Abstract
Background
Coronary flow reserve (CFR, an integrated measure of focal, diffuse and small vessel coronary artery disease, CAD), identifies patients at risk for cardiac death. We sought to determine the association between CFR, angiographic CAD, and cardiovascular outcomes.
Methods and Results
Consecutive patients (n=329) referred for invasive coronary angiography after stress testing with myocardial perfusion positron emission tomography (PET), were followed (median 3.1 years) for cardiovascular death and heart failure admission. The extent and severity of angiographic disease was estimated using the CAD prognostic index (CADPI), and CFR measured noninvasively by PET. A modest inverse correlation was seen between CFR and CADPI (r=−0.26, p<0.0001). After adjusting for clinical risk score, ejection fraction, global ischemia, and early revascularization, CFR and CADPI independently associated with events (hazard ratio for unit decrease in CFR, 2.02; 95%CI 1.20-3.40, p=0.008, and for 10-unit increase in CADPI, 1.17; 95%CI 1.01-1.34, p=0.032). Subjects with low CFR experienced rates of events similar to that of subjects with high angiographic scores, and those with low CFR and/or high CADPI showed highest risk of events (p=0.001). There was a significant interaction (p=0.039) between CFR and early revascularization by CABG, such that patients with low CFR who underwent CABG, but not PCI, experienced event rates comparable to those with preserved CFR, independently of revascularization.
Conclusions
CFR associated with outcomes independently of angiographic CAD, and modified the effect of early revascularization. Diffuse atherosclerosis and associated microvascular dysfunction may contribute to the pathophysiology of cardiovascular death and heart failure, and impact upon the outcomes of revascularization.
Keywords: coronary disease, ischemia, diffuse atherosclerosis, coronary flow reserve, revascularization
Diffuse coronary atherosclerosis is highly prevalent among patients with known or suspected coronary artery disease (CAD),1 increases the severity of inducible myocardial ischemia (beyond the effects of epicardial coronary obstruction),2 and identifies patients at high risk for serious adverse events, including cardiac death.1, 3-5 These associations are evident across heterogeneous-risk cohorts, including patients with diabetes.6 Coronary flow reserve (CFR, calculated as the ratio of hyperemic to rest absolute myocardial blood flow) is a measure of coronary vasomotor dysfunction, which integrates the hemodynamic effects of epicardial coronary stenosis, diffuse atherosclerosis, and microvascular dysfunction on myocardial tissue perfusion.2
Coronary angiography is a cornerstone of modern cardiovascular care, but its ability to identify physiologically and prognostically important coronary stenoses in stable ischemic heart disease remains controversial.2, 7 Recent randomized trials8, 9 did not show an event-free survival benefit for the addition of coronary revascularization to guideline-directed medical therapy, whereas an approach using fractional flow reserve-guided revascularization10 to identify lesion-specific ischemia was beneficial. To date, no studies have investigated the relative contributions of noninvasive measures of CFR and luminal angiographic CAD on cardiovascular outcomes, particularly as related to revascularization. We utilized the validated CAD prognostic index (CADPI)11 to quantify the extent and severity of epicardial CAD. We hypothesized that global CFR, as quantified by noninvasive positron emission tomography (PET), and overall luminal angiographic disease, as estimated by CADPI, would show limited correlation, and that CFR would be associated with the risk of future cardiovascular events independently of anatomic score and revascularization.
Methods
Study Population
Study participants were consecutive patients clinically referred for invasive coronary angiography within 90 days after stress myocardial perfusion PET at Brigham and Women's Hospital between 2006 and 2012. Indications for testing most commonly included evaluation for chest pain, dyspnea or their combination. Patient history and medication use were ascertained at time of PET imaging. From a cohort of 841 patients, those with prior CABG, left ventricular ejection fraction (LVEF) <40%, or clinical diagnosis of heart failure were excluded, leaving a final cohort of 329 individuals. The median time from PET to invasive angiography was 2.6 (IQR 0.3-13.5) days, reflecting that both diagnostic evaluations occurred in the same tertiary care center and were coordinated, when possible, for optimal care delivery. Any patients with an intervening cardiovascular event or revascularization between PET and angiography were excluded. A pretest clinical score integrating age, sex, type of chest pain, prior history of myocardial infarction, presence of diabetes, hyperlipidemia, current smoking, and electrocardiographic abnormalities into a pretest probability of obstructive angiographic CAD was calculated as previously described.12 Early revascularization with CABG or percutaneous coronary intervention (PCI), considered to be triggered by imaging results, was defined as occurring within 90 days of PET.5 The study was approved by the Partners Healthcare Institutional Review Board, and conducted in accordance with institutional guidelines.
PET Imaging
Patients were imaged with a whole-body PET–computed tomography scanner (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, WI) using 82Rubidium (1480-2200 MBq) or 13N-ammonia (700-900 MBq) as a flow tracer at rest and pharmacologic stress, as previously described.13 Computed tomography was used for attenuation correction only. For semi-quantitative assessment of myocardial scarring and ischemia, 17-segment visual interpretation of gated myocardial perfusion images was performed by experienced operators using a standard five-point scoring system.14
Summed rest and difference (stress – rest) scores were converted to percent myocardium by dividing by the maximum score of 68.15 For each of these variables, higher scores reflect larger areas of myocardial scar or ischemia, respectively. Rest LVEFs were calculated from gated myocardial perfusion images with commercially available software (Corridor4DM; Ann Arbor, Michigan). Absolute global myocardial blood flow (MBF, in mL/min/g) was quantified at rest and at peak hyperemia using automated factor analysis and a validated two-compartment kinetic model, as previously described.13 Per-patient global CFR was calculated as the ratio of stress to rest absolute MBF for the whole left ventricle. MBF and CFR values were not clinically available to referring physicians. Radiation exposure per study was ≤4.6 mSV. Quantitative measures of CFR were obtained in patients undergoing PET myocardial perfusion at no additional clinical cost, imaging time or radiation exposure.
