Despite the recent advances in the treatment of blood cancers, the great majority of malignancies of the B-lymphocyte lineage, including CLL, NHL and MM, remain incurable. A key reason for the difficulties to treat B-lineage neoplasms more effectively is the support for tumor cells provided by the tumor microenvironment (TME). Overcoming TME-dependent tumor promotion and disease relapse is thus an important goal of therapy.1 The TME is also a prime target for new approaches to tumor prevention, because it has an important role in neoplastic B cell and plasma cell (PC) development.2 However, strategies to specifically target the TME are often complicated by uncertainty as to whether the tumor-promoting factor considered for molecular targeting is indeed produced by nonmalignant bystander cells, as opposed to tumor cells. In circumstances in which the factor may be generated by both sources, such as interleukin-6 (IL-6) in MM,3,4 we do not know whether the TME-derived portion is critical for myeloma cells and, therefore, worth exploring for new TME-targeted therapies.
We developed an experimental model system to address this issue, using adoptive B cell transfer as the principal research tool. To illustrate the utility of this new method, we evaluated the role of IL-6 in plasma cell tumor (PCT) development in mice. We chose IL-6 because it drives PC neoplasia in both humans and mice and can be produced by both tumor and stromal cells.5 Additionally, despite the early recognition of its critical importance for MM, the promise of IL-6 as a therapeutic target in myeloma has not yet been translated into tangible clinical benefits.6 Here we demonstrate that stromal cell-derived ‘paracrine’ IL-6 is critical for PCT, whereas B cell-derived ‘autocrine’ IL-6 is dispensable. This finding provides proof of principle that the complex pathophysiological interactions of malignant PCs and the TME can be genetically dissected by means of adoptive B cell transfer in mice.
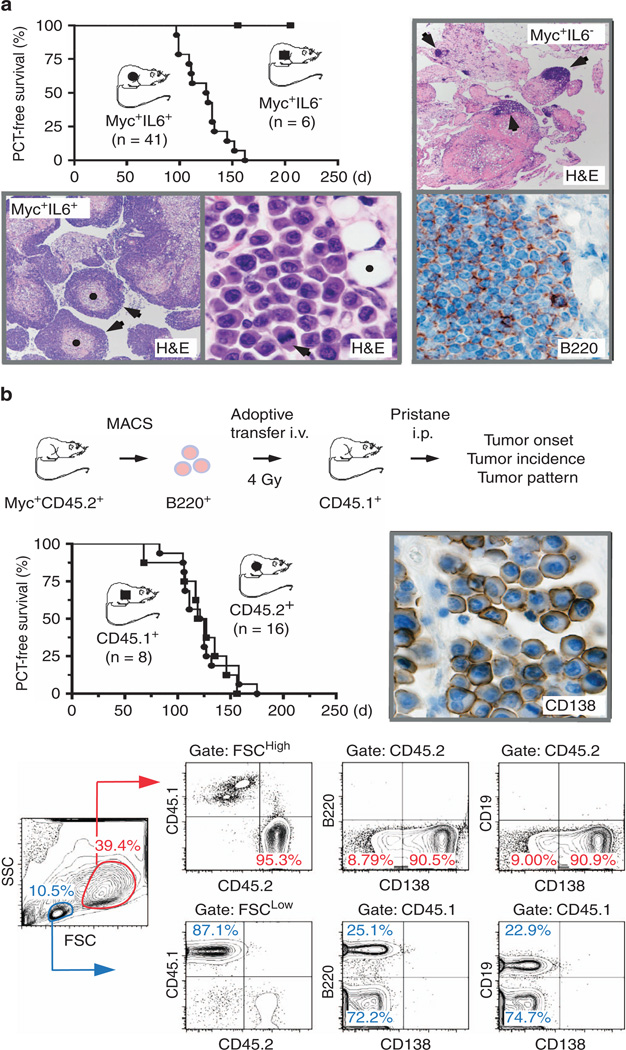
To determine the biological significance of IL-6 for inflammation-dependent PCT in mice, IL-6-deficient (IL6−) Myc-transgenic (Myc+)7 mice (n = 6) were treated i.p. with pristane. IL-6-proficient (IL6+) Myc+ mice were included as controls (n = 41). Both groups of mice were monitored for PCT until they reached 220 days of age. Tumor development in Myc+IL6+ mice was fully penetrant (100% PCT incidence) and rapid (126 days median survival), similar to results from a previous study on PCT that relied on a different Myc transgene.8 In striking contrast, none of the Myc+IL6− mice developed PCT (Figure 1a). This finding extended published results on the resistance of IL-6-deficient BALB/c (C) mice to PCT9 in demonstrating that tumor resistance was maintained in the presence of the tumor-promoting Myc transgene. We conclude that IL-6 is essential for PCT development, even in the mice genetically engineered to rapidly undergo neoplastic PC development with complete penetrance.
