75–S78. doi: 10.1098/rsbl.2003.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on bias and variance. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Brazner JC, Etges WJ. Pre-mating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. II. Effects of larval substrates on time to copulation, mate choice, and mating propensity. Evolutionary Ecology. 1993;7:605–624. [Google Scholar]
- Carlson JR. Olfaction in Drosophila: from odor to behavior. Trends in Genetics. 1996;12:175–180. doi: 10.1016/0168-9525(96)10015-9. [DOI] [PubMed] [Google Scholar]
- Caswell H. Phenotypic plasticity in life-history traits: Demographic effects and evolutionary consequences. American Zoologist. 1983;23:35–46. [Google Scholar]
- Caswell H. Stage, age and individual stochasticity in demography. Oikos. 2009;118:1763–1782. [Google Scholar]
- Cobb M. What and how do maggots smell? Biological Reviews. 1999;74:425–459. [Google Scholar]
- Cooper KW. Normal spermatogenesis in Drosophila. In: Demerec M, editor. Biology of Drosophila. John Wiley & Sons; New York: 1950. pp. 1–61. [Google Scholar]
- de Belle JS, Hilliker AJ, Sokolowski MB. Genetic localization of foraging (for): A major gene for larval behavior in Drosophila melanogaster. Genetics. 1989;123:157–164. doi: 10.1093/genetics/123.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhães JP, Budovsky A, Lehmann G, et al. The Human Ageing Genomic Resources: online databases and tools for biogerontologists. Aging Cell. 2009;8:65–72. doi: 10.1111/j.1474-9726.2008.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone S, Coelho C, Roy S, VijayRaghavan K, White K. ERECT WING, the Drosophila member of a family of DNA binding proteins is required in imaginal myoblasts for flight muscle development. Development. 1995;121:31–39. doi: 10.1242/dev.122.1.31. [DOI] [PubMed] [Google Scholar]
- Escalante A, Benado M. Predation on the cactophilic fly, Drosophila starmeri, in the columnar cactus, Pilosocereus lanuginosus. Biotropica. 1990;22:48–50. [Google Scholar]
- Etges WJ. Divergence in cactophilic Drosophila: The evolutionary significance of adult ethanol metabolism. Evolution. 1989;43:1316–1319. doi: 10.1111/j.1558-5646.1989.tb02579.x. [DOI] [PubMed] [Google Scholar]
- Etges WJ. Direction of life history evolution in Drosophila mojavensis. In: Barker JSF, Starmer WT, MacIntyre RJ, editors. Ecological and Evolutionary Genetics of Drosophila. Plenum; New York: 1990. pp. 37–56. [Google Scholar]
- Etges WJ. Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. Evolution. 1992;46:1945–1950. doi: 10.1111/j.1558-5646.1992.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Etges WJ. Genetics of host-cactus response and life-history evolution among ancestral and derived populations of cactophilic Drosophila mojavensis. Evolution. 1993;47:750–767. doi: 10.1111/j.1558-5646.1993.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Etges WJ. Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. IV. Correlated responses in behavioral isolation to artificial selection on a life history trait. The American Naturalist. 1998;152:129–144. doi: 10.1086/286154. [DOI] [PubMed] [Google Scholar]
- Etges WJ. No boundaries: genomes, organisms, and ecological interactions responsible for divergence and reproductive isolation. Journal of Heredity. 2014;105:756–770. doi: 10.1093/jhered/esu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etges WJ, Ahrens MA. Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. V. Deep geographic variation in epicuticular hydrocarbons among isolated populations. The American Naturalist. 2001;158:585–598. doi: 10.1086/323587. [DOI] [PubMed] [Google Scholar]
- Etges WJ, de Oliveira CC. Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. X. Age-specific dynamics of adult epicuticular hydrocarbon expression in response to different host plants. Ecology and Evolution. 2014 doi: 10.1002/ece3.1088. doi:10.1002/ece3.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etges WJ, de Oliveira CC, Noor MAF, Ritchie MG. Genetics of incipient speciation in Drosophila mojavensis. III. Life history divergence and reproductive isolation. Evolution. 2010;64:3549–3569. doi: 10.1111/j.1558-5646.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- Etges WJ, Heed WB. Sensitivity to larval density in populations of Drosophila mojavensis: Influences of host plant variation on components of fitness. Oecologia. 1987;71:375–381. doi: 10.1007/BF00378710. [DOI] [PubMed] [Google Scholar]