Coronary Angiography
All patients underwent selective coronary angiography using standard clinical techniques, with two or more projections obtained per vessel distribution, and angles of projection optimized for cardiac position. In each patient, the CADPI was adapted and quantified as previously described.11 Luminal diameter stenoses of the major epicardial coronary arteries were clinically graded by subjective visual consensus of experienced operators on an ordinal scale, and applied to the CADPI classification in blinded fashion. The CADPI classification is a hierarchical index (0-100) that assigns overall prognostic weights to increasing percent stenoses (50-100%) in one-, two-, or three-vessel classification, with higher weights for proximal left anterior descending (LAD) or left main (LM) artery involvement (Supplemental Table 1).
Outcomes
Patients were followed for a median of 3.1 years (IQR 1.7-4.3) for the occurrence of major adverse cardiovascular events (MACE), including death, cardiovascular death, and hospitalization for heart failure or myocardial infarction. The pre-specified primary endpoint was a composite of cardiovascular death and heart failure hospitalization. Selection of the primary endpoint was informed by emerging data suggesting a role for subtle cardiac structural abnormalities in predicting cardiovascular death and especially incident heart failure,16-18 whereas obstructive CAD classically associates with myocardial infarction and revascularization. Pre-specified secondary analyses were performed for a composite endpoint of all-cause death and heart failure hospitalization, and also for cardiovascular death and hospitalization for heart failure or myocardial infarction. Ascertainment of clinical endpoints was determined by blinded adjudication of the longitudinal medical record, Partners Healthcare Research Patient Data Registry, the Social Security Death Index, and the National Death Index by two independent cardiologist members of the Clinical Endpoints Committee. For an event to be classified as nonfatal admission for heart failure or myocardial infarction, discharge with a primary hospitalization cause of heart failure or myocardial infarction, respectively, was required. The date of the last consultation was used to determine follow-up. All patients not meeting a clinical endpoint had >30 days of follow-up.
Statistical Analysis
Baseline characteristics are reported as rates with percentages (%) for categorical variables and medians with interquartile ranges (IQR) for continuous variables. We used Fisher's-exact test and the Wilcoxon rank-sum test to assess differences in dichotomous and continuous baseline characteristics. CFR, rather than MBF, was defined as the primary variable of interest because of the clinical convenience of a ratio, as well as the known association between CFR and outcomes.1, 3-5 For simplicity in the descriptive display, we selected median CFR of 1.6 as a cutpoint. This value, lower than the all-comer cutpoint of 2,2 is consistent with the more comorbid population referred for coronary angiography. Where indicated (and for modeling) we report values of CFR as a continuous variable. Spearman's correlation was used to describe the association between the CFR and CADPI. Similar results were obtained after logarithmic transformation, and results are presented untransformed for ready clinical applicability.
Cumulative event-free survival curves for the primary endpoint were compared across dichotomous categories of CFR median (<1.6 vs. ≥1.6) and CADPI clinical cutpoint of ≥37 vs. <37 using the log-rank test. The CADPI cutpoint was selected to reflect a >70% stenosis in >1 major epicardial coronary artery, a clinically actionable threshold for revascularization; this is also the cutpoint at which a survival benefit has been demonstrated previously for revascularization.11 Where indicated (and for modeling) we report values of CADPI as a continuous variable.
Cox proportional-hazards models were used to examine the association between CFR, CADPI and outcome events after controlling for effects of clinically important covariates. Data were censored at the time of the last visit. Model development was tested on the primary endpoint, and the final model was applied to the secondary endpoints already described. Univariate associations were tested, and Cox models sequentially added age, sex, medical history, medications, pretest clinical score, imaging and angiography variables, with collinearity index used to check for linear combinations among covariates and the Akaike information criterion assessed to avoid overfitting, with final covariates chosen based on clinical knowledge. The proportional hazards assumption was evaluated using martingale residuals. The final model with CFR and CADPI was adjusted for pretest clinical score, global LV ischemia (summed difference score), time-dependent variables of early revascularization, and stratified by binary category of LVEF (<50% vs. ≥50%), as LVEF showed mild departure from proportionality. Adjusted event-free survival was plotted using survival probabilities from the Cox model, and stratified by categories of impaired CFR and elevated CADPI. Interaction terms for CFR and CADPI, and CFR and revascularization strategy, were tested for significance in the adjusted model.
In an exploratory analysis, we stratified patients by revascularization across medians of CFR, to better visualize differences in outcomes across categories of no revascularization, and revascularization with PCI or CABG. Poisson regression was performed to compute annualized event rates of the primary endpoint, after adjusting for pretest clinical score, LVEF, global LV ischemia and CADPI, to evaluate for the effect of baseline CFR on revascularization benefit. Model fit was assessed using the goodness-of-fit chi-squared test, with a non-significant result indicating adequate fit. Event-free survival curves for the primary endpoint of cardiac death and heart failure admission were compared across dichotomous categories of CFR median and revascularization using the log-rank test, and also plotted after adjusting for pretest clinical score, LVEF, global LV ischemia and CADPI. To increase power for display of revascularization subgroups, curves for revascularized patients were then plotted for all-cause death and heart failure admission. A p-value of <0.05 was considered to indicate statistical significance, and all tests were two-sided. The SAS analysis system, version 9.3, was used for all analyses (SAS Institute).
