SUMMARY
Formins catalyze nucleation and growth of actin filaments. Here we study the structure and interactions of actin with the FH2 domain of budding yeast formin Bni1p. We built an all-atom model of the formin dimer on an Oda actin filament 7-mer and studied structural relaxation and inter-protein interactions by molecular dynamics simulations. These simulations produced a refined model for the FH2 dimer associated with the barbed end of the filament and revealed electrostatic interactions between the formin knob and actin target-binding cleft. Mutations of two formin residues contributing to these interactions (R1423N, K1467L or both) reduced the interaction energies between the proteins, and in coarse-grained simulations the formin lost more inter-protein contacts with an actin dimer than with an actin 7-mer. Biochemical experiments confirmed a strong influence of these mutations on Bni1p-mediated actin filament nucleation, but not elongation, suggesting that different interactions contribute to these two functions of formins.
INTRODUCTION
Formins are large (120-220 kDa) multi-domain proteins that mediate the nucleation and growth of actin filaments. They are widely expressed in eukaryotes, helping to regulate the architecture of the actin cytoskeleton, and thus play an important role in cellular processes such as morphogenesis, cytokinesis and adhesion (Goode and Eck, 2007). The dimeric formin homology 2 (FH2) domain stabilizes filament nuclei consisting of two or three actin subunits and remains processively associated with the elongating filament barbed end by reliably dissociating from the second-to-last actin subunit and transferring onto a newly incorporated subunit (Higashida et al., 2004; Kovar et al., 2006; Kovar and Pollard, 2004; Mizuno et al., 2011). FH2-bound filament barbed ends typically elongate slower than free barbed ends, a phenomenon known as “gating”, which has been proposed to arise from conformational fluctuations between a polymerization-competent “open” state, and a polymerization-incompetent “closed” state (Kovar et al., 2006; Paul and Pollard, 2008; Vavylonis et al., 2006). Polyproline motifs in the adjacent flexible FH1 domain bind profilin-actin complexes (Chang et al., 1997; Watanabe et al., 1997) and increase the rate of polymerization by rapid, diffusion-mediated delivery of actin to the FH2-bound barbed end (Kovar et al., 2006; Kovar et al., 2003; Romero et al., 2004). The FH1 and FH2 domains are flanked by regulatory domains, which control their activity by interactions with signaling factors such as Rho GTPases (Watanabe et al., 1997). Here we focus on interactions of the formin FH2 domain with actin.
A crystal structure of the FH2 domain of the S. cerevisiae formin Bni1p (Xu et al., 2004) revealed a ring-like structure with the two FH2 domains arranged head-to-tail (Figure 1). In this arrangement, the “lasso” region at the FH2 N-terminus binds the “post” site at the C-terminus of the other subunit (see supplemental Table S1 for definitions of features). A largely unstructured “linker” peptide connects the lasso region to the main FH2 structure (Xu et al., 2004). A co-crystal of Bni1p FH2 with actin (Otomo et al., 2005) provided important insights into the interaction of the FH2 domain with the actin filament, although the actin molecules in the co-crystal were arranged with two-fold rotational symmetry of 180° rather than the 167° twist in actin filaments (Oda et al., 2009). Furthermore, the linker peptide connected the FH2 domains in the co-crystal in a continuous chain around the two-fold array of actins, rather forming head-to-tail dimers. Manual rearrangement of the linker peptide created a model of a head-to-tail FH2 dimer around a 180° twist actin filament (Figure 1) (Otomo et al., 2005). In this model the knob and post regions of the FH2 dimer contact the three actins with one FH2 (the leading FH2) closer to the barbed end of the “filament” than the other trailing FH2. FH2 “knobs” made strong contacts with the groove at the barbed end of actin, a region called the target-binding cleft (TBC) owing to interactions with a host of proteins (Dominguez, 2004, 2007; Dominguez and Holmes, 2011).
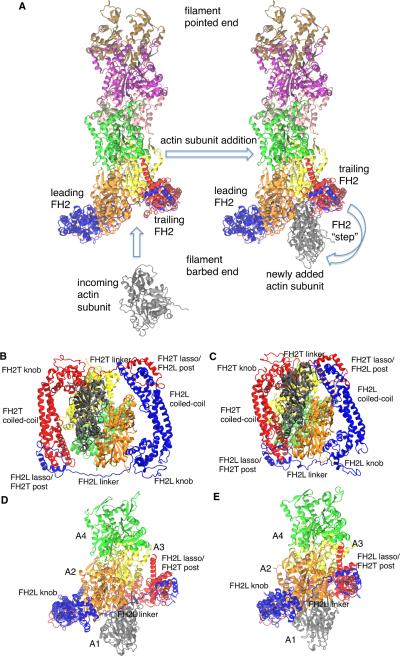
Figure 1. Comparison of the initial model and the refined model from all atom molecular dynamics simulation of a dimer of Bni1p FH2 domains associated with the barbed end of an actin filament.
The initial model was constructed by aligning the FH2 and actins from the Otomo crystal structure (Otomo et al., 2005) on the end of the Oda actin filament structure (Oda et al., 2009). This structure relaxed during 160 ns of all atom molecular dynamics simulation. Snapshots are from the 160 ns WT simulation. (A) Schematic ribbon diagrams for addition of an actin subunit (grey) to an actin filament barbed end with a bound dimer of FH2 domains (red and blue). On the left an incoming actin subunit is shown approaching the “open” conformation of the actin filament. On the right, the newly added subunit is incorporated onto the barbed end of the filament, ready for the trailing FH2 domain to take a step along the filament. We simulated the state on the right in panel (A), prior to the step of the trailing FH2 domain. (B-E) Ribbon diagrams of (blue) the leading FH2 domain and (red) and trailing FH2 domain with four of the seven actin subunits from the actin 7-mer. (B) and (C) are initial and final views down the central filament axis, and (D) and (E) are initial and final views of the system from the side. Also see Figure S1, S2, S3 and Table S1, S2, and S3 for additional structural information.
These structural insights led to proposals for the mechanisms of gating and processive elongation. Gating was proposed to arise from a rapid equilibrium of the subunits at the barbed end between an open state with a 167° twist favorable for elongation and a closed state with an unfavorable twist structure such as the 180° conformation in the co-crystal (Paul and Pollard, 2009). To explain processive elongation the trailing FH2 domain was proposed to dissociate from the filament and translocate towards the barbed end either before (Otomo et al., 2005; Zigmond et al., 2003) or after the new actin subunit adds to the barbed end (Paul and Pollard, 2009). However, the structure of the FH2 dimer bound to the end of a filament with 167° twist angle was not known.
We used molecular dynamics simulations to investigate the interactions of the Bni1p FH2 dimer with the actin filament. We started with a model of the 167° twist actin filament with a Bni1p FH2 dimer bound at the barbed end based on the actin filament structure solved by X-ray fiber diffraction (Oda et al., 2009) and intermolecular contacts observed in the Bni1p FH2/actin co-crystal (Otomo et al., 2005). Our atomistic molecular dynamics (MD) simulations refined the structure by bringing the FH2 dimer into much more intimate contact with the four actin subunits at the barbed end of a filament. The new model revealed electrostatic interactions between the FH2 domains and actin, especially interactions of residues R1423 and K1467 of Bni1p with the TBC and DNase I binding D-loop of actin. Substitution of these residues in simulations changed the electrostatic interaction energy between the FH2 knobs and the actin TBC. In simulations of coarse-grained (CG) models these substitutions had limited effects on interactions between the FH2 knobs and the TBC's of actin heptamers (7-mers), but reduced contacts with an actin dimer. Experimental observations showed that substitutions for R1423 and K1467 compromise nucleation by Bni1p FH2 domains but have no effect on the elongation rate or the processive association with the growing barbed-end of the actin filament. Taken together, our results suggest electrostatic interactions between the filament barbed end and residues R1423 and K1467 of the FH2 domain influence the nucleation activity of Bni1p, and that nucleation and elongation depend on different sets of intermolecular interactions.
