Abstract
Objective
The objective of this study was to examine the dependence of item memory and relational memory on medial temporal lobe (MTL) structures. Patients with amnesia, who either had extensive MTL damage or damage that was relatively restricted to the hippocampus, were tested, as was a matched comparison group. Disproportionate relational memory impairments were predicted for both patient groups, and those with extensive MTL damage were also expected to have impaired item memory.
Method
Participants studied scenes, and were tested with interleaved two-alternative forced-choice probe trials. Probe trials were either presented immediately after the corresponding study trial (lag 1), five trials later (lag 5), or nine trials later (lag 9) and consisted of the studied scene along with a manipulated version of that scene in which one item was replaced with a different exemplar (item memory test) or was moved to a new location (relational memory test). Participants were to identify the exact match of the studied scene.
Results
As predicted, patients were disproportionately impaired on the test of relational memory. Item memory performance was marginally poorer among patients with extensive MTL damage, but both groups were impaired relative to matched comparison participants. Impaired performance was evident at all lags, including the shortest possible lag (lag 1).
Conclusions
The results are consistent with the proposed role of the hippocampus in relational memory binding and representation, even at short delays, and suggest that the hippocampus may also contribute to successful item memory when items are embedded in complex scenes.
Keywords: Hippocampus, Medial Temporal Lobe, Relational Memory, Item Memory, Short-Term Memory
INTRODUCTION
The critical role of medial temporal lobe (MTL) structures in long-term declarative memory (Cohen & Squire, 1980; Cohen, 1984), including expressions of memory for facts and events, is now well established in the literature. Indeed, it seems that there is even emerging consensus in support of the view that anatomically distinct MTL subregions make qualitatively different contributions to declarative memory. Several groups have pointed to a critical role for the hippocampus in binding together relationships among items that co-occur in the context of scenes or events, and have further proposed that structures in the parahippocampal region (particularly perirhinal cortex) support memory for individual items (cf. Brown & Aggleton, 2001; Davachi, 2006; Eichenbaum, Otto, & Cohen, 1994; Diana, Yonelinas, & Ranganath, 2007; Eichenbaum, Yonelinas, & Ranganath, 2007; Mayes, Montaldi & Migo, 2007). Particularly strong support for this type of functional differentiation has been reported in the neuroimaging (e.g., Awipi & Davachi, 2008; Davachi, Mitchell, & Wagner, 2003; Giovanello, Schnyer, & Verfaellie, 2004; Hannula, Libby, Yonelinas, & Ranganath, 2013; Kӧhler, Danckert, Gati, & Menon, 2005; Staresina & Davachi, 2008, 2009) and animal literatures (e.g., Wan, Aggleton, & Brown, 1999; Winters, Forwood, Cowell, et al., 2004; see Aggleton, Brown & Albasser, 2012 for review), but some notable exceptions have been documented as well (e.g., Gold, Hopkins, & Squire, 2006; Gold, Smith, Bayley, et al., 2006; Stark & Squire, 2003; Stark, Bayley, & Squire, 2002).
Here, we focus on some of the methodological issues that may complicate testing of this view of functional dissociation. One particularly vexing methodological issue concerns assuring that item memory and relational memory can be assessed as purely as possible, without significant contamination of one by the other, while also assuring that they can be assessed as equivalently as possible, without differing too much in task difficulty.
Many experiments entail having participants study arbitrarily paired items (e.g., flower-pencil, canister-pillow, treasure-photograph), and then have them attempt to distinguish studied from novel materials (flower vs. paste; a test of item memory) or intact from recombined pairs (flower-pencil vs. canister-photograph; a test of relational or associative memory) when recognition memory tests are administered. Using such an approach in work conducted with amnesic patients, Giovanello, Verfaellie, & Keane (2003) and Turriziani, Fadda, Caltagirone, & Carlesimo (2004) reported disproportionate deficits in relational memory compared to item memory (see also Stark et al.2002; Gold et al., 2006). But, these tasks differ not only with regard to what type of information is being tested (and the memory processes needed to access and use them), but also how much information is needed to perform the tasks. As long as the stimuli and tasks used to test various proposed forms of memory are different, there are multiple ways to explain any observed dissociation in performance. That so much of the neuropsychological evidence distinguishing between forms of memory has been dependent on dissociations between different tasks or conditions is discussed in much greater detail elsewhere (Ryan & Cohen, 2003).
To circumvent this problem in our own previous work, we have presented participants with a single set of materials (scenic images) under a single instructional set, and have used eye movements to measure memory indirectly (Ryan, Althoff, Whitlow, & Cohen, 2000; Ryan & Cohen, 2004). One set of eye movement measures was sensitive to scene repetition, providing an index of memory for items (i.e., whole scenes), whereas another set of eye movement measures was sensitive to manipulations of the relations among elements in the same scenes, providing an index of relational memory. Amnesic patients failed selectively to show the normal effects of relational manipulations in their patterns of viewing. Given the use of a single class of materials and a single instructional set, this observed dissociation across eye movement measures provided particularly powerful evidence in support of the view that the hippocampal system is specialized for relational memory.
However, in the eye movement work mentioned above, and in a subsequent behavioral study (Hannula, Tranel, & Cohen, 2006), successful performance on tests of relational memory required retention of very specific information – memory for spatial relationships among items embedded in a scene context – whereas memory for the scenes themselves may have been based on representations of fairly general, gist-based information (e.g., red bathroom, girl’s bedroom). Therefore, differences in the complexity or amount of information in the to-be-retained representations may have contributed to, or even given rise to, the observed patterns of spared and impaired performance.
A primary objective in the current experiment was to mitigate this issue by making the materials and instructions used to test item and relational memory more comparable than has been done in past work. To this end, participants studied a series of scenes (e.g., a bedroom scene), and memory was tested with 2-alternative forced-choice displays that contained an originally studied scene and a manipulated version of that scene. The manipulated scenes were identical to those viewed previously except that a “critical item” (e.g., a laundry basket) was either replaced with a different exemplar (e.g., a different laundry basket) or had changed locations with respect to other items in the scene. When test trials were presented participants were instructed to identify the scene that had been studied from the two alternatives. Thus, performance on tests of item and relational memory both hinged on representations of specific information about a critical object, either about its precise physical form (so as to be able to distinguish one laundry basket from another) or about its relative location in the scene (for a similar approach using neuroimaging methods see Kӧhler, Crane & Milner, 2002). The instructional set was identical regardless of whether item or relational memory was being tested, and test trials, interleaved at short- and long-lags among the study trials, were always 2-alternative forced-choice.
Two well-characterized groups of amnesic patients were recruited to participate in this experiment (see Table 1) – one group with MTL damage limited largely to the hippocampus (HC) and a second group with MTL damage extending beyond the hippocampus into the adjacent parahippocampal region (HC+). Three predictions were made: 1) To the extent that the hippocampus is the critical structure for processing and representation of relations among items, both amnesic groups should be disproportionately impaired on the test of relational memory as compared to the test of item memory, particularly at long lags. Further, assuming comparable damage to the hippocampus in the two amnesic groups, relational memory performance should be similarly affected in patients with more circumscribed damage (HC group) as in patients with more extensive MTL damage (HC+ group). 2) To the extent that structures in the parahippocampal region can support memory for individual items, patients with extensive MTL damage should also show significant impairment on the test of item memory, and should have poorer item memory than HC patients, whose item memory should be relatively preserved. 3) Finally, memory impairments in these patients should be evident even at the shortest lag, when corresponding study and test trials occur in immediate succession, as in our previous work (Hannula et al., 2006; see also Cashdollar, Malecki, Rugg-Gunn, et al., 2009; Hartley, Bird, Chan, et al., 2007; Olson, Page, Moore, et al., 2006; Warren, Duff, Jensen et al., 2012; Watson, Voss, Warren et al., 2013; Yee, Hannula, Tranel, & Cohen, 2014a).
Table 1.
