Abstract
Importance
Previous evidence has implicated corticostriatal abnormalities in the pathophysiology of psychosis. Although the striatum is the primary target of all efficacious antipsychotics, the relationship between its functional connectivity and symptomatic reduction remains unknown.
Objective
The present study examined the longitudinal effect of treatment with second-generation antipsychotics on functional connectivity of the striatum during the resting state in patients experiencing a first-episode of psychosis.
Design
Prospective, controlled study, with medications administered in double-blind, randomized manner.
Setting
Clinical research center
Participants
Twenty-four patients with first-episode psychosis and twenty-four healthy participants, matched for age, sex, education, and handedness.
Interventions
Patients were scanned at baseline and after 12 weeks of treatment with either risperidone or aripiprazole. Their symptoms were evaluated with the Brief Psychiatric Rating Scale at baseline and follow-up. Healthy participants were scanned twice with a 12 week interval.
Main Outcome Measures
Functional connectivity of striatal regions was examined using a seed-based approach. Changes in functional connectivity of these seeds were compared with reductions in ratings of psychotic symptoms.
Results
As psychosis improved, we observed an increase in functional connectivity between striatal seed regions and the anterior cingulate, dorsolateral prefrontal cortex, and limbic regions such as the hippocampus and anterior insula. Conversely, a negative relationship was observed between reduction in psychosis and functional connectivity of striatal regions with structures within the parietal lobe.
Conclusions and Relevance
Our results indicate that corticostriatal functional dysconnectivity in psychosis is a state-dependent phenomenon. Increased functional connectivity of the striatum with prefrontal and limbic regions may be a biomarker for improvement in symptoms associated with antipsychotic treatment.
Disruptions in corticostriatal circuitry have been implicated in the pathophysiology of schizophrenia. Early proposals linked schizophrenia with decreases in dopamine in the prefrontal cortex, and excessive dopamine in the striatum1,2. Although elevated striatal dopamine has been shown in patients with schizophrenia3, their unaffected relatives4, and in individuals who are at-risk for developing psychotic symptoms5, corticostriatal relationships have also been demonstrated to play a central role in psychosis. Early positron emission tomography (PET) work found that severity of psychotic symptoms correlated with abnormal patterns of blood flow in limbic and prefrontal cortical regions6. Studies in schizophrenia using functional MRI (fMRI) have reported abnormal corticostriatal activation during reward7,8, and executive processing9,10. Evidence from functional connectivity and multimodal studies have shown altered corticostriatal circuitry in chronic patients with schizophrenia11,12, and in patients with prodromal psychotic symptoms13,14. Of note, a recent family-based study suggested that altered functional connectivity between striatum and cortical regions may represent a risk phenotype in patients with first-episode psychosis and in their relatives15.
Despite the evidence implicating corticostriatal links in psychosis, there is a paucity of data directly examining the relationship between corticostriatal functional connectivity and the clinical effects of antipsychotic agents. Structures of the striatum are of particular interest when considering the effects of treatment since they harbor the largest density of dopamine D2 receptors16. Although antipsychotic drugs vary in their potency and effect on cortical and subcortical functions, all known antipsychotic agents bind to the D2 receptor17. A few studies have used a longitudinal study design to examine the effects of antipsychotic treatment with network-based analyses18–20, and during reward processing21, but do not directly address the question of symptom-related changes in striatal connectivity.
In the present study our goal was to examine the relationship between changes in striatal circuitry and reduction in psychotic symptoms after treatment with antipsychotic medications. We used a prospective study design in which resting state fMRI scans were collected in a cohort of patients with first episode schizophrenia and a matched healthy comparison group at two time points. Scans in the patient group were collected at baseline and after twelve weeks of treatment with a second-generation antipsychotic; healthy comparison participants were also scanned with a twelve week interval.
