Abstract
Objective
Resting heart rate (RHR) is an established predictor of myocardial infarction (MI) and mortality, but the relationship between variation in RHR over a period of several years and health outcomes is unclear. We evaluated the relationship between long-term variation in RHR and the risks of incident MI and mortality among older adults.
Methods
1,991 subjects without cardiovascular disease from the Cardiovascular Health Study were included. RHR was taken from resting electrocardiograms at the first five annual study visits. RHR mean, trend, and variation were estimated with linear regression. Subjects were followed for incident MI and death until December 2010. Hazard ratios (HRs) for RHR mean, trend, and variation are reported for differences of 10 bpm, 2 bpm/yr, and 2 bpm respectively.
Results
262 subjects had an incident MI event (13%) and 1326 died (67%) during 12 years of median follow up. In primary analyses adjusted for cardiovascular risk factors, RHR mean (HR 1.12; 95% confidence interval [CI], 1.05-1.20) and variation (HR 1.08; 95% CI, 1.03-1.13) were associated with the risk of death while trend was not. None of the RHR variables were significantly associated with the risk of incident MI events, however confidence intervals were wide and the MI associations with RHR variables were not significantly different from the mortality associations. Adjusting for additional variables did not affect estimates, and there were no significant interactions with sex.
Conclusion
Variation in RHR over a period of several years represents a potential predictor of long-term mortality among older persons free of cardiovascular disease.
INTRODUCTION
Resting heart rate (RHR) is an established prognostic marker for mortality and cardiovascular disease among the general population [1, 2, 3, 4], the elderly [5, 6], and individuals with a variety of conditions including coronary heart disease (CHD) [7, 8] and congestive heart failure (CHF) [9, 10]. A recent population-based study found that an increase in RHR over a ten year period was associated with increased mortality [11]. However, the use of one or two observations to approximate the average and trend in RHR may result in measurement error [12].
The potential for measurement error to bias estimates of association with health outcomes has long been recognized in the blood pressure literature [13]. Furthermore, variation of a measurement over time can be quantified and may yield additional prognostic information. Using repeated blood pressure measurements from four stroke prevention clinical trials, Rothwell and colleagues demonstrated that long-term variation in blood pressure over a period of several years was a more powerful predictor of stroke and CHD than the average blood pressure [14]. This analytic framework -- using repeated measurements over a period of several years to estimate the mean, trend, and long-term variation for each person -- has not yet been applied to evaluate the association of RHR with the long-term risks of health outcomes such as mortality and myocardial infarction (MI).
We used data from the first five yearly study visits of a prospective population-based cohort study of older adults to estimate these RHR elements and their associations with two outcomes, incident MI and death. We sought to replicate the finding of an association between RHR trend and CHD risk, and we hypothesized that greater long-term variation in RHR is associated with a higher risk of incident MI and death, independent of the RHR mean and trend.
METHODS
Study Design
The Cardiovascular Health Study (CHS) is a prospective population-based cohort study of risk factors for CHD and stroke in older adults [15]. In 1989-1990, 5,201 non-institutionalized participants over the age of 65 were recruited from four field centers. In 1992-1993, 687 additional minority participants were recruited. At the baseline examination, information on risk factors and medication use was collected [16], and a variety of measurements were made: seated and supine blood pressure and heart rate, ankle-arm systolic blood pressures, height and weight, laboratory tests including creatinine and fasting cholesterol, 12-lead resting electrocardiogram (ECG), duplex carotid ultrasound, and echocardiography. Until 1999, medication data, blood pressure, heart rate, ECGs, and information from various questionnaires were collected at yearly clinic visits. The analyses described in this manuscript have been approved by the CHS Publications & Presentations Committee. The University of Washington Human Subjects Division does not consider theses analyses, which use de-identified data on CHS participants, to be Human Subjects research.
For these analyses, the baseline period comprised the first five yearly clinic visits. To avoid selection bias and confounding, participants were excluded if they died, had prevalent cardiovascular disease, used medications that directly affect heart rate (inhaled anticholinergics, beta-adrenergic agonists, beta blockers, and non-dihydropyridine calcium channel blockers), had an implanted pacemaker, or had a missing RHR measurement during the baseline period. Prevalent cardiovascular disease included angina, previous MI, angioplasty, coronary artery bypass surgery, stroke, transient ischemic attack, carotid endarterectomy, claudication, peripheral arterial bypass surgery, CHF, atrial fibrillation, or the use of digoxin or nitrates [17]. Subclinical cardiovascular disease at baseline was defined as the presence of an ankle brachial index < 0.9, left ventricular hypertrophy on any ECG, or carotid artery stenosis ≥ 25% [18].
RHR Variables
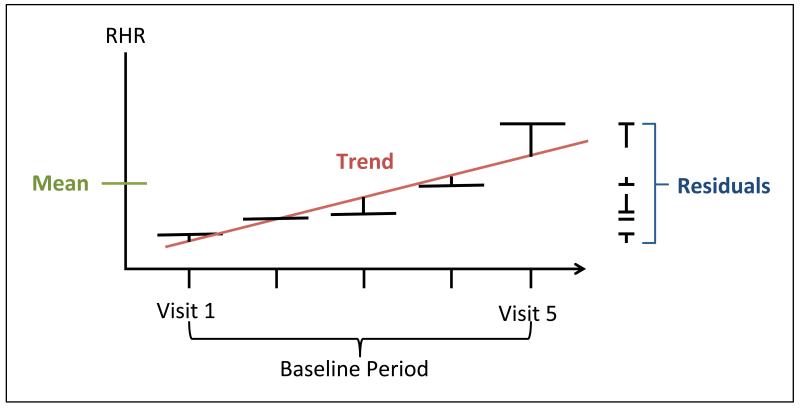
RHR measurements during the baseline study visits were taken from resting 12-lead ECGs.[15] When ECG data were missing, RHR assessed by palpation over a 30-second interval was used. For each participant, linear regression was used to estimate mean, trend, and variation in RHR (Figure 1). RHR trend is the slope of the line of best fit and RHR variation is the standard deviation of the five residuals around the line of best fit.