Results
Baseline Characteristics
Distribution of baseline characteristics is shown in Table 1. The median (IQR) age of patients in the overall cohort was 67 (59-75) years, 42.6% were women, 76.0% were white, and median pretest clinical score was 58.2% (28.4-84.8). Nearly a third of patients had prior myocardial infarction, 31.9% had prior PCI, and 58.7% underwent revascularization by either PCI or CABG within 90 days of PET imaging. Compared to patients with CFR ≥1.6 (n=166), those with CFR <1.6 (n=163) were older, had more co-morbidities and higher use of cardiovascular medications, showed increased amounts of ischemia and scar on noninvasive imaging, with higher CADPI scores on coronary angiography and higher rates of early revascularization.
Table 1.
Baseline Characteristics of Patients by Low versus High Coronary Flow Reserve.
| Characteristic | Coronary Flow Reserve* | |||
|---|---|---|---|---|
| Overall (N = 329) | <1.6 (n = 163) | ≥1.6 (n = 166) | P† | |
| Demographic characteristics | ||||
| Age‡, y (IQR) | 67 (59-75) | 69 (61-78) | 64 (57-71) | <0.001 |
| Female sex (%) | 140 (42.6) | 76 (46.6) | 64 (38.6) | 0.15 |
| White race (%) | 250 (76.0) | 117 (71.8) | 133 (80.1) | 0.09 |
| Body mass index‡, kg/m2 | 29.9 (26.3-34.5) | 29.9 (26.2-34.7) | 29.9 (26.6-34.4) | 0.72 |
| Pretest clinical score‡§, % | 58.2 (28.4-84.8) | 60.3 (31.0-85.6) | 57.7 (20.9-83.1) | 0.09 |
| Medical history | ||||
| Myocardial infarction (%) | 108 (32.8) | 63 (38.7) | 45 (27.1) | 0.03 |
| Percutaneous coronary intervention (%) | 105 (31.9) | 49 (30.1) | 56 (33.7) | 0.48 |
| Peripheral arterial disease (%) | 48 (14.6) | 28 (17.2) | 20 (12.1) | 0.21 |
| Diabetes mellitus (%) | 132 (40.1) | 75(46.0) | 57 (34.3) | 0.03 |
| Hypertension (%) | 290 (88.2) | 156 (95.7) | 134 (80.7) | <0.001 |
| Dyslipidemia (%) | 241 (73.3) | 129 (79.1) | 112 (67.5) | 0.02 |
| Current smoker (%) | 29 (8.8) | 16 (9.8) | 13 (7.8) | 0.56 |
| Chronic obstructive lung disease (%) | 45 (13.7) | 18 (11.0) | 27 (16.3) | 0.20 |
| Renal hemodialysis (%) | 11 (3.3) | 10 (6.1) | 1 (0.6) | <0.01 |
| Medications | ||||
| Antiplatelet therapy (%) | 253 (76.9) | 127 (77.9) | 126 (75.9) | 0.70 |
| Statin (%) | 231 (70.2) | 124 (76.1) | 107 (64.5) | 0.02 |
| Beta-blocker (%) | 229 (69.6) | 126 (77.3) | 103 (62.1) | <0.01 |
| Angiotensin inhibitor (%) | 149 (45.3) | 71 (43.6) | 78 (47.0) | 0.07 |
| Nitroglycerin (%) | 58 (17.6) | 33 (20.3) | 25 (15.1) | 0.25 |
| Diuretic (%) | 108 (32.8) | 64 (39.3) | 44 (26.5) | 0.02 |
| Insulin (%) | 62 (18.8) | 33 (20.3) | 29 (17.5) | 0.57 |
| Noninvasive imaging parameters | ||||
| Left ventricular ejection fraction‡, % | 57 (50-65) | 57 (49-64) | 57 (52-65) | 0.31 |
| Left ventricular scar‡, % | 0 (0-2.9) | 0 (0-5.9) | 0 (0-1.5) | <0.01 |
| Left ventricular ischemia‡, % | 10.3 (5.9-16.2) | 11.8 (7.4-20.6) | 7.4 (4.4-13.2) | <0.001 |
| Rest myocardial blood flow‡, ml/g/min | 1.0 (0.8-1.2) | 1.0 (0.8-1.3) | 0.9 (0.7-1.1) | <0.001 |
| Stress global myocardial blood flow‡, ml/g/min | 1.6 (1.1-2.0) | 1.3 (1.0-1.7) | 1.8 (1.4-2.3) | <0.001 |
| Coronary flow reserve‡ | 1.6 (1.2-2.0) | 1.2 (1.1-1.5) | 2.0 (1.8-2.4) | <0.001 |
| Rubidium-82 radiopharmaceutical, % | 293 (89.1) | 147 (90.2) | 146 (88.0) | 0.60 |
| Invasive angiography and early revascularization¶ | ||||
| Coronary artery disease prognostic index# | 32 (23-48) | 37 (23-56) | 32 (0-42) | <0.001 |
| Any early revascularization¶ (%) | 193 (58.7) | 106 (65.0) | 87 (52.4) | 0.03 |
| Percutaneous coronary intervention (%) | 157 (47.7) | 85 (52.2) | 72 (43.4) | 0.12 |
| Coronary artery bypass surgery (%) | 39 (11.9) | 22 (13.5) | 17 (10.2) | 0.40 |
Coronary flow reserve is stratified by median values.
The P-value is for the comparison between groups, and is based on the Fisher's-exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
Continuous variables are presented as medians (interquartile ranges).
Pretest clinical score is the pretest probability of >70% stenosis in ≥1 major coronary artery on angiography.12
Early revascularization is defined as within 90 days of noninvasive imaging. Three patients underwent both percutaneous coronary intervention and coronary artery bypass grafting.
