Abstract
A functional epidermal skin barrier requires the formation of a cornified envelope from terminally differentiating keratinocytes. During this process, multiple genetic and environmental signals coordinately regulate protein expression and tissue differentiation. Here we describe a critical role for hypoxia-inducible factors (HIFs) in the regulation of filaggrin expression and skin barrier formation. Similar to other mammalian tissues, fetal epidermis in mice is normally O2-deprived. Simultaneous deletion of Hif1a and Hif2a in murine epidermis revealed defects in keratinocyte terminal differentiation and epidermal barrier formation. Mice lacking Hif1a and Hif2a in the epidermis exhibited dry flaky skin, impaired permeability barrier, and enhanced sensitivity to cutaneous allergens. These defects were correlated with stratum granulosum attenuation and reduced filaggrin expression. Hypoxic treatment of primary keratinocytes induced filaggrin (Flg) gene expression in a HIF1α- and HIF2α-dependent manner, suggesting that one mechanism by which Hif1a and Hif2a loss causes epidermal barrier defects in mice lies in Flg dysregulation. Therefore, low O2 tension is an essential component of the epidermal environment that contributes to skin development and function.
Introduction
The epidermis, together with hair follicles, sebaceous glands, and dermal connective tissue, forms the largest organ in the body. Skin performs many important functions, including thermoregulation, sensory perception, immunity, and protection from physical trauma. The protective function of the epidermis derives mainly from its most superficial epithelial layer, the cornified envelope. This barrier is constantly regenerated from differentiating keratinocytes, and abnormalities in this process have been associated with a variety of skin diseases such as ichthyosis, psoriasis, and atopic dermatitis (Irvine et al. 2011).
Skin homeostasis consists of a coordinated process whereby dividing basal keratinocytes detach from the basement membrane, commit to terminal differentiation, and eventually slough off the body surface (Simpson et al. 2011). The course of epidermal development can be delineated spatially and morphologically, as well as by the expression of specific keratin intermediate filaments at distinct differentiation stages. For example, basal keratinocytes express keratin 5 (KRT5) and keratin 14 (KRT14), whereas keratin 1 (KRT1) and keratin 10 (KRT10) are expressed in the spinous and lower granular layers in newly differentiating keratinocytes (Blanpain and Fuchs 2009). Terminally differentiated keratinocytes in the upper granular layer and cornified envelope express cornification proteins such as involucrin (IVL), loricrin (LOR), and filaggrin (FLG). Notably, filaggrin binds intermediate filaments in the upper granular layer, thereby condensing the keratinocyte cytoskeleton into a strong, flattened matrix (Irvine et al. 2011). Other cornified envelope proteins bind this matrix and become crosslinked to epidermal sphingolipids. These changes confer structural integrity and barrier properties on the epidermis.
Numerous regulatory and signaling pathways govern epidermal specification, differentiation, and cornification. For example, Wnt and BMP signaling maintain epidermal stem cell self-renewal (Chen et al. 2012; Lim et al. 2013). Notch and p63 mediated transcription programs control the transition from basal to suprabasal keratinocyte cell fate (Nguyen et al. 2006; Williams et al. 2011), while formation of the cornified envelope is regulated by transcription factor pathways involving KLF4 and IKKα (Gareus et al. 2007; Sen et al. 2012). The epidermal microenvironment is also an important determinant of keratinocyte differentiation: cornified envelope formation is regulated by extracellular calcium gradients, as well as steroid hormone levels (Kömüves et al. 2000; Tu et al. 2012).
The epidermal microenvironment is further characterized by low oxygen (O2) availability. Studies in humans and rodents have demonstrated that O2 saturation in adult epidermis ranges from 0.5% to 5% (Evans et al. 2006). The transcriptional response to low O2 is mediated primarily by hypoxia inducible factors (HIFs) (Keith et al. 2012). HIFs are heterodimeric proteins comprised of an O2-labile subunit (HIF1α or HIF2α) and constitutively-expressed HIF-β subunit, also known as aryl hydrocarbon receptor nuclear translocator (ARNT). HIF1α activity in the epidermis is important in cutaneous O2 sensing, skin innate immunity, wound healing, and melanoma transformation (Elson et al. 2000; Bedogni et al. 2005; Boutin et al. 2008; Peyssonnaux et al. 2008). In comparison, little is known about the function of HIF2α in the skin. However, both HIF1α and HIF2α have well-characterized roles in the determination and differentiation of other O2-deprived tissues such as the placenta, hippocampal neurons, skeletal muscle, and bone (Dahl et al. 2005; Amarilio et al. 2007; Mazumdar et al. 2010; Majmundar et al. 2012; Rankin et al. 2012).
In this study, we investigated the effect of epidermal O2 levels on keratinocyte differentiation. O2-deprived keratinocytes specifically stimulated Flg expression in a HIF1α- and HIF2α-dependent manner. Consistent with other mammalian tissues, low-O2 conditions occurred naturally in the skin during murine development. This ‘hypoxic’ state correlated with epidermal expression of HIF1α and HIF2α. Epidermal deletion of Hif1a and Hif2a led to reduced Flg expression, decreased corneocyte integrity, and impaired epidermal barrier function. Similar to Flg mutant animals, loss of Hif1a and Hif2a in the epidermis resulted in ichthyosiform skin and percutaneous sensitization to a common skin allergen. Consequently, HIF is an essential regulator of keratinocyte differentiation and epidermal barrier function.
