Abstract
Purpose
To evaluate the T2 relaxation time of lactate (Lac) in brain tumors and the correlation of the T2 and concentration with tumor grades.
Methods
Eight pairs of the subecho time sets of point-resolved spectroscopy were selected between 58 and 268 ms, with numerical and phantom analyses, for Lac T2 measurement. In-vivo spectra were acquired from 24 subjects with gliomas (13 low grade and 11 high grade) and analyzed with LCModel using numerically-calculated basis spectra. The metabolite T2 relaxation time was obtained from monoexponential fitting of the multi-TE signal estimates versus TE. The metabolite concentration was estimated from the zero-TE extrapolation of the T2 fits.
Results
The Lac T2 was estimated to be approximately 240 ms, without a significant difference between low and high grade tumors. The Lac concentration was estimated to be 4.1±3.4 and 7.0±4.7 mM for low and high grades respectively, but the difference was not significant.
Conclusion
The Lac T2 was similar among gliomas regardless of their tumor grades. This suggests that the T2 value from this study may be applicable to obtain the T2 relaxation-free estimates of Lac in a subset of brain tumors.
Keywords: Lactate, T2, Brain tumors, Glioma, 1H MRS, 3T, PRESS (point-resolved spectroscopy)
INTRODUCTION
Proton MR spectroscopy of lactate (Lac) may be characterized by the 3CH3 proton resonance at 1.31 ppm and the 2CH proton resonance at 4.1 ppm (1). The 1.31 ppm resonance, which is J coupled to the 4.1 ppm resonance with J = 6.93 Hz, gives rise to a relatively large signal and thus is commonly focused in 1H MRS measurement of Lac. In many types of brain tumors, lipids are substantially increased and consequently in vivo detection of the Lac 1.31 ppm resonance is hampered by the CH2-chain proton signals of lipids in short-TE MRS. This spectral overlap can be overcome by means of spectral editing (2) or post-acquisition data processing (3). The Lac 1.31 ppm resonance can also be effectively separated from lipids using long TEs of point-resolved spectroscopy (PRESS), at which the Lac resonance becomes an inverted or positive doublet while the broad lipid signal is extensively attenuated due to the effects of its relatively short T2 (4,5). Given the use of long TE for Lac detection in many prior studies, the information on Lac T2 relaxation is valuable for quantification of metabolite concentration. The Lac T2 relaxation time in brain tumors has not been extensively studied to date.
In the current study, we report measurements of proton T2 relaxation times of Lac as well as choline, creatine and N-acetylaspartate in brain tumors at 3T, achieved using a single-voxel PRESS sequence. Eight TEs in the range of 58 – 268 ms were selected from computer simulations that included the effects of the PRESS slice-selective radio-frequency (RF) and gradient pulses. The selected TEs were validated in a phantom solution. In vivo T2 relaxation times were evaluated from mono-exponential fitting of the LCModel estimates of the metabolite signals versus TE. The metabolite concentrations were estimated from the zero-TE extrapolation of the T2 fits. Preliminary data from subjects with brain tumors were analyzed for testing the correlation of metabolite T2s and concentrations with tumor grade.
METHODS
Twenty-four patients with gliomas (16 males and 8 females; age range 25 – 70, median age of 39 years old) were recruited for measurement of brain metabolite T2. The tumor grades and types were determined by the histopathological analysis on surgical specimen according to the World Health Organization (WHO) criteria. The set of gliomas comprised 13 low grade gliomas that were WHO grade II (9 oligodendrogliomas, 3 astrocytomas and 1 oligoastrocytoma) and 11 high grade gliomas that were WHO grade III (4 oligodendrogliomas, 3 astrocytomas and 3 oligoastrocytomas) or grade IV (1 glioblastoma). The protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. Written informed consent was obtained prior to the scans.