Coronary artery disease prognostic index (CADPI) is a hierarchical index (0-100) assigning prognostic weights to increasing percent stenoses (50-100%) in one-, two-, or three-vessel classification, with higher weights for proximal left anterior descending or left main artery involvement. CADPI 0 (<50% stenosis), 37 (>70% stenosis in >1 major epicardial coronary artery).11
Distribution of Coronary Flow Reserve by Angiographic Disease
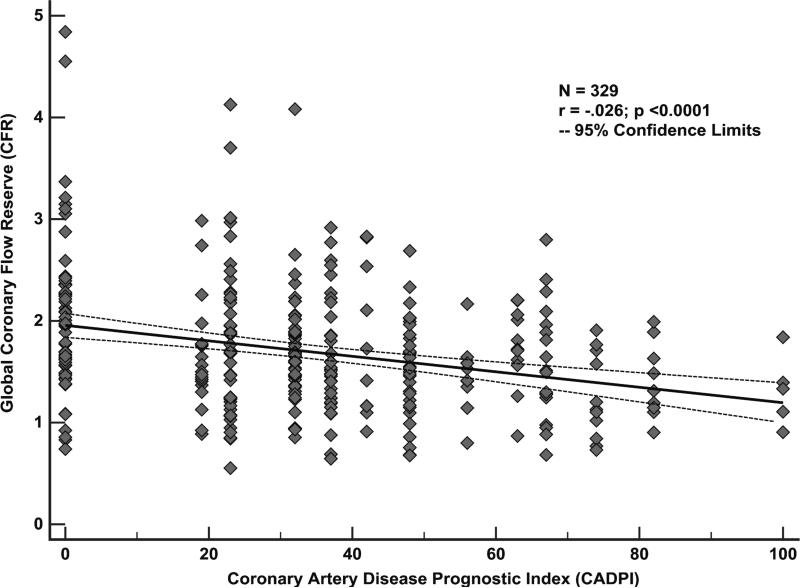
As expected, there was a significant but limited inverse correlation between global CFR and the CAD angiographic score, as assessed by CADPI, (r= −0.26, p<0.0001), likely reflecting that CFR is a measure of not only the effects of epicardial CAD, but also diffuse atherosclerosis and microvascular dysfunction on myocardial tissue perfusion. A scatter plot of CFR versus CADPI values, shown in Figure 1, illustrates a wide range of CFR values even among those subjects with CADPI of 0 [reflecting angiographically normal or non-obstructive (<50%) stenosis in the epicardial coronary arteries].
Figure 1.
Association between coronary flow reserve (CFR) and the extent and severity of angiographic disease. A significant but modest inverse correlation (r= −0.26, p<0.0001) was seen between CFR and coronary artery disease prognostic index (CADPI), a hierarchical score of angiographic disease, reflecting the role of CFR as an integrated measure of the effects of epicardial coronary artery disease, as well as diffuse atherosclerosis and associated microvascular dysfunction, on myocardial tissue perfusion. A wide range of CFR values was seen even among those subjects with CADPI of 0 [reflecting angiographically normal or non-obstructive (<50%) stenosis in the epicardial coronary arteries].
Coronary Flow Reserve, Angiographic Disease, and Clinical Events
Cardiovascular Death or Heart Failure Admission
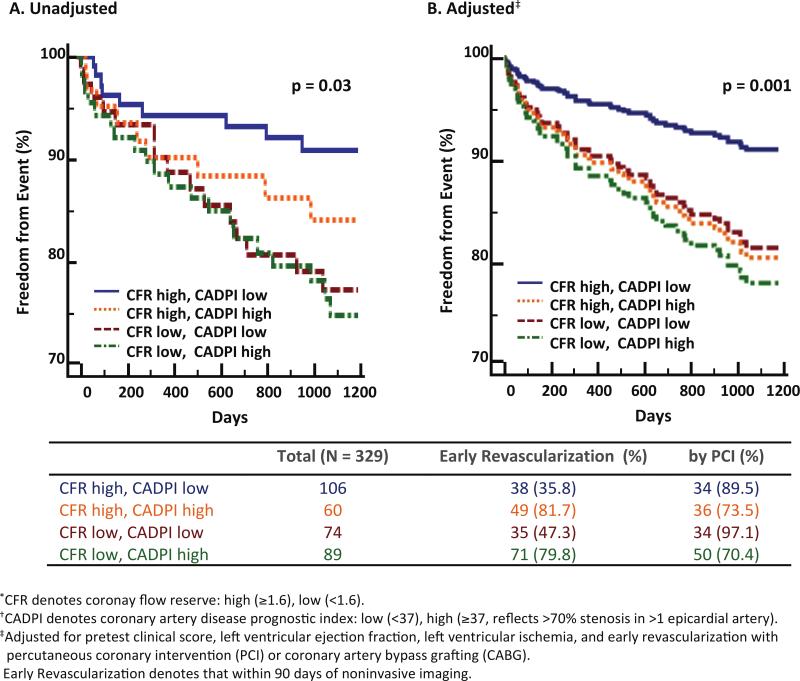
During follow-up, 64 subjects met the primary composite endpoint of cardiovascular death or heart failure admission, including 31 deaths (Supplemental Table 2). Freedom from cardiovascular death or heart failure was significantly different for subgroups stratified by CFR and angiographic score (log-rank p=0.03). Subjects with low CFR, independently of angiographic disease score, suffered higher rates of MACE, while those with high CFR and low angiographic score experienced the greatest freedom from events (Figure 2A).
Figure 2.
Freedom from cardiovascular death or heart failure admission according to coronary flow reserve (CFR) and angiographic score (CADPI). Freedom from cardiovascular death or heart failure admission differed significantly among subgroups stratified by CFR and CADPI, such that patients with low CFR, independently of angiographic disease score, suffered higher rates of events (overall p=0.03). In adjusted analysis, patients with low CFR experienced rates of events similar to that of patients with high CADPI, and those with low CFR and/or high CADPI showed highest cumulative incidence of events (adjusted overall p=0.001).
