Abstract
Background
The combination of low-dose radiation therapy with poly (ADP-ribose) polymerase (PARP) inhibition has been shown to enhance anti-tumor efficacy through potentiating DNA damage. We combined low-dose fractionated whole abdominal radiation (LDFWAR) with escalating doses of veliparib (ABT-888), a small molecule PARP inhibitor, in patients with peritoneal carcinomatosis from advanced solid tumor malignancies.
Methods
Patients were treated with veliparib (80mg-320mg daily) for a total of 3 cycles. LDFWAR consisted of 21.6Gy in 36 fractions, 0.6 Gytwice daily on days 1 and 5 for weeks 1-3 of each cycle. Circulating tumor cells (CTCs) were collected and evaluated for γ-H2AX. Quality of life (QoL) was assessed using the EORTC-QLQ-C30 questionnaire.
Results
Twenty-two patients were treated. Treatment-related grade 3 and 4 toxicites included lymphopenia (68%), anemia (9%), thrombocytopenia (14%), neutropenia (4%), leukopenia (9%), ascites (4%), vomiting (4%) and dyspnea (4%). No objective responses were observed. Disease stabilization (≥24 wks) was observed in 7 patients (33%). Median PFS was 4.47 months and mOS was 13.04 months. In the subset of 8 ovarian and fallopian cancers (OV), mPFS was 6.77 months and mOS was 17.54 months compared to mPFS 2.71 months and mOS 13.01 months in others. Patients with OV had better QoL over time than those with other cancers. An increased percentage of γ-H2AX-positive CTCs was observed in a subset of patients (3/6 with >2 CTCs at baseline).
Conclusions
Combined veliparib and LDFWAR is a well-tolerated regimen that resulted in prolonged disease stability for some patients with advanced solid tumors and carcinomatosis, particularly in the OV subpopulation.
Introduction
Peritoneal carcinomatosis presents a difficult clinical challenge, with significant morbidity as well as poor prognosis (1-3). Whole abdominal radiation has not often been used because of toxicity concerns (4-8). However, laboratory data suggest that using low-dose fractionated radiation therapy as a chemosensitizer might improve efficacy with only a minimal increase in treatment toxicity (8,9). Phase I data combining chemotherapy with low-dose fractionated whole abdominal radiation (LDFWAR) in patients with advanced small bowel, pancreatic and ovarian cancers have demonstrated good tolerability [10,11].
The poly (ADP-ribose) polymerases (PARP) are an essential group of enzymes in base excision (BER) DNA repair that are swiftly activated by cells in response to DNA damage (10). PARP-1 and PARP-2 localize to the sites of DNA damage and catalyze the transfer and polymerization of poly (ADP-ribose) (PAR) (11-13). Increased PARP activity is awell-described mechanism by which tumor cells avoid apoptosis caused by DNA damaging agents; it has been linked to drug resistance and the ability of tumor cells to withstand genotoxic stress [18-20].
PARP inhibitors interrupt the catalytic effects of PARP (14). PARP inhibition has been exploited particularly in cancers with BRCA mutations (15,16). However, even in the absence of BRCA1/2 mutations, it has been shown that PARP inhibitors may function as sensitizing agents for chemotherapy and radiation therapy that cause DNA damage (17,18). Preclinical studies have shown that the inhibition of PARP enhances the cytotoxic effects of radiation as well (19-26).
Based on these preclinical and clinical data, we hypothesized that LDFWAR with PARP inhibition might be a tolerable combination and provide clinical benefit to patients with peritoneal carcinomatosis, a group of patients with minimal therapeutic options (8,9,27,28).
Patients and Methods
Study Design
The primary objective of this multi-institutional phase I study was to assess the safety profile of the combination of veliparib and LDFWAR in patients with advanced solid tumor malignancies and peritoneal carcinomatosis. Secondary objectives included assessment of the antitumor effect and evaluation of quality of life (QoL). Serial circulating tumor cell (CTC) analysis of γ-H2AX levels in these cells were included as exploratory objectives.
Eligibility Criteria
Eligible patients had an unresectable or metastatic solid tumor malignancy with the presence of peritoneal carcinomatosis documented either via imaging, operative notes, clinical notes or symptoms. Measureable disease was not required as an eligibility criterion. Extra-abdominal disease was permitted so long as peritoneal disease was dominant. Patients had adequate organ function, an Eastern Cooperative Oncology Group (ECOG) performance status of ≤1 and a life expectancy of greater than 3 months. Exclusion criteria included prior abdominal radiation therapy (prior pelvic radiation was acceptable as long as there was no overlap between radiation fields), previous malignant bowel obstruction (except if at diagnosis) or uncontrolled ascites. The protocol was approved by the institutional review boards of the participating institutions, and written informed consent was obtained from all patients prior to performing study-related procedures in accordance with federal and institutional guidelines.
Drug Administration, Radiation Therapy and Dose-Escalation Procedures
Veliparib was provided by the Cancer Therapy Evaluation Program (CTEP) through a Clinical Trials Agreement between Abbott Laboratories and the NCI Division of Cancer Treatment and Diagnosis. Patients were treated with veliparib by mouth in 4 escalating doses [dose levels (DL) 1-4: 40mg PO BID (DL1), 80mg PO BID (DL2), 120mg PO BID (DL3) and 160mg PO BID (DL4)]. Patients received veliparib on days 5-21 of the first 28-day cycle and on days 1-21 of the subsequent 2 cycles. LDFWAR was delivered using anterior and posterior open fields, in two daily fractions of 60 cGy on days 1 and 5 (minimum 4 hours between fractions) for weeks 1-3 of each cycle, with posterior kidney shielding used to keep kidney doses < 20 Gy. The field borders were as follows: superiorly 1 cm above the dome of the diaphragm at the patient's maximum comfortable expiration and inferiorly either at the inferior border of the obturator foramina or 2 cm below the lowest extension of disease. Lateral borders extended at least 2 cm beyond skin. In some cases and extended source to skin distance (SSD) was needed to cover the entire area.
The trial was amended during the accrual period to allow ovarian/fallopian tube cancer patients who had obtained substantial benefit from the treatment to continue on single-agent veliparib at a dose of 400 mg PO BID until progression of diseaseat the discretion of the principal investigator. These patients were required to either have a BRCA mutation or a strong family history of BRCA-associated malignancies.
