Abstract
Although the causes of low back pain are poorly defined and indistinct, degeneration of the intervertebral disc is most often implicated as the origin of pain. The biochemical and mechanical changes associated with degeneration result in the discs’ inability to maintain structure and function, leading to spinal instability and ultimately pain. Traditionally, a clinical exam assessing functional range-of-motion coupled with T2-weighted MRI revealing disc morphology are used to evaluate spinal health; however, these subjective measures fail to correlate well with pain or provide useful patient stratification. Therefore, improved quantification of spinal motion and objective MRI measures of disc health are necessary. An instantaneous helical axis (IHA) approach provides rich temporal three-dimensional data describing the pathway of motion, which is easily visualized. Eighteen cadaveric osteoligamentous lumbar spines (L4-5) from throughout the degenerative spectrum were tested in a pure moment fashion. IHA were calculated for flexion-extension and lateral bending. A correlational study design was used to determine the relationship between disc measurements from quantitative T2* MRI and IHA metrics. Increased instability and out-of-plane rotation with diminished disc health was observed during lateral bending, but not flexion-extension. This new analysis strategy examines the entire pathway of motion, rather than simplifying spinal kinematics to its terminal ends of motion and provides a more sensitive kinematic measurement of disc health. Ultimately, through the use of 3D dynamic fluoroscopy or similar methods, a patient's functional IHA in lateral bending may be measured and used to assess their disc health for diagnosis, progression tracking, and treatment evaluation.
Keywords: Helical Axes, Kinematics, Magnetic Resonance Imaging, T2* (T2 star), Disc Degeneration
Introduction
Low back pain is one of the most prevalent health complaints in the US, with an estimated 70-85% of the population developing back pain at some point in their life, creating a significant financial burden (Andersson, 1999). Although many causes of low back pain are poorly defined and indistinct, intervertebral disc (IVD) degeneration remains the primary cause for such symptoms. Throughout the degenerative process the proteoglycans in the nucleus pulposus are cleaved, resulting in decreased water content, hydrostatic pressure, and disc height (Urban and Roberts, 2003). These changes in the biochemical composition diminish the mechanical competency of the disc, thus altering the interaction between the nucleus and annulus during loading (Adams et al., 1996). The discs’ inability to maintain structure and function may cause spinal instability leading to discogenic pain, nerve root pinching, or cord occlusion(Adams, 2004). The traditional definition of clinical spinal instability, as defined by Panjabi, is “the loss of normal pattern of spinal motion” (Panjabi, 2003).
Routine clinical exams for back pain include a functional exam, assessing the patient's spine motion and the presentation of pain, as well as diagnostic imaging of the intervertebral disc. Degeneration is traditionally evaluated using conventional T2 weighted sagittal magnetic resonance imaging (MRI) using a qualitative grading system assessing hydration levels and disc height, such the technique described by Pfirrmann (Pfirrmann et al., 2001). It has been shown that these categorical grading systems are not sensitive enough to detect early signs of degeneration, likely due to their subjectivity, and rarely provide clinically useful insights (Arana et al., 2010; Raininko et al., 1995). Therefore, emerging quantitative MRI techniques have recently been published, which avoid the pitfalls associated with qualitative measures by probing the biochemical content and structural integrity of the tissue (Ellingson et al., 2013a; Johannessen et al., 2006; Lotz et al., 2012; Mwale et al., 2008). These techniques may have a profound impact on the treatment of disc degeneration, especially with their ability to detect and quantify the subtle changes occurring at the early stages in the degeneration process.
It is of equal importance to understand the effects of diminished disc health on the functional mechanics and stability of the lumbar spine. In fact, this topic has been the focus of several studies including in vivo, in vitro, and in silico experiments (Ellingson et al., 2013a; Mimura et al., 1994; Natarajan et al., 2006; Passias et al., 2011). The conventional Kirkaldy-Willis model of spinal stability throughout degeneration describes a progressive increase in range of motion (RoM), until re-stabilization and a drop in RoM (Kirkaldy-Willis and Farfan, 1982). However, there still remains a lack of congruence in published literature, especially in the RoM exhibited. There is conflicting evidence supporting RoM either increases or decreases with worsening degeneration (Ellingson et al., 2013a; Fujiwara et al., 2000; Kettler et al., 2011; Tanaka et al., 2001). These contradicting results suggest RoM is not an adequate measure of spinal health due to its lack of sensitivity. The ratio of neutral zone to range of motion (NZR), a measure of joint instability or laxity, holds greater consensus in the literature. The NZR has been shown to increase with worsening degeneration (Ellingson et al., 2013a; Mimura et al., 1994; Panjabi, 1992; Zhao et al., 2005). RoM and NZR are scalar metrics that define kinematic endpoints, however the spine can move in infinite pathways of motion to reach those endpoints, and therefore are not sufficient in describing the quality of spinal motion. Also, NZR is a kinetic measure, which is unable to be measured in vivo. The center of rotation (COR) offers a more in-depth description of spinal motion (Gertzbein et al., 1985; Pearcy and Bogduk, 1988). The increased migration of the COR has been shown to be a biomarker in moderately degenerative discs and Spondylolisthesis, but even this metric simplifies the complex coupled motion of the spine into only two-dimensions (Schneider et al., 2005; Seligman et al., 1984). However, this analysis strategy is not adequate for complex motions in three-dimensions. Extending the COR to show the three-dimensional axis of rotation, rather than just a pivot point, can be obtained by computing the instantaneous helical axis (IHA). An IHA analysis approach provides rich temporal three-dimensional data describing the pathway of motion, which is easily visualized. IHA patterns have been used as a metric of stability in other joints and been employed to qualitatively describe the kinematics of the spine and the efficacy of implant devices (Duck et al., 2003; Grip and Häger, 2013; Kettler et al., 2004; Schmidt et al., 2008). The quantification of spinal instability or the off of the spine's ability to maintain its patterns of displacement under physiologic loads, is of high clinical importance, and previous analysis strategies have fallen short. It is paramount, therefore, to understand normal spinal motion to assess dysfunction in those motion patterns.