RESULTS AND DISCUSSION
Structural Changes in the Bni1p/Actin System
Our simulated all-atom system consisted of the Bni1p FH2 dimer bound to actin subunits A2 and A3 of an actin 7-mer, with an additional subunit (A1) at the barbed end of the filament to represent the “newly added” actin subunit before the step of the trailing FH2 domain (Figure 1A). We assessed the overall stability of the system using the root mean-squared deviation (RMSD) of the alpha-carbon atoms of the complex (Figure S1A in the Supplemental Information (SI)). Starting from a snapshot at the end of the wild type (WT) simulation we continued five simulations, two with WT formin (WTa and WTb) for 40 ns each, and three with amino acid substitutions for 50 ns each. Their RMSDs are stable like the initial WT structure over that time (Figure S1A).
The initial structure of the WT Bni1p/actin system relaxes during the simulation, resulting in conformational rearrangements that bring both the leading (FH2L) and trailing (FH2T) FH2 domains closer to the filament axis, allowing their knob and post/lasso regions to contact the actin filament simultaneously (Figures 1, S2, and S3). The linkers of both FH2L and FH2T shorten progressively (Figure S1B) as the FH2 domains move into closer contact with actin (compare Figure 1B with 1C, and Figure 1D with 1E). As a result, the buried surface area increases at all interfaces between the FH2 domains and actin subunits A1 to A3 over the course of the simulation (Table S2). The majority of these increases are ~ 250 Å2 or greater, with the largest change occurring at the FH2L-A2 protein interface, and the smallest change occurring at the FH2L-A3 interface. Tables S2 and S3 provide more detail regarding the surfaces involved with these changes in solvent accessible surface area (SASA) during the simulations.
While the formin FH2 domains move in towards the filament axis, their orientation with respect to one another around the filament axis does not change dramatically, as the alpha-helical domains between the knob and lasso of each FH2 domain remain roughly parallel to one another (Figure 1B, C). However, as FH2T moves in towards the filament, the C-terminal helix of its post domain makes some contacts with actin A4, which are not present in the Otomo crystal structure where the FH2 domains make contacts with only three actin subunits (Figure S4C, D). The interactions of the FH2T lasso with actin monomer A1 in our refined model of the head-to-tail FH2 dimer are missing in the Otomo structure owing to the arrangement of the FH2 domains in a continuous chain in the crystal (Figures 1 and 2).
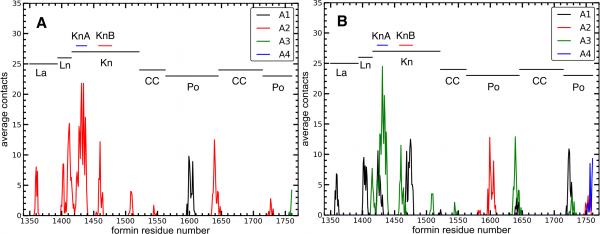
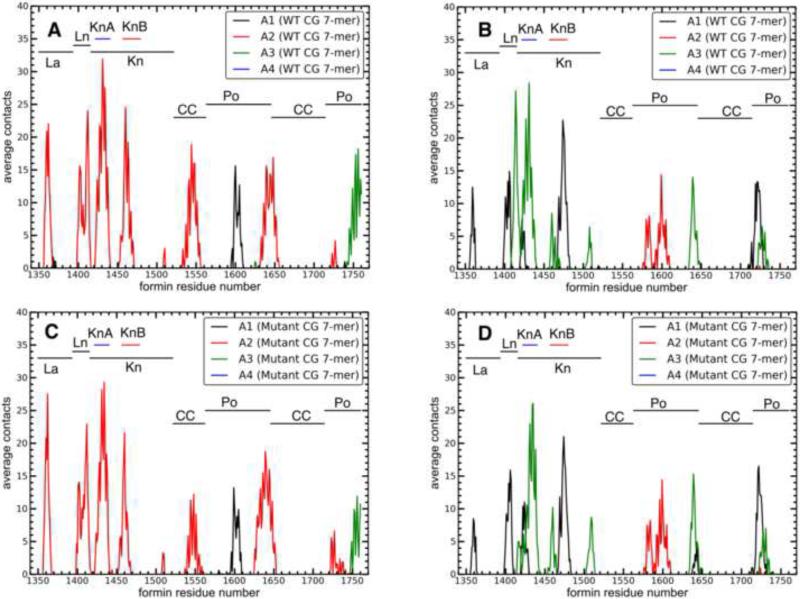
Figure 2. Contacts between formin residues and actin in the refined structure of the Bni1pFH2/actin filament seven-mer.
Number of average contacts between each residue in (A) FH2L and (B) FH2T and each actin subunit calculated as described in the main text. A contact is defined as 12 Å between alpha-carbons of two residues. The regions of Bni1p are labeled with horizontal lines. See also Table S1 for the label definitions, and also Figure S4.
Our simulations support a key assumption of virtually all models for processive stepping of FH2 domains during filament elongation, namely that the linkers between the helical domains of FH2 are flexible as first suggested by comparing crystal structures (Xu et al., 2004). The linker connecting the lasso to the FH2 knob (Figure 1) is very flexible in our simulations: the FH2L linker changed position with a RMSD of ~8.8 Å during the simulation, while the RMSD of the FH2T linker passed through a maximum of ~7.1 Å before ending at ~4.1 Å (Figure S1B). The distance between the alpha-carbon atoms of residues A1393 and Q1416 (the last residue in the lasso and first residue in the knob at either end of the linker) decreased from ~41 Å to ~38 Å in FH2T, and from ~65 Å to ~44 Å in FH2L (Figure S1B).
On the other hand, both the FH2L and FH2T linkers form extensive interactions with the actin filament by the end of the WT simulations. The FH2L linker buries 620 Å2 of SASA and forms four salt-bridges with actin A2: actin residue E2 with Bni1p residue K1417 (31.1%), D3 with K1412 (62.0%), E99 with R1402 (87.5%), and K359 with E1403 (69.9%). The numbers in parentheses are percentages of time that the salt-bridges formed averaged over the last 20 ns of all three WT simulations. The FH2T linker buries 440 Å2 of SASA and forms a salt-bridge between actin A1 D363 and FH2T R1402 (78.6%). Therefore, Bni1p must break a number of contacts between the linker and actin for the post and knob domains to detach from actin A3 and step onto the new subunits A1 at the end of the filament.