In a univariable model, the cumulative probability of freedom from MACE was significantly associated with CFR (hazard ratio per unit decrease in CFR, 2.17; 95% confidence interval (CI), 1.34-3.52; p=0.002), but did not meet statistical significance for angiographic score (hazard ratio per 10 unit increase in CADPI, 1.10; 95% CI, 0.99-1.21; p=0.07). Association of CFR with MACE was driven by MBF at peak hyperemia, and not by MBF at rest. The addition of clinically important covariates into the model, including pretest clinical score, LVEF strata, global LV ischemia, and time-dependent early revascularization with CABG or PCI led to significant associations with MACE (hazard ratio for CFR, 2.02; 95% CI 1.20-3.40, p=0.008, and for CADPI, 1.17; 95% CI 1.01-1.34, p=0.03) (Table 2). Inclusion of global LV ischemia or scar into the adjusted model did not significantly alter results, suggesting that CFR is a more sensitive measure of myocardial tissue perfusion and ischemia than semi-quantitative perfusion scores. In adjusted analysis, subjects with low CFR experienced rates of events similar to that of subjects with high angiographic scores, and those with low CFR and/or high CADPI showed the highest cumulative incidence of events (p=0.001) (Figure 2B). CFR thus associated with cardiovascular death and heart failure admission independently of angiographic score, and CFR and/or angiographic score identified patients at highest risk of events.
Table 2.
Association between Coronary Flow Reserve, Luminal Angiographic Severity and Clinical Events.
| Outcome | Univariable Model Hazard Ratio (95% CI) | Multivariable Model* Hazard Ratio (95% CI) | ||
|---|---|---|---|---|
| CFR† | CADPI‡ | CFR† | CADPI‡ | |
| Cardiovascular death or heart failure§ | 2.17 (1.34-3.52) | 1.10 (0.99-1.21) | 2.02 (1.20-3.40) | 1.17 (1.01-1.34) |
| All-cause death or heart failure§ | 1.91 (1.29-2.83) | 1.05 (0.97-1.15) | 1.64 (1.08-2.48) | 1.15 (1.03-1.29) |
| Cardiovascular death, heart failure§ or myocardial infarction¶ | 1.90 (1.23-2.93) | 1.13 (1.03-1.24) | 1.63 (1.02-2.59) | 1.22 (1.08-1.38) |
Includes pretest clinical score, left ventricular ejection fraction, left ventricular ischemia, revascularization with percutaneous coronary intervention or coronary artery bypass grafting within 90 days of noninvasive imaging, coronary flow reserve, and coronary artery disease prognostic index. There is a significant interaction between coronary flow reserve and revascularization with coronary artery bypass grafting, (P=0.04 for cardiovascular death or heart failure, P=0.006 for cardiovascular death, heart failure or myocardial infarction).
CFR denotes coronary flow reserve (per -1 unit).
CADPI denotes coronary artery disease prognostic index (per +10 units).
Admission for heart failure.
Admission for myocardial infarction.
Death or Admission for Heart Failure or Myocardial Infarction
In secondary analyses, we tested the association between CFR on two additional and related composite endpoints of i) all-cause death or heart failure hospitalization, and ii) cardiovascular death, or hospitalization for heart failure or myocardial infarction. Ninety and 74 subjects met these secondary endpoints, respectively. Our results confirmed that CFR remained significantly associated with these additional major clinical adverse events, independently of luminal angiographic score (Table 2).
Interactions of CFR, Angiographic Disease, and Early Revascularization on Outcomes
Although there was no apparent interaction between global CFR and overall angiographic score, there was a significant interaction between global CFR and revascularization by CABG (p for interaction=0.04) on the primary endpoint of cardiovascular death and heart failure admission. This interaction was additionally significant (p=0.006) on the more inclusive secondary endpoint of cardiovascular death and admission for heart failure or myocardial infarction.
To better visualize the interaction of CFR and early revascularization on outcomes, we performed an exploratory analysis of event-free survival stratified by CFR and revascularization. Approximately half (53.8%) of primary endpoint events occurred in subjects that underwent early revascularization; of the nine subjects meeting the clinical endpoint within 60 days of catheterization, seven (77.8%) had undergone revascularization. Supplemental Table 3 displays the distribution of baseline characteristics by early revascularization type, and shows that the major difference between groups was in the severity of CADPI (67 vs. 37 for CABG as compared to PCI, p>0.001).
