Abstract
Skeletal muscle fibrosis is marked by increases in tissue stiffness and collagen content. However, only a very weak correlation exists between collagen content and stiffness in skeletal muscle. Recently, it has been hypothesized that collagen crosslinking explains tissue stiffness in fibrotic skeletal muscle. Therefore, we addressed this hypothesis by correlating tissue stiffness with lysyl-pyridinoline, hydroxylysyl-pyridinoline, and pentosidine collagen crosslinks. Stepwise regression revealed that, separate or together, collagen crosslinks did not correlate with tissue stiffness. Our result demonstrates that increased tissue stiffness in skeletal muscle fibrosis is not simply explained by increased collagen crosslinks and/or collagen crosslink density. We suggest that collagen organization may affect tissue stiffness. Alternatively, changes in other extracellular matrix components or specific structural geometry could dictate tissue stiffness.
Introduction
Fibrosis results when skeletal muscle is damaged and the regenerative process fails to recapitulate normal development. Skeletal muscle fibrosis is a significant clinical problem that arises in numerous myopathies, including muscular dystrophy (Lieber and Ward, 2013). Additionally, skeletal muscle fibrosis can occur as a result of skeletal muscle trauma or in the case of brain injury, such as in stroke patients. Given that skeletal muscle fibrosis is an abundant clinical problem, a concrete understanding of the condition is critical for developing therapies.
Skeletal muscle fibrosis is the abnormal accumulation of extracellular matrix (ECM) between myofibers, specifically expressed as increased collagen content (Lieber and Ward, 2013). Apart from biochemical changes, biomechanical changes are also observed in skeletal muscle, cardiac and liver fibrosis (Carrión et al., 2010; Jalil et al., 1989; Lieber and Ward, 2013). While collagen content and tissue stiffness increase with fibrosis, there is not a strong correlation between these two values (Chapman et al., 2014; Smith and Barton, 2014). Interestingly, a recent cardiac study showed that collagen crosslinks, not collagen abundance, dictated tissue stiffness (López et al., 2012), suggesting that collagen crosslinks may explain increased tissue stiffness in muscle fibrosis. Additionally, a study conducted in dystrophic chickens demonstrated that inhibition of excessive lysyl oxidase activity, an enzyme responsible for collagen crosslinking, decreased muscle stiffness (Feit et al., 1989).
Collagen crosslinks are formed both enzymatically and non-enzymatically. Enzymatic collagen crosslinks are formed when lysyl oxidase reacts with free lysyl or hydroxylysyl side chains within collagen fibrils (Alberts et al., 2008), resulting in lysyl-pyridinoline (LP) and hydroxylysyl-pyridinoline (HP) crosslinks, respectively. Non-enzymatic crosslinks, such as pentosidine (PE), are created when glucose reacts with lysine and the resulting compound is oxidized (Paul and Bailey, 1996). We hypothesize that increased collagen crosslinks explain increased tissue stiffness in skeletal muscle fibrosis.
In this study, we used our recently described nesprin-desmin double knockout mouse (DKO) model of skeletal muscle fibrosis (Chapman et al., 2014). DKO mice had a six-fold increased tissue stiffness and a two-fold increased collagen content. Surprisingly, when we regressed tissue stiffness against collagen content, there was no significant correlation (Fig. S1 of Chapman et al 2014). This suggested that another factor, such as collagen crosslinks, could explain DKO skeletal muscle’s increased stiffness. Thus, the purpose of this study was to use multiparametric analysis to determine the role (if any) of HP, LP and/or PE collagen crosslinks in determining muscle stiffness in this transgenic model.