Figure 1.
Diagram of resting heart rate (RHR) measurements from baseline study visits. RHR trend is the slope of the line of best fit and RHR variation is the square root of the variance from the five residuals (the root mean squared error).
Outcomes
The two primary outcomes for these analyses were incident MI events and deaths. Follow up began at the last baseline study visit [19]. Participants were asked every 6 months about cardiovascular events and hospitalizations through December 31, 2010. Centers for Medicare and Medicaid Services data were also used to identify hospitalizations. Discharge summaries, lab results, and serial ECGs were obtained for all hospitalizations. Deaths were identified through surveillance calls, scheduling calls for annual visits, and local obituaries. The identification of MI was based on an algorithm that included symptoms of chest pain, increased cardiac enzymes, and ECG changes [19]. Fatal events that did not meet criteria for MI were classified as fatal CHD-related deaths if participants had chest pain within 72 hours of death or had a history of ischemic heart disease, in the absence a known non-atherosclerotic cause. An events committee adjudicated all events. Analyses of MI events included non-fatal MIs, fatal MIs, and CHD-related deaths. Deaths were classified as fatal MIs, and CHD-related deaths, or non-CHD related.
Analysis
For the entire study population, repeated measures ANOVA was used to partition the total variance in all baseline RHR measurements into between-subject and within-subject components of variance. The within-subject component was divided by the total variance to estimate the proportion of RHR variation in the entire study sample explained by changes from measurement to measurement within individuals. Correlation coefficients between pairs of the three RHR exposure variables were calculated.
Cox regression was used to estimate hazard ratios (HRs) for the associations of the three RHR exposure variables with outcomes. In the first set of analyses, adjustments were made for age, sex, and race. In the second (primary) set of analyses, adjustments were also made for cardiovascular risk factors: current smoking (yes/no), treated diabetes mellitus (yes/no), systolic blood pressure (continuous), high-density lipoprotein cholesterol level (continuous), and the presence of subclinical cardiovascular disease (yes/no). In the third set of analyses, additional adjustments were made for: marital status (married/other), education (completed high school/no), self-reported health status (very good or excellent/other), regular moderate or high-intensity exercise (yes/no), body mass index (continuous), serum creatinine (continuous), 15-foot walk speed (continuous), and grip strength (continuous). Each regression model included all three RHR exposure variables simultaneously, so that estimates for one RHR variable (e.g. variation) were adjusted for the other two (e.g. mean and trend). In sensitivity analyses, subjects with any RHR observation from manual palpation and the first 6 months of observation were excluded.
Potential interactions of sex and subclinical cardiovascular disease with the various RHR variables were assessed using interactions terms in the primary analysis models. Potential interactions of RHR mean with RHR variation were also assessed. In exploratory analyses, associations of RHR variables with different components of the primary outcomes were evaluated in the primary analysis models: incident MI events included incident MIs and CHD-related deaths; deaths were classified as CHD-related (including fatal MI) or non-CHD-related.
Because there were far fewer MI events than deaths, the power to detect RHR variable associations with MI events was limited compared with the power to detect mortality associations. Therefore, the presence of significant associations with mortality but absence of significant associations with MI events could be due to limited power rather than heterogeneity of the underlying associations. To assess whether associations of RHR variables with the two primary outcomes were different, we calculated ratios of HRs using coefficient estimates from models for each outcome that adjusted for cardiovascular risk factors and constructed confidence intervals from percentiles of bootstrap distributions of the log HR ratios [20]. This method was also used to assess the statistical evidence for differences in associations of RHR variables with the different components of the primary outcomes. The assumption of proportional hazards was evaluated using plots of scaled Schoenfeld residuals. Stata (Version 11) and R (Version 3.1.0) [21] were used to perform all analyses.
RESULTS
Study Population
Out of 5,888 total CHS participants, 585 died before the end of the baseline period, 2,119 had prevalent cardiovascular disease, 719 were on medications that affect heart rate, and 4 had a pacemaker. Of the remaining 2,461 participants, 1,991 had RHR measurements at each baseline study visit and were included in these analyses (Supplemental Figure 1). 33 of the 9,955 total RHR observations (0.3%) were from manual palpation.
Age at baseline ranged from 68 to 96 years. During follow up over a median of 12.4 years, 262 (13%) experienced an incident MI event and 1326 (67%) died. Of the incident MI events, 189 were nonfatal MIs, 40 were fatal MIs, and 33 were CHD-related deaths. 132 (10%) of the deaths were fatal MI events or CHD-related deaths, 59 of which were recurrent CHD events.
RHR Variables
The mean (and standard deviation) of the RHR mean, trend, and variation were 64.6 (8.4) bpm, 0.1 (2.0) bpm/year, and 4.4 (2.5) bpm. Using repeated measures ANOVA, within-individual variation in RHR over time accounted for 29% of the total study population variation. The strongest correlation between RHR variables occurred between RHR mean and RHR variation (r = 0.21, Supplemental Figure 2).
Participants in the highest tertile of mean RHR were less educated, reported worse health, exercised less, had higher blood pressure and cholesterol, and had more hypertension and diabetes mellitus (Table 1). In contrast, there was little difference in the characteristics of participants among the different tertiles of RHR trend and RHR variation.
TABLE 1.