The aim of this study was to investigate the potential for IHA patterns to be used as a biomarker for spinal health and stability. It is hypothesized that the IHA vectors will display greater off-axis, or out-of-plane, rotation and a larger variability in their orientation with in worsening degeneration. It is also hypothesized that the center of rotation will exhibit greater migration and a larger variability in the migration with worsening degeneration.
Methods
Eighteen fresh-frozen osteoligamentous lumbar spines (L3-S1) were acquired from the University of Minnesota Bequest Program (age: 53.2±15.5 years; range: 21-71 years). Specimens were first examined using magnetic resonance imaging protocols to evaluate intervertebral disc health, and then biomechanically exercised in flexion, extension, and lateral bending in a pure moment fashion. A correlational study design was used to examine the relationship between instantaneous helical axis patterns of the lumbar spine and intervertebral disc degeneration, based on MR imaging.
Assessment of Spinal Health
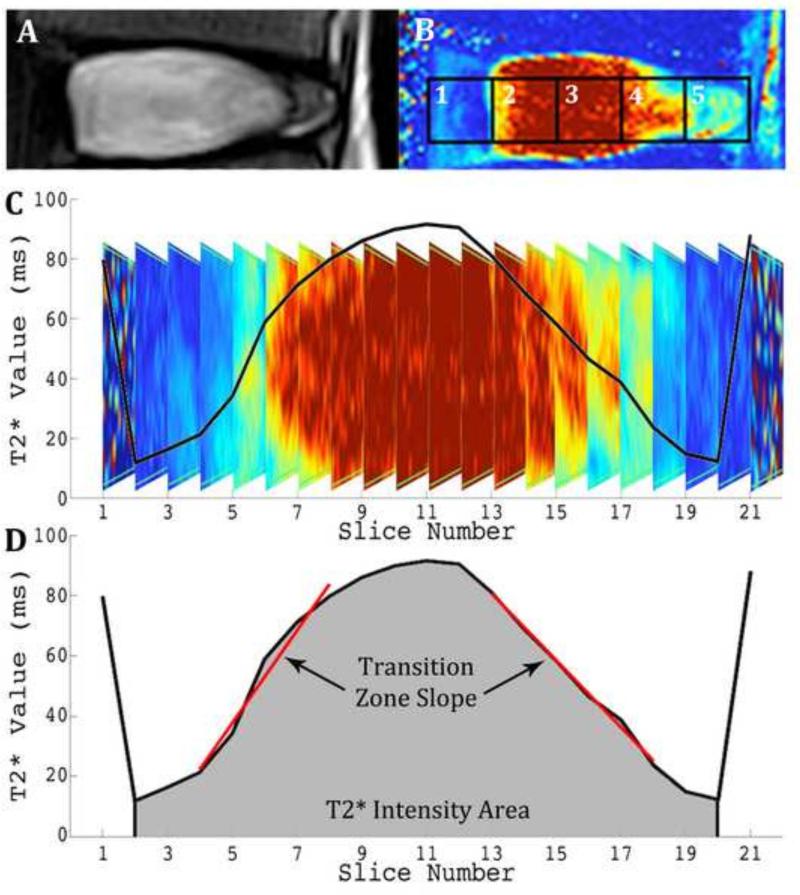
All MR imaging was performed on a Siemens 3T scanner (Magnetom Trio; Siemens Healthcare). Quantitative T2* (T2 star) relaxation maps [TR(ms): 500; TE(ms): 4.18, 11.32, 18.46, 25.60, 32.74, 39.88; Voxel Size(mm): 0.5x0.5x3.0, Slices: 33] were obtained. T2* relaxation times have been shown to be strongly correlated to the proteoglycan content and biomechanical properties, including the residual stress and strain, of the intervertebral disc (Ellingson et al., 2014). The nucleus pulposus relaxation time, T2* Intensity Area, and the Transition Zone Slope were measured in accordance with our previously published work, where the average T2* relaxation time of the central ROI (ROI 3) was evaluated through all sagittal slices across the coronal plane. These metrics are displayed in Figure 1 (Ellingson et al., 2013a). The slope of the transition zone (ms/mm) between the AF and NP on each side was linearly regressed and then averaged. Landmarks for transition zone were from 12% to 35% of the disc width from the lateral margin, as it is approximately symmetrical about the border between the AF and NP (O'Connell et al., 2007). All regressions resulted in an R2 value of greater than 0.90. This metric quantified the distinction between the NP and AF. This quantitative analysis strategy removes the bias and subjectivity inherent in classical grading schemes and has been shown to correlate better with classical biomechanics measurements of the spine than traditional Pfirrmann grading (Ellingson et al., 2013a). Specifically, the Transition Zone Slope was shown to be the strongest predictor of disc health, and therefore will be used as the primary metric for disc health for this study.
Figure 1. Quantification of imaging parameters, transition zone slope, and T2* intensity area, from T2* relaxation profile in the coronal plane of region of interest (ROI) 3.
A: Traditional T2 weighted MR image of healthy disc. B: Same disc imaged with T2* protocol and five ROI's identified. C: Coronal profile of healthy disc with plot of average T2* relaxation time (ms) overlaid. D: Corresponding quantification of transition zone slope and T2* intensity area of the healthy disc. Reprinted with permission (Ellingson et al., 2013).
Biomechanical Testing
Subsequent biomechanical tests were performed on each specimen, embedded in polymethylmethacrylate and exercised in a six-axis Spine Kinetic Simulator (8821 Biopuls, Instron, Norwood, MA) (Wheeler et al., 2011). Pure moments of ±7 Nm with no axial preload (0 N) were applied with the spine. The testing apparatus allowed for unconstrained displacement inferiorly to minimize shear forces utilizing a passive X-Y table (resistance less than 0.1 N). After three cycles of preconditioning, four cycles of bending in flexion / extension and lateral bending was performed sinusoidally (0.015 Hz). The last full cycle of each bending direction, fully unloaded to fully loaded, was used for analysis. Load and moment data were collected on two six-axis load cells (AMTI M4380, Watertown, MA), one positioned superiorly and one inferiorly. A four ball infrared reflecting marker set was attached to each vertebral body and relative motion of each functional unit was recorded at 100 Hz using a 3D visual motion analysis 5-camera system (Vicon MX-F40NIR, Vicon Motion Systems, Centennial, CO). The anterior, left-lateral, and right-lateral most points along the superior ridge of the L5 vertebrae were digitized to establish the local coordinate system.