Methods
Passive mechanics
Passive mechanical testing of skeletal muscle bundles from wild-type (WT) [n=10], nesprin-1 knockout (nesprin−/−) [n=8], desmin knockout (desmin−/−) [n=10] and nesprin-1/desmin double knockout (DKO) [n=13] mice was conducted as previously described (Fridén and Lieber, 2003). Briefly, tibialis anterior muscles were dissected, placed in a glycerol storage solution [(in mM): K-propionate (170), K3EGTA (5), MgCl2 (5.3), imidazole (10), Na2ATP (21.2), NaN3 (1), glutathione (2.5), leupeptin (0.05) and 50% (vol/vol) glycerol] and stored at −20°C for up to two-weeks. For mechanical testing, samples were placed into relaxing solution [pCa 8.0 and pH 7.1 containing (in mM): imidazole (59.4), KCH4O3S (86), Ca(MSA)2 (0.13), Mg(MSA)2 (10.8), K3EGTA (5.5), KH2PO4 (1), leupeptin (0.05) and Na2ATP (5.1)]. Muscle bundles were dissected, placed into a mechanical testing chamber and secured with 10–0 monofilament suture to a force transducer (Aurora Scientific 405A; Aurora, ON, Canada) on one side and a fixed titanium pin connected to a rotational bearing (Newport MT-RS; Irvine, CA, USA) on the other. Mechanical testing began by removing bundle slack and measuring slack force and length. Sarcomere length was monitored throughout experiments by laser diffraction. A stress-relaxation protocol was implemented by incrementally increasing sarcomere length by 0.25 μm/stretch and stress-relaxing for 3 minutes, after which stress decay has been shown to be minimal (Meyer et al., 2011). Bundles were stretched to a sarcomere length of 4.0 μm, or until failure, whichever occurred first. After each 3-minute period, force and sarcomere length were recorded. Stress was calculated by dividing force by bundle cross-sectional area assuming each bundle was an isovolumic cylinder (Smith et al., 2011). Tangent stiffness was then determined by calculating the slope of the stress-sarcomere length plot at a sarcomere length of 3.2 μm.
Collagen Crosslinks
HP, LP and PE concentrations of TA muscle samples were determined as previously described (Bank et al., 1997). These samples were derived from adjacent portions of the same muscle samples used for mechanical testing. Tissue was hydrolyzed in 6M hydrochloric acid at 110°C for 20 hours. After hydrolysis, samples were dried in a vacuum desiccator, redissolved, and then purified using 0.22 μm spin-X centrifuge tube filters (Costar, Corning, NY).
The HPLC column (TSK gel ODS-80Tm, 4.6 mm I.D. × 15 cm packed with 5 μm particles, TOSOH Bioscience, Japan) was equilibrated with 0.15% (v/v) HFBA in 24% (v/v) methanol. Samples were then injected into the HPLC system. Elution of crosslinks and a pyridoxine internal standard was achieved at 40°C at a flow rate of 1.0 mL/min in two steps. Fluorescence was monitored at 0–22 min, 295/400 nm; 22–45 min, 328/378 nm (gain 100; band width 18 mm). Elution of HP was achieved at 9.8 minutes, LP at 12.1 minutes, and PE at 21.5 minutes.
For hydroxyproline analysis, samples were dried and incubated for 10 min at room temperature in 20 μL of derivatization solution [methanol: water: triethylamine: phenylisothiocyanate in a 7:1:1:1 ratio + lyophilized hydroxy-L-proline (20 μg/ml)]. Samples were then dried and dissolved in 100 μL of reconstitution buffer (5mM Na2HPO4 in acetonitrile, pH 7.4). Samples were then injected in the HPLC system. Hydroxyproline content was determined using an HPLC column (Waters Spherisorb ODS-80. 4.6 mm I.D. ×25 cm 2–5 μm particles, All Tech, Deerfield, IL) equilibrated with a 6:4:90 solution of acetonitrile: water: 140 mm sodium acetate trihydrate buffer, pH 6.4 for 15 min at a flow rate of 1 ml/min. Elution of the hydroxyproline was achieved at 9 minutes.
Statistical Methods
Stepwise regression analysis was performed using IBM SPSS Statistics (Armonk, NY) to determine which parameters (collagen content, HP, LP, and/or PE if any) could predict muscle tissue stiffness. Tissue stiffness was the dependent variable, while collagen content, HP, LP and PE were independent variable. Stepwise criteria were as follows: probability of F-to-enter ≤0.05, and a probability of F-to-remove ≥0.1.