- Etges WJ, Heed WB. Remating effects on the genetic structure of female life histories in populations of Drosophila mojavensis. Heredity. 1992;68:515–528. doi: 10.1038/hdy.1992.74. [DOI] [PubMed] [Google Scholar]
- Etges WJ, Johnson WR, Duncan GA, Huckins G, Heed WB. Ecological genetics of cactophilic Drosophila. In: Robichaux R, editor. Ecology of Sonoran Desert Plants and Plant Communities. University of Arizona Press; Tucson: 1999. pp. 164–214. [Google Scholar]
- Etges WJ, Klassen CS. Influences of atmospheric ethanol on adult Drosophila mojavensis: Altered metabolic rates and increases in fitness among populations. Physiological Zoology. 1989;62:170–193. [Google Scholar]
- Fanara JJ, Fontdevila A, Hasson E. Oviposition preference and life history traits in cactophilic Drosophila koepferae and D. buzzatti in association with their natural hosts. Evolutionary Ecology. 1999;13:173–190. [Google Scholar]
- Fernandes J, Bate M, VijayRaghavan K. Development of the indirect flight muscles of Drosophila. Development. 1991;113:67–77. doi: 10.1242/dev.113.1.67. [DOI] [PubMed] [Google Scholar]
- Fiedler TJ, Hudder A, McKay SJ, et al. The transcriptome of the early life history stages of the California sea hare Aplysia californica. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2010;5:165–170. doi: 10.1016/j.cbd.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filchak KE, Etges WJ, Besansky NJ, Feder JL. Ecological genetics of host use in diptera. In: Yeates DK, Wiegman BM, editors. The Evolutionary Biology of Flies. Columbia University Press; 2005. pp. 340–370. [Google Scholar]
- Fishilevich E, Domingos AI, Asahina K, et al. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Current Biology. 2005;15:2086–2096. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Fogleman JC, Danielson PB. Chemical interactions in the cactus-microorganism-Drosophila model system of the Sonoran Desert. American Zoologist. 2001;41:877–889. [Google Scholar]
- Fogleman JC, Danielson PB, MacIntyre RJ. The molecular basis of adaptation in Drosophila: the role of cytochrome P450s. Evolutionary Biology. 1998;30:15–77. [Google Scholar]
- Fogleman JC, Heed WB. Columnar cacti and desert Drosophila: the chemistry of host plant specificity. In: Schmidt JO, editor. Special Biotic Relationships of the Southwest. Univ. New Mexico Press; Albuquerque: 1989. pp. 1–24. [Google Scholar]
- Fogleman JC, Kircher HW. Differential effects of fatty acid chain length on the viability of two species of cactophilic Drosophila. Comparative Biochemistry and Physiology. 1986;83A:761–764. [Google Scholar]
- Fogleman JC, Starmer WT, Heed WB. Larval selectivity for yeast species by Drosophila mojavensis in natural substrates. Proceedings of the National Academy of Sciences (USA) 1981;78:4435–4439. doi: 10.1073/pnas.78.7.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastil RG, Phillips RP, Allison EC. Reconnaissance geology of the state of Baja California. Geological Society of America Memoir. 1975;140:1–170. [Google Scholar]
- Gerber B, Stocker RF. The Drosophila larva as a model for studying chemosensation and chemosensory learning: A review. Chemical Senses. 2007;32:65–89. doi: 10.1093/chemse/bjl030. [DOI] [PubMed] [Google Scholar]
- Ghosh D. Resampling methods for variance estimation of singular value decomposition analyses from microarray experiments. Functional & Integrative Genomics. 2002;2:92–97. doi: 10.1007/s10142-002-0047-5. [DOI] [PubMed] [Google Scholar]
- Gibson G. The environmental contribution to gene expression profiles. Nature Reviews Genetics. 2008;9:575–581. doi: 10.1038/nrg2383. [DOI] [PubMed] [Google Scholar]