To interrogate the functional networks of the striatum, we employed a seed-based approach first proposed by Di Martino et al 200822. We tested the effect of antipsychotic treatment on the functional circuitry of striatum by comparing changes in connectivity of our striatal seed regions and changes in psychotic symptomatology. Our primary hypothesis was that functional connections with cortical regions would be strengthened and normalized as psychotic symptoms resolve, specifically with prefrontal and limbic regions that have been implicated to be impaired in schizophrenia. In order to test this hypothesis, we first needed to create functional maps from our healthy comparison group at baseline. We hypothesized that our functional maps will replicate the anteroposterior separation of positive and negative striatal correlations observed previously22. At baseline, we expected to see frontostriatal decoupling in our patients relative to the healthy comparison group, which would then normalize as a function of successful treatment. By contrast, we did not expect to see changes associated merely with the passage of time.
Methods
Participants
Patients aged 15 to 40 years old with first-episode psychosis (FEP) underwent resting state fMRI scanning and symptom ratings at baseline and after 12 weeks of treatment with either risperidone or aripiprazole as a part of an NIMH-funded, double blind, randomized control trial (R01MH060004). FEP includes a variety of diagnostic categories; our investigation was limited to subjects with first-episode schizophrenia-spectrum disorders (i.e. schizophrenia, schizophreniform disorder, schizoaffective disorder, or psychotic disorder, not otherwise specified). All patients were required to have 2 weeks or less of cumulative lifetime exposure to antipsychotics to enter the clinical trial. A healthy comparison (HC) group was also scanned at two time points with a 12-week interval (Table 1). Patient diagnoses were based on the Structured Clinical Interview for Axis I Diagnostic and Statistical Manual-IV Disorders (SCID), supplemented by information from clinicians and, when available, family members. After complete description of the study to the participants, written informed consent (written assent and written parental/guardian consent for those under age 18 years) was obtained as per a protocol that was approved by the Institutional Review Board of the North Shore-Long Island Jewish Health System. Additional details regarding exclusion criteria for our study participants are available in the eAppendix in the Supplement.
Table 1.
Baseline Demographics and Clinical Ratings
| Characteristic | First-Episode Psychosis (N=24) | Healthy Comparison Group (N=24) |
|---|---|---|
| Age (years) | ||
| Mean | 21.4a | 20.0 |
| SD | 5.0 | 3.1 |
| Gender (number) | ||
| Male | 17b | 17 |
| Female | 7 | 7 |
| Years of Education | ||
| Mean | 12.3a | 13.0 |
| SD | 1.8 | 2.5 |
| Handedness (Edinburgh) | ||
| Mean | 0.76a | 0.51 |
| SD | 0.32 | 0.58 |
| Interval between scans (days) | ||
| Mean | 87.3a | 89.1 |
| SD | 4.9 | 6.7 |
| Prior antipsychotic exposure (days) | ||
| Mean | 4.5 | -- |
| SD | 6.1 | -- |
| BPRS total score -- baseline | ||
| Mean | 43.3 | -- |
| SD | 9.3 | -- |
| BPRS total score – follow-up | ||
| Mean | 27.3a | -- |
| SD | 6.9 | -- |
| PSx rating -- baseline | ||
| Mean | 11.0 | -- |
| SD | 2.6 | -- |
| PSx rating – follow up | ||
| Mean | 5.6a | -- |
| SD | 2.6 | -- |
No significant difference (p<0.05) from value for comparison group (two-tailed t test).
No significant difference (p<0.05) from value for comparison group (Chi-square test).
After providing informed consent, all patients received double blind treatment with either risperidone (dose range: 1–6 mg) or aripiprazole (5–30 mg) for 16 weeks. Details regarding supplemental medications allowed in our patients are available in the eAppendix in the Supplement. Clinical ratings were administered weekly for the first month, and then every two weeks thereafter until week 12. To evaluate psychotic symptoms, the Brief Psychiatric Rating Scale - Anchored version (BPRS-A) was used. For our analyses, we were concerned only with symptoms reflective of psychosis. As such, we utilized three items from the BPRS-A that assess positive psychotic symptoms -- unusual thought content, hallucinations, and conceptual disorganization -- in order to obtain a measure of psychotic symptoms (PSx), termed by previous studies, as a thought disturbance rating23.
Resting state fMRI Image Acquisition
All fMRI exams were conducted on a 3T scanner (GE Signa HDx). Further details are provided in the eAppendix in the Supplement. During resting state scanning, participants were asked to close their eyes and instructed not to think of anything in particular. All participants were spoken to between scan sequences to ensure they were not asleep, and no behavioral differences were observed between groups during scanning.