Characteristics of participants by tertiles of resting heart rate variables.
| RHR Mean, bpm | RHR Trend, bpm/yr | RHR Variation, bpm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 41 - 60 (N=660) |
60 - 68 (N=673) |
68 - 100 (N=658) |
−13.8 - −0.7 (N=662) |
−0.7 - 0.9 (N=666) |
0.9 - 11.7 (N=663) |
0.3 - 3.0 (N=658) |
3.0 - 4.8 (N=673) |
4.8 - 25.0 (N=660) |
| Age, years, mean (SD) | 75 (5) | 76 (5) | 76 (5) | 75 (5) | 76 (5) | 76 (5) | 75 (5) | 75 (5) | 76 (5) |
| Female, n (%) | 361 (55) | 436 (65) | 473 (72) | 412 (62) | 430 (65) | 428 (65) | 415 (63) | 449 (67) | 406 (62) |
| Race, n (%) | |||||||||
| White | 578 (88) | 590 (88) | 572 (87) | 561 (85) | 583 (88) | 596 (90) | 592 (90) | 586 (87) | 562 (85) |
| Black | 77 (12) | 79 (12) | 81 (12) | 94 (14) | 80 (12) | 63 (10) | 63 (10) | 83 (12) | 91 (14) |
| Other | 5 (1) | 4 (1) | 5 (1) | 7 (1) | 3 (0) | 4 (1) | 3 (0) | 4 (1) | 7 (1) |
| Married, n (%) | 476 (72) | 480 (71) | 414 (63) | 465 (70) | 458 (69) | 447 (67) | 458 (70) | 470 (70) | 442 (67) |
| Education, n (%) | |||||||||
| Less than high school | 118 (18) | 143 (21) | 194 (30) | 157 (24) | 141 (21) | 157 (24) | 141 (21) | 154 (23) | 160 (24) |
| Graduated high school | 189 (29) | 194 (29) | 178 (27) | 199 (30) | 189 (28) | 173 (26) | 177 (27) | 214 (32) | 170 (26) |
| More than high school | 353 (54) | 336 (50) | 286 (43) | 306 (46) | 336 (50) | 333 (50) | 340 (52) | 305 (45) | 330 (50) |
| Self-reported health, n (%) | |||||||||
| Fair or poor | 58 (9) | 79 (12) | 113 (17) | 85 (13) | 72 (11) | 93 (14) | 64 (10) | 79 (12) | 107 (16) |
| Good | 271 (41) | 283 (42) | 295 (45) | 270 (41) | 285 (43) | 294 (44) | 289 (44) | 268 (40) | 292 (44) |
| Very good or excellent | 331 (50) | 311 (46) | 250 (38) | 307 (46) | 309 (46) | 276 (42) | 305 (46) | 326 (48) | 261 (40) |
| Moderate- or high-intensity exercise, n (%) |
399 (60) | 321 (47) | 320 (49) | 358 (54) | 371 (56) | 370 (56) | 397 (61) | 347 (52) | 355 (54) |
| Smoking status, n (%) | |||||||||
| Current | 61 (9) | 64 (10) | 64 (10) | 58 (9) | 70 (11) | 61 (9) | 50 (8) | 70 (10) | 69 (10) |
| Past | 326 (49) | 321 (48) | 290 (44) | 304 (46) | 317 (48) | 316 (48) | 319 (48) | 296 (44) | 322 (49) |
| Never | 273 (41) | 288 (43) | 304 (46) | 300 (45) | 279 (42) | 286 (43) | 289 (44) | 307 (46) | 269 (41) |
| Family history of MI, n, (%) | 183 (30) | 178 (29) | 158 (27) | 169 (28) | 169 (28) | 181 (30) | 176 (29) | 176 (29) | 167 (28) |
| Body mass index, kg/m2, mean (SD) |
26 (4) | 26 (4) | 27 (5) | 26 (5) | 26 (4) | 27 (5) | 26 (4) | 26 (5) | 27 (5) |
| Systolic blood pressure, mmHg, mean, (SD) |
132 (19) | 132 (10) | 134 (18) | 133 (19) | 133 (19) | 131 (18) | 133 (20) | 133 (18) | 132 (18) |
| Diastolic blood pressure, mmHg, mean (SD) |
69 (10) | 71 (10) | 71 (11) | 71 (10) | 70 (10) | 71 (11) | 70 (10) | 70 (10) | 71 (11) |
| Total cholesterol, mg/dL, mean (SD) |
199 (36) | 202 (36) | 204 (37) | 202 (37) | 200 (35) | 203 (36) | 201 (36) | 201 (35) | 203 (37) |
| LDL cholesterol, mg/dL, mean (SD) |
130 (33) | 132 (35) | 130 (36) | 131 (36) | 130 (34) | 130 (34) | 130 (34) | 131 (34) | 131 (35) |
| HDL cholesterol, mg/dL, mean (SD) |