- Gillespie JH, Turelli M. Genotype–environment interactions and the maintenance of polygenic variation. Genetics. 1989;121:129–138. doi: 10.1093/genetics/121.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley BR, Brooks AN, Carlson JW, et al. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471:473–479. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AP, Lewontin RC. A study of reaction norms in natural populations of Drosophila pseudoobscura. Evolution. 1982;36:934–948. doi: 10.1111/j.1558-5646.1982.tb05464.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson L. Lifetime reproductive success and heritability: empirical support for Fisher's fundamental theorem. The American Naturalist. 1986;128:761–764. [Google Scholar]
- Hamilton WD. The moulding of senescence by natural selection. Journal of Theoretical Biology. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Heed WB. Ecology and genetics of Sonoran Desert Drosophila. In: Brussard PF, editor. Ecological Genetics: The Interface. Springer-Verlag; New York: 1978. pp. 109–126. [Google Scholar]
- Heed WB. Central and marginal populations revisited. Drosophila Information Service. 1981;56:60–61. [Google Scholar]
- Heed WB. The origin of Drosophila in the Sonoran Desert. In: Barker JSF, Starmer WT, editors. Ecological Genetics and Evolution: The Cactus-Yeast-Drosophila Model System. Academic Press; Sydney: 1982. pp. 65–80. [Google Scholar]
- Heed WB, Mangan RL. Community ecology of the Sonoran Desert Drosophila. In: Ashburner M, Carson HL, Thompson JN, editors. The Genetics and Biology of Drosophila. Academic Press; New York: 1986. pp. 311–345. [Google Scholar]
- Hodgins-Davis A, Townsend JP. Evolving gene expression: from G to E to G x E. Trends in Ecology & Evolution. 2009;24:649–658. doi: 10.1016/j.tree.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protocols. 2009;4(1):44–45. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Istock CA, Zisfein J, Vavra KJ. Ecology and evolution of the pitcher-plant mosquito. 2. The substructure of fitness. Evolution. 1976;30:535–547. doi: 10.1111/j.1558-5646.1976.tb00931.x. [DOI] [PubMed] [Google Scholar]
- Jaureguy LM, Etges WJ. Assessing patterns of senescence in Drosophila mojavensis reared on different host cacti. Evolutionary Ecology Research. 2007;9:91–107. [Google Scholar]
- Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent CF, Daskalchuk T, Cook L, Sokolowski MB, Greenspan RJ. The Drosophila foraging gene mediates adult plasticity and gene-environment interactions in behaviour, metabolites, and gene expression in response to food deprivation. PLoS Genetics. 2009;5:e1000609. doi: 10.1371/journal.pgen.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SN, Rhee J-H, Song Y-H, et al. Age-dependent changes of gene expression in the Drosophila head. Neurobiology of Aging. 2005;26:1083–1091. doi: 10.1016/j.neurobiolaging.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Kiontke K, Felix M-A, Ailion M, et al. A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evolutionary Biology. 2011;11:339. doi: 10.1186/1471-2148-11-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsos AC, Blass C, Meister S, et al. Life cycle transcriptome of the malaria mosquito Anopheles gambiae and comparison with the fruitfly Drosophila melanogaster. Proceedings of the National Academy of Sciences. 2007;104:11304–11309. doi: 10.1073/pnas.0703988104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama S, Ueda R, Suda T, Nishihara S, Matsuura ET. Involvement of Drosophila Sir2-like genes in the regulation of life span. Genes & Genetic Systems. 2006;81:341–348. doi: 10.1266/ggs.81.341. [DOI] [PubMed] [Google Scholar]
- Landis GN, Abdueva D, Skvortsov D, et al. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proceedings of the National Academy of Sciences (USA) 2004;101:7663–7668. doi: 10.1073/pnas.0307605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebo M, Sanders L, Sun F, Arbeitman M. Somatic, germline and sex hierarchy regulated gene expression during Drosophila metamorphosis. BMC Genomics. 2009;10:80. doi: 10.1186/1471-2164-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitis DA. Before senescence: the evolutionary demography of ontogenesis. Proceedings of the Royal Society B: Biological Sciences. 2011;278:801–809. doi: 10.1098/rspb.2010.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan RL. Reproductive behavior of the cactus fly, Odontoloxozus longicornis, male territoriality and female guarding as adaptive strategies. Behavioral Ecology and Sociobiology. 1979;4:265–278. [Google Scholar]