Image analysis and Preprocessing
We used FSL (http://www.fmrib.ox.ac.uk) and AFNI (http://afni.nimh.nih.gov/afni) based script libraries from the 1000 Functional Connectomes Project (http://www.nitrc.org/projects/fcon_1000 35) for preprocessing of resting-state scans (“fcon scripts”). Details regarding preprocessing steps are provided in the eAppendix in the Supplement.
Functional connectivity analyses
To test the functional connectivity of subregions of the striatum within the putamen, caudate nucleus, and nucleus accumbens, we used a seed based approach. We utilized methods described in Di Martino et al. 200822. The central coordinates of the regions of interest (ROIs) were taken from that study and used to create 3.5 × 3.5 × 3.5 mm, spherical ROIs. ROIs were defined, bilaterally, in: dorsal caudate (DC) (x = ±13, y = 15, z = 9), ventral caudate (VSS) (x = ±10, y = 15, z = 0), ventral caudate/nucleus accumbens (VSI) (x = ±9, y = 9, z = −8), dorsal rostral putamen (DRP) (x = ±25, y = 8, z = 6), dorsal caudal putamen (DCP) (x = ±28, y = 1, z = 3), and ventral rostral putamen (VRP) (x = ±20, y = 12, z = −3).
Once ROIs were defined, AFNI based scripts from the 1000 Functional Connectomes Project were used to create correlation maps for each participant for all 12 of our ROIs. Mean activity time courses were extracted from each seed region. Whole-brain voxel-wise correlation maps for each ROI were created with the extracted waveform as a reference. The resulting correlation maps were Z-transformed.
For group level analyses we used SPM5 (http://www.fil.ion.ucl.ac.uk/spm). One sample t-tests were performed with group level correlation maps for each ROI in our baseline HC group. Results were visualized at p<0.05, uncorrected, for both positive and negative correlations for each of our ROIs. A relatively liberal threshold was used for these initial analyses, as these were used to create masks for subsequent analyses testing our primary hypothesis. We found good separation of networks with our ROIs in both the positive and negative directions, consistent with results from Di Martino et al. 200822. Results for all other analyses were considered significant if they surpassed a threshold of p<0.05, corrected for false discovery rate (FDR), by the standard function provided with the SPM package24. Baseline and follow up scans within each group and between groups were compared by two sample t-tests in SPM.
To compare changes in PSx ratings with longitudinal changes in functional connectivity of our striatal ROIs in our FEP group, we subtracted the baseline scan from the follow-up scan (Follow-up - baseline) using FSLMATHS. This image -- representing the change in correlation at each voxel -- was then taken into a group level multiple regression analysis with reduction in PSx (baseline PSx - follow-up PSx) as a regressor. This analysis was performed separately for each ROI, explicitly masked within the binary mask from the corresponding HC network. To visualize our correlations, we extracted and plotted data from the most significant voxel within clusters that surpassed our threshold for significance.
Results
Demographics
A total of 24 FEP patients and an age-, sex-, education-, and handedness-matched cohort of 24 HC participants were included in the present study (Table 1). Patients had a median exposure of one day to antipsychotic medication prior to being scanned (Mean [SD] = 4.5 [6.1]; Table 1). Eleven patients were treated with aripiprazole and thirteen patients were treated with risperidone. Due to the limited power we did not perform analyses to separate drug specific effects (see eAppendix for Supplement).
Baseline correlations
All twelve of our striatal seed ROIs (six per hemisphere) showed well-delineated patterns of positive and negative functional connectivity (Figure 1), similar to those described by Di Martino and colleagues22. Across all seeds, we observed a general anteroposterior pattern of correlative activity: positive correlations were limited to more frontal regions and negative correlations were generally observed in more posterior regions (Figure 1). Additionally we observe laterality of functional maps. In particular, the DC showed ipsilateral functional connectivity with more dorsal prefrontal regions.
Figure 1. Locations of seed regions and their representative connectivity maps.