Patient Demographics and Standardized Test Scores
| Patient | Etiology | Age | Yrs Ed | Hand | Sex | WAIS-III | WMS-III | CFT | ||
|---|---|---|---|---|---|---|---|---|---|---|
| VIQ | PIQ | FSIQ | GMI | |||||||
| HC Group | ||||||||||
| 1606 | Anoxia | 58 | 12 | R | M | 94 | 89 | 91 | 66 | 11 |
| 1846 | Anoxia | 42 | 14 | R | F | 89 | 79 | 84 | 57 | 6 |
| 2144 | Anoxia | 56 | 12 | R | F | 102 | 94 | 99 | 56 | 3 |
| 2363 | Anoxia | 49 | 16 | R | M | 112 | 83 | 98 | 73 | 5 |
| 2563 | Anoxia | 50 | 16 | L | M | 91 | 105 | 102 | 75 | 7 |
| HC+ Group | ||||||||||
| 1951 | HSE | 53 | 16 | R | M | 105 | 128 | 106 | 57 | 4 |
| 2308 | HSE | 49 | 16 | L | M | 95 | 78 | 98 | 45 | 0 |
| 6001 | CHI | 50 | 16 | R | M | 83 | 113 | 95 | 49 | 0 |
Yrs Ed, Years of Education; Hand, Handedness;HSE, Herpes Simplex Encephalitis; CHI, Closed Head Injury
WAIS-III, Wechsler Adult Intelligence Scale-III; VIQ, Verbal Intelligence Quotient; PIQ, Performance Intelligence Quotient; FSIQ, Full-Scale Intelligence Quotient; WMS-III, Wechsler Memory Scale-III; GMI, General Memory Index; CFT, Complex Figure Task. The VIQ, PIQ, FSIQ, and GMI yield mean scores in the normal population of 100 with a standard deviation of 15. Maximum score for CFT (30-minute delayed recall raw score)is 36.
METHODS
Participants
Participants were eight patients (six men, two women) with amnesia and eight neurologically intact comparison participants each matched to one of the patients individually for gender, handedness, age (mean age = 50.9 and 50.4 for patients and comparison participants, respectively; t(14)=.84, p=.20), and years of education (mean education = 14.8 and 15.3 years for patients and comparison participants, respectively; t(14)=.61, p=.51; see Table 1). Seven of the amnesic patients were drawn from a registry established and maintained by the Division of Behavioral Neurology and Cognitive Neuroscience at the University of Iowa, and one patient (6001) was seen at Washington University in St. Louis. The group of healthy comparison participants was recruited from the Champaign-Urbana community via advertisements placed in local newspapers.
For five of the patients (HC group), and as described in more detail elsewhere (Allen et al., 2006; Hannula, et al., 2006; Buchanan et al., 2005; Warren et al., 2012; Watson et al., 2013), amnesia was secondary to an anoxic event and structural MRI scans, obtained from four patients, confirmed bilateral hippocampal volume reductions. Significant loss was also evident for a subset of these individuals in the parahippocampal gyrus (i.e., parahippocampal, perirhinal, and entorhinal cortices considered together), but these reductions were less extensive than corresponding volume changes in the hippocampus, and were much less than parahippocampal gyrus volume changes in the HC+ group (described below). A coronal MRI scan through the hippocampus for patient 1606, which shows hippocampal volume changes bilaterally can be seen in Bechara et al. (1995), and high-resolution structural MRI scans for patient 1846 can been seen in Warren et al. (2012). The remaining patient (2563) has contraindications to MRI scanning, but visual inspection of CT scans suggests focal hippocampal damage. Studentized residuals, estimates of brain volume integrity relative to a healthy matched comparison group, are provided in Table 2 for each patient (see Allen et al., 2006 for more detail about how these estimates were obtained) along with performances on the tests of relational and item memory from the current investigation.
Table 2.
Medial Temporal Lobe Volume Reductions and Behavioral Performance (Percent Correct) on Tests of Item and Relational Memory
| Patient | HC | Relational Memory | Patient | PHG | Item Memory | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lag 1 | Lag 5 | Lag 9 | mean | gray | white | Lag 1 | Lag 5 | Lag 9 | mean | |||
| HC Group | HC Group | |||||||||||
| 1606 | −3.99 | 93 | 55 | 60 | 69 | 1606 | −2.46 | −2.36 | 92 | 89 | 78 | 86 |
| 1846 | −4.23 | 84 | 50 | 37 | 57 | 1846 | −1.28 | −2.19 | 93 | 79 | 83 | 85 |
| 2144 | −3.92 | 96 | 64 | 80 | 80 | 2144 | −1.22 | 0.65 | 100 | 90 | 86 | 92 |
| 2363 | −2.64 | 100 | 63 | 56 | 73 | 2363 | −2.26 | −0.37 | 92 | 96 | 80 | 89 |
| 2563 | N/A | 100 | 92 | 96 | 96 | 2563 | N/A | N/A | 97 | 96 | 89 | 94 |
| HC+ Group | HC+ Group | |||||||||||
| 1951 | −6.95 | 93 | 64 | 44 | 67 | 1951 | -- | -- | 96 | 83 | 80 | 86 |
| 2308 | -- | 72 | 50 | 42 | 55 | 2308 | −4.56 | −5.16 | 83 | 59 | 74 | 72 |
| 6001 | N/A | 96 | 34 | 67 | 66 | 6001 | N/A | N/A | 96 | 83 | 62 | 80 |
HC, hippocampus; PHG, parahippocampal gyrus; --, not enough intact tissue to acquire an accurate measurement; N/A, no available data
Measures of brain volume are studentized residuals calculated relative to brain volumes obtained from a healthy comparison group (see Allen et al., 2006 for details). The cutoff for statistically reliable volume reduction was a studentized residual of −2.00 (p<.05).
For the remaining three patients (HC+ Group), amnesia was secondary to herpes simplex encephalitis (patients 1951 and 2308) or closed-head injury (patient 6001). Structural MRI examinations completed for all three patients revealed extensive bilateral MTL damage, including the hippocampus and surrounding medial temporal lobe cortical structures. Detailed information about patient 1951, including an evaluation of high resolution structural MRI scans, is provided in Feinstein et al. (2010). As reported by these authors, right-lateralized MTL structures (i.e., the hippocampus and adjacent MTL cortical structures including the perirhinal, parahippocampal, and entorhinal cortices) are completely destroyed. While there is some sparing on the left, complete loss of tissue is evident in the anterior hippocampus and the entorhinal cortex, and it is estimated that approximately two-thirds of the perirhinal cortex is compromised. In contrast, the left parahippocampal cortex seems to be relatively intact, though there is not enough residual tissue in the parahippocampal gyrus to provide a meaningful quantitative analysis of volume reduction relative to a healthy comparison group (see Table 2). Damage is also evident in the orbitofrontal cortex, amygdala, insula, anterior cingulate, temporal pole, and the basal forebrain; as reported for MTL structures, damage is more extensive in the right hemisphere, though the amygdala is completely destroyed bilaterally.
As was the case for patient 1951 (described above), patient 2308 has a bilateral, but asymmetric lesion; here, however, the damage is more extensive in the left hemisphere. On the left side of the brain the entire medial temporal region is compromised, including the hippocampus proper, adjacent MTL cortical structures (i.e., entorhinal, perirhinal, and parahippocampal cortices), and the amygdala. The temporal pole is completely destroyed, and the damage extends posteriorly to include the anterior one-fifth to one-third of the superior, middle, inferior, and fourth temporal gyri. There is also some damage to the basal forebrain on the left. Right-sided damage is restricted to the anterior medial temporal region, including the amygdala and the anterior portion of the hippocampus. In this case, hippocampal damage is so extensive that it is not possible to provide a meaningful quantitative measure of any remaining tissue relative to normative data. Estimates of volume reduction in the parahippocampal gyrus, which is partially intact on the right, are provided in Table 2. The pole and all lateral parts of the temporal lobe are spared, as is the basal forebrain (see Cavaco, Feinstein, van Twillert & Tranel, 2012 for structural MRI scans acquired from this individual).