Figure 1.
Adoptive transfer of Myc-transgenic B cells gives rise to peritoneal PCT. (a) Line graph to the upper left indicating that IL-6-deficient Myc+IL6− mice (see Supplementary Methods for details) are resistant to pristane-induced peritoneal PCT. Myc+IL6− mice (n = 6; horizontal line with squares) and Myc+IL6+ mice (n = 41; descending line with circles) were treated on day 1 with a single i.p. injection of pristane and then monitored for accumulation of ascites, an easy-to-detect feature of incipient PCT. Tumor diagnosis was established by collecting a drop of peritoneal fluid (using abdominal paracentesis) and finding ≥50 characteristic, large, hyperchromatic PCs on a cytofuge slide stained with May–Grünwald–Giemsa. Median tumor-free survival of Myc+IL6− mice (undefined) and Myc+IL6+ mice (126 days) was significantly different by rank log assay (P < 0.0001). Histopathologic analysis of H&E-stained tissue sections of mesenteric oil granulomas obtained from tumor-bearing Myc+IL6+ mice (two images below the graph) showed heavy infiltration with neoplastic PCs. The low-power view on the left (×4 original magnification) demonstrates several granulomas (pink tissue indicated in 2 case by black dots) that have been infiltrated, in the typical centripetal pattern of peritoneal PCT, by dense, ring-like tumor masses (indicated in the same two cases by black arrows). The high-power view to the right (×100) depicts typical features of malignant PCs, including large amounts of cytoplasm, eccentric nuclei with marginated chromatin and prominent nucleoli. It also depicts three small pristane vacuoles to the upper right (one is marked by black dot) and a tumor cell undergoing mitosis (black arrow). Comparable tissue sections from Myc+IL6− mice (two images on the right) revealed scattered foci of small lymphocytes (indicated by arrows in × 4 H&E image, top) identified as B cells upon immunostaining with antibody to B220 (×100, bottom). Frank PCT or plasmacytic foci, the typical precursor lesions of these neoplasms, were not seen. (b) Presented at top is a schematic overview of the experimental approach to PCT induction using adoptive transfer of Myc-transgenic B cells. Myc+B220+CD45.2+ splenocytes were transferred i.v. to sublethally irradiated CD45.2+ (n = 16) or CD45.1+ (n = 8) mice treated 7 days later with 0.2 ml pristane i.p. B cell-reconstituted mice were monitored for PCT as described in panel A. Median tumor-free survival of these mice (123 days in both groups) was not different from survival of IL6+ hosts (114 days) shown in panel A. Shown to the right of the mouse survival graph is a representative image of a CD45.2+ peritoneal PCT that developed in a CD45.1+ host after immunostaining for syndecan-1 (CD138; 100x). The bottom panel presents flow cytometric data of ascites cells from a typical PCT-bearing CD45.1+ host, indicating that small FSCLow cells (blue gate in the forward and side scatter diagram) are mostly of host origin (87.1%) and consist approximately of 25% B220+CD19+ B cells and 75% B220−CD19− non-B cells. The latter include neutrophils, monocytes, eosinophils and other types of inflammatory peritoneal exudate cells (not shown). Large FSCHigh cells (red gate) are predominantly of donor origin (95.3%) and comprise ~90% CD138+B220−CD19− PCs. The fact that peritoneal PCT is a localized disease with limited if any spread of tumor cells outside the abdominal cavity is illustrated in Supplementary Figure 1C.
To evaluate the relative importance of B cell-derived ‘autocrine’ IL-6 and TME-derived ‘paracrine’ IL-6 for PCT, it was necessary to develop an experimental model system in which either source of the cytokine could be genetically eliminated. To do this, we evaluated whether Myc+ B cells adoptively transferred to sublethally irradiated hosts would undergo neoplastic PC development. Myc+B220+CD45.2+ splenocytes were isolated at ≥95% purity (Supplementary Figure 1A) and transferred to whole body-irradiated CD45.2+ (n = 16) or CD45.1+ (n = 8) mice treated 1 week later with i.p. pristane to induce the granulomatous tissue wherein PCTs arise (Figure 1b, top). Tumor development was complete and equally rapid (123 days median onset) in both groups of B cell-reconstituted hosts (Figure 1b, center left). Invariably, the neoplasms were confined to the peritoneal cavity (Supplementary Figure 1B) and expressed CD138 (Figure 1b, center right). Flow cytometric analysis using specific antibodies to CD45.2 (donor) and CD45.1 (host) demonstrated that PCTs were of donor cell origin and consisted predominantly of mature CD138+B220−CD19− PCs (Figure 1b, bottom). B-lymphocytes, which are also present in tumor-bearing ascites, were of donor type (Supplementary Figure 1C). These results indicated that the ‘adoptive transfer’ model of PCT is as effective in terms of tumor induction as the parental model using Myc+IL6+ mice (Figure 1a).