We enrolled successive cohorts of 3 patients each using a standard 3+3 design (29). Dose escalations occurred no sooner than 4 weeks after the last patient on the dose level had begun therapy. DLTs were defined as any grade 4 toxicity; any grade 3 toxicity with the exception of nausea, vomiting or diarrhea that improved to grade ≤ 2 within 3 days of receiving maximal medical support and any grade 3 electrolyte abnormality that did not correct to grade ≤ 2 within 48 hours. Asymptomatic lymphopenia or leukopenia of any grade was not considered to be a DLT.
Clinical Evaluation and Safety Assessment
Patients underwent a history and physical examination, performance status assessment and vital signs, blood work, and EKG at baseline. A baseline CT scan with contrast (unless contraindicated) was required within 28 days of beginning study treatment. While on study treatment during weeks 1-3 of each 4 week cycle, patients underwent weekly evaluations including brief history and physical examinations, adverse event evaluation, vital signs, CBC and chemistries. Adverse events (AEs) were classified/graded weekly according to the NCI Common Terminology Criteria of Adverse Events, version 4.0. Response and/or progression was assessed every 8 weeks by CT scan using RECIST 1.1 criteria (30), including by clinical and radiological assessment in cases wherein carcinomatosis was obviously present but no discreet lesions >1.0cm were available for RECIST response evaluation.
Circulating Tumor Cells
Our exploratory, translational hypothesis was that γ-H2AX would increase from baseline with DNA-damaging radiation and the increase would be greater with combination ABT-888 and radiation than just radiation alone. Blood draws for CTCs were taken at baseline, on cycle 1 day 1 following the first radiation dose, at cycle 1 day 3, at cycle 1 day 5 pre-radiation dose, and on cycle 1 day 12. Samples were evaluated for number of CTCs and for γ-H2AX positivity. Specimens were only evaluated if there was ≥1 CTC in the sample. Samples were analyzed using the Cell Search Circulating Tumor Cell Epithelial Kit (Veridex Cat. No. 7900000) and Control Kit, as per the manufacturer's protocol.
Quality of Life Assessment
QoL as measured by the EORTC-QLQ-C30 was assessed at baseline and then every 2 cycles. Only patients who remained on treatment completed the follow-up questionnaires.
Statistical Considerations
Proportions are reported with exact 95% binomial confidence intervals. Event time distributions were estimated with the method of Kaplan and Meier (31) and compared using the log-rank statistic (32) and the proportional hazards regression model (33). Quality of life (QoL) at baseline and during cycle 2 of treatment was assessed with the European Organization for the Research and Treatment of Cancer (EORTC) core quality of life questionnaire, QLQ-C30. Item scores were linearly transformed to a 0 to 100 scale with the five functional scales and global QoL coded so that higher scores represent a better level of functioning while symptom scales were coded so that higher scores correspond to worsening of symptoms. There was no imputation of missing data. Changes in QoL and subdomains of the QLQ-C30 standardized questionnaire pre-treatment and during cycle 2 of treatment were analyzed with paired t-tests. QoL comparisons between independent groups were made with two sample t-tests.
Results
Patients and Treatment
Twenty-two patients were enrolled in the study between September 8th, 2011 and August 16th, 2013. Of the 22 patients, 8 were men and 14 were women with a median age of 58 (range, 40-86). Patients had received a median of 4 prior anti-cancer therapies (range, 1-7). The baseline characteristics and demographics of the patients are further summarized in Table 1.
Table 1. Baseline Characteristics of All Treated Patients.
| Characteristic | Dose Level 1 (40 mg BID) | Dose Level 2 (80 mg BID) | Dose Level 3 (120 mg BID) | Dose Level 4 (160 mg BID) | All Dose Levels |
|---|---|---|---|---|---|
| N = 3 | N = 6 | N = 6 | N = 7 | N = 22 | |
| Age – yr | |||||
| Median | 65 | 57 | 59 | 56 | 58 |
| Range | 62-86 | 50-75 | 40-78 | 42-74 | 40-86 |
| Sex – no (%) | |||||
| Male | 2 (66) | 0 | 3 (50) | 3 (43) | 8 (36) |
| Female | 1 (33) | 6 (100) | 3 (50) | 4 (57) | 14 (63) |
| Ethnicity –no (%) | |||||
| Caucasian | 1 (33) | 5 (83) | 4 (66) | 5 (71) | 15 (68) |
| African American | 2 (66) | 1 (17) | 3 (14) | ||
| Asian | 0 | 0 | 1 (17) | 1 (14) | 2 (9) |
| Hawaiian/Pacific Islander | 0 | 0 | 0 | 1 (14) | 1 (4) |
| Mixed Race | 0 | 0 | 1 (17) | 0 | 1 (4) |
| ECOG performance status – no (%) | |||||
| 0 | 0 | 0 | 3 (50) | 2 (29) | 5 (23) |
| 1 | 3 (100) | 6 (100) | 3 (50) | 5 (71) | 17 (77) |
| Primary Site of Disease –no (%) | |||||
| Colon | 1 (33) | 0 | 0 | 3 (43) | 4 (18) |
| Ovary | 0 | 4 (66) | 2 (33) | 1 (14) | 7 (32) |
| Peritoneal Mesothelioma | 1 (33) | 0 | 0 | 0 | 1 (4) |
| Peritoneal low-grade adenomucinous | 1 (33) | 0 | 0 | 0 | 1 (4) |
| Urachus | 0 | 1 (17) | 0 | 0 | 1 (4) |
| Pancreas | 0 | 1 (17) | 0 | 0 | 1 (4) |
| Stomach | 0 | 0 | 1 (17) | 0 | 1 (4) |
| Appendix | 0 | 0 | 1 (17) | 0 | 1 (4) |
| Fallopian Tube | 0 | 0 | 1 (17) | 0 | 1 (4) |
| Small Bowel | 0 | 0 | 1 (17) | 0 | 1 (4) |
| Duodenum | 0 | 0 | 0 | 1 (14) | 1 (4) |
| Cholangiocarcinoma | 0 | 0 | 0 | 2 (29) | 2 (9) |
| Disease Stage –no (%) | |||||
| II | 0 | 0 | 1 (17) | 0 | 1 (4) |
| III | 0 | 0 | 0 | 1 (14) | 1 (4) |
| IV | 3 (100) | 6 (100) | 5 (83) | 3 (43) | 17 (77) |
| Unknown | 0 | 0 | 0 | 3 (43) | 3 (14) |
| Prior Therapies - no | |||||
| 1 | 2 (66) | 3 (50) | 1 (17) | 2 (29) | 8 (36) |
| 2-3 | 0 | 0 | 3 (50) | 5 (71) | 8 (36) |
| 4 or more | 1 (33) | 3 (50) | 2 (33) | 0 | 6 (27) |
Of the 22 patients, 8 had a primary ovarian or fallopian cancer. Two patients with primary peritoneal cancers (primary peritoneal mesothelioma and disseminated peritoneal adenomucinosis) were not included in this group. Two patients were BRCA 1/2 mutation carriers, two were known mutation negative. Of the four other patients for whom BRCA status was unknown, family history did not reveal a strong signal of BRCA-associated malignancies in 3 of the patients. The fourth patient withdrew consent, so family history was unable to be obtained.