Helical Axis Computation
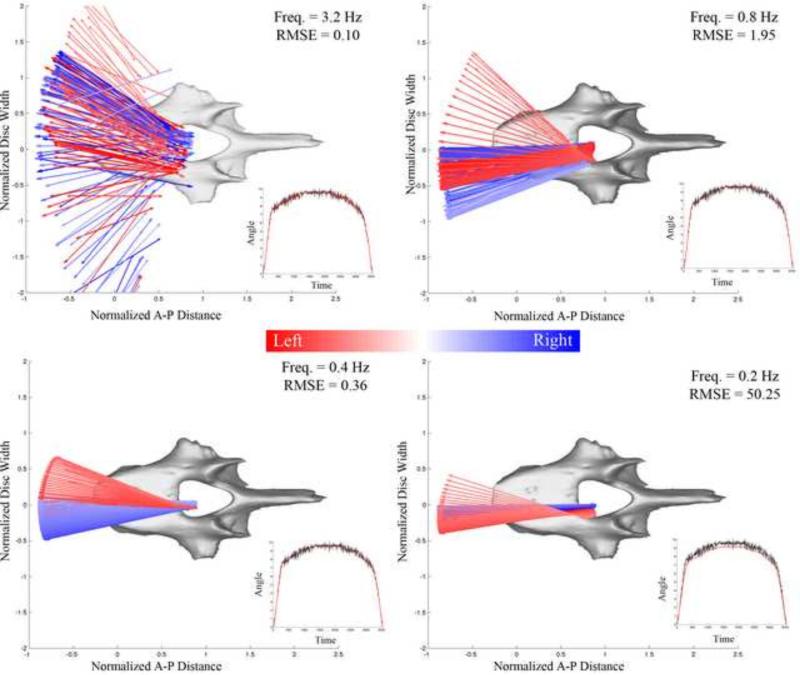
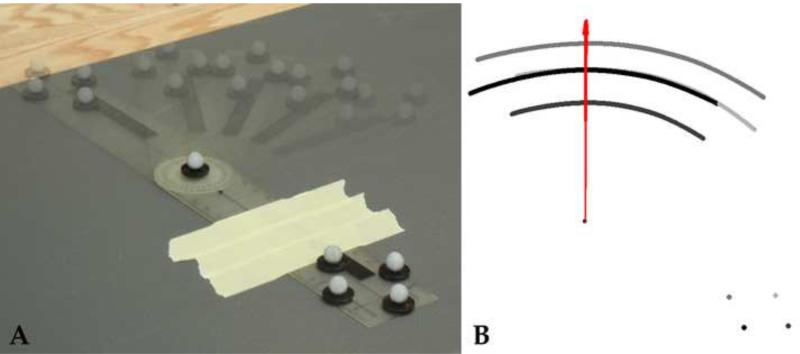
The helical axes were computed over a 0.05 degree increment for flexion, extension, and lateral bending (Spoor and Veldpaus, 1980; Woltring et al., 1985). Due to the potential noise inherent in such a small step size, a sensitivity analysis for the calculation of the IHA was first conducted on a healthy L4-L5 intervertebral disc during lateral bending (Figure 2). The axes were assessed based on the low-pass cutoff frequency used in a fourth order forward-backward Butterworth filter. The low-pass cutoff frequencies presented herein are 3.2, 0.8, 0.4, and 0.2 Hz. The root mean square of the error (RMSE) was measured between the angular displacement with and without filtering. Following this analysis the kinematic data was filtered using a fourth order low-pass Butterworth filter with a cutoff frequency of 0.4 Hz, which is similar to other published values (Anderst et al., 2013; Duck et al., 2003; Woltring et al., 1994). In order to assess the accuracy of the HA orientation and COR position, a goniometer with reflective markers was dynamically rotated approximately 90 degrees (Figure 3). HA were calculated using the filtering parameters and step size determined above and compared to the marker placement at the center of rotation of the goniometer. The measured COR distance error was 1.04 ± 0.33 mm and orientation error was less than 0.1%.
Figure 2. Helical Axis Sensitivity Analysis.
The helical axes were calculated at 0.05 degree increments using varying low-pass cutoff frequencies in a forward-backward Butterworth filter. The frequencies displayed are 3.2, 0.8, 0.4, and 0.2 Hz. The root mean squared error (RMSE) was measured between the angular displacement with and without filtering. Larger frequencies displayed stray vectors that were located outside the disc space and oriented in a sporadic fashion—not representative of physiologic motion. 0.2 Hz filtering drastically lowered the angular displacement, resulting in an RMS error of 50.25. Therefore, a cut-off frequency of 0.4 Hz was chosen for analysis.
Figure 3. Accuracy of Helical Axis Orientation and Center of Rotation Location.
In order to assess the accuracy of the HA orientation and COR position, a goniometer with reflective markers was dynamically rotated approximately 90 degrees (A). Helical Axes were calculated using a fourth order low-pass Butterworth filter with a cutoff frequency of 0.4 Hz and step size of 0.05 degrees. The measured COR distance error was 1.04 ± 0.33 mm and orientation error was less than 0.1%. Vectors are displayed in red, where the reflective markers are shown in shades of gray throughout motion (B).
The current study focused on only the L4-L5 level to avoid the possibility of boundary condition errors caused by the testing apparatus being attached to the superior and inferior most vertebral body. Each axis was computed at every 0.05 degree increments in the primary plane of bending. This was smallest step size for which stable helical axes could be calculated. The COR was defined where the IHA crosses the mid-sagittal plane (x, z plane) for flexion/extension and the coronal plane at the anterior margin of the L5 vertebral body (y, z plane) for lateral bending. Only the migration in the medial-lateral direction and anterior-posterior direction were analyzed for lateral bending and flexion/extension, respectively.