- Markow T, Gallagher PD, Krebs RA. Ejaculate-derived nutritional contribution and female reproductive success in Drosophila mojavensis (Patterson and Crow). Functional Ecology. 1990;4:67–73. [Google Scholar]
- Markow TA. Mating systems of cactophilic Drosophila. In: Barker JSF, Starmer WT, editors. Ecological Genetics and Evolution: The Cactus-Yeast-Drosophila Model System. Academic Press; Sydney: 1982. pp. 273–287. [Google Scholar]
- Matsuo T, Sugaya S, Yasukawa J, Aigaki T, Fuyama Y. Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biology. 2007;5:e118. doi: 10.1371/journal.pbio.0050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzkin LM. The molecular basis of host adaptation in cactophilic Drosophila: Molecular evolution of Glutathione-S-transferase (Gst) in Drosophila mojavensis. Genetics. 2008;178:1073–1083. doi: 10.1534/genetics.107.083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll SA, Murphy CT, Zou S, et al. Comparing genomic expression patterns across species identifies shared transcriptional profile in aging. Nature Genetics. 2004;36:197–204. doi: 10.1038/ng1291. [DOI] [PubMed] [Google Scholar]
- Merkey AB, Wong CK, Hoshizaki DK, Gibbs AG. Energetics of metamorphosis in Drosophila melanogaster. Journal of Insect Physiology. 2011;57:1437–1445. doi: 10.1016/j.jinsphys.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Mukai M, Kitadate Y, Arita K, Shigenobu S, Kobayashi S. Expression of meiotic genes in the germline progenitors of Drosophila embryos. Gene Expression Patterns. 2006;6:256–266. doi: 10.1016/j.modgep.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Newby BD, Etges WJ. Host preference among populations of Drosophila mojavensis that use different host cacti. Journal of Insect Behavior. 1998;11:691–712. [Google Scholar]
- Oliveira DCSG, Almeida FC, O'Grady PM, et al. Monophyly, divergence times, and evolution of host plant use inferred from a revised phylogeny of the Drosophila repleta species group. Molecular Phylogenetics and Evolution. 2012;64:533–544. doi: 10.1016/j.ympev.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Olsson SB, Linn CE, Jr., Roelofs WL. The chemosensory basis for behavioral divergence involved in sympatric host shifts. I. Characterizing olfactory receptor neuron classes responding to key host volatiles. Journal of Comparative Physiology A. Neuroethology, Sensory, Neural, and Behavioral Physiology. 2006;192:279–288. doi: 10.1007/s00359-005-0069-2. [DOI] [PubMed] [Google Scholar]
- Orzack SH, Tuljapurkar S. Population dynamics in variable environments. VII. The demography and evolution of iteroparity. American Naturalist. 1989;133:901–923. [Google Scholar]
- Pletcher SD, Macdonald SJ, Marguerie R, et al. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Current Biology. 2002;12:712–723. doi: 10.1016/s0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- Polak M. Effects of ectoparasitism on host condition in the Drosophila-Macrocheles system. Ecology. 1998;79:1807–1817. [Google Scholar]
- Ponnapalli SP, Saunders MA, Van Loan CF, Alter O. A higher-order generalized singular value decomposition for comparison of global mRNA expression from multiple organisms. PLoS ONE. 2011;6:e28072. doi: 10.1371/journal.pone.0028072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price T, Schluter D. On the low heritability of life-history traits. Evolution. 1991;45:853–861. doi: 10.1111/j.1558-5646.1991.tb04354.x. [DOI] [PubMed] [Google Scholar]
- Remolina SC, Chang PL, Leips J, Nuzhdin SV, Hughes KA. Genomic basis of aging and life-history evolution in Drosophila melanogaster. Evolution. 2012;66:3390–3403. doi: 10.1111/j.1558-5646.2012.01710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick DN. The impact of predation on life history evolution in Trinidadian guppies: the genetic components of observed life history differences. Evolution. 1982;36:1236–1250. doi: 10.1111/j.1558-5646.1982.tb05493.x. [DOI] [PubMed] [Google Scholar]
- Reznick DN, Bryant MJ, Roff D, Ghalambor CK, Ghalambor DE. Effect of extrinsic mortality on the evolution of senescence in guppies. Nature. 2004;431:1095–1099. doi: 10.1038/nature02936. [DOI] [PubMed] [Google Scholar]