Results and Discussion
Developing murine epidermis is naturally low in O2
Murine epidermis begins to differentiate by day 14 of gestation (E14) from a single layer of epidermal keratinocytes, such that maturing epidermal layers expressing late differentiation-specific keratins are present by E16 (Byrne et al. 1994). The cornified envelope, which is the most superficial layer, undergoes further maturation between E18 and birth (P0) (Candi et al. 2005). To characterize the epidermal O2 environment during this developmental process, we injected animals with the O2-sensitive dye pimonidazole. Positive pimonidazole staining indicated that the epidermis encounters low O2 levels (<2%) between E16 and P0 (Figure 1a). This finding is consistent with O2 electrode measurements in sheep embryos, which showed that ovine epidermis is hypoxic between days 60 and 120 of gestation (Scheid et al. 2002). Low O2 levels in the newborn epidermis correlated with HIF1α and HIF2α protein expression (Figure 1b). HIF1α and HIF2α were also stabilized in primary keratinocytes when cultured at 0.5% O2 (Figure 1c). These results suggested that murine epidermal development occurs in an O2-deprived setting.
Figure 1. Murine epidermis develops under naturally low O2 tension.
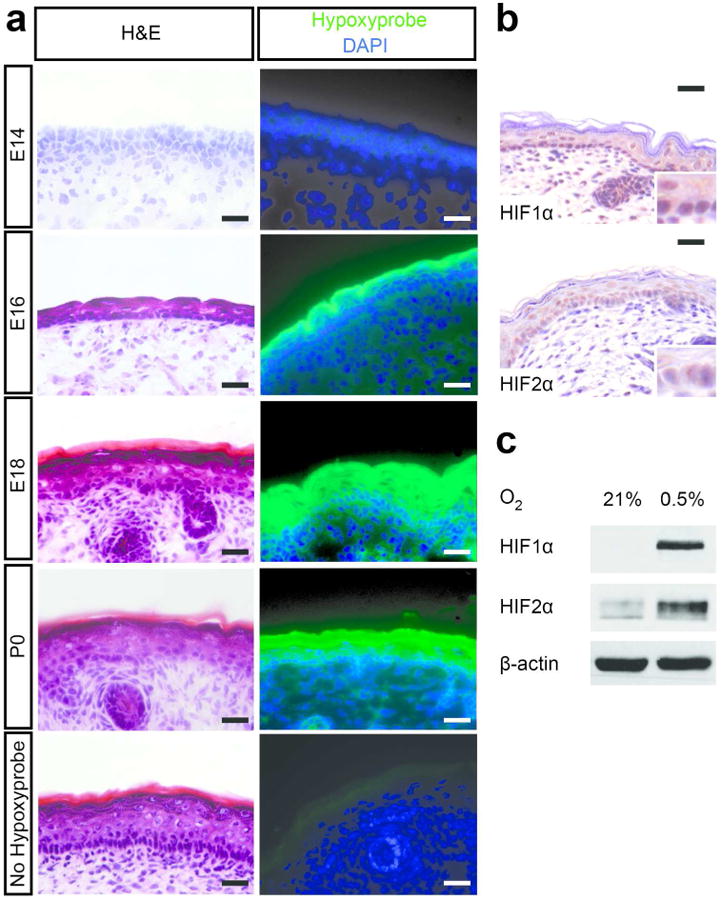
(a) Hypoxyprobe was injected into pregnant mice at E14, E16, E18, and P0 pups. Frozen skin sections were stained using hematoxylin and eosin, anti-hypoxyprobe-FITC (green), and DAPI (blue). Uninjected mice of the same age were used as negative controls. (b) Skin from wild type P2 pups were immunostained for HIF1α and HIF2α. Inserts (400×) highlight nuclear immunostaining. (c) Primary murine keratinocytes were grown at 21% or 0.5% O2 for 48 hours and cell lysates immunoblotted for HIF1α and HIF2α. Scale bar, 50 μm.
Interestingly, pimonidazole staining appears stronger in more superficial epidermal layers (Fig. 1a). This disparity is most apparent at E16, but diminishes by the time of birth. In contrast, HIF immunostaining is comparable between basal and suprabasal nuclei (Fig. 1b). This finding indicates that HIF1α and HIF2α are stabilized in the epidermis below a certain threshold of hypoxia. However, the mechanisms by which low O2 tension is maintained in the epidermis are poorly understood. Possible causes of O2 deprivation in the superficial epidermis include the absence of vascularization as well as low O2 saturation in amniotic fluid (Bejar et al. 1971). Additionally, non-O2-dependent processes such as PI3K/AKT signaling or the generation of reactive oxygen species in proliferating keratinocytes may contribute to HIF stabilization in the basal epidermis.
Hypoxic keratinocytes stimulate filaggrin expression
To determine the effect of low O2 on epidermal development, we examined the expression of early and late differentiation genes in keratinocytes cultured under hypoxia. Keratin 1 (Krt1) and keratin 10 (Krt10) are expressed in early differentiating cells, whereas loricrin (Lor), involucrin (Ivl), and filaggrin (Flg) expression are induced at the terminal stage of epidermal differentiation. Transcriptional upregulation of Flg and the related filaggrin-2 (Flg2) gene was observed in hypoxic primary keratinocytes at the mRNA and protein level (Figure 2a-b). Although mRNA levels of early epidermal markers Krt1 and Krt10 were somewhat reduced after hypoxic culture, KRT10 protein levels and that of another early epidermal marker, KRT5, were unchanged (Figure 2a-b). Treatment with 2-oxoglutarate-dependent dioxygenase inhibitors, including dimethyloxalylglycine (DMOG) and deferoxamine (DFO), which inhibit HIFα degradation post-translationally, also induced filaggrin expression (Figure 2c). Similar upregulation of filaggrin mRNA and protein was observed in hypoxic primary human keratinocytes (Figure 2d), indicating that this response to low O2 tension is conserved.
Figure 2. Hypoxia induces filaggrin expression.