The remaining patient (6001) also has a bilateral lesion, and like patient 2308, the lesion is asymmetric with more extensive left-sided damage, particularly in the lateral part of the temporal lobe. In the left hemisphere damage extends posteriorly from the temporal pole and includes the amygdala, hippocampus proper, entorhinal cortex, and perirhinal cortex. Posterior aspects of the parahippocampal gyrus appear to be relatively intact on visual inspection. Lateral temporal damage is extensive and includes approximately the anterior two-thirds of the superior, middle, and inferior temporal gyri. In the right hemisphere, damage also extends posteriorly from the temporal pole and includes the hippocampus proper, perirhinal cortex, and the amygdala. The most posterior one-third of the parahippocampal gyrus appears to be relatively intact. Lateral temporal damage is limited to approximately the anterior one-third of the temporal lobe, with the superior, middle and inferior temporal gyri all compromised within that extent. Ventrolateral prefrontal cortex damage is also evident, particularly on the left, and the lateral ventricles are enlarged bilaterally (see also Demery, Hanlon, & Bauer, 2001).
All of the patients tested here had memory impairments that were sufficiently severe to interfere with daily life, including preventing them from being employed since the onset of their amnesia. Each patient completed neuropsychological testing to confirm selective memory impairment disproportionate to any decline in general cognitive or intellectual functioning, and exhibited severe impairment on standardized tests of memory.
The performance of the HC Group on the Wechsler Memory Scale-III was at least 25 points lower than their performance on the Wechsler Adult Intelligence Scale-III (mean Full Scale IQ minus General Memory Index = 29.4), and the performance of the HC+ Group on the Wechsler Memory Scale-III was at least 46 points lower than their performance on the Wechsler Adult Intelligence Scale-III (mean Full Scale IQ minus General Memory Index = 49.3). All of the patients were also severely impaired on the Complex Figure Test after a 30-minute delay (mean performance of the HC and HC+ Groups was 6.4 and 1.3, respectively, out of 36). See Table 1 for the individual scores on these tests.
The procedures used in this experiment were approved by the ethics committees at the University of Illinois, the University of Iowa, and Washington University in St. Louis, and informed consent was obtained from each participant.
Stimuli and Design
The stimuli were 96 unique rendered scenes that were created using Punch! Home Design Software©, and sized to 800x600 pixels. Three versions of each scene, the original scene and two manipulated versions, were created, producing a total of 288 stimuli. One item in each of the original scenes was designated a “critical item”, and in the manipulated versions of those scenes it was either: (1) replaced with a different item exemplar – i.e., item manipulation, or (2) occupied a different, albeit equally plausible, spatial location – i.e., relational manipulation (see Figure 1). Each critical item and the corresponding alternative item exemplar were presented in the context of just one scene, and across scenes, the critical item moved equally often from left (in the original scene) to right (in the manipulated scene) and from right to left when a relation change occurred.
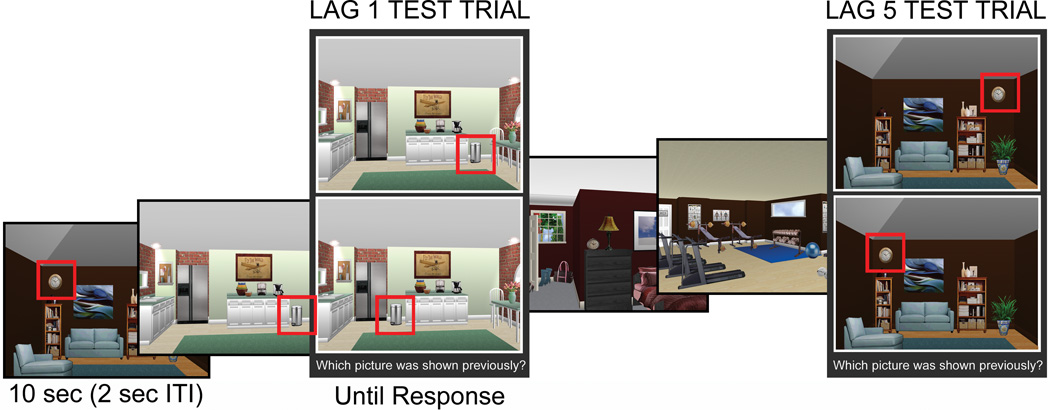
Figure 1.
Example of an original scene along with manipulated versions of that scene. During the item test, the original scene (left) was presented along with a scene in which the critical item was replaced with a different item exemplar (middle). During the relational test, the original scene (left) was presented along with a scene in which the critical item had changed positions relative to other scene elements (right), constituting a change in the relations among items embedded in the original scene.
There were two experimental blocks, each consisting of 24 study trials and 24 probe trials. Memory for items and memory for the relations among items were tested separately in the first and second experimental blocks, respectively. A continuous recognition task was employed in which probe trials were presented systematically among the study trials. Probe trials either appeared immediately after the corresponding study trial (lag 1), five stimuli later (lag 5), or nine stimuli later (lag 9); see Figure 2.
Figure 2.
Study trials and test trials were systematically interleaved during each experimental block. Test trials were presented among the study trials at short (lag 1), medium (lag 5), or long (lag 9) lags. A scene and the corresponding orienting question were presented for 10 seconds on every study trial, and test trials were visible until a response was made. A fixation cross, presented for 2 seconds, separated one trial from the next. The examples shown here test memory for relationships among items; red boxes highlighting the critical items are for illustrative purposes only.
On every study trial, the original scene was presented along with an orienting question which drew the viewer’s attention to the “critical item” in that scene, and encouraged processing relevant to the type of probe trial that would follow. The “item” question was constructed to elicit processing of the features of the critical item itself, whereas the “relational” question was constructed to elicit processing of the spatial relationships between the critical item and other items in the scene. For the scene in Figure 1, the question meant to orient the participant to the item itself was, “Is the blanket blue?” In contrast, the question meant to orient the participant to the position of the critical item in relation to other items in the scene was, “Is the blanket on the chair?”. Half of the scenes were associated with item questions that elicited “yes” responses and relational questions that elicited “no” responses, while the remainder were associated with orienting questions that elicited the opposite responses.
On every probe trial, the original scene was presented along with a manipulated version of that scene in a 2-alternative forced-choice format. The orienting questions were created so that the response given during the study trial would be uninformative for the purpose of responding on probe trials. That is, participants could not rely on the responses they provided during the study trials to disambiguate the original scene from the manipulated version of that scene when probe trials were presented.
Scenes were randomly assigned to lists, and counterbalancing was conducted such that each list rotated across conditions (item vs. relation), lags (1, 5, and 9) and blocks (block 1 and block 2). For relational changes, the critical item moved from left to right and from right to left equally often at each lag and within each experimental block for every participant.
Procedure
After the experimenter had obtained informed consent, participants were seated in front of the computer monitor and the task instructions were provided. Participants were told that a series of scenes (e.g., a bedroom scene, a café) would be presented on the computer screen, each with a question, and that the experimenter would read the question aloud. They were told that the question would require a yes/no response, and that it could be answered based on information presented in the picture. Additionally, participants were told that two pictures would be presented together on some of the trials, and that when this was the case they were to identify the picture that they had seen previously. It was emphasized that the pictures would be nearly identical, but that one was an exact match of a scene presented earlier, and that the other was a manipulated version of that scene – they were to choose the exact match. Participants provided verbal responses that were recorded by the experimenter via button press. The instructions were repeated between experimental blocks.
Each trial began with the presentation of a centrally located fixation cross, which remained on the screen for 2 seconds. During study trials, a single scene was presented for 10 seconds along with the associated orienting question. Participants were to respond to this question while the scene was still visible, and generally did not find it difficult to comply. During probe trials, two scenes were presented and the pair remained on the screen until the participant made their response. Participants were allowed to take a break between the first and second experimental blocks as needed.
All of the participants completed this experiment four times in four separate sessions; each scene was used twice, once in the item condition and once in the relational condition, in separate sessions. Altogether, there were 32 item trials per lag and 32 relational trials per lag for each participant. Debriefing was provided at the end of the final session.
Statistical analyses
The orienting questions had the intended effect, with participants’ answers indicating that they had processed the significant features (item condition) or spatial position (relational condition) of the critical item for the majority of trials. Those trials on which participants responded incorrectly (7.17% and 5.48% of the trials for amnesic patients and comparison participants, respectively) or failed to make a response (8.74% and 2.28% of the trials for amnesic patients and comparison participants, respectively) were eliminated from subsequent analyses.