56 (15) | 57 (16) | 57 (17) | 57 (15) | 57 (15) | 57 (16) | 56 (15) | 57 (16) | 57 (16) |
| Creatinine, mg/dL, mean (SD) | 1.0 (0.2) | 1.0 (0.3) | 0.9 (0.3) | 1.0 (0.2) | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.2) | 0.9 (0.3) | 1.0 (0.3) |
| Treated hypertension, n (%) | 162 (25) | 193 (29) | 226 (34) | 209 (32) | 195 (29) | 177 (27) | 184 (28) | 183 (27) | 214 (33) |
| Diabetes, n (%) | 43 (7) | 72 (11) | 89 (14) | 68 (10) | 55 (8) | 84 (13) | 60 (9) | 71 (11) | 76 (12) |
| Treated | 27 (4) | 43 (6) | 48 (7) | 34 (5) | 32 (5) | 52 (8) | 31 (5) | 41 (6) | 46 (7) |
| Untreated | 19 (3) | 29 (4) | 41 (6) | 34 (5) | 23 (3) | 32 (5) | 29 (4) | 30 (4) | 30 (5) |
| Subclinical CVD, n (%) | 252 (40) | 306 (45) | 265 (40) | 279 (42) | 282 (42) | 272 (41) | 281 (43) | 274 (41) | 278 (42) |
| ABI < 0.9 | 27 (4) | 42 (6) | 47 (7) | 39 (6) | 40 (6) | 37 (6) | 32 (5) | 43 (6) | 41 (6) |
| Carotid stenosis > 25% | 241 (37) | 281 (42) | 239 (37) | 254 (39) | 258 (39) | 249 (38) | 260 (40) | 246 (37) | 255 (39) |
| Left ventricular hypertrophy | 18 (3) | 20 (3) | 21 (3) | 22 (3) | 20 (3) | 17 (3) | 22 (3) | 19 (3) | 18 (3) |
| Medications, n (%) | |||||||||
| Aspirin | 16 (2) | 15 (2) | 17 (3) | 17 (3) | 11 (2) | 20 (3) | 16 (2) | 13 (2) | 19 (3) |
| Statin | 24 (4) | 24 (4) | 40 (6) | 23 (3) | 29 (4) | 36 (5) | 26 (4) | 33 (5) | 29 (4) |
| ACE inhibitor | 45 (7) | 46 (7) | 64 (10) | 59 (9) | 48 (7) | 48 (7) | 43 (7) | 44 (7) | 68 (10) |
| Dihydropyridine CCB | 20 (3) | 31 (5) | 31 (5) | 25 (4) | 18 (3) | 39 (6) | 23 (4) | 31 (5) | 28 (4) |
| Diuretic | 82 (12) | 103 (15) | 129 (20) | 108 (16) | 100 (15) | 106 (16) | 91 (14) | 103 (15) | 120 (18) |
Data were missing on > 1% of participants for: family history of MI (9%), total cholesterol (3%), LDL cholesterol (2%), and body mass index (2%). Abbreviations: bpm = beats per minute, BP = blood pressure, CVD = cardiovascular disease, HDL = high density lipoprotein, LDL = low density lipoprotein, MI = myocardial infarction.
Associations with Outcomes
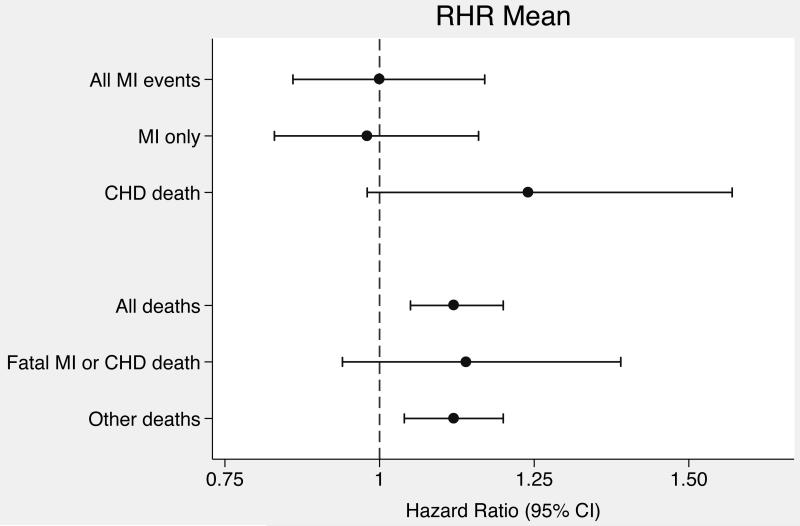
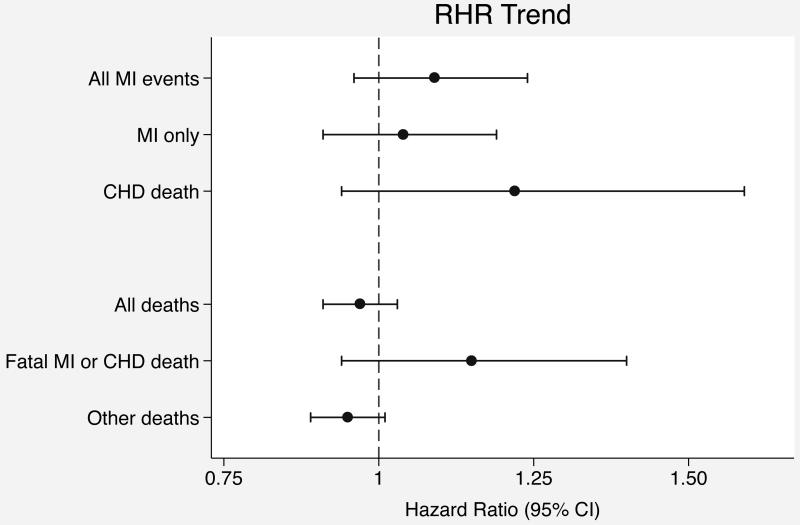
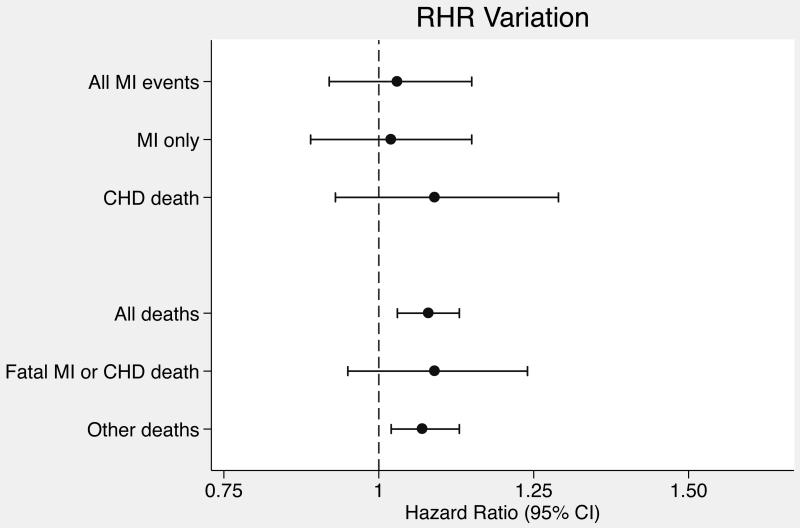
Hazard ratios for associations of RHR mean, trend, and variation with outcomes were standardized to units of 10 bpm, 2 bpm/year, and 2 bpm respectively, which were the population standard deviations for each RHR variables. In both the minimally-adjusted and the primary analyses adjusted for cardiovascular risk factors, RHR trend and variation were positively associated with incident MI, but confidence intervals were wide and included 1.0 (Table 2). On the other hand, RHR mean and RHR variation were significantly associated with death while RHR trend was not. To place these findings in context with other established cardiovascular disease risk factors, in the mortality analysis adjusted for cardiovascular risk factors, the associations for RHR mean (HR 1.12; 95% CI, 1.05-1.20) and variation (HR 1.08; 95% CI, 1.03-1.13) were similar in magnitude to the association for systolic blood pressure (HR 1.11; 95% CI, 1.05-1.17) standardized to the study population standard deviation (19 mmHg). Adjusting for additional variables did not affect RHR association estimates for either primary outcome (Table 2). Forest plots of RHR variable associations with the primary outcomes are displayed in Figure 2.