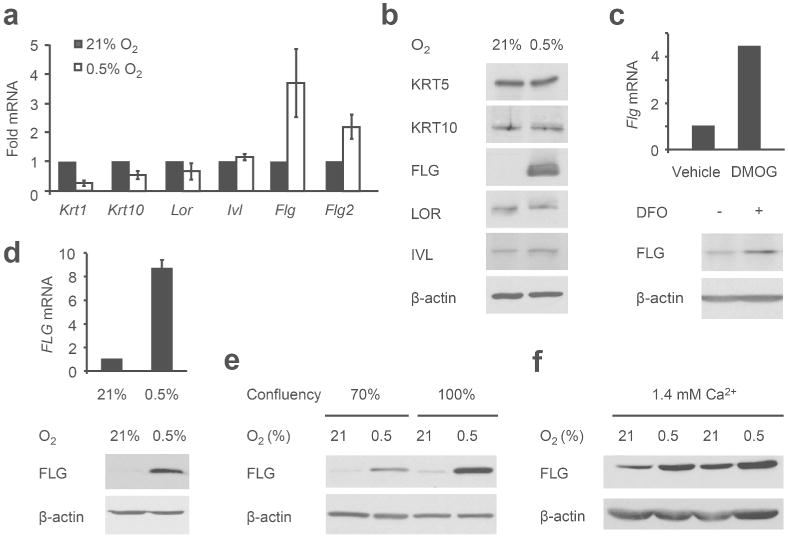
(a, b) Primary murine keratinocytes were grown at 21% or 0.5% O2 for 48 hours. (a) Epidermal gene expression was assessed by qRT-PCR and (b) cell lysates immunoblotted for epidermal proteins as indicated. (c) Primary murine keratinocytes were grown in 1 mM DMOG or 100 μM DFO for 48 hours and analyzed for filaggrin transcripts (upper panel) and protein (lower panel). (d) Primary human keratinocytes were grown as above and FLG expression evaluated. (e) Primary murine keratinocytes were grown at 21% or 0.5% O2 for 48 hours to 70% or 100% confluency and lysates immunoblotted for filaggrin. (f) Primary murine keratinocytes were grown at 1.4 mM Ca2+ at 21% or 0.5% O2 for 48 hours and lysates immunoblotted for filaggrin.
Although filaggrin expression in cultured keratinocytes is known to be induced by cell confluency and extracellular calcium (Resing et al. 1993), hypoxic treatment of primary keratinocytes led to elevated filaggrin accumulation in both subconfluent and confluent cultures (Figure 2e). Similarly, hypoxia further increased filaggrin expression even at high calcium concentrations (Figure 2f). We concluded that O2 deprivation induces filaggrin expression; furthermore, this effect is independent of known environmental regulators of epidermal development.
Filaggrin expression is HIF-dependent
To characterize the requirement for HIF in hypoxic Flg induction, we generated mice lacking HIF1α and HIF2α in the epidermis using the Cre transgene driven by the keratin 14 (Krt14) promoter, expressed in the basal epidermis (Dassule et al. 2000). Mice lacking either HIF1α or HIF2α in the epidermis have been described previously and do not develop epidermal abnormalities (Boutin et al. 2008; Rezvani et al. 2011). Krt14-Cre+Hif1afl/wtHif2afl/fl mice were crossed to produce control (Krt14-Cre+Hif1afl/wtHif2afl/fl) or epidermis-specific double knockout mice (DKO; Krt14-Cre+Hif1afl/flHif2afl/fl) (Supplementary Figure S1a). Control mice, which lack epidermal HIF2α, are phenotypically indistinguishable from wild type animals (data not shown).
In the absence of Hif1a and Hif2a, epidermal filaggrin expression in ear, trunk, and tail skin was significantly reduced (Figure 3a and data not shown). Consistent with a role for HIF in transcriptional regulation of Flg, DKO epidermis exhibited lower levels of Flg transcripts, profilaggrin, and filaggrin monomers (Figure 3b). Flg induction was similarly abrogated in hypoxic primary keratinocytes lacking Hif1a and Hif2a (Figure 3c and Supplementary Figure S1b). Primary keratinocytes lacking Arnt also did not express filaggrin under hypoxic conditions (Figure 3d). These results indicated that HIF is required for filaggrin expression both in vitro and in vivo.
Figure 3. Filaggrin expression is HIF-dependent.
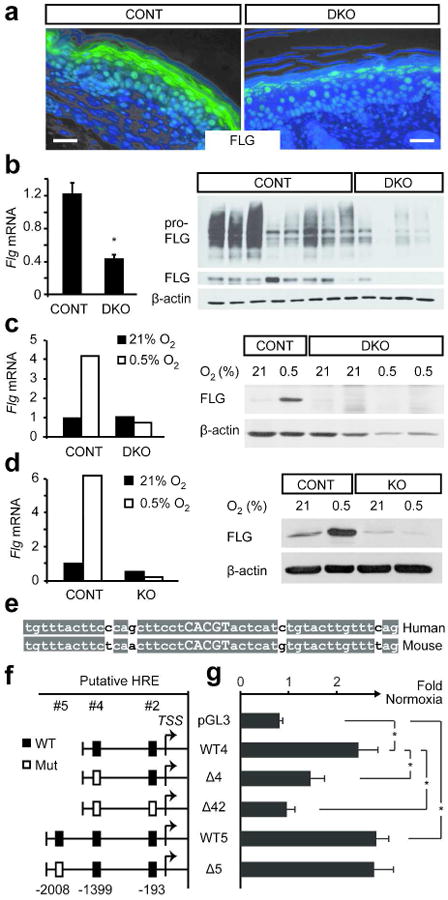
(a) Ear skin from control and DKO pups were immunostained for filaggrin. Scale bar, 50 μm. (b) Tail skin from control and DKO pups were analyzed for filaggrin transcripts (left) and protein (right). (c, d) Primary murine keratinocytes from control (CONT) and DKO pups (c) or KO pups lacking Arnt (d) were grown at 21% or 0.5% O2 for 48 hours and analyzed for filaggrin transcripts (left) and protein (right). (e) Alignment of the hypoxia response element (HRE) at position -193 and flanking sequences indicates high mammalian conservation. (f) Fragments of the human FLG promoter were cloned into pGL3 luciferase reporter. Rectangles indicate putative HREs. (g) Primary human keratinocytes transfected with promoter construct and Renilla luciferase were exposed to 21% or 0.5% O2 for 30 hours. Luciferase activity under hypoxia was normalized to normoxic values. Data are presented as the mean of three independent experiments. *, p<0.05
Filaggrin accumulation is regulated by the epidermal serine proteases caspase 14 (CASP14) and serine protease inhibitor matriptase (PRSS8), which cleave profilaggrin to monomeric filaggrin (List et al. 2003; Denecker et al. 2007). Casp14 and Prss8 expression was unchanged in DKO epidermis (Supplementary Figure S1c). However, the expression of Spink5, an inhibitor of epidermal serine proteases, was increased (Supplementary Figure S1c). Of note, SPINK5 deficiency is correlated with increased monomeric filaggrin in the epidermis (Chavanas et al. 2000). Increased Spink5 expression in DKO mice may therefore inhibit the conversion of profilaggrin to monomeric filaggrin.