To further assess the suitability of the adoptive transfer model for studies on PCT, we investigated the biological properties of Myc+ B cells in greater depth. Myc+ and normal B cells developed comparably in the bone marrow (Supplementary Figure 2A) but exhibited changes in the proportions of follicular, transitional and B1a B cells in the spleen (Supplementary Figure 2). Despite this developmental difference, Myc+ and normal B cells harbored identical levels of the IL-6 receptor (IL-6R) and were both capable of secreting IL-6 in response to stimulation (Supplementary Figure 3A). Myc+ and normal B cells engrafted similarly in the spleens of mice not treated with pristane (Supplementary Figure 3B) and failed to develop PCT during an observation period of 220 days (Supplementary Figure 3C). These results underlined the importance of peritoneal inflammation for tumor development (Supplementary Figure 4) and supported the use of Myc+ B cells for a comparison of the efficacy with which autocrine and paracrine IL-6 promote inflammation-dependent PCT.
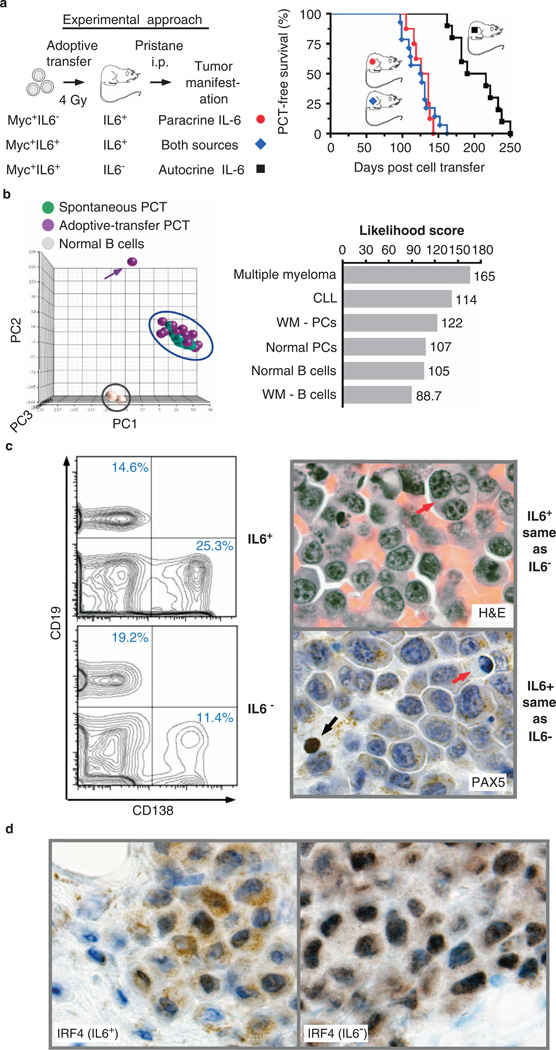
Transfer of Myc+IL6− or Myc+IL6+ B cells to IL6+ hosts resulted in both cases in fully penetrant tumor development with median onsets of 131 days (n = 8) and 126 days (n = 14), respectively, (P = 0.976; Figure 2a). Microarray-based global gene expression profiling of tumors from adoptively transferred mice demonstrated (i) a close match with the gene expression program of PCT that arose spontaneously in double-transgenic IL6Myc mice7 (Figure 2b, left) and (b) greater pathway-based similarity with human myeloma than with other malignant or normal human B-lineage cells (Figure 2b, right). Transfer of Myc+IL6+ B cells to IL6− recipients (n = 10) was associated with a significant delay in tumor onset (203 days median) relative to IL6+ hosts from this study (131 days; P < 0.001) or the study presented in Figure 1b (123 days; P < 0.001). Preliminary comparisons of neoplasms from IL6− and IL6+ hosts demonstrated no differences in CD138 expression as assessed by flow cytometry, PAX5 expression determined by immunohistochemistry, and a range of histopathologic features observed on examination of H&E-stained tissue sections (Figure 2c). However, the tumors differed in IRF4 immunoreactivity patterns, which were dominated by nuclear and coarse cytoplasmic staining in cases from IL6− and IL6+ mice, respectively (Figure 2d). We conclude that TME-derived IL-6 is important for PCT development, whereas B cell-derived IL-6 is dispensable (Figure 2a). Paracrine sources of IL-6 may also be critical for tumor development in previously developed mouse models of human MM driven by a widely expressed IL6 transgene7 (Supplementary Figure 5).