TABLE 2.
Hazard ratios for resting heart rate variables and the risk of incident myocardial infarction events and death.
| Events | Subjects | Person-years | RHR Mean, HR (95% CI) |
RHR Trend, HR (95% CI) |
RHR Variation, HR (95% CI) |
|
|---|---|---|---|---|---|---|
| Any incident MI event | ||||||
| Minimally-adjusted | 262 | 1991 | 22,372 | 1.07 (0.92-1.25) | 1.12 (0.99-1.28) | 1.01 (0.90-1.13) |
| CV risk factor-adjusted | 260 | 1971 | 22,173 | 1.00 (0.86-1.17) | 1.09 (0.96-1.24) | 1.03 (0.92-1.15) |
| Fully-adjusted | 259 | 1941 | 21,920 | 0.99 (0.85-1.16) | 1.08 (0.95-1.23) | 1.01 (0.90-1.14) |
| Death from any cause | ||||||
| Minimally-adjusted | 1326 | 1991 | 23,324 | 1.14 (1.07-1.22) | 0.98 (0.92-1.04) | 1.07 (1.03-1.13) |
| CV risk factor-adjusted | 1311 | 1971 | 23,122 | 1.12 (1.05-1.20) | 0.97 (0.91-1.03) | 1.08 (1.03-1.13) |
| Fully-adjusted | 1286 | 1941 | 22,862 | 1.08 (1.01-1.16) | 0.98 (0.91-1.04) | 1.06 (1.02-1.12) |
Each regression model included all three resting heart rate (RHR) exposure variables, and hazard ratios adjusted for cardiovascular risk factors represent the primary analyses (bolded). Hazard ratios for RHR mean, RHR trend, and RHR variation are per units of 10 bpm, 2 bpm/year, and 2 bpm respectively. Minimally-adjusted models were adjusted for age, sex, and race; CV risk factor-adjusted models were also adjusted for current smoking, treated diabetes mellitus, systolic blood pressure, high-density lipoprotein cholesterol level, and the presence of subclinical cardiovascular disease; and fully-adjusted models were also adjusted for marital status, education, self-reported health status, regular moderate or high-intensity exercise, body mass index, serum creatinine, 15-foot walk speed, and grip strength. CI = confidence interval, CV = cardiovascular, HR = hazard ratio.
Figure 2.
Forest plots of hazard ratios for resting heart rate variable associations with outcomes. (A) Hazard ratios for RHR mean. (B) Hazard ratios for RHR trend. (C) Hazard ratios for RHR variation.
Excluding subjects with any RHR observation from manual palpation and excluding the first 6 months of observation affected estimates only slightly (Supplemental Tables 1 and 2). No significant interactions with sex or subclinical cardiovascular disease status were identified (P > 0.05 for all interaction terms). There was marginal statistical evidence of an interaction between RHR mean and variation in the mortality analysis (P = 0.052): RHR variation was more strongly associated with mortality among subjects with mean RHR at or below the median value (HR 1.13; 95% CI, 1.05-1.22) than among subjects with mean RHR above the median (HR 1.03; 95% CI, 0.97-1.09). Associations of RHR variables with the components of the primary outcomes are shown in Table 3 and Figure 2. Plots of Schoenfeld residuals did not demonstrate violations of the proportional hazards assumption.
TABLE 3.