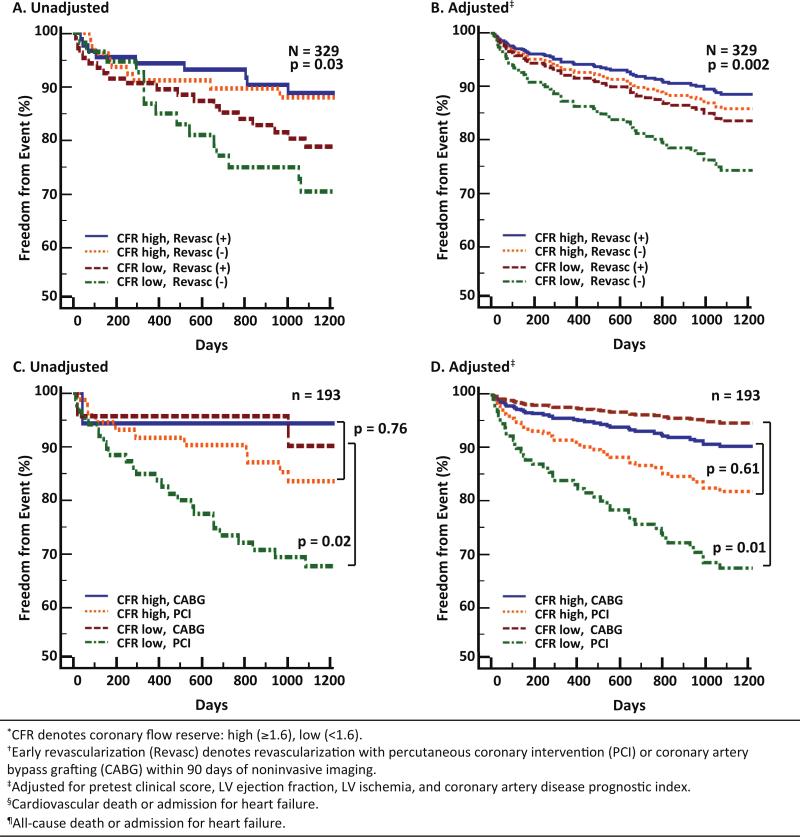
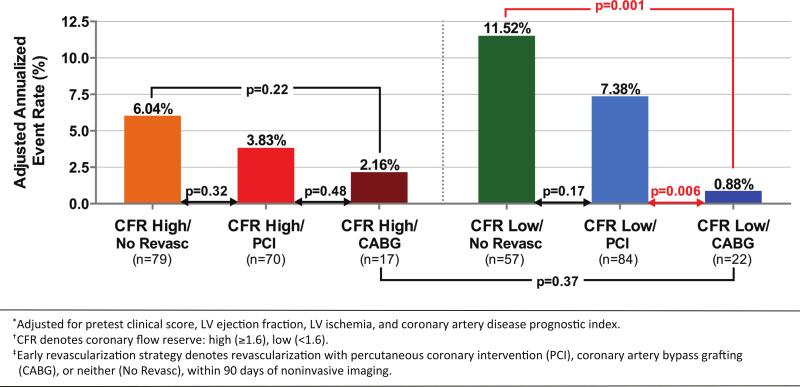
Unadjusted and adjusted freedom from cardiovascular death or heart failure was significantly different for subgroups stratified by CFR and early revascularization (log-rank p=0.03, p=0.002 after adjusting for pretest clinical score, LVEF, LV ischemia, and CADPI). Subjects with high CFR, independently of revascularization, experienced lower rates of MACE, while those with low CFR who did not undergo revascularization suffered the highest rate of events (Figure 3A, 3B). In the subgroup of patients who underwent revascularization, there was no difference in event-free survival for those with high CFR who underwent CABG or PCI (log-rank p=0.76, adjusted p=0.61). Among patients with low CFR, however, only those who underwent CABG, as compared with those who underwent PCI alone, experienced lower rates of events (log-rank p=0.02, adjusted p=0.01) (Figure 3C, 3D). This is further illustrated in Figure 4, which shows that patients with low CFR who underwent CABG had adjusted annualized event rates that were similar, and possibly better than, those with high CFR who underwent CABG. In contrast, patients with low CFR who underwent PCI showed event rates that were not statistically different from those with low CFR who did not undergo revascularization. In the patients with high CFR, there was no difference in event rates between those who did and did not undergo revascularization, by either CABG or PCI.
Figure 3.
Freedom from events according to coronary flow reserve (CFR) and early revascularization. Freedom from cardiovascular death or heart failure admission differed significantly among subgroups stratified by CFR and revascularization (overall log-rank p=0.03, adjusted p=0.002) (A, B). Patients with high CFR, independently of revascularization, experienced lower rates of events, while those with low CFR who did not undergo revascularization suffered the highest rate of events. In the subgroup of patients who underwent revascularization (C, D), there was no difference in event-free survival among those with high CFR (log-rank p=0.76, adjusted p=0.61), but in those with low CFR, only those who also underwent coronary artery bypass grafting, versus percutaneous coronary intervention, experienced lower rates of events (log-rank p=0.02, adjusted p=0.01).
Figure 4.
Adjusted annualized rates of cardiovascular death and heart failure admission among patients referred for coronary angiography, by coronary flow reserve (CFR) and early revascularization strategy [coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), or neither]. No difference in event rates was seen in patients with high CFR (orange, red, maroon), regardless of revascularization strategy pursued. In patients with low CFR, those who underwent CABG (dark blue) had lower event rates than those who underwent PCI (light blue, p=0.006) or no revascularization (green, p=0.001), and similar event rates to those with high CFR who underwent CABG (maroon). Annualized event rates were adjusted for pretest clinical score, left ventricular ejection fraction, left ventricular ischemia and coronary artery disease prognostic index.
Discussion
We demonstrated that, although global CFR is only modestly associated with the overall extent and severity of angiographic disease, both low CFR and high CADPI independently associate with adverse clinical events. In addition, global CFR modified the effect of revascularization in this cohort, such that only patients with low CFR appeared to benefit from revascularization, and only if the revascularization included CABG. Implied in these data is the possibility that invasive revascularization in certain patients, i.e. with preserved CFR, may contribute to increased events. The apparent discrepancy between angiographic appearance of coronary lesions and their physiologic significance has been attributed to limitations in the resolution of X-ray angiography,7, 19, 20 and its inadequacy to characterize microvascular disease and/or diffuse coronary atherosclerosis,2, 21 a nearly ubiquitous finding in autopsy and intravascular ultrasound studies of patients with CAD.22, 23 Thus, a stenosis that does not produce angina in one patient (with otherwise normal coronary arteries, or outward remodeling and/or robust downstream collaterals) might result in severe functional limitation, chronic low-level ischemia and myocardial remodeling in another (with diffuse atherosclerosis and/or microvascular disease). Furthermore, angina caused by a small versus large ischemic area may carry a different prognosis and associated risk-benefit profile with revascularization such that angina itself may be an inadequate biomarker of risk.