MR experiments were carried out on a whole-body 3T scanner (Philips Medical Systems) using a body coil for RF transmission and an 8-channel phased-array head coil for reception. The in-vitro test was conducted on a spherical phantom (6 cm diameter; pH = 7.0) which included Lac (20.0 mM), N-acetylaspartate (NAA) (10.0 mM), creatine (Cr) (9.0 mM), and phophorylcholine (PCh) (3.3 mM). Data were acquired from a 2×2×2 cm3 voxel, using a PRESS sequence, at TE = 58, 88, 118, 148, 178, 208, 238 and 268 ms (TR = 9 s). The second subecho time TE2 was varied while TE1 was kept constant at 32 ms. Volume localization was obtained using a 9.8 ms 90° RF pulse (bandwidth = 4.3 kHz) and two 13.2 ms 180° RF pulses (BW = 1.3 kHz) at an RF field intensity (B1) = 13.5 µT. For in vivo experiments in glioma patients, the tumor was identified with T2-weighted fluid attenuated inversion recovery (T2w-FLAIR) imaging. Water-suppressed PRESS data were obtained from a voxel (size 8 – 12 mL) within the tumor mass, with 16 signal averages at each of the eight TEs. Acquisition parameters included; TR = 2 s, number of sampling points = 2048, and spectral width = 2500 Hz. In 11 patients, spectra were acquired additionally from the normal-appearing contralateral brain. A vendor-supplied four pulse variable flip angle scheme was used for water suppression. First and second order shimming for the selected volume was carried out using FASTMAP (6). The carrier frequency of the PRESS RF pulses was set at 2.5 ppm. In addition, unsuppressed PRESS water signals were acquired with 2 signal averages at each TE.
In data processing, the residual eddy current artifacts in metabolite data were minimized using the PRESS water signals, followed by correction for field drift effects using choline (3.21 ppm) or NAA (2.01 ppm) as reference. Data were apodized using a 1-Hz exponential and 1-Hz Gaussian function to remove potential artifacts in the later part of the free-induction decay. Spectral fitting was performed with LCModel (7), using a basis function that contained in-house calculated spectra of 18 brain metabolites. These included Lac, tNAA (NAA + N-acetyl-aspartyl-glutamate), tCr (creatine + phosphocreatine), tCho (glycerophosphorylcholine + phosphorylcholine), glutamate, glutamine, 2-hydroxyglutarate, γ-aminobutyric acid, myo-inositol, glycine, taurine, scyllo-inositol, aspartate, ethanolamine, phosphorylethanolamine, alanine, glutathione, and citrate. The basis spectra were numerically calculated including the effects of the PRESS volume-localizing RF and gradient pulses, according to a product-operator based transformation-matrix method described in a prior paper (8). In brief, for each metabolite spin system, a product-operator transformation matrix, which represents the density operator evolution during an RF pulse, was created for each of the PRESS 90° and 180° RF pulses and used for calculating the density operator at the end of the PRESS multiple TE sequences. The spatial resolution of the simulation was set at 1% (i.e. 0.01 = sample length / number of pixels / slice thickness), with 200 pixels within a sample length 2-fold longer than the slice thickness. In addition, dual-echo Lac spectra were calculated, using very short (1 ns) RF pulses (without volume localization), for comparison with the PRESS volume-localized simulations. Published chemical shift and J coupling constants were used for the simulations (1). The LCModel built-in functions were used for fitting of macromolecule and lipid signals. The spectral fitting was conducted between 0.5 and 4.0 ppm. The T2 relaxation times of Lac, tCho, tNAA, tCr and water were evaluated by fitting the LCModel estimates of the signals to a monoexponential function of TE, exp(−TE/T2). The zero-TE magnetization was obtained by extrapolating the exponential fit to TE = 0. Assuming identical T1 between metabolites and water in tumors and healthy brain, the metabolite concentration was estimated with reference to water at 42 M, similarly as in a prior study (8). Unpaired two-tailed t-test was used to evaluate the statistical significance of the T2 and concentration differences among normal-appearing brain, low grade, and high grade tumor groups. Statistical significance was declared for p-value ≤ 0.05.