Hazard ratio ratios for associations of resting heart rate variables with different outcomes.
| RHR Mean | RHR Trend | RHR Variation | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | HRR (95% CI) | HR (95% CI) | HRR (95% CI) | HR (95% CI) | HRR (95% CI) | |
| Primary outcomes | ||||||
| Any incident MI event | 1.00 (0.86-1.17) | - | 1.09 (0.96-1.24) | - | 1.03 (0.92-1.15) | - |
| Death | 1.12 (1.05-1.20) | - | 0.97 (0.91-1.03) | - | 1.08 (1.03-1.13) | - |
| Death vs any incident MI event | - | 1.12 (0.96-1.29) | - | 0.89 (0.77-1.01) | - | 1.05 (0.95-1.17) |
| Type of incident MI event | ||||||
| Incident MI | 0.98 (0.83-1.16) | - | 1.04 (0.91-1.19) | - | 1.02 (0.89-1.15) | - |
| CHD-related death | 1.24 (0.98-1.57) | - | 1.22 (0.94-1.59) | - | 1.09 (0.93-1.29) | - |
| CHD-related death vs incident MI | - | 1.27 (0.97-1.64) | - | 1.17 (0.93-1.56) | - | 1.08 (0.89-1.30) |
| Cause of death | ||||||
| Fatal MI or CHD-related death | 1.14 (0.94-1.39) | - | 1.15 (0.94-1.40) | - | 1.09 (0.95-1.24) | - |
| Death from other causes | 1.12 (1.04-1.20) | - | 0.95 (0.89-1.01) | - | 1.07 (1.02-1.13) | - |
| Death from other causes vs fatal MI or CHD-related death |
- | 0.98 (0.80-1.21) | - | 0.83 (0.67-1.02) | - | 0.99 (0.86-1.15) |
All analyses are adjusted for cardiovascular risk factors. Hazard ratios and hazard ratio ratios for RHR mean, RHR trend, and RHR variation are per units of 10 bpm, 2 bpm/year, and 2 bpm respectively. CI = confidence interval, CV = cardiovascular, HR = hazard ratio, HRR = hazard ratio ratio.
Comparisons of Associations with Different Outcomes
To determine whether associations of RHR variables with incident MI events were different from associations of RHR variables with mortality, we calculated ratios of HRs for the two outcomes and estimated confidence intervals (Table 3). For all RHR variables, there was no strong statistical evidence of different associations for the two primary outcomes. The evidence was strongest for RHR trend: the ratio of HRs for death compared with MI was 0.89 (95% CI, 0.77-1.01). For the ratios of HRs comparing different components of the primary outcomes, the statistical evidence for differences in associations was similarly weak.
DISCUSSION
In this prospective cohort study of older adults free of cardiovascular disease, we found that the mean and long-term variation in RHR, but not the trend, were associated with the risk of death. These risk estimates were similar in magnitude to the risk estimate for systolic blood pressure, an established cardiovascular risk factor. Incident MI events were less frequent than deaths, resulting in wide confidence intervals for HR estimates, and none of the RHR variables were significantly associated with incident MI risk. However, there was little statistical evidence that the incident MI associations were different from the mortality associations with RHR variables. These analyses adjusted for not only established cardiovascular risk factors such as smoking and hypertension, but also other predictors of mortality including education, self-reported health status, physical activity, and grip strength.
Our findings are consistent with the results of large population-based epidemiologic studies from the 1980s, in which increased RHR was associated with an increased risk of incident CHD and death from both CHD- and non-CHD-related causes [1, 2, 3]. At least 36 additional studies had evaluated RHR and mortality with nearly all reporting similar associations [22]. In most of these studies, RHR was measured only once and the highest quantiles were compared with the lowest, which estimates associations only at the extremes of the exposure distribution, limiting generalizability [22, 23]. For example, non-CHS investigators have used a publically available CHS dataset, which has limited information on covariates and outcomes, to evaluate the association of baseline RHR with mortality over 8 years of follow up. In that analysis, the highest quartile of RHR (mean 81 bpm) was associated with a 2.2-fold increase risk of death compared with the lower quartile (mean 53 bpm) [24].
Recent studies have used two RHR measurements several years apart to evaluate change in RHR as a prognostic marker among persons free of prevalent cardiovascular disease. Jouven and colleagues found that an increase in RHR of > 4 bpm over a 4-year period compared with an increase of −3 to 4 bpm was associated with a 19% increase in mortality (HR 95% CI, 1.04-1.37) [25], and Nauman and colleagues found that an increase in RHR from < 70 bpm to > 85 bpm over a ten-year period compared with a RHR that remained < 70 bpm was associated with a 1.5-fold increase in mortality (HR 95% CI, 1.2-1.9) [11]. The associations with CHD-related death were even stronger in both of these studies. In our study, we evaluated a continuous distribution of RHR trend derived from multiple measurements, which is likely to be a more sensitive analytic method. Nonetheless, the upper bound of the 95% confidence interval for the mortality HR was only 1.03, a null result. We also failed to detect a significant association with the risk of incident MI events, but the confidence intervals are wide and our results are consistent with as much as much as a 24% increased risk per 2 bpm increase in RHR per year. Our failure to replicate the previous findings may be due to differences in the study populations, limited power in our study, or a lack of a true association.
In our study, there was strong evidence that greater long-term variation in RHR was associated with an increased risk of death, but no significant association with an increased risk of incident MI events was identified. In a previous study of blood pressure variation that made use of repeated measurements, Rothwell and colleagues also found that RHR variation across several study visits was not associated with the risk of CHD events, and they did not evaluate mortality associations [14]. The lack of association between long-term RHR variation and primarily non-fatal CHD events in both our study and the Rothwell study may have been due to similarly limited power rather than true differences in the underlying associations of RHR variation with CHD events and with mortality. The long-term variation in RHR evaluated in our study is not the same as beat-to-beat heart rate variability (HRV) during a single episode of measurement [26], which is a marker of autonomic nervous system activity that has been evaluated previously in the Cardiovascular Health Study [27].