FLG promoter contains hypoxia response elements
Nucleotide analysis of the human FLG promoter for HIF binding sites matching the consensus sequence (A/G)CGTG identified three putative hypoxia response elements (HREs) at 193, 1399, and 2008 bases upstream of the FLG transcriptional start site. The proximal consensus site at -193 is highly conserved between mammals (Figure 3e). Fragments of the FLG promoter containing these HRE sequences were cloned into a luciferase expression vector and transfected into primary keratinocytes (Figure 3f). In cells transfected with wild type promoter sequences (WT4 and WT5), luciferase activity was increased under hypoxic conditions relative to normoxic control (Figure 3g). Sequential mutagenesis of the two proximal HREs (Δ4 and Δ42) reduced hypoxic reporter activity progressively (Figure 3g). In contrast, mutation of the most distal HRE (Δ5) had no effect on hypoxic stimulation of reporter activity (Figure 3g). Therefore, the FLG promoter contains two functional binding sites for HIF1α and/or HIF2α.
Surprisingly, chromatin immunoprecipitation did not reveal HIF1α or HIF2α occupancy at the human FLG promoter (data not shown). While we cannot exclude the possibility that hypoxic stimulation of FLG is an indirect consequence of HIF activity, promoter mutagenesis experiments clearly demonstrated that functional binding sites exist for HIF1α and HIF2α. Therefore, it is likely that the interaction between HIF and the FLG promoter lies beneath the threshold of detection by ChIP, implying that the proximal HREs represent consensus sequences possessing relatively weak HIF/DNA interactions.
HIF-deficient mice develop skin abnormalities
DKO mice were indistinguishable from control littermates at birth. However, 4 to 5 days later (P4-P5), DKO mice exhibited dry, flaky skin on their extremities (Figure 4a). By P8, DKO mice had developed thickened and diminutive external ears, constricted hind digits, and hyperkeratotic tail constrictions (Figure 4b). This set of phenotypes is associated with epidermal barrier defects in other mouse models and phenocopies the flaky tail (ft) mice, which possess a spontaneous mutation in the Flg gene (Lane 1972; Presland et al. 2000; Fallon et al. 2009). Adult DKO mice retained underdeveloped external ears and dry, flaky tail skin (data not shown).
Figure 4. Impaired epidermal permeability barrier and corneocyte integrity in DKO mice.
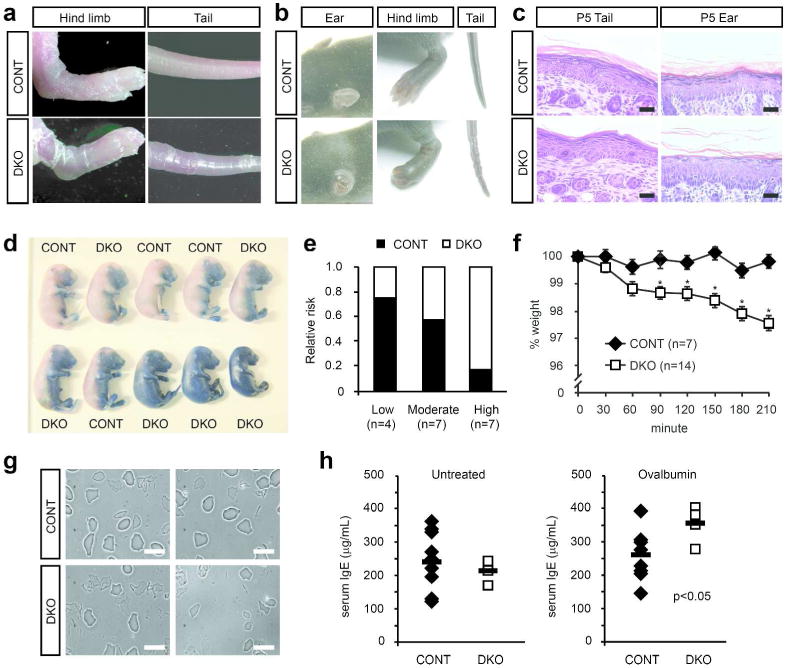
(a) Flaky skin on hind limbs and tails of DKO P5 pups compared to control litttermates. (b) Thick, shortened pinna of the ear, taut skin of the feet, and constricted tail skin in DKO P8 pups compared to control littermates. (c) DKO epidermis stained with hematoxylin and eosin shows attenuated stratum granulosum compared to control epidermis. Scale bar, 50 μm. (d) Control (CONT) and DKO littermates were stained with toluidine blue dye at E17. (e) Embryos were categorized into groups of low, moderate, or high permeability and results from multiple experiments quantified. (f) Newborn CONT and DKO mice were weighed at regular intervals as indicated. *, p<0.05 compared to CONT. (g) Corneocytes from CONT or DKO P3 epidermis were sonicated and visualized under a microscope. Scale bar, 40 μm. (h) CONT (n=9) and DKO (n=4) littermates were treated topically with ovalbumin for 5 days × 3 cycles and serum IgE levels determined after treatment using ELISA (right). Controls (left) are untreated animals.