RESULTS
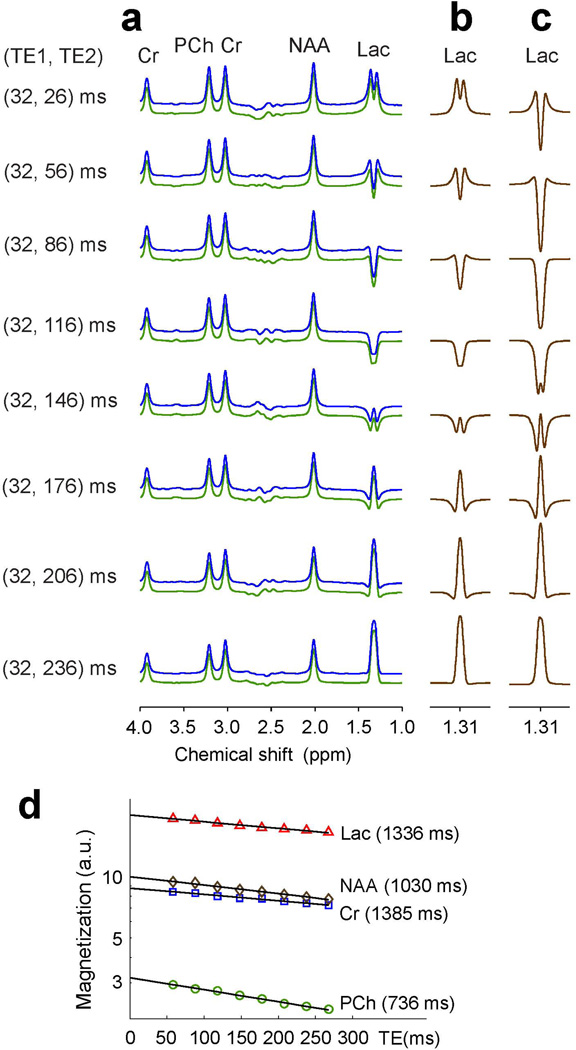
In the phantom solution, due to the high mobility of molecules and consequently long T2 relaxation times, the singlets of PCh, Cr and NAA were slightly decreased as TE increased from 58 ms to 268 ms (Fig. 1a). For Lac, the signal intensity and pattern were both varied with increasing TE largely due to the J coupling effects. For a singlet linewidth of 6 Hz, the Lac CH3 resonance at 1.31 ppm was a positive doublet at TE = 58 ms, with minimal J evolution. However, the resonance exhibited a negative single-peak pattern at TE = 118 and 148 ms, and with further J evolution the Lac signal became a positive peak at TE = 238 and 268 ms, whose amplitudes were larger than those at shorter TEs in the phantom solution. When the Lac 1.3 ppm signal from the phantom was compared with PRESS volume-localized simulation, the multiplet pattern showed good agreement (Fig. 1b). The phantom spectra were well reproduced by LCModel fits. The LCModel estimates of Lac, PCh, Cr and NAA were all decreased monoexponentially with increasing TE from 58 to 268 ms (Fig. 1d), with the phantom T2 estimation of 1336, 736, 1385 and 1030 ms, respectively. The zero-TE signal estimates of the solutes, obtained from the extrapolation of the T2 fits, reproduced closely the prepared phantom concentration ratio (20.1:3.2: 8.8: 10.1 vs. 20: 3.3: 9: 10). For comparison, the Lac signals which were calculated for a dual-echo sequence with instantaneous RF pulses (without volume localization) were substantially different than experiment in terms of signal intensity and pattern (Fig. 1c).
Figure 1.
(a) In vitro spectra at eight pairs of the first and second subecho times TE1 and TE2 of PRESS are presented together with LCModel fits and residuals. The phantom contained Lac (20 mM), PCh (3.3 mM), Cr (9 mM), and NAA (10 mM). Spectra were broadened to singlet linewidth (FWHM) of 6 Hz. (b, c) Numerically-calculated Lac 1.31 ppm signals at the 8 TEs. Spectra were calculated incorporating the PRESS slice-selective RF and gradient pulses in (b), and calculated for a hard RF pulse dual-echo sequence in (c). The Lac signals were scaled with respect to calculated Cr signal for the same Lac-to-Cr concentration ratio as in the phantom solution, neglecting T2 effects. (d) Phantom T2 evaluation of Lac, PCh, Cr, and NAA by monoexpoential fitting of LCModel estimates versus TE. The estimated T2 values are shown in brackets.