The proportion of correct responses (i.e., correct identification of the previously viewed scene from among the 2-alternatives that were provided) made on the remaining trials was calculated for each participant at each lag. Due to possible violations of the homogeneity of variance associated with binary data summarized as proportions or percentages, an arcsine transformation was performed and statistical tests were conducted using the transformed data. Further, to improve the equality of variance, a correction was applied if performance was perfect (i.e., proportion correct = 1.0) prior to applying the arcsine transformation. The formula used to correct perfect scores was as follows: (n-1/4)/n, where n = total number of trials. Effect sizes (i.e., Cohen’s d) are reported and were calculated based on the subtraction of means across groups divided by the pooled standard deviation for contrasts of interest. Because we were working with small samples sizes, and there was relatively little variability in comparison group performance, all of the repeated measures ANOVAs were calculated using a nonparametric statistical approach (as reported by Konkel and colleagues, 2008; see also Olson et al., 2006). As was done by Konkel and colleagues, all of the reported repeated measures ANOVAs were calculated initially using the scores obtained from each participant in each condition of interest. Subsequently, scores were randomly assigned to individual participants and conditions of interest without replacement, and a new repeated measures ANOVA was performed. This procedure was repeated 10,000 times to generate a nonparametric distribution of F-values for main effects and interactions. An effect based on the originally obtained scores was considered statistically reliable if the F-value fell within the top 5% of the distribution for a given effect. The reported p-values indicate where the originally obtained F-values fell in the calculated distribution.
RESULTS
Performances of Comparison Participants Matched to HC and HC+ Patients were Statistically Equivalent
Results of a repeated measures ANOVA with the factors condition (item, relational) and lag (1, 5, and 9) indicated that there were no differences in performance between comparison participants matched to HC and HC+ patients (main effect of group: F(1,6)=.05, p=.82; group×condition interaction: F(1,6)=.75, p=.42; group×lag interaction: F(2,12)=.73, p=.50; group×condition×lag interaction: F(2,12)=.76, p=.49). Therefore, the comparison participant data are combined for all subsequent analyses.
Item Changes are Not Easier to Recognize than Relational Changes
A critical methodological contribution of this work was the assessment of memory for items and memory for relationships among items using materials, instructions, and procedures that were better matched across conditions than has been done in past work. Findings show that performance on tests of item and relational memory were comparable for the comparison participants (98.0 and 97.5 percent correct, respectively; t(7)=.56, p=.62), though near-ceiling results made it impossible to detect any small differences in performance that may have been present. To determine whether or not performance was well-matched across experimental conditions when the memory test was more difficult, a control experiment was conducted at the University of Wisconsin-Milwaukee (UWM).
After providing informed consent in a manner approved by the local institutional review board, sixteen students from the UWM community completed four interleaved study-test block sequences. Forty novel scenes were presented in each study block, and then, during a corresponding test block, participants were instructed to indicate whether the same scenes, presented one at a time and in random order, were repeated (20 trials/block) or manipulated (20 trials/block). For half of the participants manipulated scenes contained an item change and for the remaining participants, manipulated scenes contained a change in spatial relationships among scene elements as described above (see Stimuli and Design). The decision to use a between-subjects design was made to maximize the number of trials in a given condition for analyses that will be reported elsewhere.
For current purposes, analyses were limited to the subset of scenes that were used in the current investigation, and comparisons were made between groups. A statistically reliable difference in performance was evident across groups (i.e., conditions). Participants were less able to distinguish repeated from manipulated scenes when one of the items was replaced with a different exemplar (corrected recognition=61.10%, SD=12.20%; d'=1.83) than when one of the items moved to a new location (corrected recognition=76.23%, SD=10.83%, d'=2.58; t's(14)≥2.62, p’s=.02, Cohen’s d=1.31 for corrected recognition and d' scores, respectively). These results suggest that it is more difficult to detect item changes than to detect relational changes using the scenes that were developed for the experiment that is reported here. If anything, this difference should work against us and make it more difficult to obtain evidence for disproportionate relational memory impairments among amnesic patients in the current investigation.
Disproportionate Relational Memory Impairments are Evident Whether MTL Damage is Limited or Extensive
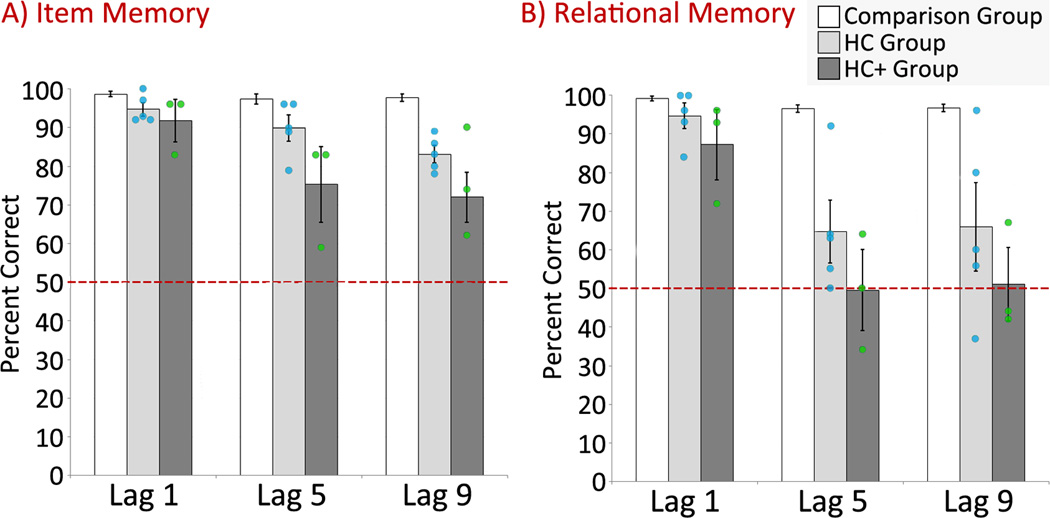
It was predicted that both HC and HC+ patients would be disproportionately impaired on the test of relational memory (vs. item memory) relative to comparison participants, and that impaired performance would be evident even when test trials were presented immediately after corresponding study trials (i.e., lag 1). In addition, it was predicted that HC+ patients would also be impaired on the test of item memory. To test these predictions, two between-groups repeated measures ANOVAs with the factors condition (item memory, relational memory) and lag (1, 5, and 9) were calculated using the non-parametric approach described earlier. One of these compared performances of HC patients to the comparison group and another compared performances of HC+ patients to the comparison group. Results showed that both HC and HC+ patients performed more poorly than comparison participants (main effects of group: F(1,11)=28.04, p=.001 and F(1,9)=113.05, p<.001, respectively), with performance disproportionately impaired on the test of relational memory (condition×group interactions: F(1,11)=8.09, p=.02 and F(1,9)=23.16, p=.001, respectively), and at longer lags (lag×group interactions: F(2,22)=22.94, p<.001 and F(2,18)=28.00, p<.001, respectively). There was also a condition×lag×group interaction when HC patients were compared to controls (F(2,22)=4.94, p=.01). This result was a consequence of a lag-based decline in performance that was greater on the test of relational memory than the test of item memory among HC patients (condition×lag interaction: F(2,8)=6.47, p=.02). Despite this difference in the magnitude of decline across conditions, performance did fall off significantly as a function of lag relative to the healthy comparison group in both experimental conditions (relational memory: lag×group interaction: F(2,22)=16.67, p<.001; item memory: lag×group interaction: F(2,22)=6.04, p=.01). This outcome is consistent with previous findings that show a precipitous drop in relational memory performance at longer lags in the same group of patients (Hannula et al., 2006). The 3-way interaction was not statistically reliable for HC+ patients (F(2,18)=.94, p=.41; see Figure 3); instead, the decline in performance across lags was similar on tests of item and relational memory (condition×lag interaction: F(2,4)=.69, p=.55).
Figure 3.
Percent correct on the tests of item (a) and relational memory (b) for the comparison group (white bars), for patients with damage limited in large part to the hippocampus (light gray bars), and for patients with extensive MTL damage (dark gray bars). The performance of individual patients from the HC and HC+ groups are illustrated with blue and green circles, respectively. Standard error bars are plotted around the mean and the dashed line represents chance performance.
