Abstract
IMPORTANCE
In individuals with human immunodeficiency virus 1 (HIV-1) infection who are receiving antiretroviral therapy (ART), factors that promote full immune recovery are not well characterized.
OBJECTIVE
To investigate the influence of the timing of ART relative to HIV-1 infection on normalization of CD4+ T-cell counts, AIDS risk, and immune function.
DESIGN, SETTING, AND PARTICIPANTS
Participants in the observational US Military HIV Natural History Study with documented estimated dates of seroconversion (EDS) who achieved virologic suppression with ART were evaluated. Markers indicative of immune activation, dysfunction, and responsiveness were determined. Responses to hepatitis B virus (HBV) vaccine, an indicator of in vivo immune function, were also assessed. The timing of ART was indexed to the EDS and/or entry into the cohort. The CD4+ counts in HIV-1–uninfected populations were surveyed.
MAIN OUTCOMES AND MEASURES
Normalization of CD4+ counts to 900 cells/μL or higher, AIDS development, HBV vaccine response, as well as T-cell activation, dysfunction, and responsiveness.
RESULTS
The median CD4+ count in HIV-1–uninfected populations was approximately 900 cells/μL. Among 1119 HIV-1–infected participants, CD4+ normalization was achieved in 38.4% vs 28.3% of those initiating ART within 12 months vs after 12 months from the EDS (P = .001). Incrementally higher CD4+ recovery (<500,500–899, and ≥900 cells/μL) was associated with stepwise decreases in AIDS risk and reversion of markers of immune activation, dysfunction, and responsiveness to levels approximating those found in HIV-1–uninfected persons. Participants with CD4+ counts of 500 cells/μL or higher at study entry (adjusted odds ratio [aOR], 2.00; 95% CI, 1.51–2.64; P < .001) or ART initiation (aOR, 4.08; 95% CI, 3.14–5.30; P < .001) had significantly increased CD4+ normalization rates compared with other participants. However, even among individuals with a CD4+ count of 500 cells/μL or higher at both study entry and before ART, the odds of CD4+ normalization were 80% lower in those initiating ART after 12 months from the EDS and study entry (aOR, 0.20; 95% CI, 0.07–0.53; P = 001). Initiation of ART within 12 months of EDS vs later was associated with a significantly lower risk of AIDS (7.8% vs 15.3%; P = .002), reduced T-cell activation (percent CD4+HLA-DR+ effector memory T cells, 12.0% vs 15.6%; P = .03), and increased responsiveness to HBV vaccine (67.9% vs 50.9%; P = .07).
CONCLUSIONS AND RELEVANCE
Deferral of ART beyond 12 months of the EDS diminishes the likelihood of restoring immunologic health in HIV-1–infected individuals.
The goal of antiretroviral therapy (ART) in patients with human immunodeficiency virus-1 (HIV-1) infection has focused primarily on achieving an undetectable plasma HIV viral load (VL), because failure to achieve this virologic landmark is associated with highly impaired immune recovery.1–3 Durable VL suppression is readily attainable with potent and well-tolerated ART, shifting attention to the goal of optimal reconstitution of a severely compromised immune system, which is the central pathogenic feature of HIV infection.1,4–7 However, a specific CD4+ T-cell count as a target for optimal immunologic health has not been validated, nor has an interval from infection to ART initiation that promotes this goal been established.
In clinical practice, an increase in the CD4+ count to 500 cells/μL or higher while receiving ART is typically regarded as optimal immune recovery.2,8–10 However, our group11 previously showed that in individuals without HIV infection, the median CD4+ count is approximately 900 cells/μL. This observation raised the possibility that HIV-infected persons with CD4+ counts less than 900 cells/μL while receiving VL-suppressive ART may remain immunologically compromised. Substantiating this finding, individuals with CD4+ counts between 500 and 750 cells/μL who are receiving ART have an increased risk of AIDS compared with those having higher CD4+ counts.12
In the present study, we tested the hypothesis that normalization of CD4+ counts (≥900 cells/μL), compared with attainment of lower CD4+ counts during VL-suppressive ART, is associated with (1) mitigated AIDS risk; (2) reduced T-cell activation and exhaustion, which are factors predictive of adverse clinical outcomes (death, AIDS, and non-AIDS comorbidities)1,12–14; and (3) enhanced T-cell responsiveness to T-cell trophic cytokines such as interleukin 7 (IL-7), a key player in T-cell homeostasis.15 We tested our hypothesis in the US Military HIV Natural History Study (NHS), a large observational cohort of individuals with HIV infection in which most participants have estimated dates of seroconversion (EDS).16–19
The results of the study in the NHS cohort affirmed our hypothesis, prompting us to identify actionable items that physicians and public health policymakers could undertake to facilitate and promote CD4+ normalization. Earlier vs later ART is traditionally defined by whether ART is initiated before or after CD4+ counts have declined below a specific threshold (eg, 500 cells/μL), rather than the duration of HIV infection before initiation of ART.2,3,20,21 However, our group’s11 prior analyses of the San Diego Primary HIV Infection Cohort indicated that initiating ART as soon as possible after acquiring HIV, especially within 12 months of infection, greatly promoted CD4+ normalization.
Few patients seek care during primary HIV infection. Thus, to validate as well as to extend our group’s11 previous finding made in a primary infection cohort to a more real-life clinical setting, we examined participants in the NHS and determined whether initiating ART after 12 months of the EDS and/or study entry was associated with 4 primary outcomes: reduced CD4+ normalization, increased AIDS risk, increased T-cell activation, and impaired in vivo functional immune responses.
Methods
NHS Cohort and Study Design
The NHS is a prospective, multicenter, observational study of HIV-infected active duty military personnel and beneficiaries (spouses, dependents, and retired military personnel) from the Army, Navy/Marines, and Air Force. The NHS participants have free access to health care and are evaluated approximately every 6 months; cohort characteristics are as described previously16–19 and in the eMethods in the Supplement. The Uniformed Services University of the Health Sciences institutional review board approved this study. All participants enrolled in this study provided written informed consent. They did not receive financial compensation.
The NHS participants (N = 5402) were evaluated using clinical data from August 20, 1986, to November 16, 2010. The 4 primary outcomes were evaluated in the following NHS subsets. First, CD4+ normalization and AIDS outcomes were examined in 1119 participants with an EDS who met the inclusion/ exclusion criteria (Figure 1). Participants who did not achieve VL suppression while receiving ART were excluded, since the muted CD4+ recovery in these individuals would confound the analyses. Second, immune markers representative of T-cell activation, dysfunction, and responsiveness were assessed in 124 participants (66 with an EDS) who were receiving VL-suppressive ART (eFigure 1 and eTable 1 in the Supplement). Third, to model the effect of the duration of untreated HIV infection on the integrity of functional immune responses in vivo, we evaluated the serologic response to hepatitis B virus (HBV) vaccine in 374 participants who received HBV vaccine only after HIV-1 diagnosis (eFigure 1, eTable 2, and eTable 3 in the Supplement).
Figure 1. Study Participants From the US Military HIV (Human Immunodeficiency Virus) Natural History Study (NHS) Evaluated for Outcomes of Normalization of CD4+ T-Cell Counts and AIDS.