In a meta-analysis of hypertension clinical trials, effects of hypertension therapies on blood pressure variation accounted for more of the treatment effect on stroke prevention than effects on the mean blood pressure [28]. This finding, together with the strong epidemiologic evidence of associations with stroke and CHD risks, suggests that long-term variation in blood pressure may be pathogenic [29]. In contrast, a causal mechanism by which long-term variation in RHR might lead to cardiovascular events or death remains unclear. Since the early epidemiologic reports, it has been postulated that an increased RHR reflects sympathetic overactivity and autonomic imbalance, which can precipitate adverse cardiovascular events through a variety of mechanisms including increased myocardial work, platelet activation leading to thrombosis, and arrhythmia [2, 30]. Heart rate is also marker of physical fitness and perhaps general health [31]. Some have argued for a direct role of heart rate in the pathogenesis of atherosclerosis [32, 33, 34], although several lines of evidence argue against this. First, meta-analyses of hypertension clinical trials have shown that beta-blockers are inferior to other therapies that do not have a heart rate-lowering effect (e.g. thiazide diuretics) for the prevention of CHD [35, 36]. Second, in a clinical trial of persons with stable CHD, the drug ivabradine, which has pure heart rate-lowering activity and no effect on blood pressure or myocardial contractility, failed to reduce the risk of the primary outcome, even among those with an elevated RHR at baseline [37]. Lastly, the association of RHR with health outcomes is nonspecific: several epidemiologic studies have demonstrated that an elevated RHR is associated with the risk of cancer mortality [38], in one case as strongly as with cardiovascular mortality [39].
Our study had several strengths: RHR measurements from resting ECGs, centrally adjudicated outcomes ascertained through active surveillance, high quality data on cardiovascular risk factors collected prospectively, and adjustment for a large number of variables that may have accounted for RHR associations with mortality in previous studies. Also, our analytic method allowed us to simultaneously evaluate the associations of several measures of long-term variation in RHR with health outcomes using information from repeated measurements, reducing measurement error.
There were several limitations. In an attempt to avoid confounding, we excluded persons with prevalent cardiovascular disease and who used medications that affect the heart rate, limiting generalizability. The number of incident MI events was much lower than the number of deaths, resulting in low power to detect associations. Also, with only five RHR measurements, we are limited in our ability to distinguish different patterns of RHR variation, such as consistently variability across visits or having a single low or high measurement. Lastly, although we adjusted for a number of predictors of mortality, including socioeconomic factors, self-reported health, walking speed, and grip strength, the associations between RHR and mortality could reflect unmeasured confounding.
Although the prognostic relevance of RHR is well established, the relationship between long-term changes in RHR and health outcomes is not. In this prospective cohort study of older adults, variation in RHR over a period of four years was a strong predictor of mortality while the trend in RHR was not, independent of known cardiovascular risk factors and average RHR. These findings require replication, and populations that include younger adults and persons with established cardiovascular disease should also be studied.
Supplementary Material
KEY QUESTIONS.
What is already known on this subject?
Previous studies have established that that a single measurement of resting heart rate is an important predictor of mortality and coronary heart disease, but the relationship between long-term variation in heart rate and health outcomes has not been well studied.
What does this study add?
In a population of older adults free of cardiovascular disease, we used repeated measurements to estimate for each subject the average, trend, and variation in heart rate over a four-year period, and we estimated associations of these variables with the risks of myocardial infarction and death. We found that both average heart rate and the variation in heart rate were strongly associated with the risk of death, and the magnitude of these associations was similar to the association for systolic blood pressure, a well-established cardiovascular risk factor. Resting heart rate variables were not associated with the risk of myocardial infarction, but there were few myocardial infarction events and confidence intervals were wide.
How might this impact on clinical practice?
Newer biomarkers, some of which are expensive to measure, have added little prognostic value to well-established risk factors for cardiovascular disease. Although our findings require replication, variation in the resting heart rate over a four-year period may be an important prognostic marker, independent of the average heart rate and other cardiovascular risk factors.
ACKNOWLEDGEMENTS
The authors would like to thank Brian Shirts, MD, PhD (University of Washington) for his comments during the drafting of this manuscript, which were provided without compensation.
Sponsor’s Role: Funding agencies did not influence the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Funding: This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contributions from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at https://chs-nhlbi.org/CHS-NHLBI.org. JSF was supported by NHLBI grant K08HL116640.
Footnotes
Conflicts of Interest: None
Author Contributions. All authors participated in the concept and design of the study, interpretation of data, and preparation of the manuscript. JSF and CMS conducted the analyses.