The patients with primary ovarian or fallopian tube cancer had received a median of 4 prior anti-cancer therapies (range, 1-7) and of these, a median of 1.5 platinum-containing therapeutic regimens (range, 1-4). At the time of enrollment, 4 patients were considered to platinum-sensitive and 4 were considered to be platinum-resistant (Supplemental Table 1).
The protocol allowed for a total of 3 cycles of veliparib plus LDFWAR to be administered. A total of 49 complete cycles of veliparib plus LDFWAR were administered with a mean number of cycles per patient being 2.0 (range 0-3); 50% of the patients received all 3 planned cycles. Two of these patients went on to enter the maintenance phase with full dose veliparib (400 mg BID) upon completion of therapy (for 2 cycles and 5 cycles, respectively).
Reasons for discontinuation of therapy included progression of disease (7 patients), withdrawal of consent (1 patient) and AEs (3 patients). Although not stipulated by the protocol, no patients received any further anti-cancer treatments until there was evidence of disease progression. Once progression was confirmed, six patients went on to have another line of therapy. One patient withdrew consent so follow-up data was unable to be obtained. One additional patient's follow-up treatment data was unable to be obtained despite multiple attempts.
Dose-escalation
Three patients were treated at dose level 1 without significant toxicity. Of the first 3 patients treated at dose level 2, 1 experienced protracted grade 2 thrombocytopenia that lasted 2 weeks. Therefore, 3 more patients were enrolled at this dose level. The next 3 patients were treated at dose level 3. Two patients required replacement due to early clinical progression following less than one cycle of veliparib. Therefore, 3 more patients were enrolled at this dose level in order to more fully establish a side effect profile of the regimen. At dose level 4, 7 patients were enrolled, as one patient at that dose level required replacement. A maximum tolerated dose (MTD) was not reached. A summary of dosing is found in Table 2.
Table 2. Patient Dosing and DLT Assessment.
| Dose Cohort | ABT-888 (mg days 5-21 of cycle 1 and 1-21 of subsequent cycles) | XRT (cGy days 1 and 5 weeks 1-3 for 3 cycles*) | N | Median no of Completed Cycles (range) | DLT |
|---|---|---|---|---|---|
| 1 | 40mg BID | 60 cGy | 3 | 2.66 (2-3) | 0 |
| 2 | 80mg BID | 60 cGy | 6 | 2.16 (0-3) | **1 |
| 3 | 120mg BID | 60 cGy | 6 | 2.00 (1-3) | 0 |
| 4 | 160mg BID | 60 cGy | 7 | 1.71 (1-3) | 0 |
Cycle is 28 days
Grade 2, protracted thrombocytopenia. By the original protocol, this would not have met requirement for DLT. However, this was considered a DLT at the discretion of the principle investigators.
Safety
Twenty-two patients were evaluable for toxicity (Table 3). The most common treatment-related AEs of all grades across all cohorts included lymphopenia in 68%, anemia in 45%, thrombocytopenia in 50%, leukopenia in 54%, neutropenia in 41%, nausea in 68%, diarrhea in 41% and fatigue in 41%. Grade 3 or 4 toxicities included lymphopenia in 68%, anemia in 9%, thrombocytopenia in 14%, neutropenia in 4%, leukopenia in 9%, ascites in 4%, vomiting in 4%, small bowel obstruction in 4% and dyspnea in 4%. One patient experienced pneumonitis that was possibly related to infection as a result of immunosuppression caused by veliparib; this patient elected not to be intubated or resuscitated and died due to respiratory failure.
Table 3. Adverse Events At Least Possibly Attributable to Therapy.