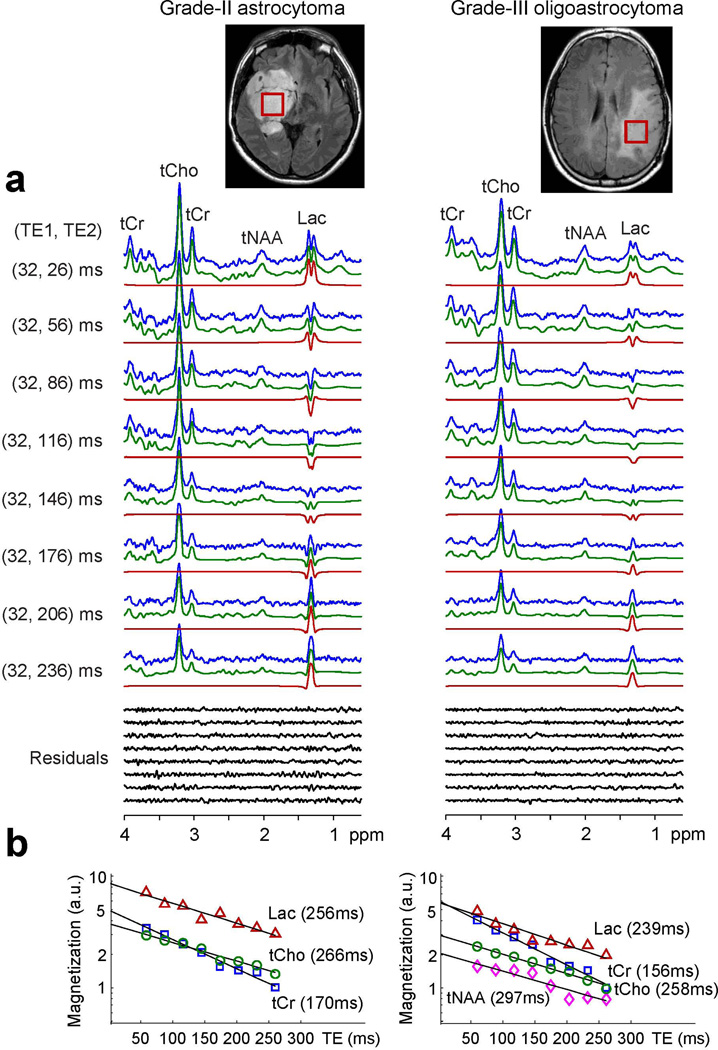
Figure 2 presents in vivo spectra at the eight TEs, obtained from astrocytoma (grade II) and oligoastrocytoma (grade III), together with voxel positioning and monoexponential T2 fitting. The tumors showed a classic pattern of metabolic abnormality; namely, increased tCho and decreased tCr and tNAA. Additionally, both tumors showed elevated Lac. The Lac 1.31 ppm signal was large and clearly discernible in all eight spectra from the tumors (Fig. 2a). For both tumors, the spectra were well reproduced by LCModel fits, without substantial chemical-shift dependence of residuals. From monoexponential fitting of the LCModel signal estimates vs. TE (Fig. 2b), T2 was estimated to be 256, 266 and 170 for Lac, tCho and tCr in the astrocytoma, and 239, 258, 156 and 297 ms for Lac, tCho, tCr and tNAA in the oligoastrocytoma, respectively. In the case of the astrocytoma, the tNAA signal to noise ratio was low and thus T2 fitting was not conducted. From the zero-TE estimates of the metabolite and water signals, the concentration was estimated to be 8.8, 3.8 and 5.0 mM for Lac, tCho and tCr in the astrocytoma and 5.8, 3.0, 5.9 and 2.1 mM for Lac, tCho, tCr and tNAA in the oligoastrocytoma, respectively.
Figure 2.
(a) In vivo spectra, at the eight (TE1, TE2) pairs of PRESS, from subjects with astrocytoma (grade II) and oligoastrocytoma (grade III) are shown together with voxel positioning (size 23×23×23 mm3) and LCModel fitting results. Spectra are normalized with respect to water signals. (b) Monoexponential T2 fitting of LCModel estimates versus TE.