Planned comparisons showed that both HC and HC+ patients were significantly impaired on the test of relational memory at all three lags, including the shortest possible lag when test trials were presented immediately after corresponding study trials (Lag 1: t(11)=2.10, p=.03, Cohen’s d=.78 and t(9)=3.43, p=.004, Cohen’s d=1.29; Lag 5: t(11)=5.94, p<.001, Cohen’s d=2.78 and t(9)=9.70, p<.001, Cohen’s d=4.35; Lag 9: t(11)=4.17, p=.001, Cohen’s d=1.90 and t(9)=10.20, p<.001, Cohen’s d=4.95, for HC and HC+ patients respectively). Performance at the two longer lags was not reliably better than chance for either group (HC patients: t’s(4)≤1.88, p’s≥.07; HC+ patients: t’s(2)≤.05, p’s≥.52). In contrast to the predicted outcomes, performances on the test of item memory were also impaired at all three lags for both groups (Lag 1: t(11)=2.58, p=.01, Cohen’s d=1.26 and t(9)=2.74, p=.01, Cohen’s d=1.20; Lag 5: t(11)=2.70, p=.01, Cohen’s d=1.23 and t(9)=4.83, p<.001, Cohen’s d=2.14; Lag 9: t(11)=7.11, p<.001, Cohen’s d=3.64 and t(9)=7.77, p<.001, Cohen’s d=3.88, for HC and HC+ patients respectively). However, in contrast to results reported for the test of relational memory, performance on the test of item memory was reliably better than chance for both groups at all three lags (all t’s(4)≥9.44, p’s<.001 and all t’s(2)≥3.03, p’s≤.05, for HC and HC+ patients respectively).
Finally, evaluation of response time data indicated that comparison participants made their recognition memory decisions in about half the amount of time required by the patients (Item Memory: comparison group = 4.08s, SD=.99; HC group = 8.11s, SD=.47; HC+ group = 9.96s, SD=1.31; Relational Memory: comparison group = 4.46s, SD=.76; HC group = 8.59, SD=.86; HC+ group = 8.79, SD=1.62). These differences were statistically reliable for both experimental conditions (HC vs. comparison group: t’s(11) ≥ 8.43, Bonferroni adjusted p’s <.001; HC+ vs. comparison group: t’s(11) ≥ 6.28, Bonferroni adjusted p’s <.001). It is important to reiterate that button presses were actually made by the experimenter (D.E.H), and that any interpretation of this outcome should be considered accordingly.
Item Recognition is Marginally Poorer among Patients with Extensive MTL Damage
It was predicted that patients in the HC and HC+ groups would perform similarly on the test of relational memory, but that HC+ patients would perform more poorly than HC patients on the test of item memory. To test this prediction, performances of patients in the HC and HC+ groups were compared directly using a non-parametric between-groups repeated measures ANOVA with the factors condition (item memory, relational memory) and lag (1, 5, and 9). Consistent with results reported above, patients performed more poorly on the test of relational memory than the test of item memory (main effect of condition: F(1,6) = 19.75, p=.005), particularly at longer lags (condition×lag interaction: F(2,12)=4.44, p=.04). However, between-group differences in performance were not statistically reliable (condition×group interaction: F(1,6)=.12, p=.74; lag×group interaction: F(2,12) = .97, p=.41; condition×lag×group interaction: F(2,12) = .19, p=.83).
Because we had predicted differences in performance across groups on the test of item memory, Bonferroni corrected post-hoc comparisons were performed despite the lack of group differences in the ANOVA. When performances on the test of item memory were compared directly for the two patient groups, there was a trend in the expected direction at the longest lag, with the HC group outperforming the HC+ group (t(6)=2.34, p=.09, Cohen’s d= 1.51). Although results were also in the expected direction for lags 1 and 5, differences in recognition memory performance were not statistically significant (t’s(6)≤2.15, p’s≥.11; see Figure 3). As expected, there were no differences between groups on the test of relational memory at any lag (t's(6)≤1.27, p’s≥.33).
DISCUSSION
The performances of two groups of amnesic patients with more or less extensive MTL damage were evaluated in the reported investigation on two tests of memory, one that emphasized memory for relationships among items embedded in scenes and another that emphasized memory for the items themselves, in the context of the same scenes. Successful performance on both tests relied on specific information about a critical object, either memory for details about its physical form (item memory) or memory for its relative location (relational memory). The design of these tasks was meant to better equate the amount and complexity of to-be-remembered information across experimental conditions, and to minimize any differences in task difficulty that might otherwise affect performance. This is a notable departure from past work (although see Pigott & Milner, 1993), which has compared memory for studied items to memory for the co-occurrence of studied items (e.g., Giovanello et al., 2003; Gold et al., 2006; Turriziani, et al., 2004; Stark et al., 2002), or memory for whole scenes to memory for detailed inter-item relationships embedded in those scenes (e.g., Ryan, et al., 2000; Hannula, et al., 2006).
As predicted, a key outcome of the reported work was evidence for disproportionate decline in performance on the test of relational memory across lags whether MTL damage was limited or more extensive. Both groups of MTL patients showed a precipitous drop in recognition memory accuracy at longer lags, greater for relational memory than for item memory, with performance on the relational memory test no better than chance when just 4 trials separated corresponding sample and probe displays (i.e., lag 5). These findings complement past reports that have shown hippocampal amnesia is associated with a selective or disproportionate deficit in long-term memory for relationships among items (e.g., Giovanello et al., 2003; Hannula et al., 2006; Konkel et al., 2008; Ryan, et al., 2000; Ryan & Cohen, 2004; Turriziani, et al., 2004), and provide additional support for the relational memory theory (e.g., Eichenbaum et al., 1994). Here, the data from the two patient groups inform us further, indicating that extensive damage to MTL structures is not necessary to elicit at-chance performance on a 2-alternative forced-choice recognition test when relational memory is being evaluated. We return to this outcome below.
The second major finding concerned contributions of structures in the parahippocampal region to memory for items. Whereas the deficit in relational memory was comparable for the two patient groups despite differences in the extent of their MTL lesions, post-hoc comparisons hinted at the possibility of differences in the integrity of item memory across groups, with marginally worse performance among HC+ patients at the longest lag (i.e., lag 9). Results were also in the predicted direction for lags 1 and 5, but these small differences in performance were not statistically reliable. Careful evaluation of individual patient performance may provide some additional insight, particularly since a subset of patients assigned to the HC group had modest volume reduction in the parahippocampal gyrus. The performance of one patient in particular (i.e., 2144) is worth noting, as she had gray and white matter parahippocampal gyrus volumes that were within normal limits (see Table 2). With just one exception (i.e., 2563, the HC patient for whom we do not have volume estimates), patient 2144 outperformed all of the remaining patients when item memory was tested. That said, it is still the case that her performance at longer lags fell short of the comparison group mean (Lag 5: control mean = 97% correct; 2144 – 90% correct; Lag 9: control mean = 98% correct; 2144 = 86% correct), a pattern of results that hints at potential hippocampal contributions to item memory. In sum, there is some evidence in favor of the predicted outcome, but the absence of a statistically reliable group difference prevents us from reaching strong conclusions about the correspondence between structures in the parahippocampal region and item memory in this experiment. That said, it is important to keep in mind that items were embedded in complex scenes – contributions of this factor, and others, to patient performance on the item memory test are considered in the discussion that follows.
Given the expectation that item memory would be relatively preserved in HC patients, it is important to consider what might account for the observed item memory impairments. However, before describing some of the factors that may have contributed to the reported outcome, it is worth reiterating that unanticipated differences in task difficulty across experimental conditions were evident in the control experiment conducted with neurologically intact college-age participants. The control experiment was designed to bring accuracy down, as the performance of healthy comparison participants in the primary investigation was near ceiling. When overall performance was reduced, it became clear that the test of memory for item identity was more difficult than the test of memory for spatial relationships – this difference in task difficulty may have contributed to the modest deficit in item memory among hippocampal amnesic patients, a possibility that could be examined in future work. With this in mind, we will now consider additional factors that may have contributed to the reported outcome.
One potential explanation for the unanticipated item memory deficit among HC patients is that the proposed functional contributions of the parahippocampal region and the hippocampus to item and relational memory, respectively, are not strictly dichotomous (e.g., Squire, et al., 2004). But, as indicated in the introduction, there is strong evidence from the animal and neuroimaging literatures in support of the view that there are qualitative differences in the types of memory processing supported by the hippocampus and adjacent structures in the parahippocampal region. Furthermore, the impairment seen here among HC patients on the item memory task was quite mild, never even approaching the chance level performance they showed on the relational memory task at longer lags.