The computed IHA were used for visualization purposes, but for statistical hypothesis testing, the IHA orientation and COR location were averaged over 1 Nm windows from −6.5 Nm to 6.5 Nm and 6.5 Nm to −6.5 Nm to evaluate both loading and unloading of the spine. This analysis resulted in 7 - 1 Nm total windows, each with a minimum of 8 IHA vectors per specimen. The standard deviations for the IHA and COR within each window were also investigated as a relative measure of the spread of the helical axes and center of rotation; a larger standard deviation would be indicative of more instability. The specific outcome measures of mean helical axis vector orientation, standard deviation of helical axis vector orientation, mean center of rotation location, and standard deviation of center of rotation location were then evaluated in a correlational study design to determine the effects of disc health. Pearson's correlation coefficient (r) along with the corresponding p-value from an F-test was utilized to statistically evaluate the hypotheses and determine if IHA analysis may have a predictive role in the assessment of disc health, where a 95% level of confidence was assumed in all statistical testing. Post-hoc corrections for multiple comparisons were performed using Benjamini-Hochberg adjusted p-values method to control for the false discovery rate (Benjamini and Hochberg, 1995). The minimum acceptable False Discovery Rate was 5%. The null hypothesis was rejected if any portion of bending was significantly altered by diminished disc health.
Results
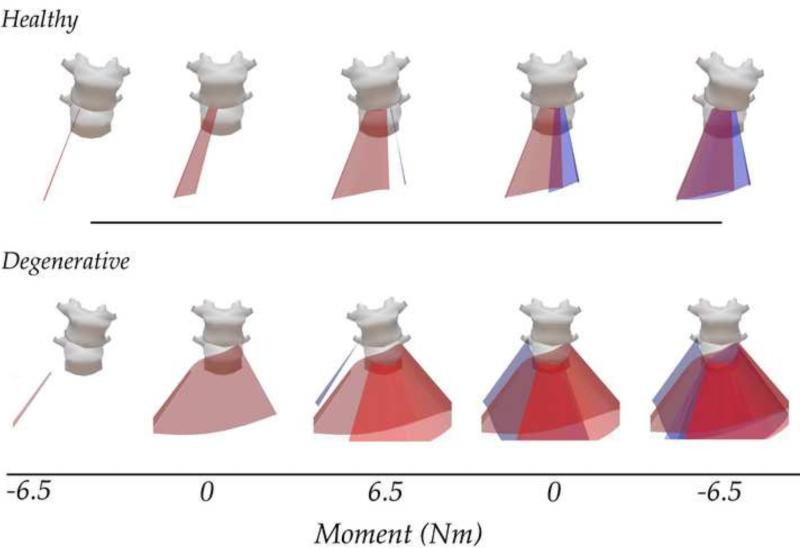
The orientation and location of the helical axes were computed for flexion, extension, and lateral bending for the L4-L5 spinal segment (18 functional spinal units) to understand the pathway of motion, and how it is affected by the quality of the intervertebral disc. Specimens of all degeneration levels exhibited overall similar motion patterns for flexion/extension and lateral bending, shown in a representative healthy specimen (Figure 4).
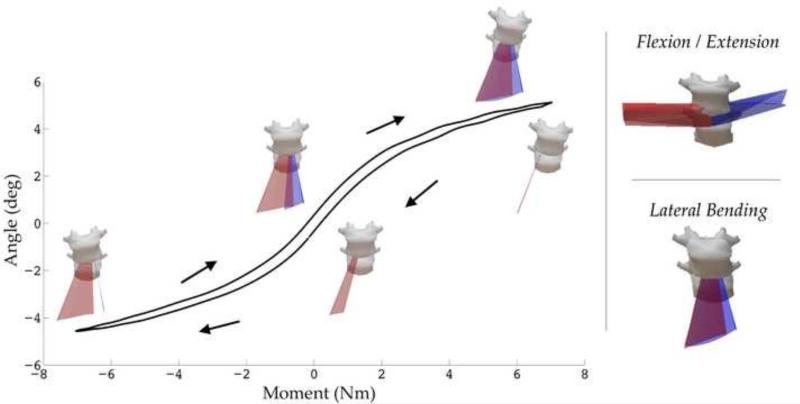
Figure 4. Representative Helical Axes from a Healthy Intervertebral Disc.
The axes for lateral bending through unloading and loading are overlaid atop a characteristic angle vs. moment plot. Lateral Bending: The red surface displays the axes for right bending and blue for left bending. Flexion / Extension: The blue surface displays the axes for flexion and red for extension.
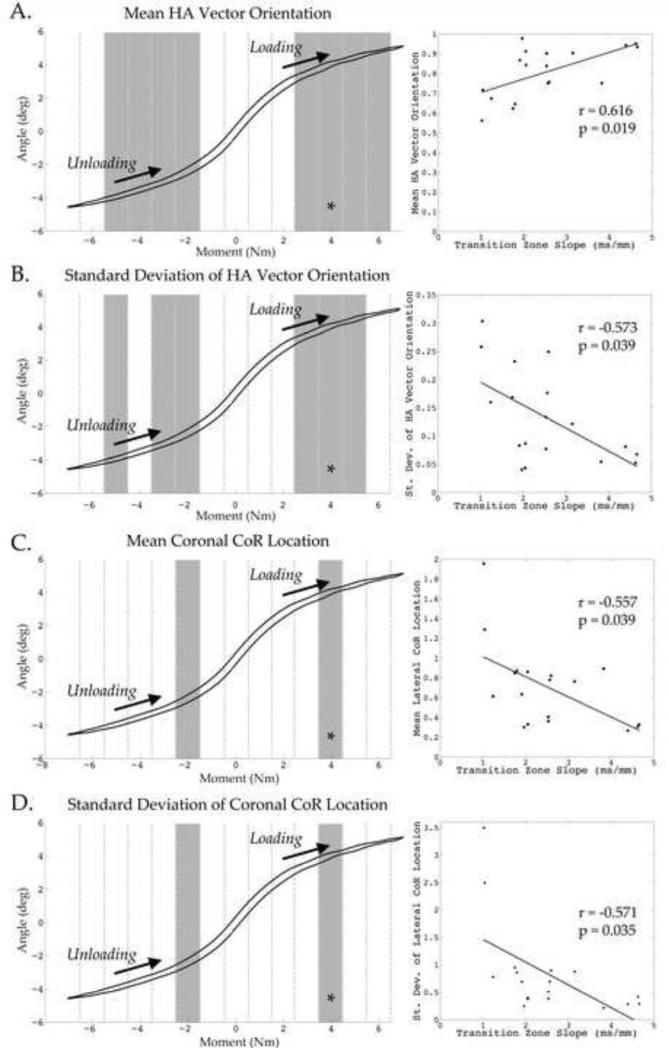
Figure 5 depicts representative IHA vector sets from a healthy and degenerative specimen. Qualitatively, these vector sets appear to be different, where more degenerative specimens displayed an exacerbated shift in IHA orientation and COR location. To investigate the relationship between disc health and measures of spinal kinematics, the transition zone slope was correlated to the mean and standard deviation of each helical axis parameter over a 1 Nm window from −6.5 Nm to 6.5 Nm through the unloading and the loading curve. The most significant changes occurred during lateral bending, displayed in Figure 6, which illustrates a representative angular displacement - moment plot broken up into 1Nm bins. The shaded region represents a significant correlation between the corresponding helical axis parameter and disc health over that 1 Nm (n=18). Adjacent to each figure is a correlational plot between the helical axis parameter and the transition zone slope (as a proxy for disc health). There were no significant correlations observed between any helical axis parameter and disc health during either flexion or extension.