One-way ANOVA with Tukey’s post-hoc tests was used to determine statistical differences in collagen content and crosslink content among the four genotypes (GraphPad Prism, La Jolla, CA). Significance level (α) was set to 0.05 in all cases.
Results
Collagen content, measured by HPLC was significantly increased in DKO mice compared with all other genotypes (p<0.05; Fig. 1). HP and PE collagen crosslinking values were significantly increased in DKO mice (p<0.05; Fig. 1). Additionally, nesprin−/− mice showed elevated levels of HP compared with wild-type, while desmin−/− mice had higher levels of HP and PE compared with wild-type animals (p<0.05; Fig. 1). Surprisingly, LP levels were significantly increased in wild-type animals compared to all other genotypes (p<0.05; Fig. 1).
Figure 1. Collagen content and collagen crosslink values in WT, nesprin−/−, desmin−/− and nesprin−/−, desmin−/− double knockout (DKO) skeletal muscle.
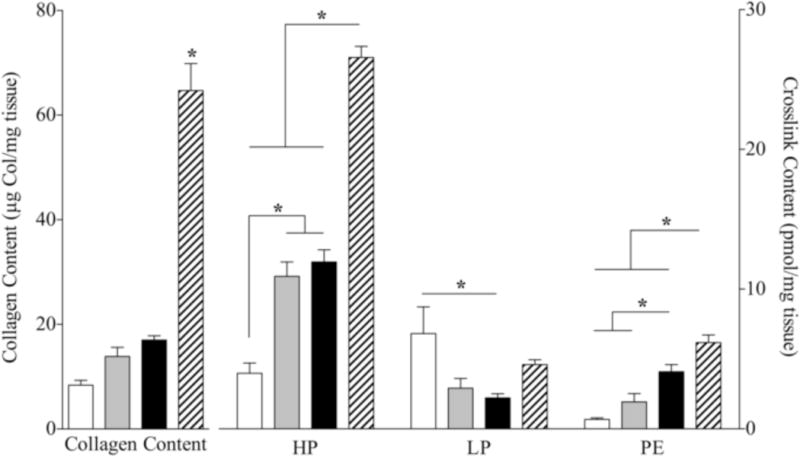
Collagen content and HP, LP and PE collagen crosslinks were assessed using HPLC. Collagen content as measured by HPLC was significantly elevated in DKO skeletal muscle. HP and PE crosslinks were significantly increased over WT and single knockout values. (*p<0.05, via one-way ANOVA)
To investigate whether collagen crosslinks were associated with tissue stiffness, crosslink data were plotted against stiffness values (Fig. 2). When tissue stiffness was plotted against collagen, HP or PE values, there was a significant correlation between stiffness and collagen and crosslink content (collagen: p<0.05, r2=0.62; HP: p<0.05, r2=0.73; PE: p<0.05, r2=0.52; Figs. 2A,B & D). However, when examining each genotype separately, this relationship disappeared (collagen: p>0.1, r2≤0.28; HP: p>0.2, r2≤0.15; LP: p>0.2, r2≤0.18; PE: p>0.1, r2≤0.22). Thus, the correlation was caused by differences between genotypes and created only a pseudocorrelation (Draper and Smith, 1981). LP crosslink content also had no correlation with tissue stiffness. These conclusions were further validated using stepwise regression. In the stepwise regression model, the only variable that entered the linear model was HP content (r2=0.73), suggesting a good relationship. However, it should be noted that when data from any single genotype were run through stepwise regression, no variables were included in the model.
Figure 2. Muscle bundle stiffness versus collagen content and collagen crosslink concentrations.