Both left and right seed regions of interest are displayed in green in first column. The corresponding positive (red) and negative (blue) functional connectivity maps of each of these seeds from the right hemisphere in the healthy comparison group at baseline are displayed in subsequent columns. Maps are shown with threshold of p <0.001, uncorrected, for the purpose of visualization. DC: caudate, VSS: ventral caudate, VSI: ventral caudate/nucleus accumbens, DRP: dorsal rostral putamen, DCP: dorsal caudal putamen, and VRP: ventral rostral putamen.
Both positive and negative masks were created from all 12 connectivity maps in our HC group at baseline with a threshold of p <0.05, uncorrected. Our subsequent analyses that involved psychotic symptom ratings were limited within these reference masks.
Between Groups Comparisons
In our between group analyses, we compared connectivity maps of all 12 ROIs at baseline in our FEP and HC groups. No results in either masked or whole-brain analyses were observed at our level of significance (p < 0.05, FDR corrected).
Between Scan Comparison
Our HC group showed no significant changes in functional connectivity in masked or whole-brain analyses of all 12 of our seed ROIs when baseline scans were compared with follow-up scans in a paired manner. Our FEP group showed only one significant, masked, finding between baseline and follow-up scans in paired comparisons: the right VSS showed increased connectivity with the thalamus on the left side, and a cluster of voxels adjacent to the seed within the nucleus accumbens on the right side (see eTable 1 in the Supplement for details).
Increase in Striatal Functional Connectivity with Treatment Response
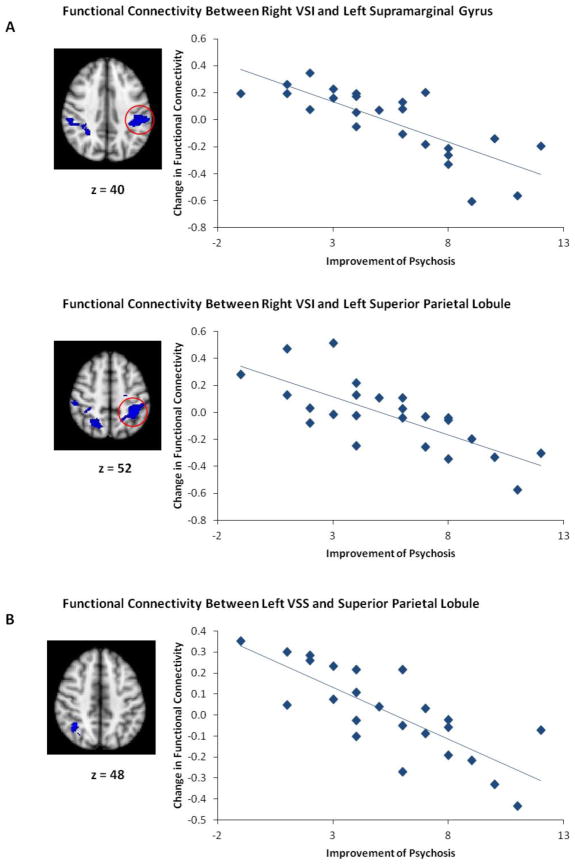
Antipsychotic treatment in our cohort of patients with FEP resulted in an overall significant reduction in positive symptoms (Mean [SD] PSx score at baseline= 11 [2.6], at 12 weeks=5.6 [2.6]; T = 7.35, p<0.0001), as measured by our PSx score, a composite of items reflective of psychosis from the BPRS. We performed multiple regression analyses in our FEP group to compare PSx at baseline and follow-up, as well as the reduction in PSx with changes in functional connectivity of each of our seed ROIs. No significant correlations were observed between PSx and functional connectivity of our ROIs at baseline or follow-up. Greater reduction in PSx showed a robust, positive correlation with increased functional connectivity between right DC and prefrontal regions that include the orbitofrontal cortex, anterior cingulate, and the right dorsolateral prefrontal cortex (Figure 2; Table 2). As psychosis resolved, the right VSI seed showed a significant increase in connectivity with a cluster of voxels located in the left hippocampus (Figure 2; Table 2). Similarly, as symptoms improved, the right VRP seed showed increased functional connectivity with anterior cingulate and right anterior insula (Figure 2; Table 2). No other seed regions showed results that survived correction for multiple comparisons. We observed no significant findings at the whole brain level outside of masks derived from our HC group.