We used simulations to test if FH2 domains influence the twist angle between actin subunits at the barbed end of the filament, as proposed to explain gating (Paul and Pollard, 2008). In a control simulation of an Oda actin filament 30-mer without formin the distribution of twist angles between subunits A1 and A2 does not change between the first and last 20 ns of the 160 ns simulation (Figure S1E, F). For the actin 7-mer with formin the twist angles do not drift significantly from 167° during the first 20 ns of simulation (Figure S1C), but by the end of the 160 ns WT simulations, the distributions of twist angles become more heterogeneous and shift towards larger values (although it should be noted we do not see a full transition to a 180° “closed” state). For example, the twist between A1-A2 becomes ~172°, indicating a flatter filament (Figure S1D). Thus the simulations may sample the influence of the formin on the rapid equilibrium between the standard twist, favorable for subunit addition in the “open state”, and a flatter closed conformation that would be less favorable for adding a subunit (Paul and Pollard, 2008). When Bni1p is bound to the end of a filament the open and closed states are equally populated (Kovar et al., 2006; Paul and Pollard, 2008; Vavylonis et al., 2006).
Salt-Bridges and Hydrogen Bonds Between the FH2 Knob Helices and the Actin Target Binding Cleft (TBC)
To form a more quantitative picture of the contacts between the FH2 domains and the actin filament in our MD simulations, we measured distances between alpha-carbons of the FH2 domains and actin subunits A1 to A4 for ten trajectory frames over the last 20 ns of the WT simulation. We defined a contact as a pair of alpha-carbons separated by <12 Å. For each Bni1p residue, we then integrated over all actin contacts to determine the time-averaged total number of contacts of each Bni1p residue with actin (Figure 2). FH2L primarily contacts actin subunit A2, although its post domain also contacts subunits A1 and A3. On the other hand, FH2T mainly contacts subunits A1 and A3, but its post domain also contacts subunits A2 and A4. The KnA and KnB helices in the knob domain contact the actin TBC and D-loop (Figures 3 and S4 A and B). The KnA and KnB helices of FH2L contact subunit A2, and these helices in FH2T contact with both subunits A1 and A3. These contacts are stronger in our equilibrated Bni1p/Oda system (Figure 2) than the starting Otomo structure (Figure S4 C and D), especially FH2T KnA/KnB (Table S1). Additionally, our equilibrated structure has a small number of contacts between actin and the Bni1p coiled-coil domain (around residue number 1550) that are absent from the starting model.
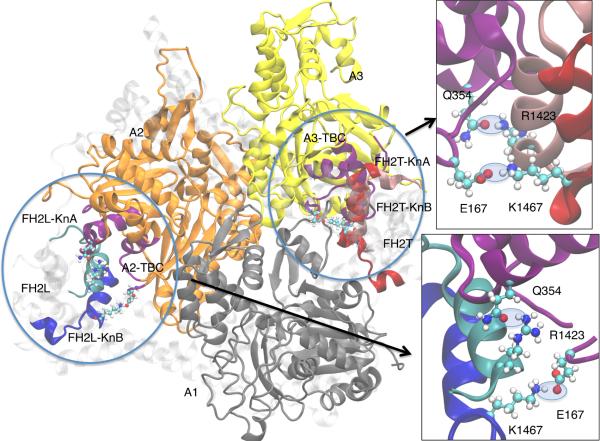
Figure 3. Salt bridges and H-bonds of Bni1p R1423 and K1467 with actin.
Ribbon diagrams of actin filament subunits A1 to A3 are shown in blue, gold and yellow, along with formin helices KnA (residues 1422-1440, cyan and pink in FH2L and FH2T respectively) and KnB (residues 1457-1479, blue and red in FH2L and FH2T respectively) The backbone of the actin TBC is shown in purple (defined as residues 22-26, 139-149, 167-169, 338-355). The insets show close-up views of the FH2T interactions with A3 (upper right) and the FH2L interactions with A2 (lower right). Salt-bridges/H-bonds are highlighted with light blue, transparent ovals. The residues comprising TBC, KnA and KnB were used to calculate the electrostatic interaction energies in Table 2 and Table S2. See also Table S5 for information about the evolutionary conservation of these residues.
The large number of contacts with actin and their proximity to the actin TBC and D-loop focused our attention on the FH2 knob helices, KnA and KnB. As actin is highly charged, we looked for salt-bridges and H-bonds between actin and charged residues in the FH2 knob helices KnA (R1423, D1424) and KnB (E1463, K1467, E1469, E1472, R1479) (Table 1).
Table 1.
Salt bridges/H-bonds between Bni1p knob helices and actin.
| Formin construct simulated | |||||||
|---|---|---|---|---|---|---|---|
| Formin residue | FH2 domain | Actin residue | Actin subunit | WT | R1423N | K1467L | R1423N/K1467L |
|
Salt-bridges
a
| |||||||
| Residues binding the TBC | |||||||
| E1463 | FH2L | R147 | A2 | 82.6c | 47.6 | 89.6 | 77.0 |
| E1463 | FH2T | R147 | A3 | 85.8 | 81.5 | 81.1 | 91.3 |
| K1467 | FH2L | E167 | A2 | 43.4 | 0 | 0 | 0 |
| K1467 | FH2T | E167 | A3 | 98.9 | 98.4 | 0 | 0 |
|
Residues binding outside the TBC | |||||||
| D1424 | FH2T | K50 | A1 | 55.2 | 93.6 | 88.1 | 98.9 |
| E1469 | FH2T | K61 | A1 | 92.6 | 99.6 | 54.8 | 5.6 |
| E1472 | FH2T | R95 | A1 | 1.1 | 0 | 0 | 90.9 |
| R1479 | FH2T | E2 | A1 | 0 | 0 | 83.0 | 0 |
| R1479 | FH2T | E100 | A1 | 88.3 | 89.1 | 70.0 | 75.4 |
|
H-bonds b | |||||||
| Residues binding the TBC | |||||||
| R1423 | FH2L | Q354 | A2 | 31.1 | 20.0 | 3.7 | 10.2 |
| R1423 | FH2T | Q354 | A3 | 18.3 | 5.3 | 0.2 | 14.4 |
| E1463 | FH2L | R147 | A2 | 97.3 | 74.6 | 96.3 | 89.4 |
| E1463 | FH2T | R147 | A3 | 96.4 | 96.4 | 97.6 | 98.8 |
| K1467 | FH2L | E167 | A2 | 33.0 | 0 | 0 | 0 |
| K1467 | FH2T | E167 | A3 | 95.1 | 95.4 | 0 | 0 |
|
Residues binding outside the TBC | |||||||
| D1424 | FH2T | K50 | A1 | 49.4 | 82.2 | 81.4 | 94.3 |
| D1424 | FH2T | Q49 | A1 | 15.0 | 0.5 | 1.0 | 1.9 |
| E1469 | FH2T | K61 | A1 | 74.8 | 82.8 | 49.3 | 4.2 |
| E1472 | FH2T | R95 | A1 | 55.8 | 5.1 | 0.1 | 92.5 |
| R1479 | FH2T | E2 | A1 | 0 | 0 | 97.1 | 0 |
| R1479 | FH2T | E100 | A1 | 94.9 | 96.0 | 87.2 | 81.8 |
The salt-bridge calculation was carried out with the Salt Bridges plugin that is provided with VMD (Humphrey, et al., 1996), using a distance cutoff of 4.0 Å between the position of oxygen and nitrogen atoms of the contributing residues.
H-bonds were calculated using a 3.5 Å distance cutoff and a 30° angle of linearity using the HBonds plugin of VMD.
All values in the table are unitless percentages, representing the percentage time that each salt-bridge and H-bond is observed.