A second possibility emphasizes the potential contribution of the parahippocampal region to item memory, even within the framework of relational memory theory. We have proposed (Eichenbaum, et al., 1994) that while the hippocampus is critically involved in binding together the constituent elements of some scene or event, and in retrieving those relationally bound representations for some time after learning, the parahippocampal region mediates the persistence of single item representations in service of hippocampal binding. Something that remains unclear is for how long, and under what circumstances, the parahippocampal region can fully maintain these representations. It is possible that with time, across several intervening items, or with increasing complexity of the context in which the item is experienced (e.g., items embedded in scenes), the hippocampus itself is recruited to take on some of this responsibility. If this were the case, then we might expect to see some decline in performance across lags among patients with hippocampal damage because they are disadvantaged compared to healthy individuals who can recruit an intact hippocampus to help maintain relevant representations. However, this decline should not be nearly as severe as the drop in performance on tasks that require relational memory processing, for which the hippocampus is the necessary substrate.
In addition to any potential hippocampal involvement consequent to the length of the retention interval or the complexity of represented information, it may be the case that the item memory impairment is related to the selectivity or purity of the tasks used here. Documenting dissociations between impaired and spared performances following MTL damage presents a formidable challenge in the neuropsychological literature. With regard to the specific dissociation being tested here, it is not completely clear how best to test the status of item and relational memory separately when so much of our knowledge is bound up in memory for relationships among items. The manner in which items may call up the information with which they were associated in the past was appreciated very early in the history of psychology, and forms the basis for a more contemporary distinction in the recognition memory literature between recollection and familiarity (cf. Yonelinas, 2002). For instance, James (1890/1918) wrote that “…objects once experienced together tend to become associated in the imagination, so that when any one of them is thought of, the others are likely to be thought of also, in the same order of sequence of coexistence as before” (p. 561).
The challenge of designing tasks that are process pure (i.e., that tap a single process exclusively) is the bane of many fields; for example, it is at the heart of major debates in the perception literature about the mere existence of implicit perception. In that literature, evidence for processing in the absence of awareness is undermined by the possibility that some degree of conscious awareness may have affected performance on the test used to measure implicit perception (see Hannula, Simons, & Cohen, 2005). Similarly, in the work reported here we cannot be sure that performance on the test of memory for items did not stand to benefit from influences of relational memory processing (see also Kan, Giovanello, Schnyer, et al., 2007). To illustrate, imagine that you have been shown the picture of the hospital room in Figure 1; your attention has been directed to the blue blanket by virtue of the associated orienting question, and after having answered the question in compliance with experimental instructions, you think of a similar blanket that you have at home, or notice that the blanket is not quite the same shade of blue as the chair or the curtains also present in the picture. On the corresponding test trial you are presented with two versions of the hospital room picture and are to identify the one that is an exact match of the picture seen earlier based on your memory for the critical item (i.e., the blue blanket). The items bear a reasonably strong resemblance to one another, and consequently neither item feels more familiar to you than the other; you do, however, successfully identify the blanket because you remember having thought about a similar one you have at home, or because you remember having observed that the blanket was not quite the same shade of blue as the curtains and the chair.
In this way, elaborative encoding and subsequent recall (or recollection) of relationships between the critical item and other items in the room, or experiences outside the context of the experiment, might contribute to one’s ability to identify the previously viewed item when the strength of the memory representation associated with the item itself fails to discriminate it from the foil (see Anderson, 1980, p. 193, for a similar example). Past work suggests that elaborative encoding strategies like these are generated spontaneously in neurologically intact individuals (Gardiner, Ramponi, & Richardson-Klavehn, 1998), and it does not seem unreasonable to assume that these relationally-bound memory representations may then contribute to successful recollection when recognition tests, like the one used here, are administered. Whether or not patients with amnesia might also spontaneously engage in elaborative encoding remains to be examined, but several investigations suggest that the hippocampus contributes critically to conscious recollection of studied content (cf. Montaldi & Mayes, 2010; Yonelinas et al., 2010). It stands to reason then, that if memory strength failed to differentiate the studied exemplar from the foil on a subset of trials, patients with amnesia would be at a distinct disadvantage relative to healthy comparison participants because they could not rely on recollection of relationally-bound memory representations to rescue performance. Future work could address questions about whether or not this explanation has any traction by having healthy participants indicate whether their recognition memory responses were driven by familiarity or recollection (see Migo, Mayes, & Montaldi, 2012) using materials like the ones developed here.
In addition to the potential absence of recollection-based performance advantages, it is important to entertain the possibility that the reported item memory impairment was due to deficient online processing of the presented alternatives when forced-choice probe trials were presented. A handful of recent investigations have implicated the hippocampus in online processing and comparison of complex materials like the scenes used here (Voss et al., 2011; Warren, et al., 2011, 2012; Yee et al., 2014b; see Lee et al., 2012 and Olsen et al., 2012 for reviews; but see Erez et al., 2013), and evaluation of our reaction time data indicates that patients were slower to respond than healthy comparison participants when test trials were administered1. This outcome may reflect slower detection of differences across scenes (e.g. because patients repeatedly visit previously evaluated parts of each picture; cf. Yee et al., 2014b), uncertainty about what was studied despite intact detection of perceptual differences across scene exemplars (i.e., a memory impairment), or some combination thereof. In the absence of additional, more sensitive measures that can index online processing of presented content (e.g., eye movement measures; see Hannula et al., 2010 for review), we cannot provide definitive evidence against contributions of defective online processing to the reported impairments. Notably though, this is not a viable explanation for the full pattern of reported results, including the unexpected item memory impairment. This is because performance on the test of item memory declined disproportionately across lags for both groups of patients. Lag-based decline could not have been a consequence of deficient online processing because demands on the comparison process remain the same whether probe displays are presented immediately after corresponding study displays (lag 1) or several trials later (lags 5 and 9). Nonetheless, it would be of interest, in future work, to evaluate more systematically whether, and to what extent, memory-based online processing deficiencies are contributing to reported outcomes in tasks that depend on the integrity of a forced-choice comparison process, as was the case here.
One last factor that deserves consideration with respect to the reported item memory outcomes concerns characteristics of the patients themselves. It may be the case, for example, that documented item memory impairments were a consequence of our having recruited patients with clinically significant memory impairments on standardized tests. In other words, memory impairments may not be dictated strictly by the nature/extent of MTL damage in our patients, but rather by the selection of patients with documented memory difficulties (cf. Holdstock et al., 2008). At least one observation suggests that this explanation is unlikely. The performance of patient 2144 on the General Memory Index was 43 points below her Full-Scale IQ score; this spread meant that she was an outlier relative to other patients in the HC group, whose FSIQ-GMI performances were separated by no more than 27 points. If selection of patients with deficient performance on standardized tests was contributing to the item memory impairment, we might expect all of the remaining HC patients to outperform 2144 on the test of item memory. Indeed, we might expect her performance to be most like the HC+ group, whose FSIQ-GMI differences ranged from 46–53. However, in contrast to these potential outcomes, 2144’s performance on the item memory test was quite good, and she was the second best performer in the HC group. As discussed earlier, this result makes good anatomical sense, as she was the only patient in the HC group whose parahippocampal gyrus volume (gray and white matter) was within normal limits. The only patient who outperformed her on the test of item memory was not eligible for MRI scanning, but based on evaluation of a CT scan, appears to have damage limited to the hippocampus. Another notable outcome of the reported results was the absence of full-blown item memory impairment among patients with extensive MTL damage. Two of these patients appear to have some residual tissue in the perirhinal cortex (1951 in the right hemisphere; 2308 in the left hemisphere), but whether or not this tissue remains viable is unclear. All three patients have relative sparing in more posterior aspects of the parahippocampal gyrus (at least unilaterally), likely corresponding to parahippocampal cortex, which itself has been implicated in memory for context (cf. Diana, Yonelinas, & Ranganath, 2012; Aminoff, Kveraga, & Bar, 2013). It is possible that a unitized (or fused) representation of the whole scene could support some residual discrimination of targets from foils, though why this compensation would not extend to the relational memory condition is unclear. In sum, the mechanism driving the relative success (i.e., above-chance performance) of HC+ patients on the test of memory for items embedded in complex scenes cannot be pinned down here, but could be evaluated in a future neuroimaging investigation.