Figure 2.
Unlike B cell-derived IL-6, TME-produced IL-6 is not expendable for PCT. (a) Experimental overview (left) and line graph depicting time course of tumor development (right) in three groups of mice. Myc+B220+ splenocytes proficient for IL-6 (Myc+IL6+) or deficient for IL-6 (Myc+IL6−) were transferred to sublethally irradiated and pristane-primed hosts that were either able (IL6+) or unable (IL6−) to produce IL-6. The B cell-reconstituted hosts were monitored for PCT as described in Figure a. Median tumor-free survival of IL6+ hosts adoptively transferred with IL6− B cells (red circles, n = 8) or IL6+ B cells (blue diamonds, n = 14) was 131 days and 126 days, respectively (P = 0.976 by log rank analysis). Median tumor-free survival of IL6− hosts adoptively transferred with IL6+ B cells (black squares, n = 10) was 203 days—a significant delay compared with both groups of IL6+ hosts (P < 0.0001). (b) Global gene expression analysis of adoptive-transfer PCTs indicating a close match with human MM based on genetic pathway comparison. Shown to the left is a three-dimensional PCA (principal component analysis) plot that demonstrates tight co-clustering (blue ellipse) of adoptive-transfer PCTs (n = 14, purple spheres) and PCTs that arose spontaneously in double-transgenic IL6 Myc mice (n = 11, green spheres). MACS-fractionated B220+ splenocytes from normal C mice (n = 3, gray spheres) were used for comparison. The three PCA dimensions (PC1-3) account for maximum variability in the microarray data. Each sphere represents an independent sample; an outlier in the adoptive-transfer group is indicated by a purple arrow. Shown to the right is a bar plot of genetic pathway-based likelihood scores of mouse PCTs (n = 24; all tumors from the blue ellipse in the PCA plot) compared with relevant B cell and PC malignancies in humans (MM, CLL, WM) or normal human cells. The numerical scores grow with increasing similarity of pathway ranking across the human–mouse species barrier; that is, the mouse PCT in this comparison is most and least similar to human MM and purified B cells from WM, respectively. (c) Flow cytometric and histological findings indicating a high degree of similarity between PCTs that arose in IL6+ vs IL6− hosts. The histograms on the left depict the abundance of neoplastic CD138+CD19− PCs among peritoneal exudate cells (ascites) in a representative PCT-bearing IL6+ host (25.3%) and IL6− host (11.4%). The images of tissue sections on the right (×100) show cytological features of tumor cells that were indistinguishable in IL6+ and IL6− hosts, using either H&E staining or immunostaining with antibody to PAX5. Note the large PC in the H&E image that contains three nuclei (red arrow) and the coarse cytoplasmic staining pattern of PCs in the PAX5 image that also includes a small B cell (strong nuclear PAX5 reactivity indicated by black arrow) that is useful for comparison. A tumor cell undergoing apoptosis (red arrow) is also indicated in the PAX5 image. (d) Discordance of PCTs from IL6+ vs IL6− hosts as revealed by tissue sections stained with antibody to IRF4. Tumor cells exhibit a more pronounced nuclear staining pattern in IL6+ hosts compared with IL6− hosts.
The chief result of this study is genetic evidence that non-malignant bystander cells in the TME are the main source of IL-6 for PCT in mice.10 Although IL-6 production by tumor precursors is sufficient to drive PCT when TME-derived IL-6 is lacking, tumor development is significant delayed as it depends on alternative, less efficient mechanisms of malignant PC transformation. These may include IL-6R-independent pathways11 driven by IL-2112 and IL-6R-dependent pathways activated by IL-11, IL-27, IL-31 and oncostatin M.13 Considering that lack of paracrine IL-6 prolonged PCT onset by ~50%, it is likely that targeting IL-6 production in human bone marrow stromal cells would slow the transition from MGUS14 to frank MM with similar efficiency, potentially preventing many cases of newly diagnosed disease. The adoptive cell transfer approach described here can be readily extended to studies on other myeloma drivers that govern complex tumor-TME interactions, such as Bruton tyrosine kinase (BTK).15 For example, complementary transfers of Myc+BTK− B cells to BTK+ hosts or Myc+BTK+ B cells to BTK− hosts may further our understanding of the specific function of BTK in myeloma cells vs osteoclasts and, thereby, provide preclinical support for the clinical testing of small-compound BTK inhibitors in myeloma.