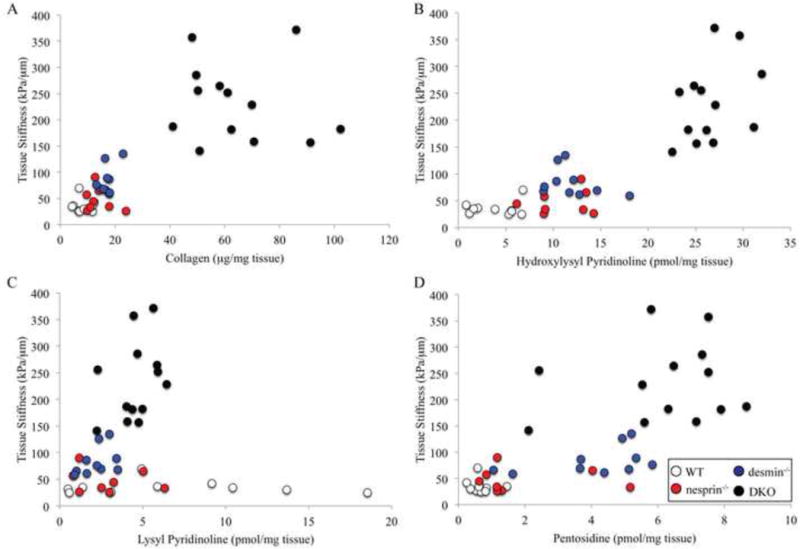
Tissue stiffness [data from Chapman, et al. 2014] was plotted against collagen content (A), HP content (B), LP content (C) and PE content (D) to determine whether these parameters correlate with tissue stiffness. Tissue stiffness correlates poorly with collagen content (p<0.05, r2=0.62) and HP (p<0.05, r2=0.73), LP (p>0.8, r2=0.0002) and PE (p<0.05, r2=0.52) collagen crosslinks. This lack of a relationship is particularly prominent when examining each genotype individually (collagen: p>0.1, r2≤0.28; HP: p>0.2, r2≤0.15; LP: p>0.2, r2≤0.18; PE: p>0.1, r2≤0.22).
Discussion
Previous reports showed that collagen content is a poor predictor of muscle stiffness (Chapman et al., 2014; Lieber and Ward, 2013; Smith and Barton, 2014). Given these reports, we determined whether collagen crosslinks dictate stiffness as has been observed in human ventricles and dystrophic avian skeletal muscle (Feit et al., 1989; López et al., 2012). We found that skeletal muscle stiffness in a murine model of fibrosis did not significantly correlate with HP, LP or PE collagen crosslinks.
HP and PE levels were significantly elevated in DKO skeletal muscle. These elevated values are reflected in the increased total collagen content in DKO muscle. Surprisingly, LP crosslinks were elevated in WT muscle over all other genotypes. It is unclear why WT muscle had elevated LP levels, but it is possible that in fibrotic muscle, there is a shift in collagen crosslinks from LP to HP. Compared to our previous study on these same mice, HPLC collagen values were slightly elevated. This discrepancy could be explained by the different methods used to assay collagen content. In our previous study, we used a colorimetric hydroxyproline assay, while in the current study we used HPLC, which may have increased specificity (Green and Reagan, 1992).
In spite of the current findings, it remains unclear which parameters in skeletal muscle ECM are responsible for dictating tissue stiffness. Although neither collagen content nor collagen crosslinks are highly correlated with tissue stiffness, collagen organization within the ECM remains a potential contributer to tissue stiffness. None of the methods used account in any way for the gross arrangement of collagen bundles which are rich in the perimysial space (Gillies and Lieber, 2011). Recently, a serial block face scanning electron microscopy method was used to reconstruct skeletal muscle ECM over hundreds of microns (Gillies et al., 2014). This technology holds great potential for determining collagen/ECM ultrastructure, and could answer questions about whether tissue stiffness is related to ECM organization. Other factors such as proteoglycans may also affect stiffness, as it has been shown that decorin and biglycan are also increased in muscle fibrosis models (Fadic et al., 2006).
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers AR061303 & T32AR0607 and by the National Institute of Child Health and Human Development under Award Number HD050837. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also acknowledge the National Science Foundation for an NSF-Graduate Research Fellowship to MAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflicts.