Our final prediction concerned potential contributions of MTL structures (namely, the hippocampus) to short-term retention of studied content. Whether, and under what circumstances, the hippocampus contributes to short-term memory is currently a matter of debate (cf. Jeneson & Squire, 2011; Yonelinas, 2013), but converging evidence from neuroimaging (e.g., Axmacher et al., 2007; Davachi & Wagner, 2002; Hannula & Ranganath, 2008; Karlsgodt et al., 2005; Nichols et al., 2006; Piekema et al., 2006; Ranganath & D’Esposito, 2001; Schon et al., 2004; Stern et al., 2001) and neuropsychological (e.g., Cashdollar et al., 2009; Hartley et al., 2007; Olson et al., 2006; Warren et al., 2012; Watson et al., 2013; Yee et al., 2014a) studies is mounting in favor of this view. Consistent with these studies, we found that amnesic patients who participated in the current experiment were impaired on the memory tests used here even when there were no intervening items between corresponding study and test displays. Similar lag 1 deficits have been reported previously in two additional experiments conducted with a subset of the patients tested here (Hannula, et al., 2006), and more recently we have documented near-chance performance when these patients participated in experiments with more conventional short-term memory demands (e.g., a standard short-term memory trial structure; Yee et al., 2014a; see also Watson et al., 2013). It is important to mention that these outcomes have not always been replicated (Baddeley et al., 2010, 2011; Jeneson et al., 2010, 2011; Shrager et al., 2008). What exactly is driving differences in reported outcomes remains to be determined, but as outlined in more detail elsewhere (Yee et al., 2014a), it is our contention that the absence of impairment in these studies may reflect the use of tasks that depend upon perceptual feature binding, supported by extra-hippocampal structures, or the formation and use of unitized or fused memory representations, which seem to depend on MTL cortical structures (e.g., Haskins et al., 2008; Quamme et al., 2007; for another recent perspective see Yonelinas, 2013).
Before concluding, it is important to consider our results in the context of the broader memory literature. First, there is notable resemblance between our investigation and earlier work conducted by Pigott and Milner (1993). As was done here, Pigott and Milner assessed memory for item identity and item position (i.e., left/right displacement), but pictures were line drawn scenes, and patients had undergone temporal lobectomies. To evaluate MTL contributions to task performance, patients were split into groups based on the location (i.e., left vs. right hemisphere) and extent (i.e., lesions limited to the hippocampal head and anterior MTL structures vs. lesions extending into the body of the hippocampus and/or posterior MTL structures) of the surgical resection. Impaired performance on both tests was reported for patients with right-lateralized lesions whether the resections were limited or extensive. Because the impairment was not worse when hippocampal resections were larger, it was proposed that memory for visual details and the spatial composition of scenes is supported by right-sided anterior temporal neocortical structures, not the hippocampus. On their face, these conclusions are strikingly different from ours, but it is important to point out that even patients with smaller resections had lesions that compromised the most anterior part of the hippocampus. As such, arguments in favor of the view that the impairment was not due to hippocampal damage may be incorrect. Indeed, recent neuroimaging investigations have reported activity differences that are especially robust in the anterior-most part of the hippocampus when tasks have relational memory processing demands (e.g., Giovanello et al., 2009; Hannula et al., 2013). Clearly, more work is needed to adjudicate among alternative interpretations of the reported work, and to identify the boundary conditions associated with hippocampal contributions to memory for scene detail.
Finally, it is worth evaluating observations of at-chance performance on the 2-alternative forced-choice test in the context of past work, and the complementary learning systems (CLS) framework. Previous studies have shown that hippocampal amnesic patients perform within normal limits on forced-choice item recognition tests, even when the studied target and the associated foil are perceptually similar (e.g., Holdstock et al., 2002), an outcome consistent with the CLS model. According to this model, the hippocampus is specialized to encode pattern separated memory representations that can support subsequent recall (pattern completion) in response to a partial cue. In contrast, the perirhinal cortex is thought to distinguish studied and novel item by virtue of differences in memory strength rather than pattern separation (cf. Norman, 2010; Norman & O’Reilly, 2003). Critically, if similar lures are used during test, the CLS model predicts that perirhinal cortex cannot support accurate performance on old/new recognition memory tests because a strong memory signal is generated by both studied items and corresponding lures; instead, successful recognition depends upon recall, and performance is hippocampus-dependent. However, if corresponding targets and lures are presented together (as in the 2-alternative forced-choice test used here), the CLS model predicts that a small, but reliable difference in cortical signal strength will support successful recognition. Because the alternatives are so similar, they both drive a familiarity signal – however, when they are presented together, the studied picture should have a slight, but sufficient advantage (i.e. greater relative memory strength), which should drive accurate responding even when the hippocampus is damaged. Under these circumstances, one might have predicted that performances on tests of item memory and relational memory would be intact in our experiment. To date, most of the empirical work and computational modeling that has tested predictions of the CLS model has focused on item, rather than scene or relational, memory (cf. Norman, 2010). How exactly this model maps on to other types of memory representations remains unclear, but our results suggest that strength-based memory processes, said to be mediated by MTL cortex, cannot support relational memory performance, even when 2-alternative forced-choice test trials are used.
General Conclusions
The experiment reported here was designed to explore memory for items and memory for the relations among items, separately, in two groups of well-characterized amnesic patients differing in the extent MTL damage. Test trials were systematically presented among study trials, and the materials, instructions, testing procedures, and level of difficulty were well matched across conditions. Hippocampal amnesia was associated with a disproportionate deficit in relational memory, regardless of the extent of MTL damage – a finding that provides strong support for the relational memory theory. There was some evidence for less severe item memory impairment when MTL damage was relatively circumscribed to the hippocampus. However, relative to the comparison group, memory for items was impaired for both HC and HC+ patients, which raises some issues about the ability to test item and relational memory in a process pure way and the challenge of tying those forms of memory unambiguously to distinct MTL regions in patients with naturally occurring lesions. Finally, impairment was observed even at the shortest lag (lag 1), with no intervening items between the study trial and the corresponding test trial. This last finding is consistent with current neuroimaging findings of possible hippocampal involvement in memory even at the short lags and delays characteristic of working memory, and with our recent studies of hippocampal amnesia, contrary to classic views of hippocampal function in the neuropsychology literature.
Supplementary Material
Acknowledgments
This work was supported by NINDS P01 NS19632 and NIDA R01 DA022549 grants to D.T., and NIMH RO1 MH062500 to N.J.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of NINDS, NIDA, or NIMH. We would like to express our gratitude to the patients and their families, and would also like to thank Alex Konkel for sharing a matlab data analysis script and Luke Jenkins for modifying it.
Footnotes
Note that participants made verbal responses, and that the corresponding button press was made by the experimenter (D.E.H). This was done to avoid any potential response mapping difficulties among patients. Results and conclusions based on response times should be considered with this in mind.