Figure 2. Functional connectivity changes with improvement in psychotic symptoms.
Key regions that show significantly (p <0.05, FDR corrected) increased functional connectivity, as symptoms improve, with seed regions in right DC in panel A, and right VSI in panel B.
Table 2.
Summary of significant differences in functional connectivity from multiple regression with change in psychotic symptoms
| Seed region | Direction of correlation | Correlated Region | Brodman n Area | Montreal Neurological Institute Coordinates | T-score | Z-score | FDR correct ed p- value |
|---|---|---|---|---|---|---|---|
| Right DC | Positive | Right dorsolateral prefrontal cortex | 9 | 30, 46, 22 | 4.98 | 4.03 | 0.006 |
| Positive | Anterior cingulate | 32 | −6, 38, 14 | 7.16 | 5.09 | 0.004 | |
| Positive | Right orbitofrontal cortex | 47 | 24, 26, −12 | 4.56 | 3.79 | 0.018 | |
| Positive | Left thalamus | −6,−8,−4 | 4.18 | 3.55 | 0.014 | ||
| Right VRP | Positive | Bilateral anterior cingulate | 32 | 14, 44, 4 | 4.72 | 3.88 | 0.039 |
| Positive | Right insula | 13 | 40, 14, −10 | 4.74 | 3.9 | 0.039 | |
| Right VSI | Positive | Left hippocampus | −34, −26, −12 | 6.17 | 4.65 | 0.029 | |
| Negative | Left superior parietal lobule | 7 | −32, −46, 50 | 5.96 | 4.55 | 0.03 | |
| Negative | Left supramarginal gyrus | 40 | −52, −32, 44 | 5.96 | 4.55 | 0.03 | |
| Negative | Right superior parietal lobule | 7 | 22 −56 56 | 5.18 | 4.14 | 0.036 | |
| Negative | Right supramarginal gyrus | 40 | 58, −28, 42 | 5.23 | 4.17 | 0.035 | |
| Left VSS | Negative | Right superior parietal lobule | 7 | 38, −58, 48 | 6.01 | 4.57 | 0.026 |
Decrease in Striatal Functional Connectivity with Treatment Response
Conversely, in several ROIs we observed significant negative correlations between symptom improvement and change in functional connectivity with posterior regions. This pattern mirrored the negative correlation maps we observe with our seeds in the HC group at baseline (Figure 1). With improvement in PSx, we observe significantly less connectivity between right VSI and bilateral superior parietal lobule and supramarginal gyrus (Figure 3; Table 2). Similarly, as psychosis improves, left VSS shows less connectivity with superior parietal lobe (Figure 3; Table 2). No additional findings were observed outside of our masks at the whole brain level.
Figure 3. Functional connectivity changes with less improvement in psychotic symptoms.
Key regions that show significant (p <0.05, FDR corrected) negative correlation between change in functional connectivity and symptom improvement, with seed regions in right VSI in panel A, and left VSS in panel B.
Discussion
We utilized resting state fMRI to examine the effects of treatment with either aripiprazole or risperidone on functional networks of striatal regions in a unique cohort of patients with first episode psychosis. Scans were collected in a longitudinal study design, pre- and post- twelve weeks of controlled treatment. With improvement of psychosis, we observed a significant increase in functional connectivity between the right dorsal caudate and several prefrontal regions that include the anterior cingulate, the right dorsolateral prefrontal cortex, and the orbitofrontal cortex. Additionally, as psychotic symptoms resolved, we observed an increase in functional connectivity between the right ventral caudate/nucleus accumbens and hippocampus, and between a seed region placed within the ventral putamen with anterior insula. Finally, we also found that as symptoms improved, the ventral caudate showed decreased functional connectivity with posterior regions. Intriguingly, these regions in superior parietal lobe and supramarginal gyrus were noted to be negatively connected with striatal regions at baseline in our HC group (Figure 1).