In the WT simulations, K1467 and E1463 establish stable salt-bridges and H-bonds with residues in the actin TBC. These interactions occur between FH2L and actin subunit A2, and between FH2T and subunit A3. Residues D1424, E1469, E1472 and R1479 of FH2L form additional H-bonds and salt-bridges to actin subunit A1. These interactions can only occur between FH2T and A1 due to its position at the barbed end of the filament, and therefore are unique to the pre-stepped state. Bni1p residues E1469 and D1424 interact primarily with residues K50 and K61 in subdomain 2 (SD2) of actin, which contains the D-loop. An H-bond is present between R1423 in KnA and Q354 in the actin TBC, occurring between both FH2L and A2, and between FH2T and A3.
We tested the importance of these salt bridges in interactions between Bni1p and actin by making point substitutions of one electrostatically interacting residue in each knob helix. We used simulations to choose R1423 in KnA, a residue that H-bonds with actin Q354 in both FH2L and FH2T. The region of actin with Q354 interacts with other actin-binding proteins, including vinculin (Golji and Mofrad, 2013), profilin (Ezezika et al., 2009; Schutt et al., 1993), myosin (Patel and Root, 2009) and cofilin (McGough et al., 1997). We chose K1467 in KnB due to its strong interactions in both FH2L and FH2T with actin residue E167, especially between FH2T and A3. E167 is implicated in interactions with the D-loop of adjacent actin subunits in actin filaments (Fujii et al., 2010; Oda et al., 2009) and with specific ions in the D-loop region (Kang et al., 2013; Kang et al., 2012). Both the R1423/Q354 H-bond and the K1467/E167 salt-bridge can form simultaneously (Figure 3).
Point Mutations Decrease the Electrostatic Interaction Energy Between the FH2 Knob Helices and Actin
We made point-substitutions at positions 1423 and 1467 in Bni1(pP1FH2)p, a construct with the FH1 polyproline track located closest to the FH2 domain, followed by the FH2 domain. This construct is stable, easily purified and its actin polymerization properties well characterized (Courtemanche and Pollard, 2012; Paul and Pollard, 2008). Substituting alanine for R1432 or K1467 produced insoluble proteins, but constructs with asparagine substituting for R1423 or leucine substituting for K1467 or both substitutions expressed well and could be purified both individually and in combination. The wild-type and all three mutant constructs had indistinguishable elution profiles during size exclusion chromatography (Figure S5A), and the purified proteins migrated as single bands corresponding to the same molecular weight by SDS- PAGE (Figure S5B). Fluorescence emission spectra of the wild-type and mutant constructs contained single peaks that coincided at 325 nm, suggesting that the tryptophan residues reside in similar hydrophobic environments and similar overall tertiary structures (Figure S5C).
Based on the WT structure at 160 ns of simulation we constructed all-atom MD models of two single amino acid mutants: (1) R1423N that perturbs KnA, and (2) K1467L that perturbs KnB. We also tested the double mutant R1423N/K1467L that compromises interactions of both KnA and KnB with actin.
Simulations lasting 50 ns show that some salt bridges and H-bonds are maintained in the mutant formins but others are disrupted (Table 1). The following pairwise interactions (forminactin) are relatively stable in all three mutant formins: E1463-R147; D1424-K50; R1479-E100; E1463-R147 in both FH2 domains; D1424-K50; and R1479-E100. On the other hand, the K1467L and R1423N/K1467L substitutions completely disrupt the 1467/167 salt-bridge. The interaction of K1467 in FH2L with E167 in A2 is also lost in the R1423N mutant, as K1467 interacts instead with E1469 within the same FH2L domain 52.2% of the time. H-bonding between R1423 in Bni1p and Q354 in actin is reduced to varying degrees compared to the WT in simulations of all three mutant formins. For the knob residues that interact with actin residues outside the TBC, E1469 (nearby to K1467) forms a salt-bridge and H-bond with K61 in subdomain 2 (SD2) of actin, and both the K1467L and R1423N/K1467L mutations reduce these interactions. The interactions between D1424 of FH2T and K50 in the D-loop of actin subunit A1 may contribute to D-loop insertion during the addition of a subunit at the barbed end.
Calculations of the total interaction energy (i.e., sum of the non-bonded terms in the MD force-field) showed that the charge mutations made interactions between KnA and KnB and the actin TBC's were less favorable (less negative) for FH2L-A2 and even more so for FH2T-A3 (Table 2 with individual contributions of KnA and KnB in Table S4). The loss of interaction energy is larger for the double mutant (Table 2). The interaction energies of actin with KnB of both FH2L and FH2T are larger than KnA, and the double mutant reduces the KnB/actin interactions more than the KnA/actin interactions (Table S4).
Table 2.
Total non-bonded interaction energya between FH2 knob helices and actin TBC
| Simulation | FH2L-knob helices/A2-TBC | FH2T-knob helices/A3-TBC |
|---|---|---|
| WT | −105.7 | −117.6 |
| R1423N | −45.8 (−59.9)b | −77.1 (−40.5) |
| K1467L | −51.5 (−54.2) | −34.1 (−83.5) |
| R1423N-K1467L | −15.4 (−90.3) | −8.9 (−108.7) |
See also Table S4.
All interaction energies are given in kcal/mol
Numbers in parentheses are Ewild-type - Emutant.
Simulations of Course-grained Models of Mutant Formins and Actin
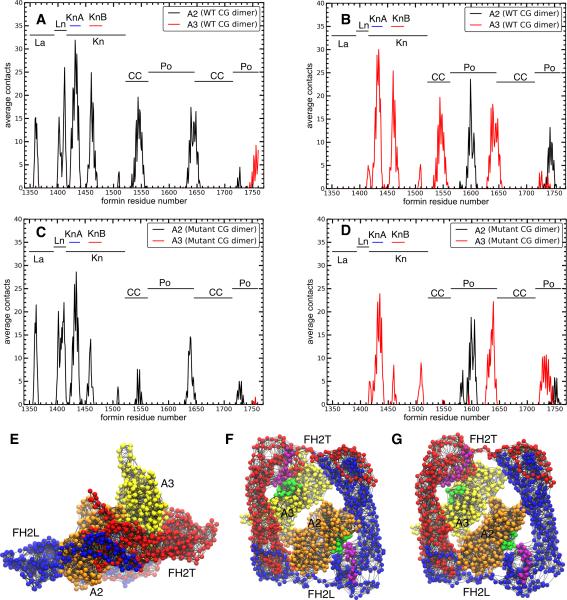
To test the effect of the point mutations on the interactions between Bni1p and actin on time scales beyond the reach of all-atom simulations, we built coarse-grained (CG) models of both WT and mutant Bni1p FH2 dimers bound to either subunits A2 and A3 of an actin 7-mer (Figure 4C) or to an actin dimer (Figure 4B). The spectrum of contacts between Bni1p and actin is very similar in CG simulations of the WT (Figures 5A and 5B) and mutant (Figures 5C and 5D) formins with the actin 7-mer, although the magnitudes of these contacts are larger in the CG simulations (Figure 5) than the all-atom simulations (Figure 2). The larger number of contacts observed in some regions of the CG simulations (for example, the additional allowed contacts between the alpha-helical domain and actin in the CG system) likely results from the absence of explicit solvent in the CG model.