REFERENCES CITED
- 1.Dyer AR, Persky V, Stamler J, et al. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol. 1980;112:736–49. doi: 10.1093/oxfordjournals.aje.a113046. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Kannel C, Paffenbarger RS, Jr., et al. Heart rate and cardiovascular mortality: the Framingham Study. American heart journal. 1987;113:1489–94. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 3.Gillum RF, Makuc DM, Feldman JJ. Pulse rate, coronary heart disease, and death: the NHANES I Epidemiologic Follow-up Study. American heart journal. 1991;121:172–7. doi: 10.1016/0002-8703(91)90970-s. [DOI] [PubMed] [Google Scholar]
- 4.Jouven X, Empana JP, Schwartz PJ, et al. Heart-rate profile during exercise as a predictor of sudden death. The New England journal of medicine. 2005;352:1951–8. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 5.Benetos A, Thomas F, Bean K, et al. Resting heart rate in older people: a predictor of survival to age 85. Journal of the American Geriatrics Society. 2003;51:284–5. doi: 10.1046/j.1532-5415.2003.51080.x. [DOI] [PubMed] [Google Scholar]
- 6.Palatini P, Casiglia E, Julius S, et al. High heart rate: a risk factor for cardiovascular death in elderly men. Archives of internal medicine. 1999;159:585–92. doi: 10.1001/archinte.159.6.585. [DOI] [PubMed] [Google Scholar]
- 7.Diaz A, Bourassa MG, Guertin MC, et al. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. European heart journal. 2005;26:967–74. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- 8.Fox K, Ford I, Steg PG, et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–21. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 9.Bohm M, Swedberg K, Komajda M, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–94. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 10.Poole-Wilson PA, Uretsky BF, Thygesen K, et al. Mode of death in heart failure: findings from the ATLAS trial. Heart. 2003;89:42–8. doi: 10.1136/heart.89.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nauman J, Janszky I, Vatten LJ, et al. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA: the journal of the American Medical Association. 2011;306:2579–87. doi: 10.1001/jama.2011.1826. [DOI] [PubMed] [Google Scholar]
- 12.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289. doi: 10.1136/bmj.c2289. [DOI] [PubMed] [Google Scholar]
- 13.MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–74. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Annals of epidemiology. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.Psaty BM, Lee M, Savage PJ, et al. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. Journal of clinical epidemiology. 1992;45:683–92. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 17.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Annals of epidemiology. 1995;5:270–7. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 18.Kuller L, Borhani N, Furberg C, et al. Prevalence of subclinical atherosclerosis and cardiovascular disease and association with risk factors in the Cardiovascular Health Study. Am J Epidemiol. 1994;139:1164–79. doi: 10.1093/oxfordjournals.aje.a116963. [DOI] [PubMed] [Google Scholar]
- 19.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Annals of epidemiology. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 20.Efron B, Tibshirani R. An Introduction to the Bootstrap. Chapman & Hall/CRC; Boca Raton, FL: 1993. [Google Scholar]
- 21.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing V; Austria: 2014. URL http://www.R-project.org/ [Google Scholar]
- 22.Palatini P, Benetos A, Grassi G, et al. Identification and management of the hypertensive patient with elevated heart rate: statement of a European Society of Hypertension Consensus Meeting. Journal of hypertension. 2006;24:603–10. doi: 10.1097/01.hjh.0000217838.49842.1e. [DOI] [PubMed] [Google Scholar]
- 23.Greenland S. Avoiding power loss associated with categorization and ordinal scores in dose-response and trend analysis. Epidemiology. 1995;6:450–4. doi: 10.1097/00001648-199507000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Hartaigh BO, Allore HG, Trentalange M, et al. Elevations in time-varying resting heart rate predict subsequent all-cause mortality in older adults. Eur J Prev Cardiol. 2014 doi: 10.1177/2047487313519932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jouven X, Empana JP, Escolano S, et al. Relation of heart rate at rest and long-term (>20 years) death rate in initially healthy middle-aged men. The American journal of cardiology. 2009;103:279–83. doi: 10.1016/j.amjcard.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 26.Nicolini P, Ciulla MM, De Asmundis C, et al. The prognostic value of heart rate variability in the elderly, changing the perspective: from sympathovagal balance to chaos theory. Pacing Clin Electrophysiol. 2012;35:622–38. doi: 10.1111/j.1540-8159.2012.03335.x. [DOI] [PubMed] [Google Scholar]
- 27.Stein PK, Sanghavi D, Sotoodehnia N, et al. Association of Holter-based measures including T-wave alternans with risk of sudden cardiac death in the community-dwelling elderly: the Cardiovascular Health Study. Journal of electrocardiology. 2010;43:251–9. doi: 10.1016/j.jelectrocard.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webb AJ, Fischer U, Mehta Z, et al. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–15. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 29.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–48. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 30.Palatini P, Benetos A, Julius S. Impact of increased heart rate on clinical outcomes in hypertension: implications for antihypertensive drug therapy. Drugs. 2006;66:133–44. doi: 10.2165/00003495-200666020-00001. [DOI] [PubMed] [Google Scholar]
- 31.Kannel WB, Wilson P, Blair SN. Epidemiological assessment of the role of physical activity and fitness in development of cardiovascular disease. American heart journal. 1985;109:876–85. doi: 10.1016/0002-8703(85)90653-2. [DOI] [PubMed] [Google Scholar]
- 32.Palatini P, Julius S. Elevated heart rate: a major risk factor for cardiovascular disease. Clinical and experimental hypertension. 2004;26:637–44. doi: 10.1081/ceh-200031959. [DOI] [PubMed] [Google Scholar]
- 33.Palatini P. Heart rate as an independent risk factor for cardiovascular disease: current evidence and basic mechanisms. Drugs. 2007;67(Suppl 2):3–13. doi: 10.2165/00003495-200767002-00002. [DOI] [PubMed] [Google Scholar]
- 34.Lang CC, Gupta S, Kalra P, et al. Elevated heart rate and cardiovascular outcomes in patients with coronary artery disease: clinical evidence and pathophysiological mechanisms. Atherosclerosis. 2010;212:1–8. doi: 10.1016/j.atherosclerosis.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 35.Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA: the journal of the American Medical Association. 2003;289:2534–44. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 36.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005;366:1545–53. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- 37.Fox K, Ford I, Steg PG, et al. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–16. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 38.Persky V, Dyer AR, Leonas J, et al. Heart rate: a risk factor for cancer? Am J Epidemiol. 1981;114:477–87. doi: 10.1093/oxfordjournals.aje.a113213. [DOI] [PubMed] [Google Scholar]
- 39.Jouven X, Escolano S, Celermajer D, et al. Heart rate and risk of cancer death in healthy men. PLoS One. 2011;6:e21310. doi: 10.1371/journal.pone.0021310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.