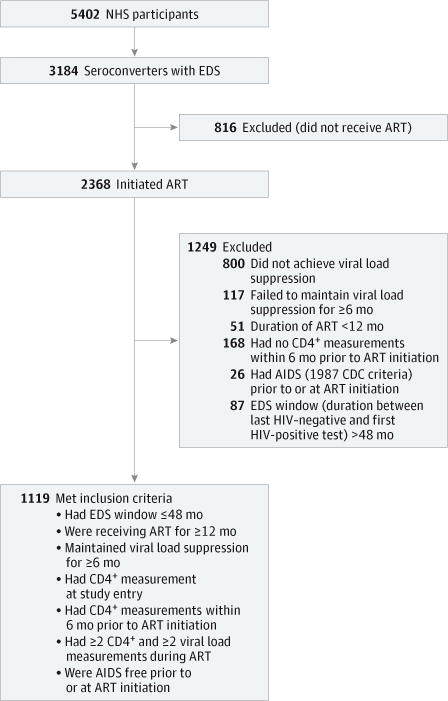
The flowchart depicts the inclusion criteria applied to the NHS participants in whom the associations of CD4+ normalization with AIDS and antiretroviral therapy (ART) timing with CD4+ normalization and AIDS were evaluated. CDC indicates Centers for Disease Control and Prevention; EDS, estimated date of seroconversion; and HIV, human immunodeficiency virus.
Study Definitions
Study entry was defined as the date when the first CD4+ count was measured after HIV diagnosis. The pre-ART CD4+ count and VL were the closest measurements recorded within 6 months before ART initiation. A CD4+ count of 500 cells/μL or more versus a count of less than 500 cells/μL at entry or pre-ART were designated as higher vs lower CD4+ counts. Viral load suppression was defined as 2 or more consecutive VL determinations of less than 400 copies/mL at least 14 days apart. The EDS was the midpoint between the dates of the last documented negative and first documented positive HIV tests. Two reference time points were used to index earlier vs later ART: the EDS, which is a fixed reference point in a patient’s infection, and date of entry into a health care system, which can be variable. Earlier vs later ART indicated an interval of 12 months or less vs more than 12 months from the EDS or study entry until ART initiation, and corresponded to shorter vs longer durations of untreated HIV infection. Study duration was predefined as 10 years from ART initiation until the loss of VL suppression or cessation of ART.
CD4+ Counts in HIV-Uninfected Persons
Three sources were used to define “normal” values of CD4+ counts. We updated our previous MEDLINE literature survey11 to May 19, 2014, of confirmed or presumed HIV-uninfected persons of European or African ancestry (eTable 4 in the Supplement), used measurements from the US National Health and Nutrition Examination Survey22 (eTable 5 in the Supplement), and used measurements from the University of California, San Diego, HIV Neurobehavioral Research Center cohort23 (eTable 6 in the Supplement).
Clinical and CD4+ Count Outcomes
AIDS was defined by both 1987 (ie, having an AIDS-defining illness) and 1993 (including CD4+ T-cell counts of <200 cells/ μL) Centers for Disease Control and Prevention criteria.24 Normalization of the CD4+ cell count was defined as achieving at least 1 CD4+ count of 900 cells/μL or more during the study period.
In Vitro Immunologic Outcomes
The levels of T-cell activation and T-cell dysfunction were assessed as the proportion of CD4+HLA-DR+ effector memory T cells (CD4+HLA-DR+ CD45RO+ CC chemokine receptor 7− T cells) and proportion of CD4+ T cells positive for programmed cell death protein-1 (PD1) (%CD4+PD1+ T cells), respectively. The level of T-cell responsiveness was assessed as the proportion of T cells responding to IL-7, based on the percentage of CD3+ T cells positive for phosphorylated signal transducer and activator of transcription (STAT5) (%CD3+pSTAT5+ T cells) after in vitro stimulation of peripheral blood mononuclear cells with IL-7. These markers were assessed in a total of 124 HIV-infected NHS participants (eFigure 1 in the Supplement) and 13 HIV-uninfected individuals matched by age and sex (eTable 7 in the Supplement), all from a prior NHS analysis.15
In Vivo Functional Immune Responses
A positive HBV vaccine response was defined as an HBV surface antibody value of 10 IU/L or more, which is a level associated with protective immunity.25 The HBV vaccine response was evaluated 1 to 12 months after the last vaccine dose.
Statistical Analysis
Associations for the time to or likelihood of achieving the first CD4+ count of 900 cells/μL or higher or the first AIDS event were determined by univariate and multivariate Cox proportional hazards and logistic regression models; the rate ratios, hazard ratios, and odds ratios (ORs) with 95% CIs were estimated. These associations were determined in all participants and after categorizing participants into subsets based on ART timing and CD4+ counts at entry and pre-ART. The KaplanMeier plots with a log-rank test, t test, or Mann-Whitney test, Wilcoxon signed rank test, and χ2 test were used where appropriate. The incidence rate ratio per 1000 person-years for time to AIDS was also calculated. Participants with a pre-ART CD4+ count of less than 200 cells/μL were excluded when the associations for AIDS 1993 criteria were computed. Additional detailed statistical methods are outlined in the eMethods in the Supplement. Statistical analyses were performed using SAS, version 9.3 (SAS Institute Inc).
Results
Normal CD4+ Count in HIV-Uninfected Persons
The median (interquartile range [IQR]) CD4+ counts in the HIV Neurobehavioral Research Center and National Health and Nutrition Examination Survey samples were 922 (741–1145) cells/μL and 904 (686–1126) cells/μL, respectively. The median of the mean CD4+ counts recorded in 27 studies comprising a total of 16 226 individuals was 988 (840–1036) cells/μL (Figure 2A). The fact that the median CD4+ counts in these sample sets closely approximated 900 cells/μL (Figure 2A and eTable 6 in the Supplement) provided the basis to consider this CD4+ level as a therapeutic target for HIV-infected persons receiving ART.
Figure 2. Clinical and Immunologic BenefitsofCD4+ T-Cell Count Normalization.
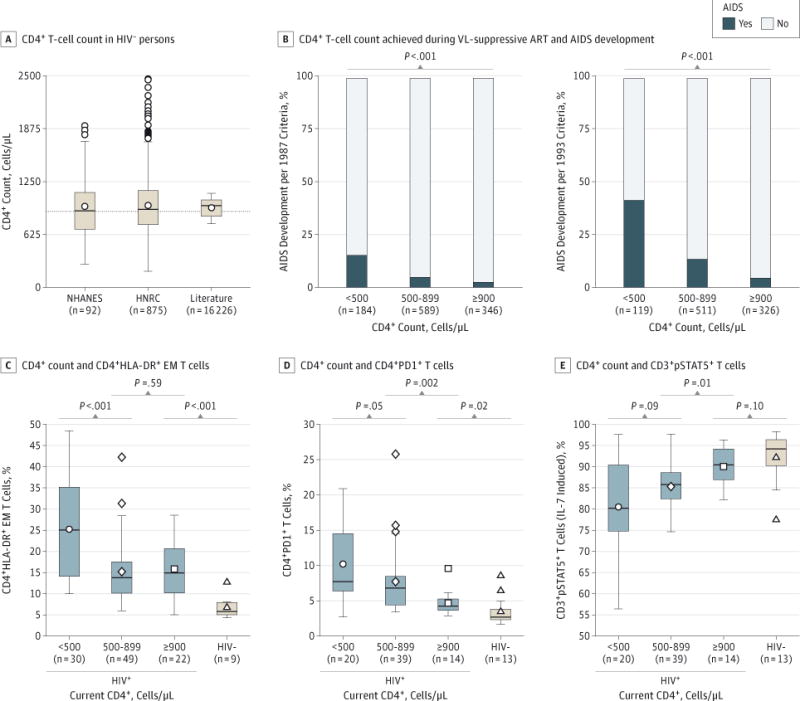
A, CD4+ T-cell counts in human immunodeficiency virus–uninfected (HIV−) persons. CD4+ T-cell counts are from the HIV Neurobehavioral Research Center (HNRC) and US National Health and Nutrition Examination Survey (NHANES) and from an updated MEDLINE literature review of 27 reports. The dashed line across the plot indicates a CD4+ T-cell count of 900 cells/μL. B, Associations between CD4+ T-cell count strata achieved on viral load (VL)-suppressive antiretroviral therapy (ART) and the proportion developing AIDS during the study period in 1119 US Military HIV Natural History Study participants. Associations for the 1993 AIDS criteria were derived from 956 NHS participants; individuals with pre-ART CD4+ counts of less than 200 cells/μL (n = 163) were excluded from these analyses. C, Association between the current CD4+ count during VL-suppressive ART and the percentage of CD4+HLA-DR+ effector memory (EM) T cells. D, Association between the current CD4+ count during VL-suppressive ART and the percentage of programmed cell death protein-1+ CD4+ (CD4+PD1+) T cells. E, Association between the current CD4+ count during VL-suppressive ART and the percentage of CD3+ T cells positive for phosphorylated signal transducer and activator of transcription 5 (CD3+pSTAT5+) T cells after ex vivo interleukin 7 (IL-7) stimulation. Levels of these markers in HIV− individuals are also shown; CD4+ T cell counts in these HIV− participants were unavailable. In panels A and C through E, the box-and-whisker plots depict the median (horizontal line in box), upper and lower quartiles (ends of box), and extremes (symbols outside of box). The symbols inside the boxes indicate the mean.