Histologically, DKO epidermis resembled ichthyosis vulgaris lesions, which are associated with loss of filaggrin (Compton et al. 2002). DKO epidermis showed attenuation of the stratum granulosum and reduced cornified envelope thickness (Figure 4c). Importantly, changes in the stratum granulosum and cornified envelope did not correlate with changes in keratinocyte proliferation or cell fate. No significant difference in the number of Ki67-positive basal keratinocytes was observed between control and DKO mice (Supplementary Figure S1d-e). KRT10, an early differentiation marker, was appropriately expressed in all suprabasal keratinocytes (Supplementary Figure S1d). These results indicated that HIF deficiency in the epidermis specifically affects keratinocyte terminal differentiation in the granular layer and cornified envelope.
DKO mice exhibit defective permeability barrier and cornified envelope integrity
Formation of the outside-to-in epidermal barrier occurs between E16 and E18 and can be visualized by permeability to toluidine blue dye (Hardman et al. 1998). Most control embryos efficiently excluded toluidine blue at E17; in contrast, many DKO embryos were completely or partially stained (Figure 4d). Because both control and DKO mice displayed some phenotypic variability, embryos were classified into those with high, moderate, or low dye permeability. DKO mice were more likely to exhibit high permeability to toluidine blue, indicative of epidermal barrier dysfunction (Figure 4e).
Inside-to-out barrier function was assessed by measuring the rate of trans-epidermal fluid evaporation. Newborn control pups maintained constant body weight over 3.5 hours, while DKO pups suffered progressive weight loss (Figure 4f). Therefore, the loss of epidermal HIF activity resulted in impaired epidermal barrier development.
We hypothesized that reduced filaggrin expression would render Hif-deficient epidermis more susceptible to mechanical stress. Corneocytes (i.e. terminally differentiated keratinocytes) were isolated from control and DKO epidermis and subjected to ultrasonic disruption. Control corneocytes remained largely intact, whereas DKO corneocytes became fragmented or destroyed (Figure 4g). These experiments indicate that cornified envelope integrity is compromised in DKO mice, resulting in epidermal barrier dysfunction.
Epidermal barrier disruption due to filaggrin deficiency results in percutaneous allergen sensitization (Palmer et al. 2006; Fallon et al. 2009). As a result, FLG mutations and deletions are common causes of atopic dermatitis and ichthyosis vulgaris (Palmer et al. 2006; Smith et al. 2006). We applied the common allergen ovalbumin (OVA) to the skin of control and DKO mice and measured serum IgE levels as a marker of atopic response. Untreated control and DKO mice showed no significant difference in baseline IgE levels (Figure 4h). However, OVA treatment significantly elevated IgE levels in DKO mice (Figure 4h). The loss of HIF activity in the epidermis may therefore create conditions favorable for developing atopic skin disease.
Comparison with Arnt-deficient mice
In the epidermis, Arnt is required for ceramide synthesis and its absence results in epidermal barrier defects in mice (Takagi et al. 2003; Geng et al. 2006). Additionally, it is required for AHR-dependent keratinocyte differentiation in culture (Takagi et al. 2003; Geng et al. 2006; Sutter et al. 2009). To characterize the role of ARNT in epidermal development in the context of hypoxic gene regulation, Krt14-Cre+ mice were crossed to Arntfl/fl mice to produce control (Krt14-Cre+Arntfl/wt) and KO (Krt14-Cre+Arntfl/fl) mice (Supplementary Figure S2a). Consistent with previous reports, Arnt KO mice were perinatal lethal. Arnt KO epidermis showed reduced FLG expression (Supplementary Figure S2b). Similar to DKO epidermis, stratum granulosum and cornified envelope thickness were also reduced (Supplementary Figure S2b). No difference between KO and control animals was observed in immunohistochemical staining for the proliferation marker Ki67 or early differentiation proteins KRT1 and KRT10 (Supplementary Figure S2c-d). Although previous studies have proposed that epidermal barrier defects in Arnt KO mice are a result of deficiencies in lipid processing, these findings suggested that reduced filaggrin expression in O2-deprived keratinocytes also contributes to this phenotype (Figure 3d).
Conclusion
In this study, we demonstrated the importance of hypoxic signaling in skin development. We showed that a low-O2 environment exists in the murine embryonic epidermis. Low-O2 conditions stabilize HIF1α and HIF2α, the absence of which impaired expression of the HIF target gene filaggrin. As a result, keratinocyte terminal differentiation and epidermal barrier formation are impaired. Interestingly, although HIF1α and HIF2α can sometimes act in distinct and opposing fashion (Keith et al. 2012), they appear to regulate Flg expression and epidermal barrier function in a redundant manner.
The link between epidermal barrier defects and atopic skin diseases became firmly established with the identification of FLG as a susceptibility gene (Palmer et al. 2006). However, only 30% of European patients with atopic dermatitis bear FLG mutations (Bieber 2008). Recent genome-wide association studies have uncovered additional gene loci that confer risk for atopic dermatitis (Esparza-Gordillo et al. 2009; Sun et al. 2011; Paternoster et al. 2012). These investigations have not identified mutations in HIF pathway genes, but it is important to note that no study has controlled for FLG status amongst their cohorts. On the other hand, it is equally possible that HIF-dependent regulation of filaggrin is required for normal development but not disease progression. Further investigation into HIF activity in patients with atopic dermatitis may enhance our understanding of the mechanisms by which the low-O2 environment of the epidermis regulates skin development and function.
Materials and Methods
Mice
Krt14-Cre+ transgenic mice were purchased from JAX. Arntfl/fl, Hif1afl/fl, and Hif2afl/fl mice have been described previously (Ryan et al. 2000; Tomita et al. 2000; Gruber et al. 2007). PCR was performed using allele-specific primers (available on request). All animal experiments were performed in compliance with Institutional Animal Care and Use Committee regulations and approved by the University of Pennsylvania institutional review board.