| Event | Level 1 (40mg BID); No (%) | Level 2 (80mg BID); No (%) | Level 3 (120mg BID); No (%) | Level 4 (160mg BID); No (%) | All Dose Levels; No (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 3 | N = 6 | N = 6 | N = 7 | N = 22 | |||||||
| GR1/2 | GR3/4 | GR1/2 | GR3/4 | GR1/2 | GR3/4 | GR1/2 | GR3/4 | GR1/2 | GR3/4 | All Grades | |
| Hematologic | |||||||||||
| Anemia | 0 | 0 | 4(66) | 0 | 2(33) | 2(33) | 2(28) | 0 | 8(36) | 2(9) | 10(45) |
| Thrombocytopenia | 1(33) | 0 | 3(50) | 1(17) | 1(17) | 2(33) | 3(43) | 0 | 8(36) | 3(14) | 11(50) |
| Neutropenia | 2(66) | 0 | 5(83) | 0 | 1(17) | 1(17) | 0 | 0 | 8(36) | 1(4) | 9(41) |
| Lymphopenia | 0 | 2(66) | 0 | 4(66) | 0 | 4(66) | 0 | 5(71) | 0 | 15(68) | 15(68) |
| Leukopenia | 3(100) | 0 | 3(50) | 1(17) | 3(50) | 1(17) | 1(14) | 0 | 10(45) | 2(9) | 12(54) |
| Gastrointestinal | |||||||||||
| Nausea | 2(66) | 0 | 5(83) | 0 | 5(83) | 0 | 3(43) | 0 | 15(68) | 0 | 15(68) |
| Bloating | 1(33) | 0 | 1(17) | 0 | 0 | 0 | 0 | 0 | 2(9) | 0 | 2(9) |
| Diarrhea | 1(33) | 0 | 3(50) | 0 | 2(33) | 0 | 3(43) | 0 | 9(41) | 0 | 9(41) |
| Constipation | 0 | 0 | 0 | 0 | 1(17) | 0 | 1(14) | 0 | 2(9) | 0 | 2(9) |
| Ascites | 0 | 0 | 0 | 0 | 0 | *1(17) | 0 | 0 | 0 | 1(4) | 1(4) |
| Abdominal Pain/Cramping | 0 | 0 | 1(17) | 0 | 0 | 0 | 1(14) | 0 | 2(9) | 0 | 2(9) |
| Dyspepsia (GERD) | 0 | 0 | 2(33) | 0 | 1(17) | 0 | 0 | 0 | 3(14) | 0 | 3(14) |
| Flatulence | 0 | 0 | 2(33) | 0 | 1(17) | 0 | 0 | 0 | 3(14) | 0 | 3(14) |
| Dysgeusia | 0 | 0 | 3(50) | 0 | 0 | 0 | 0 | 0 | 3(14) | 0 | 3(14) |
| Anorexia | 0 | 0 | 2(33) | 0 | 2(33) | 0 | *2(28) | 0 | 6(27) | 0 | 6(27) |
| Vomiting | 0 | 0 | 3(50) | 0 | 2(33) | 0 | 2(28) | *1(14) | 7(32) | 1(4) | 8(36) |
| Small Bowel Obstruction | 0 | 0 | 0 | 0 | 0 | *1(17) | 0 | 0 | 0 | 1(4) | 1(4) |
| Hepatic | |||||||||||
| AST elevation | 0 | 0 | 0 | 0 | 1(17) | 0 | 0 | 0 | 1(4) | 0 | 1(4) |
| Alk Phos elevation | 0 | 0 | 1(17) | 0 | 1(17) | 0 | 0 | 0 | 2(9) | 0 | 2(9) |
| Neurological | |||||||||||
| Headache | 0 | 0 | 1(17) | 0 | 0 | 0 | 0 | 0 | 1(4) | 0 | 1(4) |
| Dizziness | 0 | 0 | 0 | 0 | 1(17) | 0 | 1(14) | 0 | 2(9) | 0 | 2(9) |
| Neuropathy | 0 | 0 | 1(17) | 0 | 0 | 0 | 0 | 0 | 1(4) | 0 | 1(4) |
| Integumentary | |||||||||||
| Rash | 0 | 0 | 1(17) | 0 | 0 | 0 | 0 | 0 | 1(4) | 0 | 1(4) |
| Lower extremity edema | 0 | 0 | 0 | 0 | 0 | 0 | 1(14) | 0 | 1(4) | 0 | 1(4) |
| Pruritus | 0 | 0 | 1(17) | 0 | 0 | 0 | 0 | 0 | 1(4) | 0 | 1(4) |
| Dry eyes | 0 | 0 | 1(17) | 0 | 0 | 0 | 0 | 0 | 1(4) | 0 | 1(4) |
| Electrolyte Abnormalities | |||||||||||
| Hypocalcemia | 0 | 0 | 0 | 0 | 1(17) | 0 | 0 | 0 | 1(4) | 0 | 1(4) |
| Hypomagnesemia | 0 | 0 | 0 | 0 | 2(33) | 0 | 0 | 0 | 2(9) | 0 | 2(9) |
| Constitutional | |||||||||||
| Fatigue | 1(33) | 0 | 1(17) | 0 | 4(66) | 0 | 3(43) | 0 | 9(41) | 0 | 9(41) |
| Dehydration | 0 | 0 | 2(33) | 0 | 1(17) | 0 | 0 | 0 | 3(14) | 0 | 3(14) |
| Infection | |||||||||||
| Skin infection | 1(33) | 0 | 2(33) | 0 | 0 | 0 | 0 | 0 | 3(14) | 0 | 3(14) |
| Vaginal infection | 0 | 0 | 0 | 0 | 1(17) | 0 | 0 | 0 | 1(4) | 0 | 1(4) |
| Pulmonary | |||||||||||
| Dyspnea | 0 | 0 | 1(17) | 0 | 0 | *1(17) | 0 | 0 | 1(4) | 1(4) | 2(9) |
| Cough | 0 | 0 | 1(17) | 0 | 0 | 0 | 0 | 0 | 1(4) | 0 | 1(4) |
| Pneumonitis | 0 | 0 | 1(17) | 0 | 0 | *Φ1(17) | 0 | 0 | 1(4) | 1(4) | 2(9) |
Serious Adverse Event
Grade 5
Of the twenty-two patients, one experienced protracted grade 2 thrombocytopenia at dose level 2 requiring permanent discontinuation of veliparib after only 3 doses of drug during cycle 1. Although this toxicity was not considered a DLT by the definitions within the parameters of the protocol, this event was considered a DLT by the investigators at their discretion. The dosing schedule and DLT is summarized in Table 2.
Seven patients required either a temporary suspension (n=4) or permanent discontinuation (n=3) of veliparib due to an AE though not all these AEs were related to study treatment. Discontinuation of veliparib was due to multiple intolerable grade 2 AEs, small bowel obstruction, and biliary obstruction; the latter two due to disease progression, not study treatment. Dizziness, leukopenia, and thrombocytopenia were the reasons for temporary holds in veliparib.
Clinical Activity
Twenty-one patients were evaluable for disease response. Eighteen of these patients were able to be assessed for response by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (34) and the remaining 3 patients were assessed by clinical and radiological assessment of carcinomatosis (i.e. peritoneal studding and/or ascites). The remaining patient, who was treated at dose level 2, discontinued the study during cycle 1 and did not undergo response assessment.
No objective responses were observed. Twelve patients (57%) achieved stable disease throughout treatment and disease stability of ≥24 weeks (range: 24.8-101.6 weeks) was seen in 7 patients (33%) (Table 4). Median PFS and OS were calculated from date of first therapeutic intervention to date of progressive disease (Figure 1 and Figure 2). Overall, median PFS was 4.47months (range 0.46-23.26 months) and median OS was 13.04 months (range 0.82-24.27 months).