Clinical Benefits ofCD4+ Normalization
The association of CD4+ normalization with AIDS was determined in 1119 participants (Figure 1); the key characteristics of these individuals are reported in Table 1. Most participants were male (95.3%), the proportions of European Americans and African Americans were similar, and the median age at ART initiation was 31 years. The median interval between the last negative and first positive HIV test was 15.0 months. The median interval from entry to ART initiation was 6.9 months. A total of 30.9% of the participants achieved CD4+ normalization. The median duration of VL-suppressive ART was 4.7 years. Attainment of incrementally higher CD4+ counts (<500, 500–899, and ≥900 cells/μL) was associated with stepwise decrements in the proportion of participants who developed AIDS (Figure 2B).
Table 1.
Characteristics of NHS Participants Evaluated for Associations With CD4+ T-Cell Count Normalization and AIDS According to Timing of ARTa
| Characteristic | All Participants | Timing of ART From EDSb | Timing of ART From Study Entryb | |||||
|---|---|---|---|---|---|---|---|---|
| Earlier | Later | P Valuec | Earlier | Later | P Valuec | |||
| No. of participants, (%) | 1119 (100.0) | 292 (26.1) | 827 (73.9) | 645 (57.6) | 474 (42.4) | |||
| Male, No. (%) | 1066 (95.3) | 284 (97.3) | 782 (94.6) | .06 | 623 (96.6) | 443 (93.5) | .01 | |
| Ethnicity, No. (%) | .48 | .57 | ||||||
| European American | 497 (44.4) | 121 (41.4) | 376 (45.5) | 282 (43.7) | 215 (45.4) | |||
| African American | 476 (42.5) | 130 (44.5) | 346 (41.8) | 273 (42.3) | 203 (42.8) | |||
| Other | 146 (13.0) | 41 (14.1) | 105 (12.7) | 90 (14.0) | 56 (11.8) | |||
| Age at ART initiation, median (IQR), y | 31 (29–37) | 29 (24–34) | 32 (28–38) | <.001 | 30 (26–36) | 32 (28–38) | <.001 | |
| Time interval, median (IQR), mo | ||||||||
| From last documented negative to first documented positive HIV-1 test | 15.0 (9.9–24.2) | 10.3 (6.2–14.0) | 18.8 (11.7–27.7) | <.001 | 15.6 (9.7–25.1) | 15.0 (10.0–23.3) | .27 | |
| From EDS to study entry | 10.0 (6.8–15.2) | 6.4 (4.4–8.4) | 12.4 (8.4–17.7) | <.001 | 9.6 (6.3–15.1) | 10.5 (7.6–15.4) | .07 | |
| From EDS to ART initiation | 21.3 (11.7–42.8) | 7.9 (5.8–9.9) | 30.1 (18.6–56.8) | <.001 | 7.9 (5.8–9.9) | 30.1 (18.6–54.8) | <.001 | |
| From study entry to ART initiation | 6.9 (0.7–29.6) | 0.5 (0.2–1.7) | 15.8 (4.2–40.0) | <.001 | 1.1 (0.3–4.7) | 35.4 (21.9–58.7) | <.001 | |
| From ART initiation to VL suppression | 5.8 (2.1–28.9) | 4.9 (2.1–14.6) | 6.2 (2.1–37.6) | .004 | 5.1 (2.1–20.6) | 7.0 (2.2–40.0) | .01 | |
| Study entry CD4+, median (IQR), cells/μL | 456 (329–613) | 379 (275–520) | 492 (370–647) | <.001 | 381 (282–515) | 575 (447–731) | <.001 | |
| Study entry CD4+ ≥500 cells/μL, No. (%) | 483 (43.2) | 81 (27.7) | 402 (48.6) | <.001 | 176 (27.3) | 307 (64.8) | <.001 | |
| Pre-ART CD4+, median (IQR), cells/μL | 358 (271–462) | 382 (285–509) | 350 (265–449) | .01 | 364 (277–462) | 350 (264–462) | .30 | |
| Pre-ART CD4+ ≥500 cells/μL, No. (%) | 221 (19.8) | 77 (26.4) | 144 (17.4) | <.001 | 133 (20.6) | 88 (18.6) | .39 | |
| Study entry VL, median (IQR), log10 copies/mL | 4.4 (3.9–4.9) | 4.8 (4.3–5.1) | 4.3 (3.8–4.8) | <.001 | 4.7 (4.2–5.0) | 4.1 (3.6–4.6) | <.001 | |
| Pre-ART VL, median (IQR), log10 copies/mL | 4.6 (4.1–5.0) | 4.8 (4.3–5.0) | 4.5 (4.0–4.9) | <.001 | 4.7 (4.2–5.0) | 4.5 (4.0–4.9) | <.001 | |
| Duration of VL-suppressive ART, median (IQR), yd | 4.7 (2.5–8.0) | 4.4 (2.4–7.8) | 4.7 (2.6–8.1) | .23 | 4.7 (2.4–8.0) | 4.7 (2.7–8.0) | .59 | |
| Achieving CD4+ ≥900 cells/μL, No. (%) | 346 (30.9) | 112 (38.4) | 234 (28.3) | .001 | 228 (35.4) | 118 (24.9) | <.001 | |
| Achieving CD4+ ≥900 cells/μL, starting ART with CD4+ ≥500 cells/μL, No. (%) | 154 (69.7) | 64 (83.1) | 90 (62.5) | .001 | 102 (76.7) | 52 (59.1) | .005 | |
| Achieving CD4+ ≥900 cells/μL, starting ART with CD4+ <500 cells/μL, No. (%) | 192 (21.4) | 48 (22.3) | 144 (21.1) | .70 | 126 (24.6) | 66 (17.1) | .007 | |
| AIDS (1987 criteria), No. (%)e | 58 (5.2) | 8 (2.7) | 50 (6.1) | .03 | 23 (3.6) | 35 (7.4) | .004 | |
| AIDS (1987 criteria), starting ART with CD4+ ≥500 cells/μL, No. (%)f | 4 (1.8) | 2 (2.6) | 2 (1.4) | .52 | 2 (1.5) | 2 (2.3) | .67 | |
| AIDS (1987 criteria), starting ART with CD4+ <500 cells/μL, No. (%)g | 54 (6.0) | 6 (2.8) | 48 (7.0) | .02 | 21 (4.1) | 33 (8.6) | .006 | |
| AIDS (1993 criteria), No. (%)h | 127 (13.3) | 20 (7.8) | 107 (15.3) | .002 | 65 (11.7) | 62 (15.6) | .08 | |
| AIDS (1993 criteria), starting ART with CD4+ ≥500 cells/μL, No. (%)i | 11 (5.1) | 2 (2.6) | 9 (6.3) | .34 | 5 (3.8) | 6 (6.9) | .35 | |
| AIDS (1993 criteria), starting ART with CD4+ <500 cells/μL, No. (%)j | 116 (17.7) | 18 (9.9) | 98 (17.6) | .01 | 60 (14.1) | 56 (18.0) | .15 | |
Abbreviations: ART, antiretroviral therapy; EDS, estimated date of seroconversion; HIV, human immunodeficiency virus; IQR, interquartile range; NHS, US Military HIV Natural History Study; VL, viral load.