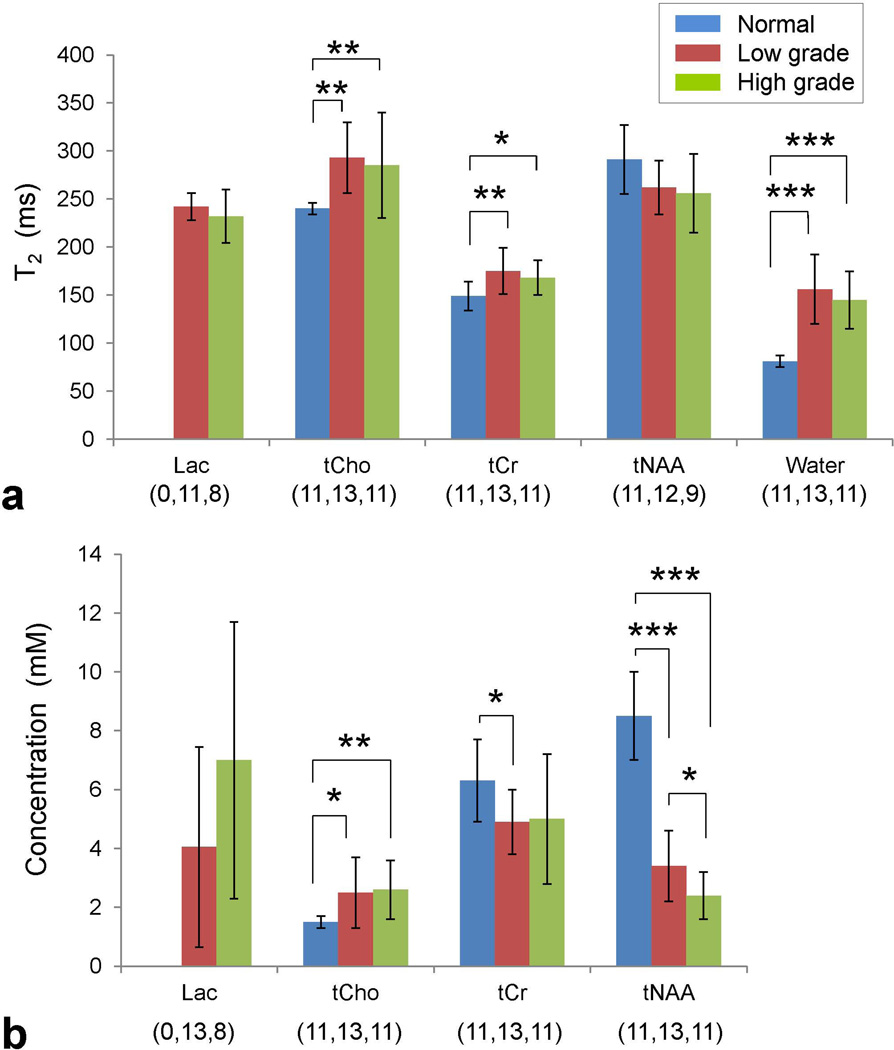
Of the 24 data sets from tumors, 19 showed well-defined Lac 1.31 ppm signal, with minimal (or negligible) lipid signals. For tumors that showed small Lac signals, T2 fitting was performed only on Lac estimates in which the ratio of the LCModel-returned Lac signal to the standard deviation of the residuals between 1.0 and 1.6 ppm was greater than 3. With this criterion, Lac T2 was evaluated with signal estimates at 6 – 8 TEs in 17 tumors, and with data at 4 and 5 TEs in 2 tumors. For the 19 tumors, the estimated Lac T2 ranged from 192 to 270 ms, with the mean of 238±21 (mean±SD). For the 11 low grade and 8 high grade tumors, the Lac T2 means were 242±14 and 232±28 ms respectively, without showing a significant difference (p = 0.3) (Fig. 3). Similarly, the water T2 was not significantly different between the low and high grade tumors (156±36 and 145±30 ms respectively; p = 0.4). The normal brain water T2 was estimated as 81±6 ms, significantly shorter than the water T2s in tumors (p = 10−6 for both low and high grades). The Lac T2 of the GBM was 229 ms. For estimation of the Lac concentration, the zero-TE magnetization was obtained from the T2 fit in 19 tumors. In 2 additional grade II tumors, in which Lac T2 was not evaluated, the Lac level was estimated using the zero-TE magnetization extrapolated, using the mean Lac T2 value (238 ms), from the Lac signal estimate at TE = 268 ms, at which the Lac signal was best defined among the TEs used. The mean concentration of Lac was estimated as 4.1±3.4 and 7.0±4.7 mM for 13 low grade and 8 high grade tumors, respectively. The Lac level in the GBM was 5.1 mM. There was a trend of higher Lac concentration in high grade than in low grade, but the difference was not significant (p = 0.1). While Lac T2 was consistently around 240 ms with a relatively small coefficient of variation (SD/mean = CV = 21/238 = 9%) in the 19 tumors, the Lac concentrations showed large variation (CV = 4.1/5.2 = 79%), indicating the presence of high heterogeneity in Lac levels across the tumors.
Figure 3.
(a) Group comparison for the mean T2s of Lac, tCho, tCr, tNAA, and water for normal appearing brain, low grade and high grade gliomas. Error bars indicate standard deviation. Statistical significance is indicated with asterisks (* for 0.01 < p ≤ 0.05; ** for 0.001 < p ≤ 0.01; *** for p ≤ 0.001). The numbers in brackets denote the sample size for the normal brain, low grade and high grade tumor groups. (b) The mean concentrations of the metabolites, estimated from zero-TE magnetization, are shown with standard deviation.