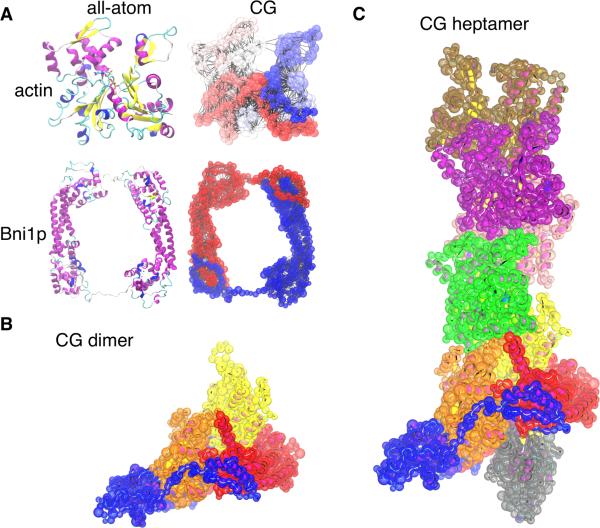
Figure 4. Coarse-grained (CG) model of formin and actin.
(A) Ribbon diagrams of the backbones of all-atom models of actin and Bni1p next to their CG hENM models. In the actin CG model, the color scale represents the residue number (dark blue is the N-terminal end, and dark red is the C-terminal end). In the formin CG model, FH2L is colored blue and FH2T is colored red. Lines connect CG sites within 10 Å, as in the hENM model. The varying CG site sizes represent the radii used for the LAMMPS excluded volume terms. (B and C) Models with the CG beads superimposed on the all-atom ribbon diagrams of the protein backbones. Bead sizes represent the excluded volume radii, as in (A). (B) Model of the CG dimer system, consisting of the formin dimer and two actin subunits extracted from the filament model. (C) Model of the formin dimer with the actin 7-mer filament.
Figure 5. Contacts between formin residues and actin in CG structures of the wild type and mutant Bni1p/actin filament heptamer systems.
Number of average contacts between each formin residue and each actin subunit in the CG heptamer systems: (A) WT FH2L; (B) WT FH2T; (C) double-mutant FH2L; and (D) double-mutant FH2T.
CG simulations of the FH2 dimer with an actin dimer gave larger differences in contacts between the WT (Figures 6A and 6B) and mutant (Figures 6C and 6D) formins than in the 7-mer system, specifically in the region of the KnB knob helix. For example, in simulations of FH2L with actin dimers both the KnB and coiled-coil domains have fewer contacts with actin in the mutant than the WT formin, and for FH2T both the helical domain and the KnB helix have fewer contacts with actin A3 in the mutant compared to WT formin. The greater loss of contacts between KnB of FH2T and A3 in the actin dimer simulation is consistent with the all-atom simulation where formin mutations reduce the total interaction energy more for the FH2T-KnB interaction with actin (Table 2 and Table S4).
Figure 6. Contacts between formin residues and actin in CG structures of WT and double mutant Bni1p/actin dimer systems.
Number of average contacts between each formin residue and each actin subunit in the CG dimer systems: (A) WT FH2L; (B) WT FH2T; (C) double- mutant FH2L; and (D) double-mutant FH2T. (E-G) CG models of Bni1p FH2 domains (FH2L blue, FH2T red) on a dimer of actin subunits (orange) A2 and (yellow) A3. The model has one site per amino acid, and every site has a charge assigned from the charge fitting procedure described in the Experimental Methods. Lines represent the elastic network bonds between CG sites. (E) Side view. (F, G) View looking from the barbed end towards the pointed end of actin. Purple spheres are the Bni1p knob helix KnB, and green spheres represent actin residues within 12 Å of the KnB residues. (F) WT system. (G) Double-mutant system.
Bni1(pP1FH2)p with Substitutions for K1423 or R1467 Elongate Actin Filaments Normally
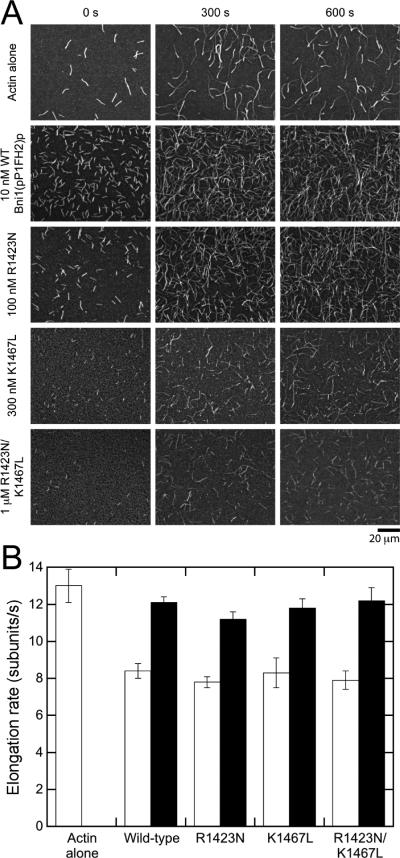
For comparison with the all-atom and CG analyses, we investigated the effects of the R1423N and K1467L substitutions in Bni1(pP1FH2)p on actin polymerization in vitro. We measured the elongation rates of filament barbed ends in the presence of wild-type or mutant formins by time-lapse total internal reflection fluorescence (TIRF) microscopy (Figure 7A). Barbed ends associated with wild-type Bni1(pP1FH2)p elongated at 8.4 subunits/s in the presence of 1.5 μM actin (33% labeled with Oregon Green on cysteine 374), compared to 13.0 subunits/s for free barbed ends (Figure 7B). This corresponds to a gating factor (defined as the ratio of polymerization rates with and without formin) of 0.6, consistent with previous reports (Kovar et al., 2006; Paul and Pollard, 2008; Vavylonis et al., 2006). Inclusion of 5 μM profilin in the reaction increased elongation mediated by Bni1(pP1FH2)p to 12.1 subunits/s, reflecting the delivery of profilin-actin from the FH1 domain, which contains a single polyproline track, to the barbed end (Figure 7B).
Figure 7. Substitutions R1423N and K1467L do not affect the elongation of actin filament barbed ends mediated by Bni1(pP1FH2)p.
Conditions: 10 mM imidazole (pH 7.0), 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 50 mM DTT, 0.3 mM ATP, 0.02 mM CaCl2, 15 mM glucose, 0.02 mg/ml catalase, 0.1 mg/ml glucose oxidase and 0.5% methylcellulose (4,000 cP at 2% (w/v)). Data were collected with TIRFM. (A) Time series of images of 1.5 μM actin (33% Oregon green-labeled) filaments growing in the presence of wild-type and mutant Bni1(pP1FH2)p constructs. The concentration of each formin is indicated. (B) Rates of barbed end elongation mediated by wild-type and mutant Bni1(pP1FH2)p in the absence (open bars) or presence (filled bars) of 5 μM profilin. For each sample, we measured the elongation of 10-20 filaments typically over a span of at least 300 s. The error bars are standard errors of the mean. See also Figures S5 and S6 for additional information about the experimental characterization of the actin/formin system.
Point-substitutions at positions 1423 and 1467, both individually and in combination, do not affect actin elongation rates mediated by Bni1(pP1FH2)p, either in the absence and presence of 5 μM profilin (Figure 7B). As seen for wild-type Bni1p constructs (Courtemanche and Pollard, 2012; Kovar and Pollard, 2004; Paul and Pollard, 2008), the fluorescence intensity of formin-bound filaments polymerized in the presence of profilin was lower than filaments with free barbed ends owing to the lower affinity of profilin for fluorescently labeled actin monomers (Vinson et al., 1998), confirming that the FH1 domain is capable of mediating polymerization (Figure S6A). Thus these substitutions do not affect FH2 domain gating or efficient delivery of profilin-actin to the barbed end via the FH1 domain.