As hypothesized, our results demonstrate that symptomatic improvement of psychosis is associated with alterations in functional connections of the striatum, a structure consistently implicated in the pathophysiology of psychosis and shared site of D2 receptor binding of all antipsychotic agents17. One region that showed robust changes in functional interactions as psychosis improved, is the dorsal caudate, also known as the associative caudate. This region has been shown to be anatomically and functionally connected to the dorsolateral prefrontal cortex22, 25, 26. A large body of evidence has linked striatum with dorsolateral prefrontal regions in psychosis12–15. Our results extend these findings and demonstrate that this link is modulated by pharmacologic intervention in a manner that correlates with symptom reduction. Additionally, our finding of significantly increased connectivity between striatal regions and dorsal anterior cingulate implicate a role for error monitoring and cognitive control in recovery from psychotic symptoms27. Our work supports previous results that demonstrate second-generation antipsychotic treatment based alterations in blood flow to the anterior cingulate28.
Psychotic phenomenology has been characterized as the result of abnormal assignment of salience to internal and external stimuli29, 30. Several brain regions from the present study that demonstrate increased striatal connectivity in response to treatment have been implicated in the normal attribution of salience. Specifically, as psychotic symptoms remit, the ventral putamen showed increased connectivity with anterior insula and anterior cingulate, both of which are regions that have been linked to the salience network 11. Findings in the present study suggest that changes in the functional coordination between striatum and prefrontal and limbic systems may influence salience processing as psychotic symptoms are reduced. In support of this hypothesis, induction of psychosis by cannabis has shown to modulate activation of caudate and prefrontal cortex during salience processing31.
Interestingly we did not observe significant baseline differences in functional connectivity of the striatum between healthy individuals and our patients with psychosis. A recent study by Fornito and colleagues suggested that frontostriatal dysconnectivity is an endophenotype for psychosis by showing decreased coupling between striatum and dorsolateral prefrontal cortex in the unaffected relatives of patients15. While our study did not address familiality, baseline differences in connectivity patterns between studies may be related to differences in clinical variables, imaging parameters, and statistical approaches.
While previous fMRI studies have reported cross-sectional evidence linking reduced striatal signal and psychotic symptomotology32–34, our study utilized a longitudinal approach to examine the effects of antipsychotic medications on network-based functional connectivity measures. To our knowledge, only one resting state fMRI study has been reported that examines antipsychotic treatment and functional connectivity in first episode schizophrenia. Lui and colleagues observed treatment-based disruption in connectivity between brain regions and functional circuits in conjunction with altered low frequency fMRI signal within cortical regions and striatum18. They did not report changes in functional circuitry that correlate with treatment efficacy, and further, the study did not specifically examine cortico-striatal connectivity with an anatomically driven approach. Additionally, one longitudinal PET study observed decreased connectivity of medial frontal cortex with hippocampus and ventral striatum after treatment19, while a task-based fMRI study reported alterations in default mode network connectivity after olanzapine treatment20. Other neuroimaging studies have also taken a longitudinal approach to test the effects of antipsychotic treatment on task-based activation, and generally report normalization of signal after treatment 35, 36.
The present study also contributes to the evolving field of biomarkers for psychotic disorders and treatment response. Previous studies have shown differential neuroimaging signals based on treatment response37. Additionally, differences in response to treatment with antipsychotic medications have been associated with polymorphisms in the gene coding for the D2 receptor38, 39. Independently, DRD2 variation has been shown to be related to functional engagement of frontostriatal circuits40. Further studies are required to clarify the association between our finding of treatment related modulation of corticostriatal interactions and genetic variation.
Limitations of the present study include a relatively modest sample size; however, our sample size is comparable to other recent studies that examined first episode schizophrenia patients at two time points21, or utilize functional connectivity15, 41,42. Larger sample sizes would be useful to examine differential effects of various antipsychotic agents. Additionally, we examined a select group of psychotic patients and are unable to extend our results to other groups of patients experiencing psychosis. Future studies are required to examine striatal connectivity in illnesses such as bipolar disorder. By examining changes in psychotic symptoms, our results focus on state dependent changes in corticostriatal circuitry that are reflective of successful treatment rather than the effect of treatment alone. Further studies are required to separate trait related abnormalities in circuitry.
Conclusions
The present study provides evidence that efficacy of treatment of psychosis with second-generation antipsychotic medications is associated on a neurophysiological level with alterations in functional corticostriatal circuitry. To the extent that psychosis successfully improves, functional connectivity between the striatum and prefrontal, as well as limbic regions, are strengthened. These data further characterize the pathophysiology of psychosis and provide support for the role of neuroimaging as a potential biomarker for clinical response.