Cell culture
Primary human epidermal keratinocytes isolated from neonatal foreskin were obtained from the Penn Skin Disease Research Center and maintained in a 1:1 mixture of K-SFM supplemented with human rEGF and BPE (Invitrogen) and Medium 154 supplemented with HKGS (Invitrogen). Primary murine keratinocytes were isolated as described in (Lichti et al. 2008) and maintained at 34°C, 8% CO2 in MCDB 153 media (Sigma) supplemented with 5% chelexed FBS (Hyclone), 100 μM ethanolamine (Sigma), 100 μM phosphorylethanolamine (Sigma), 2 mM L-glutamine (Invitrogen), 10 ng/mL human rEGF (Invitrogen), 1 μM hydrocortisone (Invitrogen), 5 μg/mL insulin (Invitrogen), 0.2 nM choleratoxin (Sigma), 2 nM 3,3′5-triiodo-L-thyronine (Sigma), and 45 μM CaCl2 (Sigma). For hypoxia treatment, cells were cultured in an InVivo2 400 hypoxia workstation (Ruskinn).
Visualization of tissue pO2
1-((2-hydroxy-3-piperdinyl)propyl)-2-nitroimidazole hydrochloride (Hypoxyprobe Inc) was injected intraperitoneally into pregnant mice at E14, E16, E18, and intraperitoneally into P0 pups. After 4 hours, embryos/pups were collected and frozen in OCT (Sakura). Skin cryosections were stained using anti-hypoxyprobe-FITC antibody (Hypoxyprobe Inc).
Histological and immunohistochemical analysis
Paraffin-embedded sections were immunostained with antibodies directed against ARNT (SC-8076; Santa Cruz), filaggrin, HIF1α (Ab2185; Abcam), HIF2α (NB100-132; Novus), IVL, Ki67 (Novocastra), KRT1, KRT6, KRT10, and LOR (Covance unless otherwise noted). Antibody binding was detected using DAB (Vector Labs) or AlexaFluor 488 anti-rabbit immunoglobulin (Invitrogen).
Quantitative RT-PCR
Gene expression was analyzed using specific primers (available on request). Expression levels of 18S rRNA and HPRT1 were used for normalization.
Western blotting
Primary antibodies used were directed against ARNT (NB100-110; Novus), β-actin (Sigma), filaggrin, IVL, HIF1α C-Term (Cayman), HIF2α (NB100-122; Novus), KRT5, KRT10, and LOR (Covance unless otherwise noted).
FLG promoter analysis
Fragments of the human FLG promoter were amplified from keratinocyte genomic DNA using specific primers (available on request) and cloned into pGL3-Basic luciferase expression vector (Promega). Individual constructs were transfected into primary human keratinocytes using FuGENE reagent (Roche). Luciferase activity was measured after normoxic or hypoxic treatment using the Dual-Luciferase Reporter Assay (Promega).
Outside-to-in permeability barrier assay
Epidermal permeability was assessed by toluidine blue staining as described in (Takagi et al. 2003) and categorized as high (dorsal staining), moderate (>50% ventral staining), or low (<50% ventral staining).
Corneocyte sonication
Cornified envelope was purified and sonicated as described in (Koch et al. 2000).
Ovalbumin treatment and serum IgE ELISA
Ovalbumin (OVA) challenge was performed as described in (Palmer et al. 2006). 20 to 50 ug of OVA (fraction V; Sigma) in PBS was applied daily to ventral skin of control or DKO littermates for five consecutive days. OVA challenge was repeated twice with intervening intervals of 7 days. Total serum IgE was measured using PharMingen antibodies (BD Biosciences).
Statistical analysis
Data are presented as mean+s.e.m. with differences between two groups analyzed for significance using Student's t test. A p-value <0.05 was considered to be significant.
Supplementary Material
Supplementary Figure S1. Epidermal Hif1a and Hif2a double knockout (DKO) mice. (a) PCR analysis of epidermal and dermal DNA with allele-specific primers indicated site-specific and efficient recombination. (b) Primary murine keratinocytes from control (CONT) or DKO P2 pups were grown at 21% or 0.5% O2 for 48 hours and lysates immunoblotted for HIF1α and HIF2α. (c) Epidermal RNA was extracted from control and DKO P3 pups and subjected to qRT-PCR using specific primers for Casp14, Prss8, and Spink5. (d) Paraffin-embedded ear sections from control (CONT) and DKO P5 pups were immunostained using the indicated antibodies. No observable differences were detected in Ki67, KRT10, and loricrin (LOR) staining intensity between CONT and DKO epidermis. Scale bar, 50 μm. (e) Quantification of Ki67-positive nuclei in the basal epidermis of CONT and DKO animals demonstrated no difference in keratinocyte proliferation.
Supplementary Figure S2. Arnt is required for hypoxic filaggrin induction. (a) PCR analysis of epidermal and dermal DNA with allele-specific primers indicated site-specific and efficient recombination in the epidermis (upper panel). Cell lysates from primary murine keratinocytes obtained from control (CONT) or KO P0 pups were immunoblotted for ARNT (lower panel). n.s., non-specific band. (b) Paraffin-embedded skin sections from control (CONT) and KO P0 pups were stained with hematoxylin and eosin or with antibodies against filaggrin. (c) Skin sections from control (CONT) and KO pups were immunostained using the indicated antibodies. No observable differences were detected in Ki67, KRT10, and loricrin (LOR) staining intensity between CONT and KO epidermis. Scale bar, 50 μm. (d) Quantification of Ki67-positive nuclei in the basal epidermis of CONT and KO animals demonstrated no difference in keratinocyte proliferation.
Acknowledgments
We thank Sarah Millar and Todd Ridky for helpful discussions and Brian Keith for his critical reading of the manuscript. Aimee Payne and Christine Marshall provided primary human keratinocytes and technical assistance with keratinocyte culture. This work was supported by grants 5P30AR057217-03 (M.C.S), 5R01HL066310-12 (M.C.S.), and by the Howard Hughes Medical Institute (M.C.S.).
Footnotes
Conflict of Interests: The authors declare no conflict of interests.