During the 10 years of follow-up, a total of 21 195 measurements of CD4+ counts and 21 139 measurements of VL were analyzed. The median number of measurements of CD4+ counts per participant was 17 (IQR, 9–25; range, 2–127), and the median number of measurements of VL per participant was 17 (IQR, 9–26; range, 2–103). The median time between consecutive measurements of CD4+ counts per participant was 102 (IQR, 76–164) days and of VL per participant, 102 (75–167) days.
Earlier ART was defined as initiating ART within 12 months of EDS or study entry; and later ART was defined as initiating ART more than 12 months after EDS or study entry.
P values were calculated with the use of the t test, Mann-Whitney test, or χ2 test as appropriate.
The duration of VL-suppressive ART was indexed from the date of starting ART.
All participants were AIDS (1987 criteria) free prior to ART initiation, and AIDS development (1987 criteria) was evaluated in these patients.
Participants starting ART with CD4+ counts of greater than or equal to 500 cells/μL were evaluated for AIDS development (1987 criteria). All of these participants were AIDS (1987 criteria) free prior to ART initiation.
Participants starting ART with CD4+ counts of less than 500 cells/μL were evaluated for AIDS development (1987 criteria). All of these participants were AIDS (1987 criteria) free prior to ART initiation.
A total of 956 of 1119 participants were AIDS free (1993 criteria) prior to starting ART and were evaluated for development of AIDS (1993 criteria) while receiving VL-suppressive ART; 257 and 558 of these participants, respectively, initiated ART earlier indexed from the EDS and the study entry.
A total of 218 of the 956 participants described aboveh started ART with CD4+ counts of greater than or equal to 500 cells/μL and were evaluated for the development of AIDS (1993 criteria); 76 and 131 of these participants, respectively, initiated ART earlier indexed from the EDS and the study entry.
A total of 738 of the 956 participants described aboveh started ART with CD4+ counts of less than 500 cells/μL and were evaluated for the development of AIDS (1993 criteria); 181 and 427 of these participants, respectively, initiated ART earlier indexed from the EDS and study entry.
Immunologic Benefits of CD4+ Normalization
The median interval from ART initiation until the measurement of immune markers indicative of T-cell activation, dysfunction, and responsiveness in 124 VL-suppressed NHS participants was 96 (IQR, 44–126) months. The median pre-ART CD4+ (373 cells/μL) and VL (log10 4.57 copies/mL) values in these individuals were similar to those in the larger group of 1119 study participants (eTable 1 in the Supplement and Table 1). The T-cell activation levels were lower in participants with CD4+ counts between 500 and 899 cells/μL compared with less than 500 cells/μL, but were similar between individuals with CD4+ counts between500 and 899 cells/μL and 900 cells/μL or more (Figure 2C). Although CD4+ strata of less than 500 cells/μL, 500 to 899 cells/μL, and 900 cells/μL or more were associated with a stepwise decrement in levels of T-cell dysfunction and increments in T-cell responsiveness, these levels differed between participants with CD4+ counts between 500 to 899 cells/μL and 900 cells/μL or more (Figure 2D and E). In participants with CD4+ counts of 900 cells/μL or more, levels of T-cell activation and dysfunction remained significantly higher compared with the levels in HIV-uninfected persons (Figure 2C and D), whereas levels of T-cell responsiveness were nearly normalized (Figure 2E).
Earlier ART and CD4+ Normalization
The conceptual framework for the remainder of the study is depicted in Figure 3A, which shows both the CD4+ trajectory and specific time intervals from infection within which initiation of ART was associated with improved CD4+ normalization rates in a primary infection cohort: 4 or 12 months from infection for participants starting ART with CD4+ counts of less than 500 cells/μL or 500 cells/μL or more, respectively.11 However, the EDS, but not date of infection, was known in the NHS cohort, and the number of NHS participants accrued within 4 months of acquiring HIV infection was likely to be very low. Therefore, in the present study, we used an interval of less than or equal to 12 months and more than 12 months between EDS or study entry and ART initiation as a classifier for earlier vs later ART. Consistent with our prior work,11 a CD4+ count of 500 cells/μL at study entry or before ART initiation was used to classify higher vs lower CD4+ counts, because it is used as a threshold for initiation of ART in most international HIV treatment guidelines.2,3
Figure 3. CD4+ Normalization Rates in 1119 US Military HIV (Human Immunodeficiency Virus) Natural History Study Participants According to Antiretroviral Therapy (ART) Timing Indexed by the Estimated Date of Seroconversion (EDS) and CD4+ T-Cell Counts at Study Entry and ART Initiation.
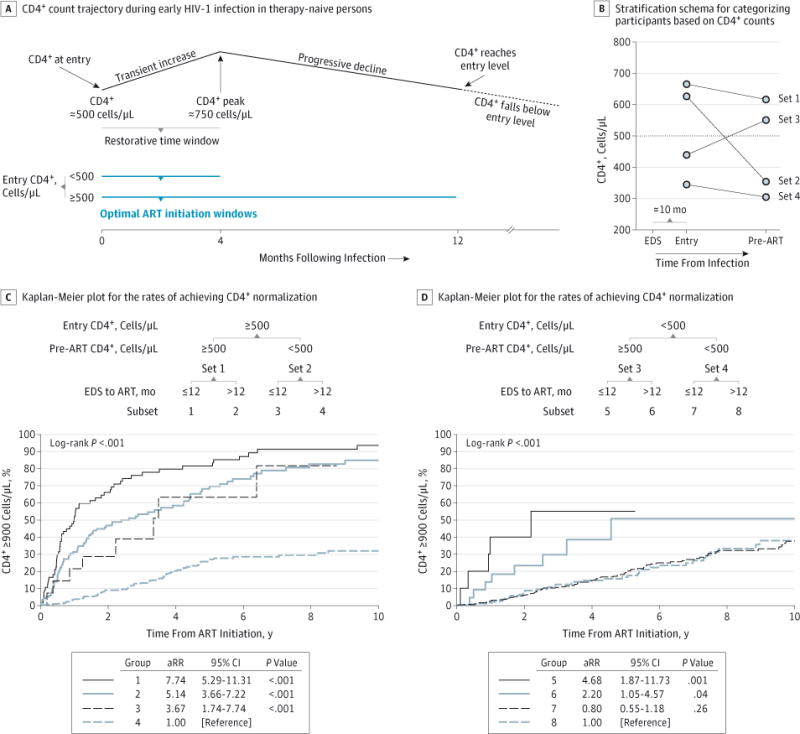
A, Schema of CD4+ trajectory in therapy-naive persons and time windows after infection within which initiation of ART is optimal for CD4+ normalization in persons presenting with lower vs higher CD4+ counts (<500 vs ≥500 cells/μL). The schema was derived based on results presented in a previous study.11 B, Stratification schema for categorizing participants into 4 sets by CD4+ counts at study entry and pre-ART The median CD4+ count at study entry and pre-ART in each of the 4 CD4+-defined sets are depicted as circles; the median interval in the 1119 NHS participants between EDS and study entry was 10 months. C and D, Kaplan-Meier plots for the rates of achieving CD4+ normalization in participants categorized according to the schema shown. The CD4-derived sets 1 to 4 shown in panel B were stratified into the indicated 8 groups depending on whether ART was initiated within or after 12 months of EDS. The rate ratio was adjusted (aRR) for calendar year of ART initiation, ART regimen, duration of viral load (VL)-suppressive ART indexed from the date of starting ART, and time from ART initiation to VL suppression.