Table 4. Patients who achieved durable disease stability (≥24 weeks): Demographics, PFS and OS.
| Patient Identifier | Primary Disease | Age – years | Prior therapies – No. | BRCA Status | Veliparib Dose – mg/day | Continued veliparib maintenance? - yes/no | PFS – mo. | OS – mo. |
|---|---|---|---|---|---|---|---|---|
| 2 | PERITONEAL MESOTHELIOMA | 65 | 2 | N/A | 80 | NO | 23.26 | 24.27 |
| 4 | URACHAL | 55 | 1 | N/A | 160 | NO | 5.42 | 22.66 |
| 7 | OVARIAN | 55 | 7 | NEGATIVE | 160 | YES | 6.76 | 10.58 |
| 8 | OVARIAN | 75 | 6 | UNKNOWN | 160 | NO | 9.72 | 17.54 |
| 9 | OVARIAN | 50 | 1 | UNKNOWN | 160 | NO | 12.78 | 18.99 |
| 13 | SMALL BOWEL | 69 | 2 | N/A | 240 | NO | 5.51 | 15.08 |
| 15 | FALLOPIAN | 63 | 4 | POSITIVE | 240 | YES | 7.91 | 8.64 |
Figure 1. Overall survival (OS) and progression-free survival (PFS): all patients by ovarian versus non-ovarian cancers.
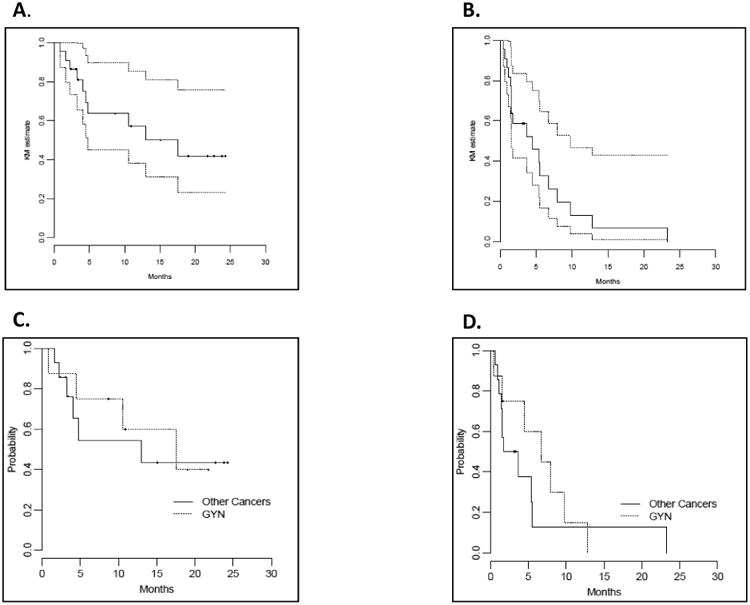
Kaplan-Meier survival curves of OS (A) and PFS (B) in all patients and OS (C) and PFS (D) by ovarian and non-ovarian cancers.
Figure 2. Progression-free survival (PFS) for evaluable patients.
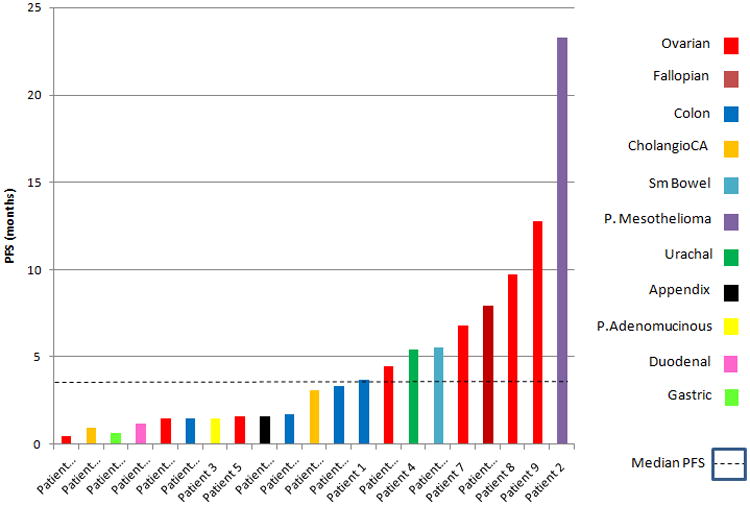
Ovarian and fallopian tube patients are differentiated by the red and maroon colored bars.
At the time of study enrollment, 16/22 patients had exclusively abdominal disease and 6/22 patients had both intra-abdominal and extra-abdominal (lung) disease. Of the 6 patients with known intra and extra abdominal disease at study onset, 3 progressed in both sites, 2 progressed in the abdomen alone and 1 progressed in the lungs only. Of the 16 patients with exclusively intra-abdominal disease at study enrollment, 1 developed new lung lesions at the time of progression while abdominal disease was stable. The remaining patients progressed in the abdomen alone (Supplemental Table 2).
In a post-hoc analysis, patients with primary ovarian/fallopian tube cancers (n=8) achieved a median PFS of 6.8 months and a median OS of 17.5 months compared to a median PFS of 2.7 months and a median OS of 13.0 months in the non-GYN cancer patients. Of these patients, the two with known BRCA mutations had PFS 4.47 months and 7.92 months, respectively, and OS 4.47 months and 8.64 months, respectively. The two patients who were known to be BRCA wild type had PFS 6.77 months and 0.46 months, respectively, and OS 10.58 month and 0.82 months, respectively.
At the data cutoff, two patients (colorectal carcinoma and cholangiocarcinoma) had not yet progressed and 10 patients (45%) had died. All deaths were disease-related with the exception of the patient above who developed pneumonitis.
Quality of Life Assessment
Twenty patients completed QoL assessment prior to starting treatment and thirteen of these patients completed a second QoL assessment at cycle 2. Of the 7 patients who did not complete the second QoL assessment at cycle 2, 4 had come off study prior to cycle 2 due to toxicity, 2 had come off study prior to cycle 2 for disease progression and 1 patient did not complete the QoL questionnaire but remained on study.