References
- Amarilio R, Viukov SV, Sharir A, et al. HIF1α regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–28. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- Bedogni B, Welford SM, Cassarino DS, et al. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell. 2005;8:443–54. doi: 10.1016/j.ccr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Bejar R, Giussi G, Casaguberta C, et al. The actual value of PO2 in human amniotic fluid. Eur J Obstet Gynecol. 1971;1:189–93. [Google Scholar]
- Bieber T. Atopic Dermatitis. N Engl J Med. 2008;358:1483–94. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–17. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin AT, Weidemann A, Fu Z, et al. Epidermal Sensing of Oxygen Is Essential for Systemic Hypoxic Response. Cell. 2008;133:223–34. doi: 10.1016/j.cell.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C, Tainsky M, Fuchs E. Programming gene expression in developing epidermis. Development. 1994;120:2369–83. doi: 10.1242/dev.120.9.2369. [DOI] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–40. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Chavanas S, Bodemer C, Rochat A, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–2. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- Chen T, Heller E, Beronja S, et al. An RNA interference screen uncovers a new molecule in stem cell self-renewal and long-term regeneration. Nature. 2012;485:104–8. doi: 10.1038/nature10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton JG, DiGiovanna JJ, Johnston KA, et al. Mapping of the associated phenotype of an absent granular layer in ichthyosis vulgaris to the epidermal differentiation complex on chromosome 1. Exp Dermatol. 2002;11:518–26. doi: 10.1034/j.1600-0625.2002.110604.x. [DOI] [PubMed] [Google Scholar]
- Dahl KDC, Fryer BH, Mack FA, et al. Hypoxia-Inducible Factors 1α and 2α Regulate Trophoblast Differentiation. Mol Cell Biol. 2005;25:10479–91. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, et al. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–85. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Denecker G, Hoste E, Gilbert B, et al. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–74. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- Elson D, Ryan H, Snow J, et al. Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000;60:6189–95. [PubMed] [Google Scholar]
- Esparza-Gordillo J, Weidinger S, Fölster-Holst R, et al. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat Genet. 2009;41:596–601. doi: 10.1038/ng.347. [DOI] [PubMed] [Google Scholar]
- Evans NT, Naylor PF. The systemic oxygen supply to the surface of human skin. Respir Physiol. 1967;3:21–37. doi: 10.1016/0034-5687(67)90020-5. [DOI] [PubMed] [Google Scholar]
- Evans SM, Schrlau AE, Chalian AA, et al. Oxygen Levels in Normal and Previously Irradiated Human Skin as Assessed by EF5 Binding. J Invest Dermatol. 2006;126:2596–606. doi: 10.1038/sj.jid.5700451. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Sasaki T, Sandilands A, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–8. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareus R, Huth M, Breiden B, et al. Normal epidermal differentiation but impaired skin-barrier formation upon keratinocyte-restricted IKK1 ablation. Nat Cell Biol. 2007;9:461–9. doi: 10.1038/ncb1560. [DOI] [PubMed] [Google Scholar]
- Geng S, Mezentsev A, Kalachikov S, et al. Targeted ablation of Arnt in mouse epidermis results in profound defects in desquamation and epidermal barrier function. J Cell Sci. 2006;119:4901–12. doi: 10.1242/jcs.03282. [DOI] [PubMed] [Google Scholar]
- Gruber M, Hu CJ, Johnson RS, et al. Acute postnatal ablation of Hif-2α results in anemia. Proc Natl Acad Sci. 2007;104:2301–6. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman MJ, Sisi P, Banbury DN, et al. Patterned acquisition of skin barrier function during development. Development. 1998;125:1541–52. doi: 10.1242/dev.125.8.1541. [DOI] [PubMed] [Google Scholar]
- Henry J, Hsu CY, Haftek M, et al. Hornerin is a component of the epidermal cornified cell envelopes. FASEB J. 2011;25:1567–76. doi: 10.1096/fj.10-168658. [DOI] [PubMed] [Google Scholar]
- Irvine AD, McLean WHI, Leung DYM. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–27. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch PJ, de Viragh PA, Scharer E, et al. Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J Cell Biol. 2000;151:389–400. doi: 10.1083/jcb.151.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kömüves LG, Hanley K, Lefebvre AM, et al. Stimulation of PPARalpha promotes epidermal keratinocyte differentiation in vivo. J Invest Dermatol. 2000;115:353–60. doi: 10.1046/j.1523-1747.2000.00073.x. [DOI] [PubMed] [Google Scholar]
- Lane PW. Two new mutations in linkage group XVI of the house mouse. Flaky tail and varitint-waddler-J. J Hered. 1972;63:135–40. doi: 10.1093/oxfordjournals.jhered.a108252. [DOI] [PubMed] [Google Scholar]
- Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WLC, et al. Interfollicular Epidermal Stem Cells Self-Renew via Autocrine Wnt Signaling. Science. 2013;342:1226–30. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List K, Szabo R, Wertz PW, et al. Loss of proteolytically processed filaggrin caused by epidermal deletion of Matriptase/MT-SP1. J Cell Biol. 2003;163:901–10. doi: 10.1083/jcb.200304161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Skuli N, Mesquita RC, et al. O(2) regulates skeletal muscle progenitor differentiation through phosphatidylinositol 3-kinase/AKT signaling. Mol Cell Biol. 2012;32:36–49. doi: 10.1128/MCB.05857-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, O'Brien WT, Johnson RS, et al. O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol. 2010;12:1007–13. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair M, Teng A, Bilanchone V, et al. Ovol1 regulates the growth arrest of embryonic epidermal progenitor cells and represses c-myc transcription. J Cell Biol. 2006;173:253–64. doi: 10.1083/jcb.200508196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BC, Lefort K, Mandinova A, et al. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20:1028–42. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CNA, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- Paternoster L, Standl M, Chen CM, et al. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat Genet. 2012;44:187–92. doi: 10.1038/ng.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Boutin AT, Zinkernagel AS, et al. Critical Role of HIF-1[alpha] in Keratinocyte Defense against Bacterial Infection. J Invest Dermatol. 2008;128:1964–8. doi: 10.1038/jid.2008.27. [DOI] [PubMed] [Google Scholar]