The median interval between the EDS and study entry was approximately 10 months; ART was initiated in 26.1% or 57.6% of the patients at 12 months or less from the EDS or study entry, respectively (Table 1). Earlier compared with later ART was associated with a significantly shorter time to VL suppression. The proportion of participants achieving CD4+ normalization was significantly greater in those starting ART earlier compared with those initiating ART later, indexed to the EDS (38.4% vs 28.3%; P = .001) or study entry (35.4% vs 24.9%; P < .001).
In univariate analyses, ART timing indexed to either the EDS or study entry, CD4+ count at study entry or pre-ART, calendar year of ART initiation, ART regimen, duration of VL-suppressive ART, and time from ART initiation to VL suppression were associated with CD4+ normalization rates (Table 2). Earlier compared with later ART indexed to the EDS or study entry and higher compared with lower CD4+ counts at entry or pre-ART were each associated with increased CD4+ normalization rates, after controlling for covariates (Table 2, models 1–4).
Table 2.
Rate of Achieving CD4+ T-Cell Count Normalization
| Model | Rate Ratio (95% CI)a |
P Value |
|---|---|---|
| Univariate | ||
| Sex, female vs male | 1.18 (0.73–1.89) | .51 |
| Ethnicity | ||
| African American vs European American | 0.92 (0.73–1.15) | .44 |
| Other vs European American | 0.80 (0.56–1.14) | .22 |
| Age at ART initiation, each increase of 1 y | 1.00 (0.99–1.02) | .86 |
| Time from EDS to ART initiation, ≤12 vs>12 mo | 1.53 (1.22–1.91) | <.001 |
| Time from entry to ART initiation, ≤12 vs >12 mo | 1.51 (1.21–1.89) | <.001 |
| Study entry CD4+, ≥500 vs <500 cells/μL | 2.45 (1.98–3.05) | <.001 |
| Pre-ART CD4+, each increase of 10 cells | 1.06 (1.05–1.06) | <.001 |
| Pre-ART CD4+, ≥500 vs <500 cells/μL | 5.91 (4.77–7.32) | <.001 |
| Pre-ART viral load, each increase of 1 log10 copies/mL | 1.05 (0.90–1.21) | .57 |
| Calendar year of ART initiation, each increase of 1 y | 1.06 (1.04–1.09) | <.001 |
| Antiretroviral regimenb | ||
| PI-based vs NNRTI-based | 1.39 (1.06–1.82) | .02 |
| Other vs NNRTI-based | 0.61 (0.47–0.80) | <.001 |
| Other vs PI-based | 0.44 (0.34–0.57) | <.001 |
| Duration of VL-suppressive ART, each increase of 1 yc | 0.88 (0.84–0.92) | <.001 |
| Time from ART initiation to VL suppression, each increase of 1 mo | 0.98 (0.97–0.98) | <.001 |
| Multivariated | ||
| Model1: time from EDS to ART initiation, ≤12 vs>12 mo | 1.32 (1.04–1.67) | .02 |
| Model 2: time from study entry to ART initiation, ≤12 vs >12 mo | 1.77 (1.38–2.26) | <.001 |
| Model 3: study entry CD4+, ≥500 vs <500 cells/μL | 2.00 (1.51–2.64) | <.001 |
| Model 4: pre-ART CD4+, ≥500 vs <500 cells/μL | 4.08 (3.14–5.30) | <.001 |
Abbreviations: ART, antiretroviral therapy; EDS, estimated date of seroconversion; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; VL, viral load.
The Cox proportional hazards model was used to estimate the rate ratio for achievement of the first CD4+ count of 900 cells/μL or more after ART initiation.
PI-based was defined as PI therapy without any NNRTI, NNRTI-based was defined as NNRTI therapy without any PI, and the other treatment options were a combination of PI and NNRTI therapy or neither of these drug classes.
Duration of VL-suppressive ART was indexed from the date of starting ART.
The covariates adjusted for in models 1 and 2 were study entry CD4+ counts 500 cells/μL or more or less than 500 cells/μL, pre-ART CD4+ count 500 cells/μL or more or less than 500 cells/μL, calendar year of ART initiation, ART regimen, length of follow-up (years), and duration of VL-suppressive ART. The covariates adjusted for in model 3 were pre-ART CD4+ counts 500 cells/μL or more or less than 500 cells/μL, and time from study entry to ART initiation within 12 months or more than 12 months, calendar year of ART initiation, ART regimen, length of follow-up (years), and duration of VL-suppressive ART. Similar results were found when the model was adjusted for time from EDS to ART initiation within 12 months or more than 12 months instead of time from entry to ART. The covariates adjusted for in model 4 were study entry CD4+ counts 500 cells/μL or more or less than 500 cells/μL; time from study entry to ART initiation within 12 months or more than 12 months, calendar year of ART initiation, ART regimen, length of follow-up (years), and duration of VL-suppressive ART. Similar results were found when the model was adjusted for time from EDS to ART initiation within 12 months or more than 12 months instead of time from entry to ART.
Participants were initially stratified into 4 sets based on the change in CD4+ counts between study entry and ART initiation referenced to a CD4+ threshold of 500 cells/μL (Figure 3B), and then into 8 subsets based on whether ART was initiated earlier vs later indexed to the EDS (Figure 3C and D) or study entry (eFigure 2 in the Supplement). In general, there was an additive effect of later ART and lower CD4+ counts at study entry and/or pre-ART on depressing CD4+ normalization rates, resulting in a hierarchal pattern of these rates (Figure 3C and D and eFigure 2 in the Supplement). First, among participants with study entry CD4+ counts of less than 500 cells/μL, earlier ART did not substantially improve CD4+ normalization rates, whereas it did so among participants with entry CD4+ counts of 500 cells/μL or more. Second, among participants with entry CD4+ counts of less than 500 cells/μL, those manifesting a spontaneous rebound to 500 cells/μL or more and having ART initiated at these higher CD4+ levels had higher CD4+ normalization rates.
To parse further the effects of progressively increasing durations of untreated infection on the likelihood of CD4+ normalization, we stratified the 4 CD4+-defined sets shown in Figure 3B into 12 subsets (subsets 1′–12′ in Figure 4). Participants were categorized according to whether they initiated ART earlier or later co-indexed to the EDS and study entry; that is, whether ART was started within12 months of both the EDS and study entry (earlier/earlier [E/E]), after 12 months from the EDS but within 12 months of study entry (later/earlier [L/E]), and after 12 months from both the EDS and study entry (later/ later [L/L]) (Figure 4).
Figure 4. Likelihood of CD4+ Normalization in 1119 US Military HIV (Human Immunodeficiency Virus) Natural History Study Participants Categorized by Duration of Untreated HIV Infection Coindexed by Estimated Date of Seroconversion (EDS) and Study Entry, as Well as CD4+ Counts at Study Entry and Antiretroviral Therapy (ART) Initiation.