Figure 5. Representative Helical Axis Patterns of Healthy (top) and Degenerative (bottom) Disc During Lateral Bending.
The vectors of the healthy disc are aligned in the anterior direction near the neutral zone and slightly shift toward end ranges of motion. This highlights the coupled motion of the lumbar spine during lateral bending. These patterns are exacerbated in the degenerative specimens, where the center of rotation migrates significantly and the orientation of the helical axis vector demonstrates significant out-of-plane bending. Animations for these are available in the supplemental material.
Figure 6. Results of Correlations between Helical Axis Parameters and Disc Health for Lateral Bending.
Shaded 1 Nm windows indicate a significant correlation between transition zone slope (proxy for disc health) and corresponding helical axis parameter. Worsening degeneration resulted in an increase in out-of-plane bending and center of rotation migration laterally. Also, increased standard deviation of HA orientation and COR suggests an increased instability with degeneration. *Indicates the window from which the adjacent correlational plot between the helical axis parameter and transition zone slope was taken. Note that each shaded region has a significant correlational plot similar to these representative plots.
The null hypotheses of this study were examined using correlational statistics. The null hypothesis that IHA vector orientation does not change with worsening degeneration was rejected by the results of lateral bending (peak correlation r = 0.616, p = 0.019). So too, the variability of the IHA orientation vector was examined to test that it did not change with advancing disc degeneration, and it was rejected for lateral bending (peak correlation r = 0.573, p = 0.024). The null hypotheses stating that the center of rotation location and its variability would not change in relation to altered disc health, as assessed by MRI, was also evaluated and the results suggest that these null hypotheses are rejected (COR location peak correlation r = 0.557, p = 0.039; COR variability peak correlation r = 0.571, p = 0.035). Therefore, the alternative hypotheses that IHA parameters are correlated with disc health remains viable, as does the possibility that these IHA parameters from lateral bending motions may be utilized in the assessment of disc health.
The primary orientation of the helical axis vector for lateral bending was found to become less aligned in the anterior direction with worsening degeneration. Therefore, as degeneration worsens there is significantly more out-of-plane rotation occurring. This was particularly prominent during the end ranges of motion, while the axes seem to be primarily aligned in the anterior direction through the neutral zone. The standard deviation of the primary component of the helical axis during lateral bending (anterior direction) provides insight into the relative ‘spread’ of the axes during motion. There was a significant correlation between disc health and the standard deviation of this vector.
Both the mean and standard deviation of the center of rotation in the lateral direction were also affected by disc health, displayed in Figure 6. With increased signs of degeneration, the COR shifted further laterally, away from the origin of the intervertebral disc, and the relative spread of the points was greater in more degenerative discs.
Discussion
The functional mechanics of the lumbar spine, such as ROM, stiffness, NZR, are altered by disc health (Ellingson et al., 2013a; Fujiwara et al., 2000; Kettler et al., 2011; Mimura et al., 1994; Panjabi, 1992; Tanaka et al., 2001; Zhao et al., 2005). However, there have been discrepancies in the literature about the changes that occur. Additionally, these two-dimensional scalar measures simplify spinal motion. In an effort to understand the pathway of motion, and how it's affected by the quality of the intervertebral disc, the orientation and location of the helical axes were computed for flexion, extension, and lateral bending for the L4-L5 spinal segment (18 functional spinal units). The helical axis is a precise three-dimensional vector that describes how a body both rotates and translates about another body; it is a useful instrument for visualization and when the HA is computed at very small intervals, the pathway of motion can be interrogated. This provides substantially more information on the kinematical description of the spinal segment. These IHA and COR patterns measured and reported herein are consistent with theoretical models (Kettler et al., 2004; Schmidt et al., 2008; White and Panjabi, 1978). For the purpose of this study and to make statistical comparisons, the IHA were averaged over a 1 Nm window through the unloading and loading profiles, this technique isolates the aspect of bending where differences arise. Classically, clinicians assess functional motion primarily in the flexion-extension direction. Interestingly, there were no significant linear correlations between any of the metrics of disc health and the helical axis patterns during flexion or extension. Changes in the helical axis orientation and COR location were observed during lateral bending, however. Although flexion and extension is the primary direction of bending in the lumbar spine, this study suggests the changes of disc health associated with degeneration may have a larger impact in the quality of motion in the coronal plane. Whereas flexion and extension have increased ligament and facet interactions, assessment of the disc alone may be clouded by these other structures. Previous studies have also indicated the importance of lateral bending for measuring and understanding spinal instability mainly because the disc is the only structure resisting motion (Ellingson et al., 2013a; Zhao et al., 2005). During lateral bending there is minimal spinal ligament involvement, therefore the disc and its structural integrity is the primary motion granting tissue. This allows for kinematic changes in response to diminished disc health to be more apparent in coronal plane motion.