Supplementary Material
Acknowledgments
Funding/Support: Supported by NIMH grants P50MH080173 to Dr. Malhotra, P30MH090590 to Dr. Kane;R01MH060004 to Dr. Robinson; and R01MH076995 to Dr. Szeszko.
Footnotes
Conflict of Interest Disclosures: Dr. Robinson has been a consultant to Asubio and Shire, and he has received grants from Bristol-Meyers Squibb, Janssen, Otsuka. Dr. Lencz is a consultant to Eli Lilly. Dr. Kane is a shareholder in Medvante, Inc., has been a consultant for Amgen, Alkermes, Bristol-Meyers Squibb, Eli Lilly, Forrest Pharmaceuticals, Genentech, H. Lundbeck Intracellular Therapeutics, Janssen Pharmaceutica, Jazz Pharmaceuticals, Johnson and Johnson, Lundbeck, Merck, Novartis, Otsuka, Pierre Fabre, Proteus, Reviva, Roche and Sunovion, and has been on the Speaker’s Bureau for Bristol-Meyers Squibb, Eli Lilly, Janssen, and Otsuka. Dr. Malhotra is a consultant to Genomind, inc. The other authors report no financial relationships with commercial interests.
Author Contributions: Study concept and design: Sarpal, Lencz, Ikuta, Argyelan, Robinson, Szeszko, Malhotra. Data Collection: Gallego, Robinson, Malhotra. Analysis and interpretation of data: Sarpal, Ikuta, Lencz, Malhotra, Argyelan. Drafting of Manuscript: Sarpal, Lencz, Malhotra. Obtained funding: Szeszko, Robinson, Malhotra, Kane. Critical Revision of Manuscript for important intellectual content: Sarpal, Lencz, Malhotra, Argyelan, Karlsgodt, Gallego, Robinson.
References
- 1.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 2.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148(11):1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 3.Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huttunen J, Heinimaa M, Svirskis T, et al. Striatal dopamine synthesis in first-degree relatives of patients with schizophrenia. Biol Psychiatry. 2008;63(1):114–117. doi: 10.1016/j.biopsych.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Fusar-Poli P, Howes OD, Allen P, et al. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch Gen Psychiatry. 2010;67(7):683–691. doi: 10.1001/archgenpsychiatry.2010.77. [DOI] [PubMed] [Google Scholar]
- 6.Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen MØ, Rostrup E, Wulff S, et al. Alterations of the brain reward system in antipsychotic naïve schizophrenia patients. Biol Psychiatry. 2012;71(10):898–905. doi: 10.1016/j.biopsych.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Koch K, Wagner G, Nenadic I, et al. Fronto-striatal hypoactivation during correct information retrieval in patients with schizophrenia: an fMRI study. Neuroscience. 2008;153(1):54–62. doi: 10.1016/j.neuroscience.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 9.Quidé Y, Morris RW, Shepherd AM, Rowland JE, Green MJ. Task-related fronto-striatal functional connectivity during working memory performance in schizophrenia. Schizophr Res. 2013;150(2–3):468–475. doi: 10.1016/j.schres.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Tu PC, Lee YC, Chen YS, Li CT, Su TP. Schizophrenia and the brain’s control network: aberrant within- and between-network connectivity of the frontoparietal network in schizophrenia. Schizophr Res. 2013;147(2–3):339–347. doi: 10.1016/j.schres.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Orliac F, Naveau M, Joliot M, et al. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res. 2013;148(1–3):74–80. doi: 10.1016/j.schres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Meyer-Lindenberg A, Miletich RS, Kohn PD, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5(3):267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 13.Fusar-Poli P, Howes OD, Allen P, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16(1):67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- 14.Dandash O, Fornito A, Lee J, et al. Altered Striatal Functional Connectivity in Subjects With an At-Risk Mental State for Psychosis. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fornito A, Harrison BJ, Goodby E, et al. Functional Dysconnectivity of Corticostriatal Circuitry as a Risk Phenotype for Psychosis. JAMA Psychiatry. 2013;70(11):1143–1151. doi: 10.1001/jamapsychiatry.2013.1976. [DOI] [PubMed] [Google Scholar]