The T2s of tCho, tCr, and tNAA were evaluated for all 24 patients. The data included 11 data sets from normal-appearing brains, 13 from low grade gliomas, and 11 from high grade gliomas. Excluding three tumors (1 grade II and 2 grade III) that showed low tNAA (as in the astrocytoma of Fig. 2a), the T2 of tNAA was evaluated in 12 grade II and 9 grade III tumors. For the normal brain, low grade, and high grade tumor groups, the tCho T2s were estimated to be 240±6, 293±37 and 285±55 ms, the tCr T2s were 149±15, 175±24 and 168±18 ms, and the tNAA T2s were 291±36, 262±28 and 256±41 ms, respectively (Fig. 3). The T2s of tCho and tCr were significantly different between normal brain and low grade tumors (p = 6×10−4 and 5×10−3, respectively) and between normal brain and high grade tumors (p = 0.005, and 0.01, respectively). The tNAA T2 in tumors was not significantly different than in normal brain. There was no significant T2 difference between the tumor grades. For the 3 tumors in which tNAA T2 was not obtained, the zero-TE magnetization of tNAA was obtained from extrapolation of the signal estimate at TE = 58 ms using the mean T2 value of low grade or high grade. For the normal brain, low grade, and high grade tumors, the tCho concentrations were estimated to be 1.5±0.2, 2.5±1.2 and 2.6±1.0 mM, the tCr levels 6.3±1.4, 4.9±1.1 and 5.0±2.2 mM, and the tNAA levels 8.4±1.5, 3.4±1.2 and 2.4±0.8 mM, respectively. The tCho and tNAA levels were both significantly different between normal brain and low grade (p = 0.02 and 10−8 respectively) and between normal brain and high grade (p < 0.005 and 10−9 respectively), but tCr was significantly different only between normal brain and low grade tumors (p = 0.01). The tNAA level was significantly lower in high grade than in low grade tumors. The mean CVs of tCho, tCr and tNAA T2s were similar between the groups (4 – 16%). In contrast, the CVs of the metabolite concentrations were large in tumors compared to those from normal brain (20 – 50% versus 10 – 20%).
DISCUSSION
The current paper reports the first T2 measurement and T2 relaxation-free estimation of Lac in subjects with brain tumors in vivo, achieved at 3T. The estimated Lac T2 was approximately 240 ms, without a significant difference between low and high grade gliomas. The mean Lac level in the tumors was about 5.2 mM, much higher than the concentration in healthy brain (< 1 mM) (1,9). The Lac concentration, estimated from zero-TE magnetization, was higher in high grade than in low grade, but the difference was not significant, most likely due to large variations in Lac levels across tumors (CV ≈ 80%). Similar variations of Lac levels were also reported in two prior 1.5T studies (5,10), in both of which the data showed a trend of increasing Lac with malignancy but no significant difference was observed between tumor grades. Our Lac estimates, which were 4.1 and 7.0 mM for low grade and high grade gliomas respectively, are in good agreement with the values reported by Howe et al. (1.5, 5.8, and 11.7 mM for grade-II astrocytomas, anaplastic astrocytomas, and glioblastomas, respectively) (5).
Three prior studies have reported the T2 relaxation times of tCho, tCr and tNAA in brain tumors; one at 3T (11) and two studies at 1.5T (4,12). A common observation among the present and two prior studies (11,12) is significantly longer T2 of tCho in tumors than in normal-appearing brain. The three studies showed a trend of longer tCho T2 in high grade tumors, without a significant difference. In contrast, in the Sijens et al. study, tCho T2 was measured to be shorter in tumors than in normal brain (4). For tNAA T2, the present study did not show significant difference between tumors and normal brain, in agreement with the prior 3T study (11), but in the prior 1.5T studies (4,12) the tNAA T2 in tumors was significantly shorter compared to normal brain. For tCr, our observation of significantly longer T2 in tumors agrees with the two prior studies (4,11).