The finding that global CFR associates with events independently of angiographic score underscores the morbidity associated with diffuse atherosclerosis and/or microvascular disease. This has been illustrated in diabetic patients, who demonstrate impaired coronary vasoreactivity even in the absence of obstructive atherosclerosis,24 and in whom absence of myocardial ischemia on noninvasive testing does not necessarily identify a lower-risk cohort.6, 25 In contrast to diabetic patients without known CAD with preserved CFR (who demonstrate very low levels of risk), diabetic patients without known CAD with impaired CFR showed a risk of cardiac death comparable to, and possibly higher than, that for nondiabetic patients with known CAD.6 The current study was not limited to diabetics (40.1% of cohort), and together with previous findings,6 suggests that impaired CFR may be a more powerful biomarker for diffuse atherosclerosis than diabetes alone.
These observations may be clinically relevant, particularly when considering that revascularization procedures based on anatomic thresholds have not reduced rates of adverse cardiovascular events in patients with stable ischemic heart disease in randomized controlled trials comparing revascularization to guideline-directed medical therapy,8, 9 or fractional flow reserve-guided percutaneous coronary intervention.10 An alternative hypothesis generated from observational15 and post hoc26 analyses proposes that there may be a threshold of ischemia above which a revascularization strategy might result in improved cardiovascular outcomes. Yet, like angiographic severity, traditional semi-quantitative measures of ischemia alone may be insufficient to risk stratify patients potentially eligible for benefit from coronary revascularization.2, 27 Furthermore, the current study raises the possibility that the type of revascularization in this context may have profound impact on optimal management strategy.
Indeed, contemporary multicenter randomized clinical trials comparing outcomes of CABG and PCI in subjects with multivessel CAD have suggested benefit in major adverse cardiovascular events with CABG,28 particularly in patients with diabetes.9, 29, 30 In the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial, the benefit of CABG, relative to PCI, on outcomes, was independent of the SYNTAX score30 (reflecting overall coronary lesion complexity), which may not be as sensitive as CFR to identify diffuse, downstream disease, particularly among diabetics. A better understanding of the relationship between diffuse coronary vascular dysfunction and CAD co-morbid conditions, including diabetes mellitus and dyslipidemia, may guide new, more effective approaches for global cardiovascular risk reduction that may achieve some of the therapeutic benefit derived from more “complete revascularization” with CABG. These findings thus identify diffuse atherosclerosis and microvascular dysfunction as potentially relevant targets for aggressive therapeutic intervention.
Exactly how impaired CFR associates with increased clinical risk independently of angiographic score, and precisely how it modifies the effect of revascularization, cannot be determined from this study. Low-level inflammation in the coronary microvasculature has been implicated as a potential driver of both coronary vasomotor dysfunction,31 and myocardial dysfunction and remodeling in heart failure with preserved ejection fraction.32 Abnormal CFR in heart failure patients correlates with diastolic load and high-sensitivity troponin release.33 Further, the observation that chronic circulating levels of high-sensitivity troponins are associated with increased incidence of cardiovascular death or heart failure (but not acute coronary syndromes) in patients with stable CAD and preserved LVEF,17, 18 highlights the potential interplay of chronic coronary vasomotor dysfunction and subclinical myocardial injury in the pathway to diastolic dysfunction and heart failure outcomes.
This study must be interpreted in the context of its single-center observational design, in which subjects were patients clinically referred for PET myocardial perfusion imaging and subsequently referred for invasive coronary angiography. CFR results were not available to referring clinicians, and thus did not affect downstream management decisions regarding catheterization or additional therapies. We included patients undergoing both 82Rubidium and 13N-ammonia myocardial perfusion PET imaging. Although we have previously published data documenting comparable MBF and CFR estimates using these two radiopharmaceuticals,13 extrapolation of these results to imaging with other PET tracers, including 15O-water and 18FFlurpiridaz (neither of which is FDA approved) will require future studies. Our relatively modest sample size limits extensive subgroup (i.e. sex, diabetes, revascularization) analysis for outcomes, and may be underpowered to detect more subtle differences in outcomes between subgroups. We excluded patients with a reduced LVEF at time of PET so as to focus on outcomes of those without already severely impaired cardiac structure. In addition, we did not assess nonfatal stroke outcomes, and intentionally avoided repeat revascularization outcomes. Despite inherent limitations with unmeasured confounding and cautions about drawing causal inferences, this work is the first to link the complementary but distinct associations of functional and anatomic coronary abnormalities with clinically meaningful cardiovascular outcomes in a high-risk, real-world patient population.
Conclusions
Global coronary flow reserve showed limited correlation with the extent and severity of angiographic disease, was associated with MACE independently of angiographic disease score, and modified the effect of revascularization on outcomes. Low CFR and/or high CADPI together identified patients at highest risk of events. A significant interaction was seen between CFR and revascularization strategy, such that patients with low CFR who underwent CABG, but not PCI alone, experienced event rates comparable to those with preserved CFR independently of revascularization. Diffuse atherosclerosis and reduced global coronary flow reserve may play a role in the pathophysiologic abnormalities leading to increased risk of cardiovascular death or heart failure, and impact upon the outcomes of revascularization. Prospective studies are needed to evaluate the ability of CFR to reclassify subsets of patients at differing levels of clinical risk (and potential for benefit), regardless of the presence of epicardial coronary obstruction on invasive angiography, or ischemia on semi-quantitative measures of relative myocardial perfusion imaging.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by National Institutes of Health Grant T32HL094301-02 (VRT).
Dr. Dorbala receives research grant support from Astellas Global Pharma Development and Bracco Diagnostics. Dr. Murthy owns equity in General Electric. Dr. Di Carli receives investigator-initiated research grant support from Gilead Sciences.
Footnotes
Disclosures
The other authors declare that they have no relationships to disclose.