The data for patients who completed at least 2 QoL assessments were analyzed for change of QoL during the treatment phase. The baseline characteristics of patients who did and did not complete both questionnaires were also compared. Based on Osoba et al, a change in score on the EORTC-QLQ-C30 of less than 10 corresponds to a “small” clinical change. Changes of 10-20 correlate with a “moderate” clinical change and changes in scores >20 correlate with “large” clinical changes [44].
Thirteen patients completed QoL questionnaires at baseline and at cycle 2; the overall average decrease in global QoL during treatment, (change of -8.9 CI[-16.23, -1.72]), was small. A moderate clinical decrease in role function occurred between baseline and cycle 2 (change of -19.23; p = 0.007; CI [-32.13, -6.33]), and moderate increases in symptoms between baseline and cycle 2 occurred in fatigue (change of +12.82; p = 0.01; CI [3.39, 22.26] and appetite loss (change of +12.82; p = 0.02; CI [2.62, 23.02]). (Supplemental Table 3).
There were no statistically significant differences between the baseline characteristics of patients who did and did not complete both questionnaires at the outset of treatment. However, in the group of patients who ultimately did not complete cycle 2, there were statistically non-significant moderate elevations in fatigue (mean difference of 16.00; p = 0.18), pain (mean difference of 16.85; p = 0.14) and appetite loss (mean difference of 16.12; p = 0.16) and non-significant large elevations in insomnia (mean difference 27.11; p = 0.09) and constipation (mean difference 20.15; p = 0.16) as compared to the group of patients who did complete cycle 2. (Supplemental Table 3).
The mean global QoL and physical function decreases in the non-GYN patients were clinically moderate, (average changes approximately -13.0) and marginally worse compared to the GYN patients. Increased fatigue was reported exclusively by non-GYN patients.
CTC Data
Many (16/22; 73%) patients were found to have ≤2 CTCs at baseline evaluation. Six patients had >2 CTCs at baseline evaluation. Of these patients, 3 had an increase in percent γ-H2AX expression after veliparib and LDFWAR had been administered together, an event that occurred between the cycle 1, day 5 cell CTC collection and the cycle 1, day 12 CTC collection. Of the 3 patients who had an increase in their percentage γ-H2AX expression, these were of the following magnitudes: +3.6%, +16.4% and +37.14%, respectively. An additional patient who had >2 CTCs at baseline had a drop in percentage γ-H2AX expression of 40% accompanied by a notable rise in total number of CTCs, possibly suggestive of treatment failure. This patient indeed had nearly immediate progressive disease. (Supplemental Figure 1).
Discussion
The treatment of patients with peritoneal carcinomatosis is an important and unmet need in oncology, as these individuals have a significantly poorer prognosis and suffer more complications than those without peritoneal disease (1,2). For example, in colorectal cancer, a recent report found that overall survival from the time of diagnosis for patients with peritoneal carcinomatosis was 12.7 months vs 17.6 months in a post-hoc analysis of 2 large randomized studies (1). Beyond a poor prognosis, these patients also suffer considerably from challenging complications of peritoneal disease, including intractable pain and vomiting from bowel obstruction as well as abdominal distension from ascites. Our goal for this study was to identify a palliative regimen that was 1) well-tolerated, 2) had a defined, short course of treatment, and 3) would then allow patients a treatment holiday while providing disease control.
We aimed for a substantially lower MTD dose of veliparib than the established monotherapy dose of 800mg PO daily. This decision was based on the phase 0 study by Kummar et al showing significant inhibition (>95%) of PAR levels in tumor biopsies at doses of 25mg and 50mg per day doses of veliparib (35). This data suggested that higher doses of veliparib were not necessary for pharmacodynamic effect and thus would possibly add toxicity without substantial benefit. Our regimen was well-tolerated by measures of toxicity and by our QoL data. The majority of grade 3 and 4 treatment-related toxicities were largely limited to myelosuppression. Non-hematologic grade 3 and 4 toxicities were seen in no more than one patient each. There was one possibly treatment-related case of fatal infectious pneumonitis that may have been caused by lymphopenia.
Regarding QoL, we interpreted our data based on a study by Osoba et al [44]. Our QoL measure revealed only moderate adverse changes in role function, fatigue and appetite during treatment. However, only patients who remained on therapy completed the serial questionnaires. Although there were no statistically significant baseline QoL differences between those who remained on therapy and those who did not, there was a trend toward increased baseline symptoms in who ultimately did not complete cycle 2. Those who discontinued therapy early, either due to progression of disease, adverse effects or a global inability to tolerate further treatment, may have had inferior QoL while on this regimen as compared to those who were able to continue the regimen. It is difficult to assess if this is due to intrinsic patient and disease factors or due to the treatment itself.
The tolerability of our regimen can at least partially be attributed to the LDFWAR. Historically, the toxicities associated with full-dose whole abdominal radiation therapy preclude its combination with systemic therapy [8, 35-38]. However, prior phase I data by Regine et al demonstrated that hyper-fractionated low-dose radiation was tolerable in patients with pancreatic cancer when combined with LDFWAR; we chose the identical dosing and fractionation schedule. In addition, in a recent study by Kunos et al, low-dose, fractionated radiation was shown to be well-tolerated and affords the combination of radiation plus systemic docetaxel therapy (36). Our data combining LDFWAR plus low-dose veliparib demonstrates similar tolerability, making it a particularly attractive option in the palliative setting.
Our CTC data is worth commentary. γ-H2AX is a marker of double-stranded DNA damage. In our exploratory analysis, we hypothesized that radiation, as a DNA-damaging agent, would increase γ-H2AX in CTCs, and that the addition of veliparib to radiation would increase γ-H2AX further. A majority of patients had ≤2 CTCs at baseline, a finding likely attributable to the epithelial cell adhesion molecule (EpCAM) capture, which would miss cells with a more mesenchymal phenotype seen in advanced cancer patients [46]. Of the 6 patients with ≥2 CTCs, 3 demonstrated an increase in γ-H2AX after veliparib and LDFWAR were combined, suggesting increased DNA damage to tumor cells. One patient had a rise in CTCs and a drop in γ-H2AX suggestive of treatment failure and this patient indeed had immediate progressive disease. This measure was exploratory and any conclusions are highly limited; further testing in larger studies of this regimen will be necessary to assess the potential validity of the hypothesis.