To determine whether the point-substitutions affect the ability of Bni1(pP1FH2)p to remain processively bound to filament barbed ends during elongation we measured elongation rates over time in the absence of profilin, where dissociation of formin from a barbed end would result in an increase in the polymerization rate. Filament elongation rates mediated by wild-type and mutant Bni1(pP1FH2)p were constant over a timespan of up to 300 s, showing that each formin remains bound to barbed ends for at least 2400 cycles of subunit addition. Therefore, substitution of residues R1423 and K1467 does not affect the processive properties of Bni1(pP1FH2)p.
Substitutions for Residues 1423 and 1467 Impair Actin Filament Nucleation by Bni1(pP1FH2)p
To estimate the effects of substitution of R1423 and K1467 on the ability of Bni1(pP1FH2)p to nucleate actin filaments, we compared the number of filaments generated over the course of 300 s in each polymerization reaction. Whereas 10 nM of wild-type Bni1(pP1FH2)p nucleated numerous filaments, much higher concentrations of the mutant formins are required to nucleate comparable numbers of filaments (Figure 7A). By visually comparing the numbers of filaments present at the start of data collection and after 300 and 600 s, we estimate that the R1423N substitution decreases nucleation by at least 10-fold, whereas the K1467L substitution appears to be more severe and decreases nucleation by at least 30-fold. Substitution of both residues causes the most dramatic effect and decreases apparent nucleation by at least 100-fold. To rule out actin monomer sequestration as the cause of the differences in the numbers of filaments produced, we measured the rate of elongation of filaments with free barbed ends in the presence of varying range of formin mutant concentrations (Figure S6B). This rate was constant in the presence of formin concentrations up to 1 μM, confirming that the low numbers of filaments arise from weak nucleation by the formin mutants, not from sequestration of actin monomers.
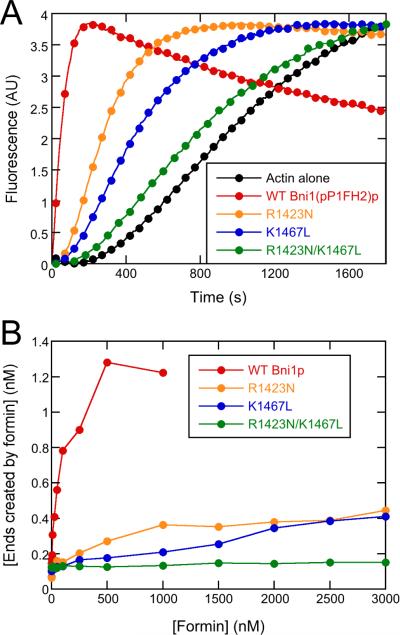
We quantified the extent of the nucleation defects caused by these substitutions by monitoring the time-course of assembly of 4 μM actin (20% pyrene-labeled) in the presence of a range of concentrations of each construct of Bni1(pP1FH2)p (Figure 8A). In these assays nucleation is the main determinant of the overall polymerization rate. Knowing the bulk polymerization rates and the elongation rates from TIRF observations (Figure 8B), one can calculate the number of filaments at each time point.
Figure 8. Substitutions R1423N and K1467L of Bni1(pP1FH2)p strongly impair actin filament nucleation.
Conditions: 10 mM imidazole (pH 7.0), 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.17 mM ATP, 0.5 mM DTT, 0.03 mM CaCl2, 1.7 mM Tris (pH 8.0), 1 part per 105 (w/v) Antifoam 204. (A) Representative time courses of spontaneous polymerization of 4 μM actin (20% pyrene-labeled) alone or in presence of 1 μM wild-type or mutant Bni1(pP1FH2)p constructs. For clarity, every 5th data point is shown. (B) Dependence of the concentration of formin-nucleated barbed ends on the concentration of Bni1(pP1FH2)p constructs. Barbed end concentrations were calculated from the elongation rates in bulk samples when half of the total actin was polymerized and the elongation rates measured in the TIRF microscopy experiments. See also Figures S5 and S6 for additional information about the experimental characterization of the actin/formin system.
Consistent with our microscopy experiments, bulk assembly assays reveal a dramatic reduction in actin assembly rates mediated by the mutant formins, with K1467L having a lower nucleation activity than R1423N, and the double-substitution having little activity. The concentrations of formin required to produce a half-maximal concentration of barbed ends are much larger for the variants than for wild-type. Thus, although substitutions for R1423 and K1467 do not affect the ability of Bni1p to stay bound to elongating filament barbed ends, these residues do contribute significantly to the interactions of Bni1p required to form actin filament nuclei.
Although we did not simulate Bni1p-mediated nucleation or polymerization directly, these results appear to be consistent with the results of the CG formin simulations where WT and double-mutant CG models of Bni1p bound to an actin 7-mer similarly, while the double mutant Bni1p had fewer contacts with the actin dimer than the CG model of WT Bni1p. Thus R1423 and K1467 are more important for electrostatic interactions with actin dimers than filaments, explaining the low nucleation activity of Bni1p with positive to neutral mutations of Bni1p knob helices. These combined computational and experimental results provide the first evidence that nucleation and elongation are separable functions of formins, and also suggest that formin FH2 domains interact differently with filament nuclei than with filament barbed ends.
Although R1423 and K1467 are not highly conserved residues among formins, many formins contain positively charged residues at positions corresponding to residues 1424, 1466 and 1468 in Bni1p (see Table S5 for representative formin sequences). As R1423 and K1467 are both located in flexible loop regions within the knob helices, it is possible that neighboring residues also located within the loops could also form salt-bridges or H-bonds with actin. Our simulations demonstrate a similar situation where a salt-bridge forms between D1424 in Bni1p and K50 in actin in the R1423N mutant (Table 1). Although further structural studies will be required to confirm the existence and mechanistic importance of salt-bridges between the FH2 domains and actin, the presence of charged residues in the knob loops of many formins suggests that electrostatic interactions contribute to the stable association between FH2 domains and small filament nuclei, and thus may confer nucleation activity to formins in general.
EXPERIMENTAL PROCEDURES
Modeling and All-Atom Simulation of the Formin/Actin System
We conducted atomistic MD simulations of a Bni1p FH2 dimer bound at the barbed end of an actin filament. The model of the FH2-capped filament barbed end derives from the crystal structure of the Bni1p FH2 domain in complex with actin (Otomo et al., 2005) and the structure of the actin filament solved using X-ray fiber diffraction by Oda et al. (Oda et al., 2009). We created the initial structure using a series of structural alignments and remodeling steps detail in the Supplemental Experimental Procedures. As indicated by structural data (Otomo et al., 2005), it is not possible to align the FH2 domain against a 167° actin filament while maintaining contacts at both the knob and lasso/post regions. Briefly, our approach was to conduct the alignment so as to preserve the binding mode of the knob, which constitutes the most extensive set of interactions between actin and formin. The configuration of the system represented the putative stage in the stepping process subsequent to actin addition, but prior to translocation of the trailing FH2 (the “pre-step” state, depicted schematically in Figure 1A). We refer to the leading and trailing FH2 domains as FH2L and FH2T and to the actin subunits in the 7-mer as A1 through A7, with A1 representing the terminal subunit at the barbed end, and A7 representing the pointed end actin subunit. We mainly discuss subunits A1 through A4, which are the only filament subunits directly accessible to Bni1p.