- 16.Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11(4):245–256. doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- 17.Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67(8):783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- 19.Bolding MS, White DM, Hadley JA, Weiler M, Holcomb HH, Lahti AC. Antipsychotic Drugs Alter Functional Connectivity between the Medial Frontal Cortex, Hippocampus, and Nucleus Accumbens as Measured by H215O PET. Front Psychiatry. 2012;3:105. doi: 10.3389/fpsyt.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambataro F, Blasi G, Fazio L, et al. Treatment with olanzapine is associated with modulation of the default mode network in patients with Schizophrenia. Neuropsychopharmacology. 2010;35(4):904–912. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen MO, Rostrup E, Wulff S, et al. Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch Gen Psychiatry. 2012;69(12):1195–1204. doi: 10.1001/archgenpsychiatry.2012.847. [DOI] [PubMed] [Google Scholar]
- 22.Di Martino A, Scheres A, Margulies DS, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18(12):2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 23.Hedlund JL, Vieweg BW. The Brief Psychiatric Rating Scale (BPRS): A comprehensive review. Journal of Operational Psychiatry. 1980;11:48–65. [Google Scholar]
- 24.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 25.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 26.Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16(10):1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- 27.Polli FE, Barton JJ, Thakkar KN, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131(Pt 4):971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- 28.Lahti AC, Holcomb HH, Weiler MA, et al. Clozapine but not haloperidol Re-establishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology. 2004;29(1):171–178. doi: 10.1038/sj.npp.1300312. [DOI] [PubMed] [Google Scholar]
- 29.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 30.Jensen J, Willeit M, Zipursky RB, et al. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33(3):473–479. doi: 10.1038/sj.npp.1301437. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharyya S, Crippa JA, Allen P, et al. Induction of psychosis by Δ9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch Gen Psychiatry. 2012;69(1):27–36. doi: 10.1001/archgenpsychiatry.2011.161. [DOI] [PubMed] [Google Scholar]
- 32.Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 33.Menon M, Schmitz TW, Anderson AK, et al. Exploring the neural correlates of delusions of reference. Biol Psychiatry. 2011;70(12):1127–1133. doi: 10.1016/j.biopsych.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 34.Sorg C, Manoliu A, Neufang S, et al. Increased intrinsic brain activity in the striatum reflects symptom dimensions in schizophrenia. Schizophr Bull. 2013;39(2):387–395. doi: 10.1093/schbul/sbr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nejad AB, Ebdrup BH, Glenthøj BY, Siebner HR. Brain connectivity studies in schizophrenia: unravelling the effects of antipsychotics. Curr Neuropharmacol. 2012;10(3):219–230. doi: 10.2174/157015912803217305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbott CC, Jaramillo A, Wilcox CE, Hamilton DA. Antipsychotic drug effects in schizophrenia: a review of longitudinal FMRI investigations and neural interpretations. Curr Med Chem. 2013;20(3):428–437. doi: 10.2174/0929867311320030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology. 2009;34(13):2675–2690. doi: 10.1038/npp.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lencz T, Robinson DG, Xu K, et al. DRD2 promoter region variation as a predictor of sustained response to antipsychotic medication in first-episode schizophrenia patients. Am J Psychiatry. 2006;163(3):529–531. doi: 10.1176/appi.ajp.163.3.529. [DOI] [PubMed] [Google Scholar]
- 39.Zhang JP, Lencz T, Malhotra AK. D2 receptor genetic variation and clinical response to antipsychotic drug treatment: a meta-analysis. Am J Psychiatry. 2010;167(7):763–772. doi: 10.1176/appi.ajp.2009.09040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertolino A, Fazio L, Caforio G, et al. Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Brain. 2009;132(Pt 2):417–425. doi: 10.1093/brain/awn248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA Psychiatry. 2013;70(8):857–868. doi: 10.1001/jamapsychiatry.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt A, Smieskova R, Aston J, et al. Brain connectivity abnormalities predating the onset of psychosis: correlation with the effect of medication. JAMA Psychiatry. 2013;70(9):903–12. doi: 10.1001/jamapsychiatry.2013.117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.