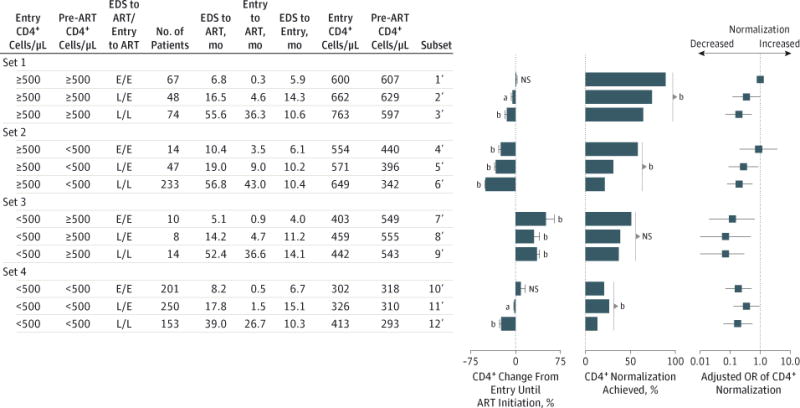
The 4 CD4-defined sets shown in Figure 3B were stratified into 12 subsets according to the entry and pre-ART CD4+ counts as well as timing of ART co-indexed from the EDS and study entry. Earlier/earlier (E/E), later/earlier (L/E), and later/later (L/L) indicate whether ART was initiated earlier (≤12 months) vs later (>12 months) from both the EDS and study entry. Median time from EDS to ART, entry to ART, and EDS to study entry as well as median CD4+ counts at entry and pre-ART are shown. P values are for the differences in the percent change in CD4+ counts between study entry and ART initiation and for the differences in the percent achieving CD4+ normalization among the indicated subsets. Odds ratios (ORs) were adjusted for covariates that were significant in univariate logistic regression analyses for the likelihood of achieving CD4+ normalization; the covariates were ethnicity, calendar year of therapy, ART regimen, time from ART to VL suppression, duration of viral load–suppressive ART, and each increase of 10 CD4+ T cells at ART initiation. NS indicates not significant. Limit lines represent 95% CI.
aP < .05.
bP < .01.
The time to ART initiation, referenced from the EDS or study entry, was progressively longer in the E/E, L/E, and L/L subsets (Figure 4). The shortest interval between the EDS and study entry was in the E/E subsets (median range, 4.0–6.7 months). Among these 12 subsets, the percentage achieving CD4+ normalization was highest in the E/E, L/E, and L/L subsets in participants with CD4+ counts of 500 cells/μL or more at both study entry and pre-ART (subsets 1′ to 3′), and this was followed by the E/E subsets in participants with study entry CD4+ counts of 500 cells/μL or more but pre-ART values of less than 500 cells/μL (subset 4′) and study entry CD4+ counts of less than 500 cells/μL but pre-ART values of 500 cells/μL or more (subset 7′).
The pre-ART CD4+ counts in the E/E, L/E, and L/L subsets in participants with CD4+ counts of 500 cells/μL or more at both study entry and pre-ART were similar (approximately 600 cells/μL) (subsets 1′–3′ in Figure 4). The CD4+ count at ART initiation was an imperfect indicator of the duration of infection, as the median intervals between the EDS and ART initiation in the E/E, L/E, and L/L subsets were 6.8, 16.5, and 55.6 months, respectively (subsets 1′–3′ in Figure 4). The odds of CD4+ normalization were lower by 65% in the L/E subset (adjusted OR [aOR], 0.35; 95% CI, 0.12–1.04; P = .06) and 80% in the L/L (aOR, 0.20; 95% CI, 0.07–0.53; P = .001) subset compared with the E/E subset, after controlling for covariates, including 10 cells/μL differences in pre-ART CD4+ counts (subsets 2′ and 3′ vs subset 1′ in Figure 4). These data indicate that it was not the numeric CD4+ value at ART initiation per se, but rather the duration of the untreated infection that is associated with CD4+ normalization. Delaying ART from the EDS was associated with a decline in immune status, as reflected by the following percentages of reduction in CD4+ counts from entry levels: nil in E/E, 5% in L/E, and approximately 15% in L/L subsets (subsets 1′–3′ in Figure 4).
Among participants with study entry CD4+ counts of 500 cells/μL or more but pre-ART values of less than 500 cells/μL (subsets 4′–6′ in Figure 4), the odds of CD4+ normalization were lower by 72% in the L/E subset (aOR, 0.28; 95% CI, 0.09–0.87; P = .03) and 80% in the L/L subset (aOR, 0.20; 95% CI, 0.08– 0.55; P < .001) compared with the E/E subset after controlling for covariates. Among participants with both entry and pre-ART CD4+ counts of less than 500 cells/μL, the proportion achieving CD4+ normalization was less than 25%, and earlier ART had minimal effects on this outcome (subsets 10′–12′ in Figure 4).
Earlier ART, AIDS, and Immune Function
Because there were few AIDS events in the present study, we aggregated all participants in the E/E, L/E, and L/L subsets, regardless of study entry or pre-ART CD4+ counts. Participants in the aggregated L/L subsets had a significantly increased AIDS risk compared with those in the E/E subsets after adjusting for covariates including the CD4+ count at study entry and ART initiation (Figure 5A and B and eTable 8 and eTable 9 in the Supplement). Earlier vs later ART indexed to the EDS was associated with significantly lower T-cell activation during VL-suppressive ART (median percentage of CD4+HLA-DR+ EM T cells, 12.0% vs 15.6%; P =.03) (Figure 5C). The likelihood of a positive seroresponse to the HBV vaccine was approximately 2-fold greater in therapy-naive participants who received the vaccine within 12 months of the EDS compared with later (57.1% vs 36.1%; P =.01) (Figure 5D), as well as in participants who received the vaccine after commencing VL-suppressive ART but started ART earlier compared with later (67.9% vs. 50.9%; P =.07) (Figure 5E) after adjusting for covariates including CD4+ count at the last vaccination (eTable 10 in the Supplement).
Figure 5. Association of Antiretroviral Therapy (ART) Timing With Development of AIDS and Duration of Untreated Infection With T-Cell Activation and In Vivo Functional Responses.
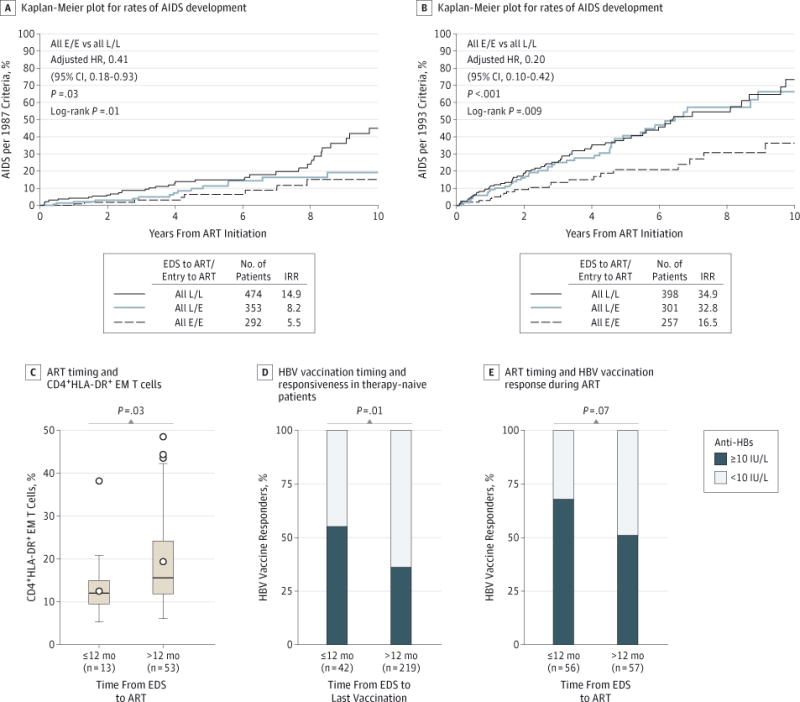
A and B, Kaplan-Meier plots depict progression to AIDS in participants who were classified to the E/E, L/E, and L/L time-indexed subsets shown in Figure 4. Incidence rate ratios (IRRs) and hazard ratios (HRs) were adjusted for the calendar year of ART, higher vs lower CD4+ counts at study entry and pre-ART, ART regimen, interval from ART initiation to viral load (VL) suppression, and duration of VL-suppressive ART. Data in panel A were derived from 1119 US Military HIV Natural History Study (NHS) participants; data in panel B were derived from 956 NHS participants because individuals with pre-ART CD4+ counts of less than 200 cells/μL (n = 163) were excluded from these analyses. C, Association of the duration between estimated date of seroconversion (EDS) and ART initiation with the percentage of CD4+HLA-DR+ effector memory (EM) T cells in participants receiving VL-suppressive ART. The box-and-whisker plots depict the median (horizontal line in box), upper and lower quartiles (ends of box), and extremes (symbols outside of box). The symbols inside the boxes indicate the mean. D, The proportion of the responders (filled box) vs nonresponders (open box) to hepatitis B virus (HBV) vaccine in HIV-infected patients who received the vaccine while they were therapy naive within or after 12 months of the EDS. E, The proportion of the responders (filled box) vs nonresponders (open box) to HBV vaccine in HIV-infected patients who received the vaccine while receiving VL-suppressive ART; patients were stratified according to whether VL-suppressive ART was initiated within or after 12 months of the EDS. Anti-HBs indicates HBV surface antibody.