References
- 1.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, Camici PG, Cerqueira MD, Chow BJ, Di Carli MF, Dorbala S, Gewirtz H, Gropler RJ, Kaufmann PA, Knaapen P, Knuuti J, Merhige ME, Rentrop KP, Ruddy TD, Schelbert HR, Schindler TH, Schwaiger M, Sdringola S, Vitarello J, Williams KA, Sr., Gordon D, Dilsizian V, Narula J. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62:1639–1653. doi: 10.1016/j.jacc.2013.07.076. [DOI] [PubMed] [Google Scholar]
- 3.Fukushima K, Javadi MS, Higuchi T, Lautamaki R, Merrill J, Nekolla SG, Bengel FM. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82rb pet perfusion imaging. J Nucl Med. 2011;52:726–732. doi: 10.2967/jnumed.110.081828. [DOI] [PubMed] [Google Scholar]
- 4.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA. Long-term prognostic value of 13n-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–156. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 5.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RS. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58:740–748. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 6.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White CW, Wright CB, Doty DB, Hiratza LF, Eastham CL, Harrison DG, Marcus ML. Does visual interpretation of the coronary arteriogram predict the physiologic importance of a coronary stenosis? N Engl J Med. 1984;310:819–824. doi: 10.1056/NEJM198403293101304. [DOI] [PubMed] [Google Scholar]
- 8.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS, Group CTR. Optimal medical therapy with or without pci for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 9.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van' t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF, Investigators FS. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 11.Mark DB, Nelson CL, Califf RM, Harrell FE, Jr., Lee KL, Jones RH, Fortin DF, Stack RS, Glower DD, Smith LR, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89:2015–2025. doi: 10.1161/01.cir.89.5.2015. [DOI] [PubMed] [Google Scholar]
- 12.Pryor DB, Shaw L, McCants CB, Lee KL, Mark DB, Harrell FE, Jr., Muhlbaier LH, Califf RM. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118:81–90. doi: 10.7326/0003-4819-118-2-199301150-00001. [DOI] [PubMed] [Google Scholar]
- 13.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Di Carli MF. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)rb pet: Comparison with (13)n-ammonia pet. J Nucl Med. 2009;50:1062–1071. doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. American Heart Association Writing Group on Myocardial S, Registration for Cardiac I. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 15.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 16.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin t detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin t using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E. Prevention of Events with Angiotensin Converting Enzyme Inhibition Trial I. A sensitive cardiac troponin t assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Carli M, Czernin J, Hoh CK, Gerbaudo VH, Brunken RC, Huang SC, Phelps ME, Schelbert HR. Relation among stenosis severity, myocardial blood flow, and flow reserve in patients with coronary artery disease. Circulation. 1995;91:1944–1951. doi: 10.1161/01.cir.91.7.1944. [DOI] [PubMed] [Google Scholar]
- 20.Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994;330:1782–1788. doi: 10.1056/NEJM199406233302503. [DOI] [PubMed] [Google Scholar]
- 21.Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333–2342. doi: 10.1161/01.cir.92.8.2333. [DOI] [PubMed] [Google Scholar]
- 22.Arnett EN, Isner JM, Redwood DR, Kent KM, Baker WP, Ackerstein H, Roberts WC. Coronary artery narrowing in coronary heart disease: Comparison of cineangiographic and necropsy findings. Ann Intern Med. 1979;91:350–356. doi: 10.7326/0003-4819-91-3-350. [DOI] [PubMed] [Google Scholar]
- 23.Nicholls SJ, Tuzcu EM, Crowe T, Sipahi I, Schoenhagen P, Kapadia S, Hazen SL, Wun CC, Norton M, Ntanios F, Nissen SE. Relationship between cardiovascular risk factors and atherosclerotic disease burden measured by intravascular ultrasound. J Am Coll Cardiol. 2006;47:1967–1975. doi: 10.1016/j.jacc.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 24.Nahser PJ, Jr., Brown RE, Oskarsson H, Winniford MD, Rossen JD. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation. 1995;91:635–640. doi: 10.1161/01.cir.91.3.635. [DOI] [PubMed] [Google Scholar]
- 25.Shaw LJ, Iskandrian AE. Prognostic value of gated myocardial perfusion spect. J Nucl Cardiol. 2004;11:171–185. doi: 10.1016/j.nuclcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O'Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE, Investigators C. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: Results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (courage) trial nuclear substudy. Circulation. 2008;117:1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 27.Mancini GB, Hartigan PM, Shaw LJ, Berman DS, Hayes SW, Bates ER, Maron DJ, Teo K, Sedlis SP, Chaitman BR, Weintraub WS, Spertus JA, Kostuk WJ, Dada M, Booth DC, Boden WE. Predicting outcome in the courage trial (clinical outcomes utilizing revascularization and aggressive drug evaluation): Coronary anatomy versus ischemia. JACC. Cardiovascular interventions. 2014;7:195–201. doi: 10.1016/j.jcin.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW, Investigators S. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 29.Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The bypass angioplasty revascularization investigation (bari) investigators. N Engl J Med. 1996;335:217–225. doi: 10.1056/NEJM199607253350401. [DOI] [PubMed] [Google Scholar]
- 30.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S, 3rd, Bertrand M, Fuster V, Investigators FT Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 31.Taqueti VR, Ridker PM. Inflammation, coronary flow reserve, and microvascular dysfunction: Moving beyond cardiac syndrome x. JACC Cardiovasc Imaging. 2013;6:668–671. doi: 10.1016/j.jcmg.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 33.Takashio S, Yamamuro M, Izumiya Y, Sugiyama S, Kojima S, Yamamoto E, Tsujita K, Tanaka T, Tayama S, Kaikita K, Hokimoto S, Ogawa H. Coronary microvascular dysfunction and diastolic load correlate with cardiac troponin t release measured by a highly sensitive assay in patients with nonischemic heart failure. J Am Coll Cardiol. 2013;62:632–640. doi: 10.1016/j.jacc.2013.03.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.