- Roff DA. Life History Evolution Sinauer Associates. Sunderland, MA.: 2002. [Google Scholar]
- Ruiz A, Heed WB, Wasserman M. Evolution of the mojavensis cluster of cactophilic Drosophila with descriptions of two new species. Journal of Heredity. 1990;81:30–42. doi: 10.1093/oxfordjournals.jhered.a110922. [DOI] [PubMed] [Google Scholar]
- Scheiner SM. Genetics and evolution of phenotypic plasticity. Annual Review of Ecology and Systematics. 1993;24:35–68. [Google Scholar]
- Smith G, Lohse K, Etges WJ, Ritchie MG. Model-based comparisons of phylogeographic scenarios resolve the intraspecific divergence of cactophilic Drosophila mojavensis. Molecular Ecology. 2012;21:3293–3307. doi: 10.1111/j.1365-294X.2012.05604.x. [DOI] [PubMed] [Google Scholar]
- Southworth LK, Owen AB, Kim SK. Aging mice show a decreasing correlation of gene expression within genetic modules. PLoS Genetics. 2009;5:e1000776. doi: 10.1371/journal.pgen.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer WT. Associations and interactions among yeasts. In: Barker JSF, Starmer WT, editors. In: Ecological Genetics and Evolution. The Cactus-Yeast-Drosophila Model System. Academic Press; Sydney: 1982. pp. 159–174. [Google Scholar]
- Starmer WT, Heed WB, Rockwood-Sluss ES. Extension of longevity in Drosophila mojavensis by environmental ethanol: Differences between subraces. Proceedings of the National Academy of Sciences (USA) 1977;74:387–391. doi: 10.1073/pnas.74.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. The evolution of life history traits: A critique of the theory and a review of the data. Annual Review of Ecology and Systematics. 1977;8:145–171. [Google Scholar]
- Steiner UK, Tuljapurkar S. Neutral theory for life histories and individual variability in fitness components. Proceedings of the National Academy of Sciences (USA) 2012;109:4684–4689. doi: 10.1073/pnas.1018096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokl J, Strutz A, Dafni A, et al. A deceptive pollination system targeting drosophilids through olfactory mimicry of yeast. Current Biology. 2010;20:1846–1852. doi: 10.1016/j.cub.2010.09.033. [DOI] [PubMed] [Google Scholar]
- Stolc V, Gauhar Z, Mason C, et al. A gene expression map for the euchromatic genome of Drosophila melanogaster. Science. 2004;306:655–660. doi: 10.1126/science.1101312. [DOI] [PubMed] [Google Scholar]
- Tennessen JM, Thummel C. Coordinating growth and maturation -Insights from Drosophila. Current Biology. 2011;21:R750–R757. doi: 10.1016/j.cub.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The-UniProt-Consortium Ongoing and future developments at the Universal Protein Resource. Nucleic Acids Research. 2011;39:D214–D219. doi: 10.1093/nar/gkq1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuljapurkar S. An uncertain life: demography in random environments. Theoretical Population Biology. 1989;35:227–294. doi: 10.1016/0040-5809(89)90001-4. [DOI] [PubMed] [Google Scholar]
- Tweedie S, Ashburner M, Falls K, et al. FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Research. 2009;37:D555–D559. doi: 10.1093/nar/gkn788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annual Review of Neuroscience. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Walsh B, Blows MW. Abundant genetic variation + strong selection = multivariate genetic constraints: A geometric view of adaptation. Annual Review of Ecoogy, Evolution, and Systematics. 2009;40:41–59. [Google Scholar]
- Wasserman M. Cytological evolution of the Drosophila repleta species group. In: Krimbas CB, Powell JR, editors. Drosophila Inversion Polymorphism. CRC Press; Boca Raton: 1992. pp. 455–552. [Google Scholar]
- Williams GC. Pleiotropy, natural selection and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Yang X, Li J, Lee Y, Lussier YA. GO-Module: functional synthesis and improved interpretation of Gene Ontology patterns. Bioinformatics. 2011;27:1444–1446. doi: 10.1093/bioinformatics/btr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Campbell TG, Stone EA, Mackay TFC, Anholt RRH. Phenotypic plasticity of the Drosophila transcriptome. PLoS Genetics. 2012;8:e1002593. doi: 10.1371/journal.pgen.1002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.