Simulations of the Bni1p/actin system were run with NAMD (Phillips et al., 2005) using the Charmm27 force-field with CMAP corrections (Mackerell et al., 2004). The 167° twist Oda 7-mer was built as described for a 13-mer filament (Chu and Voth, 2005; Pfaendtner et al., 2010; Saunders and Voth, 2011), except only 7 subunits were used and the filament was not built to be continuous across the boundary of the periodic simulation cell. The subunit structure used in the construction of the Oda 7-mer is based on the PDB entry 2ZWH (Oda et al., 2009), and included both ADP and the Mg2+ ion in the actin nucleotide cleft, in addition to waters that complete the coordination to the ion (Saunders and Voth, 2011). Additional details of the simulation protocol are described in the Supplemental Experimental Procedures.
We carried out two additional WT simulations for 40 ns each (WTa and WTb), each started from a different snapshot near the end of the long WT simulation in order to increase sampling of the various electrostatic interactions in the simulation and to further gauge stability of the Bni1p/actin structure (Figure 1A). We also carried out simulations of several Bni1p/actin mutant systems for 50 ns each (details of setup for mutant systems can be found in the Supplemental Experimental Procedures). These were R1423N, K1467L, and R1423N/K1467L. For the mutant simulations, a snapshot of the formin/actin system from the end of the long WT simulation was taken as the starting structure. All visualizations were done with VMD (Humphrey et al., 1996).
Data from a simulation of an Oda actin filament 30-mer was also used to ascertain the actin twist angle distribution for comparison with the formin-bound barbed end simulations. The initial geometry of the Oda 30-mer was identical to the Oda 7-mer, but without formin bound at the barbed end. After minimization, heating and equilibration phases, the simulation was run for approximately 160 ns. Additional details of the simulation protocol are described in the Supplemental Experimental Procedures.
Formin/Actin Coarse-Grained (CG) Model
Coarse-grained (CG) models of actin and Bni1p were created to study the long timescale behavior of small actin/Bni1p complexes (Figure 4). A residue-based model (sites for each amino acid were placed on the alpha carbon) with a hetero elastic network model (hENM) (Lyman et al., 2008) for the intra-protein interactions and charge and excluded volume interactions for inter-protein interactions was chosen. In the intra-protein hENM CG models of actin and Bni1p, two sites were connected if the distance between them was less than 10 Å. The charges, excluded volume and the hENM bond lengths and force constants were parameterized using the all-atom MD simulation data. Bni1p charges for CG wild-type simulation were parameterized from the WT simulation, and Bni1p charges for the CG mutant simulation were parameterized from the R1423N/K1467L simulation. Additional details can be found in the Supplemental Experimental Procedures.
Using the separately parameterized Bni1p and actin CG models, CG models of a Bni1p/actin 7-mer and Bni1p/actin dimer were built (Figure 4B,C). The starting orientation for Bni1p on actin in the CG model was taken from the end of the all-atom WT simulation. All CG simulations were performed with LAMMPS (Plimpton, 1995). Pairwise interactions (charge and excluded volume) were computed on graphics processing units (GPUs) as implemented in LAMMPS (Brown et al., 2012; Brown et al., 2011). The simulations were carried out at 310K in the NVT ensemble with Langevin dynamics (1000 fs damping parameter). Additional details can be found in the Supplemental Experimental Procedures.
Plasmid Construction
Bni1(pP1FH2)p (residues 1312-1766) was inserted into a pGV67 plasmid, which encodes an N-terminal GST tag, and the R1423N and K1467L mutations were made with standard cloning methods (Sambrook et al., 1989). The Supplemental Experimental Procedures describe details of the protein purification process.
Microscopy
We generated time-lapse movies of actin filaments using prism-style total internal reflection fluorescence microscopy (TIRFM) on an Olympus IX-70 inverted microscope by collecting images every 10 s with a Hamamatsu C4742-95 CCD (Orca-ER) camera and MetaMorph software (Molecular Devices, Union City, CA). Images were processed with ImageJ software (http://rsbweb.nih.gov/ij/). For each sample, we measured the rates of barbed end elongation of 10-20 filaments, typically over a span of at least 300 s.
Glass flow chambers (Kuhn and Pollard, 2005) were incubated for 1 min each with 0.5% Tween-80 in high-salt TBS (HS-TBS) (50 mM Tris-HCl, pH 7.5, 600 mM KCl), 250 nM NEM-inactivated skeletal muscle myosin in Hs-TBS, and 10% BSA (w/v) in HS-TBS, with washes of HS-TBS after each incubation step. Polymerization was initiated by mixing 1.5 μM actin monomers (33% labeled with Oregon Green) with or without 5 μM profilin and varying concentrations of formin in standard microscopy buffer (10 mM imidazole, pH 7.0, 50 mM KCl, 1.0 mM EGTA, 1 mM MgCl2, 1 mM EGTA, 0.3 mM ATP, 15 mM glucose, 50 mM DTT, 0.02 μM CaCl2, 20 μg/mL catalase, 100 μg/mL glucose oxidase, and 0.5% methylcellulose [4,000 cP at 2% (w/v)]).
Pyrene-Actin Assembly Assays
We collected time-courses of fluorescence emission with a Molecular Devices SpectraMax Gemini XPS fluorescence plate-reader using Corning 96-well flat-bottom plates. Reactions containing 4 μM actin (20% pyrene-labeled) and varying formin concentrations were polymerized in 10 mM imidazole (pH 7.0), 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.17 mM ATP, 0.5 mM DTT, 0.03 mM CaCl2, 1.7 mM Tris (pH 8.0) and 1 part per 105 (w/v) Antifoam 204. Samples were excited at 365 nm and fluorescence emission intensity was sampled every 10s at 407 nm over a period of 30-60 minutes.
We converted the fluorescence signal to polymer concentration by normalizing the maximum fluorescence signal in each trace to the final predicted actin polymer concentration, assuming a critical concentration of 0.17 μM. We calculated the concentration of barbed ends in each sample using the following equation:
| (1) |
where k+ and k- are the barbed end association and dissociation rates of actin. We calculated the polymerization rate from the slope of the change in fluorescence signal at the point where half of the actin is polymerized.
Supplementary Material
ACKNOWLEDGEMENTS
JLB, DLP, and GAV were supported by the National Science Foundation through the Center for Multiscale Theory and Simulation (grant CHE-1136709). MM was supported by funding from the National Institutes of Health under Ruth L. Kirschstein National Research Service Award (GM101848) from the NIGMS. NC and TDP were supported by NIH research grant GM-026338 (awarded to TDP) and a postdoctoral fellowship from the Leukemia and Lymphoma Society (awarded to NC). Simulations were performed using the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by NSF grant number OCI-1053575. This research also used resources of the Argonne Leadership Computing Facility (ALCF) and the ALCF-2 Early Science Program at Argonne National Laboratory, which is supported by the Office of Science of the U.S. Department of Energy under contract DE-AC02-06CH11357.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
TDP and GAV designed the research project. JLB ran all-atom simulations, and analyzed all-atom and CG simulation data. NC ran polymerization and nucleation experiments and analyzed experimental data. MM ran the CG simulations and built CG models. DLP built the initial Bni1p/actin model. All authors contributed to writing the manuscript.