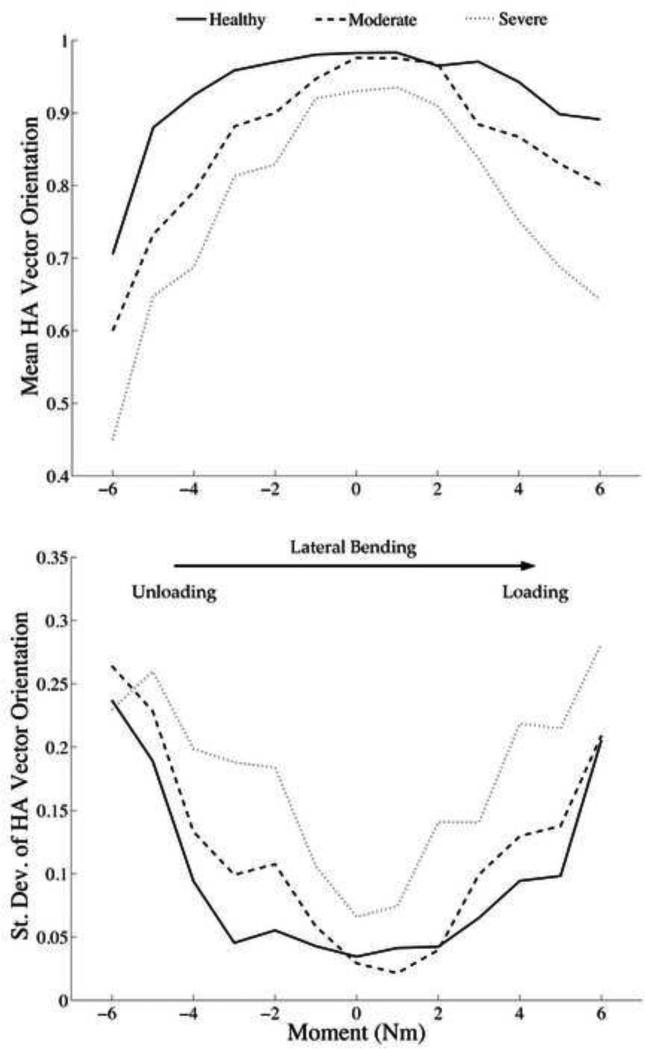
The majority of the differences in the helical axis parameters with respect to disc health occurred at the end ranges of motion, during both unloading and loading. Wherein, a healthy specimen displayed helical axes orientated nearly perpendicular to the plane of pure bending through the neutral zone. Then closer to the end range of motion there is a slight shift in HA orientation, representing interactions between both axial rotation, and flexion. Figure 7 summarizes the average helical axis orientation throughout lateral bending motion for three representative specimens of varying disc health. The orientation near the neutral zone is similar through degeneration, and then they become accentuated at the end ranges of motion.
Figure 7. Helical Axis Vector Orientation and Standard Deviation for Lateral Bending.
Three representative IHA patterns are displayed, Healthy (Transition Zone Slope = 4.39), Moderate (Transition Zone Slope = 2.56), and Severe (Transition Zone Slope = 1.02). The orientation and standard deviation near the neutral zone is similar through degeneration, and then they become accentuated at the end ranges of motion. A vector orientation of 1 indicates a pure hinge joint, as it decreases there is more out-of-plane rotation occurring. As degeneration severity increase the amount of coupled motion increases at the end ranges of motion. Additionally, there is a significant increase in the standard deviation of the vectors indicating instability. Ultimately, with degeneration there are increased out-of-plane rotations and more variability in vector direction.
The standard deviation in the HA orientation, representing the amount of variability in the direction of the vector over the 1Nm window, and the COR location increased significantly with degeneration. The standard deviations of each of these parameters perhaps are a measure of instability. The more sporadic and fluctuating the HA vectors are, the more laxity in the functional spinal unit, and therefore less stable. A similar helical axis metric has been proposed by Duck et al. evaluating elbow stability and Grip et al evaluating knee stability (Duck et al., 2003; Grip and Häger, 2013). As the intervertebral disc – the primary motion granting tissue of the spine – degenerates, there is a loss of osmotic pressure. This alters the delicate interaction between the NP and AF during loading and a loss of normal motion patterns results. Altogether, there is increased out-of- plane bending and COR migration along with increased variability in vector orientation and location. These changes are predominately near end ranges of motion. The initial motion of lateral bending, near the neutral zone, the kinematics are dictated by the disc alone, however, the interactions of the facet joints likely play a key role in the kinematics near end ranges of motion. It is thought that when the facets contact, a coupled-rotation component results.
We postulate the degree at which the facets engage is likely a function of both the quality of the disc and its height, where the diminished mechanical competency of the disc, resulting from degeneration, is responsible for the increased facet interaction and ultimately the increased out of plane rotations and increased variability in the IHA direction are exacerbated. A preliminary post-hoc analysis of our data between the IHA parameters and each: facet health (Grogan et al., 1997) and facet orientation angle (Noren et al., 1991) yielded no significant correlations. The changes in lateral bending helical axes from outside the neutral zone to the full range of motion were observed to be the strongest predictors of disc health. Therefore, the helical axis patterns, predominantly of lateral bending, are a sensitive measure related to the multi-faceted parameters of disc health. It is hypothesized that the altered mechanical properties of the intervertebral disc decreases its ability to maintain function and initiates higher facet interaction eliciting the out of plane rotations and increased variability. In other words, degeneration of the disc portends increased facet interaction irrespective of facet health and orientation. Further analysis is needed to confirm facet-loading patterns either experimentally or by the use of computer models. An additional post-hoc analysis revealed no significant correlations between age and HA parameters exemplifying the disc health predictive ability for a helical axis assessment approach.