Supplementary Material
ACKNOWLEDGEMENTS
This research was performed by TRR in partial fulfilment of the requirements for the degree Doctor of Philosophy in the Graduate Immunology Program of the University of Iowa. We thank Kristin Ness for expert mouse husbandry. This work was supported in part by NIH Predoctoral Training Grant 5T32 AI007485 (TRR), by the Intramural Research Program of the NIAID (to HCM), by NCI Core Grant P30CA086862 in support of The University of Iowa Holden Comprehensive Cancer Center, by a Senior Research Award from the Multiple Myeloma Research Foundation (to SJ), by a research award from the International Waldenström’s Macroglobulinemia Foundation (to SJ), by a NCI P50CA097274 career development award (to SJ), and by R01CA151354 from the NCI (to SJ).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
REFERENCES
- 1.Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annual Rev Pathol. 2011;6:249–274. doi: 10.1146/annurev-pathol-011110-130249. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV. Preventive strategies in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Am J Hematol. 2012;87:453–454. doi: 10.1002/ajh.23204. [DOI] [PubMed] [Google Scholar]
- 3.Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332:83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- 4.Klein B, Zhang XG, Jourdan M, Content J, Houssiau F, Aarden L, et al. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989;73:517–526. [PubMed] [Google Scholar]
- 5.Fulciniti M, Hideshima T, Vermot-Desroches C, Pozzi S, Nanjappa P, Shen Z, et al. A high-affinity fully human anti-IL-6 mAb-1339, for the treatment of multiple myeloma. Clin Cancer Res. 2009;15:7144–7152. doi: 10.1158/1078-0432.CCR-09-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chari A, Pri-Chen H, Jagannath S. Complete remission achieved with single agent CNTO 328, an anti-IL-6 monoclonal antibody, in relapsed and refractory myeloma. Clin Lymphoma Myeloma Leuk. 2013;13:333–337. doi: 10.1016/j.clml.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Duncan K, Rosean TR, Tompkins VS, Olivier A, Sompallae R, Zhan F, et al. (18)F-FDG-PET/CT imaging in an IL-6- and MYC-driven mouse model of human multiple myeloma affords objective evaluation of plasma cell tumor progression and therapeutic response to the proteasome inhibitor ixazomib. Blood Cancer J. 2013;3:e165. doi: 10.1038/bcj.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SS, Shaffer AL, Kim JS, duBois W, Potter M, Staudt LM, et al. Insertion of Myc into Igh accelerates peritoneal plasmacytomas in mice. Cancer Res. 2005;65:7644–7652. doi: 10.1158/0008-5472.CAN-05-1222. [DOI] [PubMed] [Google Scholar]
- 9.Lattanzio G, Libert C, Aquilina M, Cappelletti M, Ciliberto G, Musiani P, et al. Defective development of pristane-oil-induced plasmacytomas in interleukin-6-deficient BALB/c mice. Am J Pathol. 1997;151:689–696. [PMC free article] [PubMed] [Google Scholar]
- 10.Shacter E, Arzadon GK, Williams J. Elevation of interleukin-6 in response to a chronic inflammatory stimulus in mice: inhibition by indomethacin. Blood. 1992;80:194–202. [PubMed] [Google Scholar]
- 11.Hilbert DM, Migone TS, Kopf M, Leonard WJ, Rudikoff S. Distinct tumorigenic potential of abl and raf in B cell neoplasia: abl activates the IL-6 signaling pathway. Immunity. 1996;5:81–89. doi: 10.1016/s1074-7613(00)80312-x. [DOI] [PubMed] [Google Scholar]
- 12.Hodge LS, Ziesmer SC, Yang ZZ, Secreto FJ, Gertz MA, Novak AJ, et al. IL-21 in the bone marrow microenvironment contributes to IgM secretion and proliferation of malignant cells in Waldenstrom macroglobulinemia. Blood. 2012;120:3774–3782. doi: 10.1182/blood-2012-03-419440. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113:5418–5422. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai YT, Chang BY, Kong SY, Fulciniti M, Yang G, Calle Y, et al. Bruton tyrosine kinase inhibition is a novel therapeutic strategy targeting tumor in the bone marrow microenvironment in multiple myeloma. Blood. 2012;120:1877–1887. doi: 10.1182/blood-2011-12-396853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.