There is a paucity in literature for Lac T2 evaluation in brain tumors. One study reported Lac T2 as 83 ms in brain tumors at 1.5T, which was obtained from two patients using two TEs (135 and 270 ms) (4). Another study reported brain-tumor Lac T2 as 330 ms at 1.5T, obtained using a wavelet transformation method on single short TE data in two patients (3). In contrast, several prior studies reported Lac T2 in non-tumorous brain diseases, in which Lac was elevated. There is a large variation among the reported T2 values. Blamire et al. measured Lac T2 in stoke at 2.1T (13). From the spectra at TE = 30.4 and 270 ms, Lac T2 was estimated to be ~780 ms. In contrast, Sappey-Marinier et al. measured Lac T2 as 225 ms in a subject with chronic infarction, using data at four TEs at 2T (40, 100, 200 and 270 ms) (14). Cady et al. reported Lac T2 in developing human brain as 240 – 245 ms, obtained with a two-TE method (PRESS TE = 270 and 540 ms at 2.4T) (15). A prior study reported the Lac T2 in healthy brain as 1200 ms, which could be due to Lac predominantly originating from cerebrospinal fluids (16). Measurement of the Lac T2 in healthy brain was beyond the scope of the present study. Although brain Lac T2 may depend on field (B0) strength and the potential difference in molecular environments in the disease and during development, since the T2s of large singlet signals (i.e., tCho, tCr and tNAA) are similar between these prior studies, the large variation in the reported Lac T2 values could be due to the differences in measurement methods and possibly due to experimental errors in evaluating the J-coupled Lac resonance using a limited number of TEs.
The present study has measured Lac T2 using data at relatively large number of TEs (8 TEs), which were selected for generating large Lac peak amplitude at 1.31 ppm given the RF pulse envelopes and bandwidth. For a finite bandwidth of the PRESS 180° RF pulse, the Lac 1.31 ppm resonance gave larger signal return at asymmetric subecho times (i.e., TE1 ≠ TE2) than at symmetric subecho times for an identical total TE. Moreover, we used the TE1 < TE2 timings since these produced substantially larger peak amplitude than the TE1 > TE2 timings, especially at relatively long TEs. For instance, for the last three TEs used in this study (TE = 208, 238 and 268 ms), the Lac 1.31 ppm peak amplitudes were larger by 3.7, 2.4 and 1.5 fold at (TE1, TE2) pairs with TE1 = 32 ms than at those with TE2 = 32 ms for singlet linewidth of 6 Hz, as indicated in simulations and phantom experiments (data not shown). Since the PRESS subecho time dependence of Lac signal is due to the chemical shift displacement effects, the Lac signal variation with TE1 and TE2 is negligible when the RF bandwidth is sufficiently large.
The spectral fitting was performed between 0.5 and 4.0 ppm in the present study, excluding the Lac CH proton resonance at 4.1 ppm. Due to the large spectral distance between the Lac 1.31 and 4.1 ppm resonances, the discrepancy between the slices that were localized by the PRESS 180° RF pulses (BW = 1.3 kHz) is calculated to be 27% of the slice thickness (e.g., ~5.5 mm for 20 mm slice thickness). When the Lac concentration is not homogeneous, the relative signal strength at 1.31 and 4.1 ppm may be different than in the uniform-concentration situation. The relative peak strengths of the resonances could be affected by inhomogeneous B1. Potential errors due to these factors may be minimized by including only the 1.31 ppm resonance for Lac estimation. In addition, unsuppressed water signal from a single voxel was used for normalizing the metabolite signals that were obtained from the chemical-shift displaced voxels. With the use of RF carrier at 2.5 ppm, the voxels of Lac (1.31 ppm), tNAA (2.01 ppm), tCr (3.03 ppm), and tCho (3.21 ppm) may be displaced respectively by 2.3, 1.0, −1.0, and −1.4 mm along the directions localized by 180° RF pulses (anterior-posterior and head-foot directions). The water concentration difference between the displaced voxels was ignored in the present study. Lastly, metabolite quantification was undertaken assuming identical T1 between metabolites and water in tumors and healthy brain in the present study (TR = 2 s). The potential errors due to the differences in T1 saturation effects can be minimized using a prolonged TR in data acquisitions or correcting data for the T1 saturation effects when the T1 values are known.
In conclusion, we have measured the T2s of Lac, tCho, tCr and tNAA in glioma patients at 3T, using eight PRESS TEs between 58 and 268 ms. The Lac T2 was estimated to be about 240 ms, without showing a significant difference between low grade and high grade tumors. Also, the Lac concentration was not significantly different between the tumor grades. The T2s of tCho and tCr were significantly longer in tumors than in normal-appearing brain. The similarity of Lac T2 regardless of tumors grades suggests that the Lac T2 of this study may be applicable to obtain the T2 relaxation-free estimates of in a subset of brain tumors.
ACKNOWLEDGMENTS
This work was supported by a US National Institutes of Health grant CA159128, and by Cancer Prevention Research Institute of Texas grants RP140021-P04 and RP130427. We thank Dr. Ivan Dimitrov for technical assistance.