Limitations of this research include the use of cadaveric samples to measure lumbar kinematics wherein there was no active musculature or compressive follower load. Active musculature and/or the inclusion of a follower load would likely affect the loading patterns and ultimately the IHA patterns. Helical axes calculation is particularly susceptible to noisy data and small steps size, which can result in erroneous vectors. Therefore, care was taken into systematically decreasing step size and filtering parameters, which are included in the sensitivity analysis performed. Without accepted methods for statistically assessing IHA vectors, the rich data collected herein was reduced to component analyses for statistical purposes. The helical axes presented were determined for only one cycle of bending and does not include trial-to-trial repeatability. Additionally, since only sagittal MR images were acquired, quantification of the transition zone slope in the sagittal plane was not obtainable (methodologically a stack of sagittal images was used which resulted in a coronal plane profile) despite the potential relevance of the sagittal lane profile with flexion and extension motion.
The changes in spinal stability in response to the degenerative disc has been the objective of many previous studies, but this new analysis strategy examines the entire pathway of motion, rather than simplifying spinal kinematics to its terminal ends of motion or 2D pivot points as with the COR. These approaches do not quantify three-dimensional motion patterns, which are critical for detecting coupled motion. Further, non-planar bending profiles that exacerbate off-axis bending are influenced to a greater degree by pain and pathology than traditional planar motion and computation of the instantaneous helical axes provides a solution (Ellingson et al., 2013b). With the recent advances in fluoroscopic kinematic data, the utilization of a helical axis approach to understand in vivo spinal motion may provide beneficial information to understanding the natural kinematics of the spine and interrogate the innate coupled motion during off-axis bending. Degeneration is theorized to originate in response to altered mechanical loading of the disc and if altered loading profiles can be monitored and quantified in vivo this may have a profound impact on the early diagnosis of degeneration (Adams et al., 2014). By beginning to define the relationship between altered spinal motion and radiographic presence of degenerative changes herein, future studies may now examine if these altered spinal kinematics preceded morphologically visible changes and thus become an early detection biomarker for disc degeneration. These kinematic biomarkers may be critical for tracking the progression of disc degeneration, detecting early signs of spinal instability, implementing appropriate care strategies, as well as evaluating new biologic and arthroplasty treatment options.
Supplementary Material
Acknowledgements
Funding was provided through NIH/NIAMS grants T32 AR050938 and T32 AR056950. The authors would also like to thank Daniel F. Keefe, Ph.D. and the members of the Interactive Visualization Lab for aid with the supplemental videos.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Material
Supplemental animations are available depicting representative lateral bending helical axis profiles for both healthy and degenerative specimens.
Conflict of Interest
The authors have no conflict of interest to report.
References
- Adams MA. Biomechanics of back pain. Acupunct Med. 2004;22:178–188. doi: 10.1136/aim.22.4.178. [DOI] [PubMed] [Google Scholar]
- Adams MA, Lama P, Zehra U, Dolan P. Why do some intervertebral discs degenerate, when others (in the same spine) do not? Clinical anatomy. 2014 doi: 10.1002/ca.22404. [DOI] [PubMed] [Google Scholar]
- Adams MA, McNally DS, Dolan P. ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78:965–972. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- Anderst W, Baillargeon E, Donaldson W, Lee J, Kang J. Motion Path of the Instant Center of Rotation in the Cervical Spine During In Vivo Dynamic Flexion-Extension. Spine. 2013;38:E594–E601. doi: 10.1097/BRS.0b013e31828ca5c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana E, Royuela A, Kovacs FM, Estremera A, Sarasibar H, Amengual G, Galarraga I, Martinez C, Muriel A, Abraira V, Gil Del Real MT, Zamora J, Campillo C. Lumbar spine: agreement in the interpretation of 1.5-T MR images by using the Nordic Modic Consensus Group classification form. Radiology. 2010;254:809–817. doi: 10.1148/radiol.09090706. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- Duck TR, Dunning CE, Armstrong AD, Johnson JA, King GJW. Application of screw displacement axes to quantify elbow instability. Clinical Biomechanics. 2003;18:303–310. doi: 10.1016/s0268-0033(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Ellingson AM, Mehta H, Polly DW, Ellermann J, Nuckley DJ. Disc degeneration assessed by quantitative T2* (T2 star) correlated with functional lumbar mechanics. Spine (Phila Pa 1976) 2013a;38:E1533–1540. doi: 10.1097/BRS.0b013e3182a59453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson AM, Nagel TM, Polly DW, Ellermann J, Nuckley DJ. Quantitative T2* (T2 star) relaxation times predict site specific proteoglycan content and residual mechanics of the intervertebral disc throughout degeneration. J Orthop Res. 2014;32:1083–1089. doi: 10.1002/jor.22633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson AM, Yelisetti V, Schulz CA, Bronfort G, Downing J, Keefe DF, Nuckley DJ. Instantaneous helical axis methodology to identify aberrant neck motion. Clin Biomech (Bristol, Avon) 2013b;28:731–735. doi: 10.1016/j.clinbiomech.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara A, Lim TH, An HS, Tanaka N, Jeon CH, Andersson GB, Haughton VM. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine (Phila Pa 1976) 2000;25:3036–3044. doi: 10.1097/00007632-200012010-00011. [DOI] [PubMed] [Google Scholar]
- Gertzbein SD, Seligman J, Holtby R, Chan KH, Kapasouri A, Tile M, Cruickshank B. Centrode patterns and segmental instability in degenerative disc disease. Spine (Phila Pa 1976) 1985;10:257–261. doi: 10.1097/00007632-198504000-00014. [DOI] [PubMed] [Google Scholar]