Regarding efficacy, no objective responses were seen, but 1/3 of patients were progression-free at 24 weeks in this heavily pretreated patient population. Disease stabilization was seen across all dose levels and durable disease stabilization of ≥24 weeks was seen in 7 patients, 4 of whom had ovarian or fallopian tube cancers. In post-hoc analysis, patients in the GYN-cancer subset had an OS of 17.5 months compared to 13.0 months in the non-GYN group. Patients who demonstrated stable disease did not go on to have further anti-cancer therapy of any kind until they developed progressive disease. Of the evaluable patients, two developed interval progression of disease exclusively in the lungs while intra-abdominal disease remained stable, suggesting a possible benefit to the radiation therapy in these two patients. Overall, there was no pattern of location of treatment failure (within vs. outside of the radiation field).
Our lack of objective responses may be attributable to a heavily pretreated population, ovarian cancer patients concentrated in the lower dosing cohorts and the recently described phenomenon of PARP trapping [47]. The clinical relevance of PARP trapping has not fully been established and it is uncertain whether the doses of veliparib needed for PARP trapping are the same as those needed for PAR inhibition. We may have aimed for dose too low for that mechanism to engage.
The slightly longer PFS observed in the heavily pretreated ovarian cancer group could be a reflection of better disease biology of this histology rather than any treatment effect. The prolonged disease stability in the ovarian cancer subset is further complicated by the fact that PARP inhibitor monotherapy has been shown to be effective for patients with ovarian cancer with known BRCA mutations and the sporadic setting (15,16). Gelmon et al treated patients with serous ovarian cancer with full doses of the PARP inhibitor olaparib, regardless of BRCA status (37). In the post-hoc analysis of the Gelmon study, median time to progression (mTTP) in patients with BRCA mutation was 221 days (7.4 months) and was 192 days (6.4 months) in those without a BRCA mutation. The median PFS of 6.9 months among our patients with ovarian and fallopian tube cancer, the majority of whom had negative or unknown BRCA status,is comparable to that reported by Gelmon et al, and at significantly lower doses than its single agent MTD. Of course, our study was much smaller, and our results can only be viewed as hypothesis-generating for further evaluation of this regimen.
Any conclusions that we draw are limitedby our very small sample size, our lack of data on tumor growth kinetics prior to enrollment on this study, and by the fact that this is not a randomized, controlled trial. The overall survival in this trial, albeit a small study, is worth commentary. In pretreated ovarian cancer, the expected OS is approximately 13 months, based on multiple studies. Our small study with a heterogeneous group of tumors types had a median OS of 13.0 months overall, and 17.5 months in the ovarian subset.
Several questions regarding this regimen of low-dose veliparib plus LDFWAR remain unanswered. Would we see greater benefit, especially for ovarian cancer patients, if we escalated veliparib to its single agent dosing, and could we preserve the tolerability of the regimen in that setting? Is LDFWAR as monotherapy all that is required to stabilize disease in some patients? We are presently exploring increasing doses of veliparib in patients with ovarian, fallopian tube or peritoneal cancers with LDFWAR in an ongoing extension study, with the intention of opening a subsequent, randomized trial of veliparib with and without LDFWAR when that extension study has completed.
In conclusion, our data from this phase I trial of palliative low dose veliparib combined with LDFWAR demonstrates that this is a well-tolerated treatment regimen that may result in durable disease stabilization, especially in the ovarian cancer patient subset.
Supplementary Material
Statement of Translational Relevance.
We present “A phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy (LDFWAR) in patients with advanced solid tumor malignancies and peritoneal carcinomatosis,” a multi-institutional, phase I trial.
We pursued a novel strategy of combining two DNA-damaging modalities, both in low doses. Our primary endpoint was toxicity of this regimen. Secondary endpoints included progression free survival (PFS), overall survival (OS) and quality of life, as evaluated by the EORTC-QLQ-C30 questionnaire. As an exploratory measure, circulating tumor cells (CTCs) were serially collected from each patient at four time-points in treatment and were evaluated for expression of γ-H2AX, a marker of DNA damage.
We hypothesized that our regimen might result in a tolerable palliative regimen for patients in this challenging patient population with typically poor outcomes. To the best of our knowledge, this is the first study that combines LDFWAR with systemic therapy.