Discussion
Our results, which affirmed our study hypotheses, have 4 broad implications for the management of care for HIV-infected patients, as well as for public policy. First, if a critical goal of HIV care is restoration of immunologic health, our data indicate that normalization of CD4+ counts may be an important the rapeutic target, since attainment of this CD4+ benchmark during VL-suppressive ART was associated with maximal reductions in the AIDS risk, suppression of T-cell activation and dysfunction, and restitution of T-cell responsiveness. Second, since we found that immunologic deficits persisted, despite CD4+ normalization during VL-suppressive ART compared with HIV-uninfected persons, adjunctive therapies that specifically target these functional gaps in full immune reconstitution are needed. Potentially, these residual immunologic deficits may render HIV-infected persons susceptible to non-AIDS comorbidities.26,27
Third, the capacity for CD4+ normalization is retained if the duration of untreated infection is short and the CD4+ count at ART initiation is 500 cells/μL or more. Participants with a study entry CD4+ count of 500 cells/μL or more compared with less than 500 cells/μL had increased CD4+ normalization, but this immunologic advantage was greatly diminished if the interval between the EDS and ART initiation was more than 12 months, regardless of whether ART was subsequently initiated when CD4+ counts were still 500 cells/μL or more or deferred until the levels had declined to less than 500 cells/μL. The odds of CD4+ normalization in these latter 2 groups were 65% and 80% lower, respectively, compared with the odds in participants who initiated ART within 12 months of the EDS. Thus, the likelihood of CD4+ normalization, even among individuals with relatively preserved immune status reflected by CD4+ counts of 500 cells/μL or more at both study entry and ART initiation, was progressively lower with increasing time from the EDS.
Our group11 previously showed that the time span of 4 months since acquiring HIV infection reflects a restorative time window because during this interval CD4+ counts tend to rebound spontaneously. Initiation of ART within this restorative time window greatly promoted CD4+ normalization in individuals initiating ART when their CD4+ counts were less than 500 cells/μL. Congruent with these prior observations, among NHS participants who presented with study entry CD4+ counts of less than 500 cells/μL but manifested a spontaneous rebound in CD4+ counts, the highest odds of CD4+ normalization were in participants with the shortest interval between the EDS and initiation of ART (median, 5.1 months). Thus, in participants who experienced significant immune damage, as reflected by a rapid decrease in CD4+ counts (ie, study entry CD4+ count of less than 500 cells/μL), the likelihood of CD4+ normalization was greatest when ART was initiated closer to the restorative time window.
Fourth, a shorter duration of untreated HIV infection was associated with improved immunologic and clinical outcomes. A unique aspect of our study was evaluation of the immunologic sequelae of untreated HIV infection in vivo by using the seroresponse to HBV vaccine as an indicator of immune responses that has been independently associated with the risk of AIDS and death.25 The seroresponse to HBV vaccine was approximately 2-fold greater in untreated participants receiving the vaccine within 12 months of the EDS, as well as in participants who received the vaccine after initiating VL-suppressive ART, provided the interval from the EDS to ART initiation was less than 12 months. These data indicate that the integrity of functional immune responses is substantially compromised if the duration of untreated HIV infection is greater than 12 months. Antiretroviral therapy within 12 months of the EDS also associated with reduced T-cell activation. These beneficial immunologic effects of earlier ART, along with those reported by others,11,28–33 may together account for our finding that participants who initiated ART within 12 months of the EDS had the lowest risk of developing AIDS. This is consistent with the results of a recent clinical trial.34 An added advantage of earlier ART would be reductions in HIV transmission, especially since we observed that earlier compared with later ART was associated with a more rapid suppression of VL, which is a key determinant of transmission rates.30
Limitations of the present study include the predominance of young men in our cohort;therefore, the findings may not be generalizable to HIV-infected women or older adults. The participants were predominantly infected with HIV-1 subtype B; there fore, our results may not be applicable to populations with other HIV-1 subtypes. Treatment decisions were not randomized. In addition, we could not evaluate the long-term toxicity of ART or the beneficial effects of early ART on non-AIDS events.
Conclusions
There are significant immunologic and clinical advantages of achieving CD4+ normalization (≥900 cells/μL). Congruent with our prior work in a primary infection cohort,11 we demonstrated in the present study that there is a narrow time window after acquiring HIV infection within which commencement of ART favors CD4+ normalization. Achieving CD4+ normalization is an imminently feasible therapeutic goal, provided ART is started within 12 months of the EDS at higher CD4+ counts (≥500 cells/μL). The importance of a public health strategy that includes frequent HIV testing in persons at risk and prompt initiation of ART after diagnosis is underscored by 2 findings: the rate of unwitnessed CD4+ count decline that occurs in the interval between HIV acquisition and diagnosis cannot be predicted, and the duration of the infection cannot be predicted by the CD4+ count. This strategy may offer the best chance for rapidly terminating the progressive immune damage (eg, lymphoid tissue fibrosis31,35) that constrains optimal immune reconstitution with ART.
Supplementary Material
Acknowledgments
Funding/Support: The work was supported by the Veterans Affairs (VA) Research Center for AIDS and HIV Infection and VA Center for Personalized Medicine grant IP1 CX000875-01A1, National Institutes of Health (NIH) MERIT grant R37AI046326, and the Doris Duke Distinguished Clinical Scientist Award to Dr Ahuja. Dr Ahuja is also supported by a VA MERIT award, the Elizabeth Glaser Pediatric AIDS Foundation, the Burroughs Wellcome Clinical Scientist Award in Translational Research and the Senior Scholar Award from the Max and Minnie Tomerlin Voelcker Fund. This work was also supported by NIH grant UL1TR001120 (Clinical and Translational Science Award). Dr Le was supported by a VA Career Development Award–2. Dr Wright was supported by an Early Career Fellowship from the National Health and Medical Research Council of Australia. Support for this work was also provided in part by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense program executed through the Uniformed Services University of the Health Sciences (IDCRP-000-03). This project has been funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, NIH, under Inter-Agency Agreement Y1-AI-5072. Drs Smith, Richman, and Little were supported by NIH grants DA034978, AI43638, AI69432, MH62512, AI74621, MH097520, AI077304, AI007384, AI080193, AI106039, and AI096113 and the International AIDS Vaccine Initiative grant DMS0714991.
Dr Wright reports receiving funding for consultancy to Abbott, Gilead Sciences, MSD, and ViiV as well as funds for unrestricted research from Abbott, Boehringer Ingelheim, Cilag, Gilead, and Janssen. Dr Smith reports receiving grant funding from Pfizer and ViiV and has served as a consultant for Genprobe. Dr Richman reports acting as a consultant for Biota Pharmaceuticals, Bristol-Myers Squibb, Chimerix, Gen-Probe, Gilead, Monogram Biosciences, Prism Pharmaceuticals, and Sirenas Marine Discovery. Dr Little reports receiving grant funding from Gilead. Dr Clark reports being a cofounder, equity holder, and uncompensated member of the scientific advisory board of Genkyotex, SA.