- Grip H, Häger C. A new approach to measure functional stability of the knee based on changes in knee axis orientation. Journal of Biomechanics. 2013;46:855–862. doi: 10.1016/j.jbiomech.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Grogan J, Nowicki BH, Schmidt TA, Haughton VM. Lumbar facet joint tropism does not accelerate degeneration of the facet joints. AJNR Am J Neuroradiol. 1997;18:1325–1329. [PMC free article] [PubMed] [Google Scholar]
- Johannessen W, Auerbach JD, Wheaton AJ, Kurji A, Borthakur A, Reddy R, Elliott DM. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine (Phila Pa 1976) 2006;31:1253–1257. doi: 10.1097/01.brs.0000217708.54880.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettler A, Marin F, Sattelmayer G, Mohr M, Mannel H, Durselen L, Claes L, Wilke HJ. Finite helical axes of motion are a useful tool to describe the three-dimensional in vitro kinematics of the intact, injured and stabilised spine. Eur Spine J. 2004;13:553–559. doi: 10.1007/s00586-004-0710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettler A, Rohlmann F, Ring C, Mack C, Wilke HJ. Do early stages of lumbar intervertebral disc degeneration really cause instability? Evaluation of an in vitro database. Eur Spine J. 2011;20:578–584. doi: 10.1007/s00586-010-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop Relat Res. 1982:110–123. [PubMed] [Google Scholar]
- Lotz JC, Haughton V, Boden SD, An HS, Kang JD, Masuda K, Freemont A, Berven S, Sengupta DK, Tanenbaum L, Maurer P, Ranganathan A, Alavi A, Marinelli NL. New treatments and imaging strategies in degenerative disease of the intervertebral disks. Radiology. 2012;264:6–19. doi: 10.1148/radiol.12110339. [DOI] [PubMed] [Google Scholar]
- Mimura M, Panjabi MM, Oxland TR, Crisco JJ, Yamamoto I, Vasavada A. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine (Phila Pa 1976) 1994;19:1371–1380. doi: 10.1097/00007632-199406000-00011. [DOI] [PubMed] [Google Scholar]
- Mwale F, Iatridis JC, Antoniou J. Quantitative MRI as a diagnostic tool of intervertebral disc matrix composition and integrity. Eur Spine J. 2008;17(Suppl 4):432–440. doi: 10.1007/s00586-008-0744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan RN, Williams JR, Andersson GB. Modeling changes in intervertebral disc mechanics with degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):36–40. doi: 10.2106/JBJS.F.00002. [DOI] [PubMed] [Google Scholar]
- Noren R, Trafimow J, Andersson GB, Huckman MS. The role of facet joint tropism and facet angle in disc degeneration. Spine (Phila Pa 1976) 1991;16:530–532. doi: 10.1097/00007632-199105000-00008. [DOI] [PubMed] [Google Scholar]
- O'Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine (Phila Pa 1976) 2007;32:328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5:390–396. doi: 10.1097/00002517-199212000-00002. discussion 397. [DOI] [PubMed] [Google Scholar]
- Panjabi MM. Clinical spinal instability and low back pain. Journal of electromyography and kinesiology : official journal of the International Society of Electrophysiological Kinesiology. 2003;13:371–379. doi: 10.1016/s1050-6411(03)00044-0. [DOI] [PubMed] [Google Scholar]
- Passias PG, Wang S, Kozanek M, Xia Q, Li W, Grottkau B, Wood KB, Li G. Segmental lumbar rotation in patients with discogenic low back pain during functional weight-bearing activities. J Bone Joint Surg Am. 2011;93:29–37. doi: 10.2106/JBJS.I.01348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy MJ, Bogduk N. Instantaneous axes of rotation of the lumbar intervertebral joints. Spine. 1988;13:1033–1041. doi: 10.1097/00007632-198809000-00011. [DOI] [PubMed] [Google Scholar]
- Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- Raininko R, Manninen H, Battie MC, Gibbons LE, Gill K, Fisher LD. Observer variability in the assessment of disc degeneration on magnetic resonance images of the lumbar and thoracic spine. Spine (Phila Pa 1976) 1995;20:1029–1035. doi: 10.1097/00007632-199505000-00009. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Heuer F, Wilke HJ. Interaction between finite helical axes and facet joint forces under combined loading. Spine. 2008;33:2741–2748. doi: 10.1097/BRS.0b013e31817c4319. [DOI] [PubMed] [Google Scholar]
- Schneider G, Pearcy MJ, Bogduk N. Abnormal motion in spondylolytic spondylolisthesis. Spine (Phila Pa 1976) 2005;30:1159–1164. doi: 10.1097/01.brs.0000162400.06685.37. [DOI] [PubMed] [Google Scholar]
- Seligman JV, Gertzbein SD, Tile M, Kapasouri A. Computer analysis of spinal segment motion in degenerative disc disease with and without axial loading. Spine (Phila Pa 1976) 1984;9:566–573. doi: 10.1097/00007632-198409000-00006. [DOI] [PubMed] [Google Scholar]
- Spoor CW, Veldpaus FE. Rigid body motion calculated from spatial co-ordinates of markers. J Biomech. 1980;13:391–393. doi: 10.1016/0021-9290(80)90020-2. [DOI] [PubMed] [Google Scholar]
- Tanaka N, An HS, Lim TH, Fujiwara A, Jeon CH, Haughton VM. The relationship between disc degeneration and flexibility of the lumbar spine. Spine J. 2001;1:47–56. doi: 10.1016/s1529-9430(01)00006-7. [DOI] [PubMed] [Google Scholar]
- Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DJ, Freeman AL, Ellingson AM, Nuckley DJ, Buckley JM, Scheer JK, Crawford NR, Bechtold JE. Inter-laboratory variability in in vitro spinal segment flexibility testing. Journal of Biomechanics. 2011;44:2383–2387. doi: 10.1016/j.jbiomech.2011.06.034. [DOI] [PubMed] [Google Scholar]
- White AA, Panjabi MM. Clinical biomechanics of the spine. Lippincott; Philadelphia: 1978. [Google Scholar]
- Woltring HJ, Huiskes R, de Lange A, Veldpaus FE. Finite centroid and helical axis estimation from noisy landmark measurements in the study of human joint kinematics. J Biomech. 1985;18:379–389. doi: 10.1016/0021-9290(85)90293-3. [DOI] [PubMed] [Google Scholar]
- Woltring HJ, Long K, Osterbauer PJ, Fuhr AW. Instantaneous helical axis estimation from 3-D video data in neck kinematics for whiplash diagnostics. J Biomech. 1994;27:1415–1432. doi: 10.1016/0021-9290(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Zhao F, Pollintine P, Hole BD, Dolan P, Adams MA. Discogenic origins of spinal instability. Spine (Phila Pa 1976) 2005;30:2621–2630. doi: 10.1097/01.brs.0000188203.71182.c0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.