- Presland RB, Boggess D, Lewis SP, et al. Loss of normal profilaggrin and filaggrin in flaky tail (ft/ft) mice: an animal model for the filaggrin-deficient skin disease ichthyosis vulgaris. J Invest Dermatol. 2000;115:1072–81. doi: 10.1046/j.1523-1747.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Wu C, Khatri R, et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012;149:63–74. doi: 10.1016/j.cell.2012.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resing KA, al-Alawi N, Blomquist C, et al. Independent regulation of two cytoplasmic processing stages of the intermediate filament-associated protein filaggrin and role of Ca2+ in the second stage. J Biol Chem. 1993;268:25139–45. [PubMed] [Google Scholar]
- Rezvani HR, Ali N, Serrano-Sanchez M, et al. Loss of epidermal hypoxia-inducible factor-1α accelerates epidermal aging and affects re-epithelialization in human and mouse. J Cell Sci. 2011;124:4172–83. doi: 10.1242/jcs.082370. [DOI] [PubMed] [Google Scholar]
- Ryan HE, Poloni M, McNulty W, et al. Hypoxia-inducible Factor-1α Is a Positive Factor in Solid Tumor Growth. Cancer Res. 2000;60:4010–5. [PubMed] [Google Scholar]
- Scheid A, Wenger RH, Schäffer L, et al. Physiologically low oxygen concentrations in fetal skin regulate hypoxia-inducible factor 1 and transforming growth factor-beta3. FASEB J Off Publ Fed Am Soc Exp Biol. 2002;16:411–3. doi: 10.1096/fj.01-0496fje. [DOI] [PubMed] [Google Scholar]
- Sen GL, Boxer LD, Webster DE, et al. ZNF750 Is a p63 Target Gene that Induces KLF4 to Drive Terminal Epidermal Differentiation. Dev Cell. 2012;22:669–77. doi: 10.1016/j.devcel.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol. 2011;12:565–80. doi: 10.1038/nrm3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FJD, Irvine AD, Terron-Kwiatkowski A, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337–42. doi: 10.1038/ng1743. [DOI] [PubMed] [Google Scholar]
- Sun LD, Xiao FL, Li Y, et al. Genome-wide association study identifies two new susceptibility loci for atopic dermatitis in the Chinese Han population. Nat Genet. 2011;43:690–4. doi: 10.1038/ng.851. [DOI] [PubMed] [Google Scholar]
- Sutter CH, Yin H, Li Y, et al. EGF receptor signaling blocks aryl hydrocarbon receptor-mediated transcription and cell differentiation in human epidermal keratinocytes. Proc Natl Acad Sci. 2009;106:4266–71. doi: 10.1073/pnas.0900874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Tojo H, Tomita S, et al. Alteration of the 4-sphingenine scaffolds of ceramides in keratinocyte-specific Arnt-deficient mice affects skin barrier function. J Clin Invest. 2003;112:1372–82. doi: 10.1172/JCI18513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Sinal CJ, Yim SH, et al. Conditional Disruption of the Aryl Hydrocarbon Receptor Nuclear Translocator (Arnt) Gene Leads to Loss of Target Gene Induction by the Aryl Hydrocarbon Receptor and Hypoxia-Inducible Factor 1α. Mol Endocrinol. 2000;14:1674–81. doi: 10.1210/mend.14.10.0533. [DOI] [PubMed] [Google Scholar]
- Tu CL, Crumrine DA, Man MQ, et al. Ablation of the Calcium-Sensing Receptor in Keratinocytes Impairs Epidermal Differentiation and Barrier Function. J Invest Dermatol. 2012;132:2350–9. doi: 10.1038/jid.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley AJ, Chavanas S, Moffatt MF, et al. Gene polymorphism in Netherton and common atopic disease. Nat Genet. 2001;29:175–8. doi: 10.1038/ng728. [DOI] [PubMed] [Google Scholar]
- Williams SE, Beronja S, Pasolli HA, et al. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–8. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Epidermal Hif1a and Hif2a double knockout (DKO) mice. (a) PCR analysis of epidermal and dermal DNA with allele-specific primers indicated site-specific and efficient recombination. (b) Primary murine keratinocytes from control (CONT) or DKO P2 pups were grown at 21% or 0.5% O2 for 48 hours and lysates immunoblotted for HIF1α and HIF2α. (c) Epidermal RNA was extracted from control and DKO P3 pups and subjected to qRT-PCR using specific primers for Casp14, Prss8, and Spink5. (d) Paraffin-embedded ear sections from control (CONT) and DKO P5 pups were immunostained using the indicated antibodies. No observable differences were detected in Ki67, KRT10, and loricrin (LOR) staining intensity between CONT and DKO epidermis. Scale bar, 50 μm. (e) Quantification of Ki67-positive nuclei in the basal epidermis of CONT and DKO animals demonstrated no difference in keratinocyte proliferation.
Supplementary Figure S2. Arnt is required for hypoxic filaggrin induction. (a) PCR analysis of epidermal and dermal DNA with allele-specific primers indicated site-specific and efficient recombination in the epidermis (upper panel). Cell lysates from primary murine keratinocytes obtained from control (CONT) or KO P0 pups were immunoblotted for ARNT (lower panel). n.s., non-specific band. (b) Paraffin-embedded skin sections from control (CONT) and KO P0 pups were stained with hematoxylin and eosin or with antibodies against filaggrin. (c) Skin sections from control (CONT) and KO pups were immunostained using the indicated antibodies. No observable differences were detected in Ki67, KRT10, and loricrin (LOR) staining intensity between CONT and KO epidermis. Scale bar, 50 μm. (d) Quantification of Ki67-positive nuclei in the basal epidermis of CONT and KO animals demonstrated no difference in keratinocyte proliferation.