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Okulicz and Le contributed equally to this study. Drs Okulicz and Ahuja had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Okulicz, Le, He, Ahuja.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Okulicz, Le, He, Ahuja.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Le, He, Ahuja.
Obtained funding: Agan, Ahuja.
Administrative, technical, or material support:Okulicz, Agan, Ganesan, Ferguson, He, Ahuja.
Study supervision: Ahuja.
Conflict of Interest Disclosures: No other disclosures were reported.
Publisher's Disclaimer: Disclaimer: The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the Department of Defense, or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US government.
Additional Contributions: We thank the study participants as well as the clinical and laboratory staff that made this work possible. Puraskar Ingale, MS, Muthu Manoharan, MS, Andrew Carrillo, BS, and Ella Koppelaar, MS, assisted with the graphics, and Gabriel Catano, MD (VA Research Center for AIDS and HIV-1 Infection and VA Center for Personalized Medicine, South Texas Veterans Health Care System, San Antonio, and Department of Medicine, The University of Texas Health Science Center) reviewed the manuscript. Scott Letendre, MD, Igor Grant, MD, and Robert K. Heaton, PhD (University of California, San Diego) provided access to CD4+ T-cell data from HIV seronegative participants enrolled in the HIV Neurobehavioral Research Center (P30 MH62512). None of these individuals received financial compensation.
References
- 1.Guihot A, Bourgarit A, Carcelain G, Autran B. Immune reconstitution after a decade of combined antiretroviral therapies for human immunodeficiency virus. Trends Immunol. 2011;32(3):131–137. doi: 10.1016/j.it.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Hirnschall G, Harries AD, Easterbrook PJ, Doherty MC, Ball A. The next generation of the World Health Organization’s global antiretroviral guidance. J Int AIDS Soc. 2013;16:18757. doi: 10.7448/IAS.16.1.18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Health Organization website. http://www.who.int/hiv/pub/guidelines/arv2013. Published June 2013. Accessed June 9, 2014.
- 4.Delva W, Eaton JW, Meng F, et al. HIV treatment as prevention: optimising the impact of expanded HIV treatment programmes. PLoS Med. 2012;9(7):e1001258. doi: 10.1371/journal.pmed.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katlama C, Deeks SG, Autran B, et al. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet. 2013;381(9883):2109–2117. doi: 10.1016/S0140-6736(13)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shytaj IL, Savarino A. A cure for AIDS: a matter of timing? Retrovirology. 2013;10:145. doi: 10.1186/1742-4690-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torti C, Prosperi M, Motta D, et al. Factors influencing the normalization of CD4+ T-cell count, percentage and CD4+/CD8+ T-cell ratio in HIV-infected patients on long-term suppressive antiretroviral therapy. Clin Microbiol Infect. 2012;18(5):449–458. doi: 10.1111/j.1469-0691.2011.03650.x. [DOI] [PubMed] [Google Scholar]
- 9.Kelley CF, Kitchen CM, Hunt PW, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48(6):787–794. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodger AJ, Lodwick R, Schechter M, et al. INSIGHT SMART, ESPRIT Study Groups Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS. 2013;27(6):973–979. doi: 10.1097/QAD.0b013e32835cae9c. [DOI] [PubMed] [Google Scholar]
- 11.Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368(3):218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mocroft A, Furrer HJ, Miro JM et al. Opportunistic Infections Working Group on behalf of the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study in EuroCOORD. The incidence of AIDS-defining illnesses at a current CD4 count ≥200 cells/μL in the post-combination antiretroviral therapy era. Clin Infect Dis. 2013;57(7):1038–1047. doi: 10.1093/cid/cit423. [DOI] [PubMed] [Google Scholar]
- 13.Marin B, Thiébaut R, Bucher HC, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23(13):1743–1753. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson EM, Sereti I. Immune restoration after antiretroviral therapy: the pitfalls of hasty or incomplete repairs. Immunol Rev. 2013;254(1):343–354. doi: 10.1111/imr.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camargo JF, Kulkarni H, Agan BK, et al. Responsiveness of T cells to interleukin-7 is associated with higher CD4+ T cell counts in HIV-1-positive individuals with highly active antiretroviral therapy-induced viral load suppression. J Infect Dis. 2009;199(12):1872–1882. doi: 10.1086/598858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marconi VC, Grandits GA, Weintrob AC, et al. Infectious Disease Clinical Research Program HIV Working Group (IDCRP) Outcomes of highly active antiretroviral therapy in the context of universal access to healthcare: the US Military HIV Natural History Study. AIDS Res Ther. 2010;7:14. doi: 10.1186/1742-6405-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marconi VC, Grandits G, Okulicz JF, et al. Infectious Disease Clinical Research Program (IDCRP) HIV Working Group Cumulative viral load and virologic decay patterns after antiretroviral therapy in HIV-infected subjects influence CD4 recovery and AIDS. PLoS One. 2011;6(5):e17956. doi: 10.1371/journal.pone.0017956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krantz EM, Hullsiek KH, Okulicz JF, et al. Infectious Disease Clinical Research Program HIV Working Group Elevated CD8 counts during HAART are associated with HIV virologic treatment failure. J Acquir Immune Defic Syndr. 2011;57(5):396–403. doi: 10.1097/QAI.0b013e318221c62a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni H, Okulicz JF, Grandits G, et al. Early postseroconversion CD4 cell counts independently predict CD4 cell count recovery in HIV-1–positive subjects receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2011;57(5):387–395. doi: 10.1097/QAI.0b013e3182219113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Writing Committee for the CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171(17):1560–1569. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepherd BE, Jenkins CA, Rebeiro PF, et al. Estimating the optimal CD4 count for HIV-infected persons to start antiretroviral therapy. Epidemiology. 2010;21(5):698–705. doi: 10.1097/EDE.0b013e3181e97737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. Accessed January 13, 2014.
- 23.University of California, HIV Neurobehavioral Research Center (HNRC) https://hnrc.hivresearch.ucsd.edu/. Accessed August 6, 2014.
- 24.1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 25.Landrum ML, Hullsiek KH, O’Connell RJ, et al. Infectious Disease Clinical Research Program HIV Working Group Hepatitis B vaccine antibody response and the risk of clinical AIDS or death. PLoS One. 2012;7(3):e33488. doi: 10.1371/journal.pone.0033488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10(5):e1004078. doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 28.Jain V, Hartogensis W, Bacchetti P, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis. 2013;208(8):1202–1211. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hocqueloux L, Avettand-Fènoël V, Jacquot S, et al. AC32 (Coordinated Action on HIV Reservoirs) of the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS). Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother. 2013;68(5):1169–1178. doi: 10.1093/jac/dks533. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MS, Chen YQ, McCauley M, et al. HPTN 052 Study Team Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng M, Southern PJ, Reilly CS, et al. Lymphoid tissue damage in HIV-1 infection depletes naïve T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8(1):e1002437. doi: 10.1371/journal.ppat.1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cagigi A, Rinaldi S, Cotugno N, et al. Early highly active antiretroviral therapy enhances B-cell longevity: a 5 year follow up. Pediatr Infect Dis J. 2014;33(5):e126–e131. doi: 10.1097/INF.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 33.Fidler S, Porter K, Ewings F, et al. SPARTAC Trial Investigators Short-course antiretroviral therapy in primary HIV infection. N Engl J Med. 2013;368(3):207–217. doi: 10.1056/NEJMoa1110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. HPTN 052-ACTG Study Team Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–290. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33(6):306–314. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