REFERENCES
- Brown WM, Kohlmeyer A, Plimpton SJ, Tharrington AN. Implementing molecular dynamics on hybrid high performance computers - Particle-particle particle-mesh. Comput Phys Commun. 2012;183:449–459. [Google Scholar]
- Brown WM, Wang P, Plimpton SJ, Tharrington AN. Implementing molecular dynamics on hybrid high performance computers - short range forces. Comput Phys Commun. 2011;182:898–911. [Google Scholar]
- Chang F, Drubin D, Nurse P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J Cell Biol. 1997;137:169–182. doi: 10.1083/jcb.137.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu JW, Voth GA. Allostery of actin filaments: Molecular dynamics simulations and coarse-grained analysis. P Natl Acad Sci USA. 2005;102:13111–13116. doi: 10.1073/pnas.0503732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche N, Pollard TD. Determinants of Formin Homology 1 (FH1) domain function in actin filament elongation by formins. J Biol Chem. 2012;287:7812–7820. doi: 10.1074/jbc.M111.322958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R. Actin-binding proteins - a unifying hypothesis. Trends Biochem Sci. 2004;29:572–578. doi: 10.1016/j.tibs.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Dominguez R. A Common Binding Site for Actin-Binding Proteins on the Actin Surface. Mol Biol Intell Unit. 2007:107–115. [Google Scholar]
- Dominguez R, Holmes KC. Actin Structure and Function. Annu Rev Biophys. 2011;40:169–186. doi: 10.1146/annurev-biophys-042910-155359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezezika OC, Younger NS, Lu J, Kaiser DA, Corbin ZA, Nolen BJ, Kovar DR, Pollard TD. Incompatibility with formin Cdc12p prevents human profilin from substituting for fission yeast profilin: insights from crystal structures of fission yeast profilin. J Biol Chem. 2009;284:2088–2097. doi: 10.1074/jbc.M807073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Iwane AH, Yanagida T, Namba K. Direct visualization of secondary structures of F-actin by electron cryomicroscopy. Nature. 2010;467:724–728. doi: 10.1038/nature09372. [DOI] [PubMed] [Google Scholar]
- Golji J, Mofrad MRK. The Interaction of Vinculin with Actin. Plos Comput Biol. 2013;9 doi: 10.1371/journal.pcbi.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Higashida C, Miyoshi T, Fujita A, Oceguera-Yanez F, Monypenny J, Andou Y, Narumiya S, Watanabe N. Actin polymerization-driven molecular movement of mDia1 in living cells. Science. 2004;303:2007–2010. doi: 10.1126/science.1093923. [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graph Model. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Kang H, Bradley MJ, Elam WA, De La Cruz EM. Regulation of Actin by Ion-Linked Equilibria. Biophys J. 2013;105:2621–2628. doi: 10.1016/j.bpj.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HR, Bradley MJ, McCullough BR, Pierre A, Grintsevich EE, Reisler E, De La Cruz EM. Identification of cation-binding sites on actin that drive polymerization and modulate bending stiffness. P Natl Acad Sci USA. 2012;109:16923–16927. doi: 10.1073/pnas.1211078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124:423–435. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Kovar DR, Kuhn JR, Tichy AL, Pollard TD. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J Cell Biol. 2003;161:875–887. doi: 10.1083/jcb.200211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Pollard TD. Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces. P Natl Acad Sci USA. 2004;101:14725–14730. doi: 10.1073/pnas.0405902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn JR, Pollard TD. Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophys J. 2005;88:1387–1402. doi: 10.1529/biophysj.104.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman E, Pfaendtner J, Voth GA. Systematic Multiscale Parameterization of Heterogeneous Elastic Network Models of Proteins. Biophys J. 2008;95:4183–4192. doi: 10.1529/biophysj.108.139733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackerell AD, Feig M, Brooks CL. Extending the treatment of backbone energetics in protein force fields: Limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J Comput Chem. 2004;25:1400–1415. doi: 10.1002/jcc.20065. [DOI] [PubMed] [Google Scholar]
- McGough A, Pope B, Chiu W, Weeds A. Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J Cell Biol. 1997;138:771–781. doi: 10.1083/jcb.138.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno H, Higashida C, Yuan Y, Ishizaki T, Narumiya S, Watanabe N. Rotational movement of the formin mDia1 along the double helical strand of an actin filament. Science. 2011;331:80–83. doi: 10.1126/science.1197692. [DOI] [PubMed] [Google Scholar]
- Oda T, Iwasa M, Aihara T, Maeda Y, Narita A. The nature of the globular- to fibrous-actin transition. Nature. 2009;457:441–445. doi: 10.1038/nature07685. [DOI] [PubMed] [Google Scholar]
- Otomo T, Tomchick DR, Otomo C, Panchal SC, Machius M, Rosen MK. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature. 2005;433:488–494. doi: 10.1038/nature03251. [DOI] [PubMed] [Google Scholar]
- Patel DA, Root DD. Close proximity of myosin loop 3 to troponin determined by triangulation of resonance energy transfer distance measurements. Biochemistry. 2009;48:357–369. doi: 10.1021/bi801554m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Pollard T. The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation. Curr Biol. 2008;18:9–19. doi: 10.1016/j.cub.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AS, Pollard TD. Review of the Mechanism of Processive Actin Filament Elongation by Formins. Cell Motil Cytoskel. 2009;66:606–617. doi: 10.1002/cm.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaendtner J, Lyman E, Pollard TD, Voth GA. Structure and Dynamics of the Actin Filament. J Mol Biol. 2010;396:252–263. doi: 10.1016/j.jmb.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plimpton S. Fast Parallel Algorithms for Short-Range Molecular-Dynamics. J Comput Phys. 1995;117:1–19. [Google Scholar]
- Romero S, Le Clainche C, Didry D, Egile C, Pantaloni D, Carlier MF. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 2004;119:419–429. doi: 10.1016/j.cell.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Saunders MG, Voth GA. Water Molecules in the Nucleotide Binding Cleft of Actin: Effects on Subunit Conformation and Implications for ATP Hydrolysis. J Mol Biol. 2011;413:279–291. doi: 10.1016/j.jmb.2011.07.068. [DOI] [PubMed] [Google Scholar]
- Schutt CE, Myslik JC, Rozycki MD, Goonesekere NC, Lindberg U. The structure of crystalline profilin-beta-actin. Nature. 1993;365:810–816. doi: 10.1038/365810a0. [DOI] [PubMed] [Google Scholar]
- Vavylonis D, Kovar DR, O'Shaughnessy B, Pollard TD. Model of formin-associated actin filament elongation. Mol Cell. 2006;21:455–466. doi: 10.1016/j.molcel.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson VK, De La Cruz EM, Higgs HN, Pollard TD. Interactions of Acanthamoeba profilin with actin and nucleotides bound to actin. Biochemistry. 1998;37:10871–10880. doi: 10.1021/bi980093l. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. The EMBO journal. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YW, Moseley JB, Sagot I, Poy F, Pellman D, Goode BL, Eck MJ. Crystal structures of a formin homology-2 domain reveal a tethered dimer architecture. Cell. 2004;116:711–723. doi: 10.1016/s0092-8674(04)00210-7. [DOI] [PubMed] [Google Scholar]
- Zigmond SH, Evangelista M, Boone C, Yang C, Dar AC, Sicheri F, Forkey J, Pring M. Formin leaky cap allows elongation in the presence of tight capping proteins. Curr Biol. 2003;13:1820–1823. doi: 10.1016/j.cub.2003.09.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.