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 5. Garland Science; 2008. [Google Scholar]
- Bank RA, Beekman B, Verzijl N, Roos JADM De, Sakkee AN, Tekoppele JM. Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J Chromatogr B. 1997;703:37–44. doi: 10.1016/s0378-4347(97)00391-5. [DOI] [PubMed] [Google Scholar]
- Carrión Ja, Torres F, Crespo G, Miquel R, García-Valdecasas JC, Navasa M, Forns X. Liver stiffness identifies two different patterns of fibrosis progression in patients with hepatitis C virus recurrence after liver transplantation. Hepatology. 2010;51:23–34. doi: 10.1002/hep.23240. [DOI] [PubMed] [Google Scholar]
- Chapman MA, Zhang J, Banerjee I, Guo LT, Zhang Z, Shelton GD, Ouyang K, Lieber RL, Chen J. Disruption of both nesprin 1 and desmin results in nuclear anchorage defects and fibrosis in skeletal muscle. Hum Mol Gen. 2014;23:5879–5892. doi: 10.1093/hmg/ddu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper NR, Smith H. Applied Regression Analysis. John Wiley & Sons, Inc; New York: 1981. [Google Scholar]
- Fadic R, Mezzano V, Alvarez K, Cabrera D, Holmgren J, Brandan E. Increase in decorin and biglycan in Duchenne Muscular Dystrophy: role of fibroblasts as cell source of these proteoglycans in the disease. J Cell Mol Med. 2006;10:758–769. doi: 10.1111/j.1582-4934.2006.tb00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feit H, Kawai M, Mostafapour AS. The role of collagen crosslinking in the increased stiffness of avian dystrophic muscle. Muscle Nerve. 1989;12:486–492. doi: 10.1002/mus.880120609. [DOI] [PubMed] [Google Scholar]
- Friden J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003;26:157–164. doi: 10.1002/mus.10247. [DOI] [PubMed] [Google Scholar]
- Gillies AR, Bushong Ea, Deerinck TJ, Ellisman MH, Lieber RL. Three-Dimensional Reconstruction of Skeletal Muscle Extracellular Matrix Ultrastructure. Microsc Microanal. 2014:1–6. doi: 10.1017/S1431927614013300. [Epub Ahead of Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44:318–31. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G, Reagan K. Determination of Hydroxyproline Liquid Chromatography by High Pressure Liquid Chromatography. Anal Biochem. 1992;269:265–269. doi: 10.1016/0003-2697(92)90337-7. [DOI] [PubMed] [Google Scholar]
- Jalil JE, Doering CW, Janicki JS, Pick R, Shroff SG, Weber KT. Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ Res. 1989;64:1041–1050. doi: 10.1161/01.res.64.6.1041. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Ward SR. Cellular Mechanisms of Tissue Fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am J Physiol Cell Physiol. 2013;305:C241–C252. doi: 10.1152/ajpcell.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López B, Querejeta R, González A, Larman M, Díez J. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: potential role of lysyl oxidase. Hypertension. 2012;60:677–683. doi: 10.1161/HYPERTENSIONAHA.112.196113. [DOI] [PubMed] [Google Scholar]
- Meyer GA, McCulloch AD, Lieber RL. A nonlinear model of passive muscle viscosity. J Biomech Eng. 2011;133:091007. doi: 10.1115/1.4004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RG, Bailey AJ. Glycation of Collagen: the Basis of its Central Role in the Late Complications of Ageing and Diabetes. Int J Biochem Cell Biol. 1996;28:1297–1310. doi: 10.1016/s1357-2725(96)00079-9. [DOI] [PubMed] [Google Scholar]
- Smith LR, Barton ER. Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am J Physiol Cell Physiol. 2014;306:C889–98. doi: 10.1152/ajpcell.00383.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LR, Fowler-Gerace LH, Gerace-Fowler L, Lieber RL. Muscle extracellular matrix applies a transverse stress on fibers with axial strain. J Biomech. 2011;44:1618–1620. doi: 10.1016/j.jbiomech.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