REFERENCES
- 1.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Park I, Chen AP, Zierhut ML, Ozturk-Isik E, Vigneron DB, Nelson SJ. Implementation of 3 T lactate-edited 3D 1H MR spectroscopic imaging with flyback echo-planar readout for gliomas patients. Ann Biomed Eng. 2011;39:193–204. doi: 10.1007/s10439-010-0128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serrai H, Nadal-Desbarats L, Poptani H, Glickson JD, Senhadji L. Lactate editing and lipid suppression by continuous wavelet transform analysis: application to simulated and 1H MRS brain tumor time-domain data. Magn Reson Med. 2000;43:649–656. doi: 10.1002/(sici)1522-2594(200005)43:5<649::aid-mrm6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Sijens PE, Oudkerk M. 1H chemical shift imaging characterization of human brain tumor and edema. Eur Radiol. 2002;12:2056–2061. doi: 10.1007/s00330-001-1300-3. [DOI] [PubMed] [Google Scholar]
- 5.Howe FA, Barton SJ, Cudlip SA, Stubbs M, Saunders DE, Murphy M, Wilkins P, Opstad KS, Doyle VL, McLean MA, Bell BA, Griffiths JR. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2003;49:223–232. doi: 10.1002/mrm.10367. [DOI] [PubMed] [Google Scholar]
- 6.Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 7.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 8.Choi C, Ganji SK, Deberardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, Yang XL, Mashimo T, Raisanen JM, Marin-Valencia I, Pascual JM, Madden CJ, Mickey BE, Malloy CR, Bachoo RM, Maher EA. 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18:624–629. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi C, Coupland NJ, Kalra S, Bhardwaj PP, Malykhin N, Allen PS. Proton spectral editing for discrimination of lactate and threonine 1.31 ppm resonances in human brain in vivo. Magn Reson Med. 2006;56:660–665. doi: 10.1002/mrm.20988. [DOI] [PubMed] [Google Scholar]
- 10.Auer DP, Gossl C, Schirmer T, Czisch M. Improved analysis of 1H-MR spectra in the presence of mobile lipids. Magn Reson Med. 2001;46:615–618. doi: 10.1002/mrm.1235. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Srinivasan R, Ratiney H, Lu Y, Chang SM, Nelson SJ. Comparison of T1 and T2 metabolite relaxation times in glioma and normal brain at 3T. J Magn Reson Imaging. 2008;28:342–350. doi: 10.1002/jmri.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isobe T, Matsumura A, Anno I, Yoshizawa T, Nagatomo Y, Itai Y, Nose T. Quantification of cerebral metabolites in glioma patients with proton MR spectroscopy using T2 relaxation time correction. Magn Reson Imaging. 2002;20:343–349. doi: 10.1016/s0730-725x(02)00500-3. [DOI] [PubMed] [Google Scholar]
- 13.Blamire AM, Graham GD, Rothman DL, Prichard JW. Proton spectroscopy of human stroke: assessment of transverse relaxation times and partial volume effects in single volume steam MRS. Magn Reson Imaging. 1994;12:1227–1235. doi: 10.1016/0730-725x(94)90087-8. [DOI] [PubMed] [Google Scholar]
- 14.Sappey-Marinier D, Calabrese G, Hetherington HP, Fisher SN, Deicken R, Van Dyke C, Fein G, Weiner MW. Proton magnetic resonance spectroscopy of human brain: applications to normal white matter, chronic infarction, and MRI white matter signal hyperintensities. Magn Reson Med. 1992;26:313–327. doi: 10.1002/mrm.1910260211. [DOI] [PubMed] [Google Scholar]
- 15.Cady EB, Penrice J, Amess PN, Lorek A, Wylezinska M, Aldridge RF, Franconi F, Wyatt JS, Reynolds EO. Lactate, N-acetylaspartate, choline and creatine concentrations, and spin-spin relaxation in thalamic and occipito-parietal regions of developing human brain. Magn Reson Med. 1996;36:878–886. doi: 10.1002/mrm.1910360610. [DOI] [PubMed] [Google Scholar]
- 16.Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Localized proton NMR spectroscopy in different regions of the human brain in vivo. Relaxation times and concentrations of cerebral metabolites. Magn Reson Med. 1989;11:47–63. doi: 10.1002/mrm.1910110105. [DOI] [PubMed] [Google Scholar]