Acknowledgments
This study was supported by the following grants U01-CA-132123 (Princess Margaret Phase I Consortium) and by the P30 UMGCC Cancer Center Support Grant
References
- 1.Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(3):263–7. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klaver YL, Lemmens VE, Nienhuijs SW, Luyer MD, de Hingh IH. Peritoneal carcinomatosis of colorectal origin: Incidence, prognosis and treatment options. World journal of gastroenterology: WJG. 2012;18(39):5489–94. doi: 10.3748/wjg.v18.i39.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomassen I, van Gestel YR, Lemmens VE, de Hingh IH. Incidence, prognosis, and treatment options for patients with synchronous peritoneal carcinomatosis and liver metastases from colorectal origin. Diseases of the colon and rectum. 2013;56(12):1373–80. doi: 10.1097/DCR.0b013e3182a62d9d. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg N, Peschel RE. Postoperative abdominopelvic radiation therapy for ovarian cancer. International journal of radiation oncology, biology, physics. 1988;14(3):425–9. doi: 10.1016/0360-3016(88)90255-6. [DOI] [PubMed] [Google Scholar]
- 5.Hruby G, Bull CA, Langlands AO, Gebski V. WART revisited: the treatment of epithelial ovarian cancer by whole abdominal radiotherapy. Australasian radiology. 1997;41(3):276–80. doi: 10.1111/j.1440-1673.1997.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 6.Lindner H, Willich N, Atzinger A, Schubert-Fritschle G. Postoperative adjuvant whole abdomen irradiation in ovarian cancer. Onkologie. 1990;13(4):260–7. doi: 10.1159/000216773. [DOI] [PubMed] [Google Scholar]
- 7.Macbeth FR, Macdonald H, Williams CJ. Total abdominal and pelvic radiotherapy in the management of early stage ovarian carcinoma. International journal of radiation oncology, biology, physics. 1988;15(2):353–8. doi: 10.1016/s0360-3016(98)90015-3. [DOI] [PubMed] [Google Scholar]
- 8.Short SC, Joiner MC. Cellular response to low-dose irradiation. Clinical oncology. 1998;10(2):73–7. doi: 10.1016/s0936-6555(05)80480-7. [DOI] [PubMed] [Google Scholar]
- 9.Smith H. Quantifying radiation risks. Radiation protection dosimetry. 1999;86(4):259–62. doi: 10.1093/oxfordjournals.rpd.a032954. [DOI] [PubMed] [Google Scholar]
- 10.Petermann E, Keil C, Oei SL. Importance of poly(ADP-ribose) polymerases in the regulation of DNA-dependent processes. Cellular and molecular life sciences: CMLS. 2005;62(7-8):731–8. doi: 10.1007/s00018-004-4504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hakme A, Wong HK, Dantzer F, Schreiber V. The expanding field of poly(ADP-ribosyl) ation reactions. ‘Protein Modifications: Beyond the Usual Suspects’ Review Series EMBO reports. 2008;9(11):1094–100. doi: 10.1038/embor.2008.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADP-ribosyl) ation reactions in the regulation of nuclear functions. The Biochemical journal. 1999;342(Pt 2):249–68. [PMC free article] [PubMed] [Google Scholar]
- 13.Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. BioEssays: news and reviews in molecular, cellular and developmental biology. 2004;26(8):882–93. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 14.Chalmers AJ. The potential role and application of PARP inhibitors in cancer treatment. British medical bulletin. 2009;89:23–40. doi: 10.1093/bmb/ldp005. [DOI] [PubMed] [Google Scholar]
- 15.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 16.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 17.Chalmers AJ, Lakshman M, Chan N, Bristow RG. Poly(ADP-ribose) polymerase inhibition as a model for synthetic lethality in developing radiation oncology targets. Seminars in radiation oncology. 2010;20(4):274–81. doi: 10.1016/j.semradonc.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13(9):2728–37. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 19.Calabrese CR, Almassy R, Barton S, Batey MA, Calvert AH, Canan-Koch S, et al. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. Journal of the National Cancer Institute. 2004;96(1):56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 20.Miknyoczki SJ, Jones-Bolin S, Pritchard S, Hunter K, Zhao H, Wan W, et al. Chemopotentiation of temozolomide, irinotecan, and cisplatin activity by CEP-6800, a poly(ADP-ribose) polymerase inhibitor. Molecular cancer therapeutics. 2003;2(4):371–82. [PubMed] [Google Scholar]
- 21.Tentori L, Leonetti C, Scarsella M, Muzi A, Mazzon E, Vergati M, et al. Inhibition of poly(ADP-ribose) polymerase prevents irinotecan-induced intestinal damage and enhances irinotecan/temozolomide efficacy against colon carcinoma. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20(10):1709–11. doi: 10.1096/fj.06-5916fje. [DOI] [PubMed] [Google Scholar]
- 22.Nowsheen S, Bonner JA, Yang ES. The poly(ADP-Ribose) polymerase inhibitor ABT-888 reduces radiation-induced nuclear EGFR and augments head and neck tumor response to radiotherapy. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2011;99(3):331–8. doi: 10.1016/j.radonc.2011.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert JM, Cao C, Kim KW, Willey CD, Geng L, Xiao D, et al. Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13(10):3033–42. doi: 10.1158/1078-0432.CCR-06-2872. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer NG, James E, Wahl RL. Poly(ADP-ribose) polymerase inhibitors combined with external beam and radioimmunotherapy to treat aggressive lymphoma. Nuclear medicine communications. 2011;32(11):1046–51. doi: 10.1097/MNM.0b013e32834a369b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dungey FA, Loser DA, Chalmers AJ. Replication-dependent radiosensitization of human glioma cells by inhibition of poly(ADP-Ribose) polymerase: mechanisms and therapeutic potential. International journal of radiation oncology, biology, physics. 2008;72(4):1188–97. doi: 10.1016/j.ijrobp.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Tuli R, Surmak A, Blackford A, Leubner A, Jaffee EM, Deweese TL, et al. Effect of inhibition of poly-(ADP ribose) polymerase on gemcitabine and radiation-induced cytotoxicity of pancreatic cancer cells. 2011 Gastrointestinal Cancers Symposium. 2011 [Google Scholar]
- 27.Chalmers AJ. Poly(ADP-ribose) polymerase-1 and ionizing radiation: sensor, signaller and therapeutic target. Clinical oncology. 2004;16(1):29–39. doi: 10.1016/s0936-6555(03)00223-1. [DOI] [PubMed] [Google Scholar]
- 28.Regine WF, Hanna N, Garofalo MC, Doyle A, Arnold S, Kataria R, et al. Low-dose radiotherapy as a chemopotentiator of gemcitabine in tumors of the pancreas or small bowel: a phase I study exploring a new treatment paradigm. International journal of radiation oncology, biology, physics. 2007;68(1):172–7. doi: 10.1016/j.ijrobp.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 29.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. Journal of the National Cancer Institute. 2009;101(10):708–20. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European journal of cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–81. [Google Scholar]
- 32.Mantel N, Haenszel W. Statistical Aspects of the Analysis of Data from Retrospective Studies of Disease. Journal of the National Cancer Institute. 1959;22(4):719–48. [PubMed] [Google Scholar]
- 33.Cox DR. Regression Models and Life-Tables. J R Stat Soc B. 1972;34(2):187–+. [Google Scholar]
- 34.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada Journal of the National Cancer Institute. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 35.Kummar S, Kinders R, Gutierrez ME, Rubinstein L, Parchment RE, Phillips LR, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(16):2705–11. doi: 10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunos CA, Sill MW, Buekers TE, Walker JL, Schilder JM, Yamada SD, et al. Low-dose abdominal radiation as a docetaxel chemosensitizer for recurrent epithelial ovarian cancer: a phase I study of the Gynecologic Oncology Group. Gynecologic oncology. 2011;120(2):224–8. doi: 10.1016/j.ygyno.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. The lancet oncology. 2011;12(9):852–61. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