REFERENCES
- Aggleton JP, Brown MW, Albasser MM. Contrasting brain activity patterns for item recognition memory and associative recognition memory: insights from immediate-early gene functional imaging. Neuropsychologia. 2012;50:3141–3155. doi: 10.1016/j.neuropsychologia.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Allen JS, Tranel D, Bruss J, Damasio H. Correlations between regional brain volumes and memory performance in anoxia. Journal of Clinical and Experimental Neuropsychology. 2006;28:457–476. doi: 10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends in Cognitive Science. 2013;17:379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR. Cognitive Psychology and Its Implications. San Francisco: W. H. Freeman and Company; 1980. [Google Scholar]
- Awipi T, Davachi L. Content-specific source encoding in the human medial temporal lobe. Journal of Experiment Psychology: Learning, Memory and Cognition. 2008;34:769–779. doi: 10.1037/0278-7393.34.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, et al. Sustained neural activity patterns during working memory in the human medial temporal lobe. The Journal of Neuroscience. 2007;27:7807–7816. doi: 10.1523/JNEUROSCI.0962-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Allen R, Vargha-Khadem F. Is the hippocampus necessary for visual and verbal binding in working memory? Neuropsychologia. 2010;48:1089–1095. doi: 10.1016/j.neuropsychologia.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Jarrold C, Vargha-Khadem F. Working memory and the hippocampus. Journal of Cognitive Neuroscience. 2011;23:3855–3861. doi: 10.1162/jocn_a_00066. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociations of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and the hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Emotional autobiographical memories in amnesic patients with medial temporal lobe damage. Journal of Neuroscience. 2005;25:3151–3160. doi: 10.1523/JNEUROSCI.4735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar N, Malecki U, Rugg-Gunn FJ, Duncan JS, Lavie N, Duzel E. Hippocampus-dependent and –independent theta-networks of active maintenance. Proceedings of the National Academy of Sciences USA. 2009;106:20493–20498. doi: 10.1073/pnas.0904823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaco S, Feinstein JS, van Twillert H, Tranel D. Musical memory in a patient with severe anterograde amnesia. J Clin Exp Neuropsychol. 2012;34:1089–1100. doi: 10.1080/13803395.2012.728568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ. Preserved learning capacity in amnesia: Evidence for multiple memory systems. In: Butters N, Squire LR, editors. The Neuropsychology of Memory. New York: Guilford Press; 1984. pp. 83–103. [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Science USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. Journal of Neurophysiology. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Demery JA, Hanlon RE, Bauer RM. Profound amnesia and confabulation following traumatic brain injury. Neurocase. 2001;7:295–302. doi: 10.1093/neucas/7.4.295. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Adaptation to cognitive context and item information in the medial temporal lobes. Neuropsychologia. 2012;50:3062–3069. doi: 10.1016/j.neuropsychologia.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Science. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behavioral and Brain Sciences. 1994;17(3):449–517. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez J, Lee AC, Barense MD. It does not look odd to me: perceptual impairments and eye movements in amnesic patients with medial temporal lobe damage. Neuropsychologica. 2013;51:168–180. doi: 10.1016/j.neuropsychologia.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Rudrauf D, Khalsa SS, Cassell MD, Bruss J, Grabowski TJ, Tranel D. Bilateral limbic system destruction in man. J Clin Exp Neuropsychol. 2010;32:88–106. doi: 10.1080/13803390903066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner JM, Ramponi C, Richardson-Klavehn A. Experiences of remembering, knowing, and guessing. Consciousness & Cognition. 1998;7:1–26. doi: 10.1006/ccog.1997.0321. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Verfaellie M, Keane MM. Disproportionate deficit in associative recognition relative to item recognition in global amnesia. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(3):186–194. doi: 10.3758/cabn.3.3.186. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, Verfaellie M. Distinct hippocampal regions make unique contributions to relational memory. Hippocampus. 2009;19:111–117. doi: 10.1002/hipo.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JJ, Hopkins RO, Squire LR. Single-item memory, associative memory, and the human hippocampus. Learning & Memory. 2006;13:644–649. doi: 10.1101/lm.258406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JJ, Smith CN, Bayley PJ, Shrager Y, Brewer JB, Stark CE, Hopkins RO, Squire LR. Item memory, source memory, and the medial temporal lobe: concordant findings from fMRI and memory-impaired patients. PNAS. 2006;103:9351–9356. doi: 10.1073/pnas.0602716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Althoff RR, Warren DE, Riggs L, Cohen NJ, Ryan JD. Worth a glance: using eye movements to investigate the cognitive neuroscience of memory. Frontiers in Human Neuroscience. 2010;4:166. doi: 10.3389/fnhum.2010.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Libby LA, Yonelinas AP, Ranganath C. Medial temporal lobe contributions to cued retrieval of items and contexts. Neuropsychologia, epub ahead of print. 2013 doi: 10.1016/j.neuropsychologia.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. Journal of Neuroscience. 2008;28:116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Simons DJ, Cohen NJ. Imaging implicit perception: promise and pitfalls. Nature Reviews Neuroscience. 2005;6:247–255. doi: 10.1038/nrn1630. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. Journal of Neuroscience. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, Burgess N. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17:34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins AL, Yonelinas AP, Quamme JR, Ranganath C. Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron. 2008;59:554–560. doi: 10.1016/j.neuron.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O’Reilly RC, Norman KA. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12:341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Parslow DM, Morris RG, Fleminger S, Abrahams S, Denby C, Montaldi D, Mayes AR. Two case studies illustrating how relatively selective hippocampal lesions in humans can have quite different effects on memory. Hippocampus. 2008;18:679–691. doi: 10.1002/hipo.20427. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology: Volume I. New York: Henry Holt and Company; 1890/1918. [Google Scholar]
- Jeneson A, Mauldin KN, Hopkins RO, Squire LR. The role of the hippocampus in retaining relational information across short delays: the importance of memory load. Learning & Memory. 2011;18:301–305. doi: 10.1101/lm.2010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Mauldin KN, Squire LR. Intact working memory for relational information after medial temporal lobe damage. Journal of Neuroscience. 2010;30:13624–13629. doi: 10.1523/JNEUROSCI.2895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Squire LR. Working memory, long-term memory, and medial temporal lobe function. Learning and Memory. 2011;19:15–26. doi: 10.1101/lm.024018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan IP, Giovanello KS, Schnyer DM, Makris N, Verfaellie M. Role of the medial temporal lobes in relational memory: neuropsychological evidence from a cued recognition paradigm. Neuropsychologia. 2007;45:2589–2597. doi: 10.1016/j.neuropsychologia.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Shirinyan D, van Erp TGM, Cohen MS, Cannon TD. Hippocampal activations during encoding and retrieval in a verbal working memory paradigm. Neuroimage. 2005;25:1224–1231. doi: 10.1016/j.neuroimage.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Köhler S, Crane J, Milner B. Differential contributions of the parahippocampl place area and the anterior hippocampus to human memory for scenes. Hippocampus. 2002;12:718–723. doi: 10.1002/hipo.10077. [DOI] [PubMed] [Google Scholar]
- Köhler S, Danckert S, Gati JS, Menon RS. Novelty responses to relational and non-relational information in the hippocampus and the parahippocampal region: A comparison based on event-related fMRI. Hippocampus. 2005;15:763–774. doi: 10.1002/hipo.20098. [DOI] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel D, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. article 15. Frontiers in Human Neuroscience. 2008;2 doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Yeung LK, Barense MD. The hippocampus and visual perception. Frontiers in Human Neuroscience. 2012;6:91. doi: 10.3389/fnhum.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Science. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Migo EM, Mayes AR, Montaldi D. Measuring recollection and familiarity: improving the remember/know procedure. Consciousness and Cognition. 2012;21:1435–1455. doi: 10.1016/j.concog.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Mayes AR. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010;20:1291–1314. doi: 10.1002/hipo.20853. [DOI] [PubMed] [Google Scholar]
- Nichols EA, Kao YC, Verfaellie M, Gabrielli JD. Working memory and long-term memory for faces: Evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:604–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA. How hippocampus and cortex contribute to recognition memory: Revisiting the complementary learning systems model. Hippocampus. 2010;20:1217–1227. doi: 10.1002/hipo.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychological Review. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Olsen RK, Moses SN, Riggs L, Ryan JD. The hippocampus supports multiple cognitive processes through relational binding and comparison. Frontiers in Human Neuroscience. 2012;6:146. doi: 10.3389/fnhum.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. Journal of Neuroscience. 2006;26:4596–4601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekema C, Kessels RP, Mars RB, Petersson KM, Fernandez G. The right hippocampus participates in short-term memory maintenance of object-location associations. Neuroimage. 2006;33:374–382. doi: 10.1016/j.neuroimage.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Pigott S, Milner B. Memory for different aspects of complex visual scenes after unilateral temporal- or fontral-lobe resection. Neuropsychologia. 1993;31:1–15. doi: 10.1016/0028-3932(93)90076-c. [DOI] [PubMed] [Google Scholar]
- Quamme JR, Yonelinas AP, Norman KA. Effect of unitization on associative recognition in amnesia. Hippocampus. 2007;17:192–200. doi: 10.1002/hipo.20257. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychological Science. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Cohen NJ. Evaluating the neuropsychological dissociation evidence for multiple memory systems. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:168–185. doi: 10.3758/cabn.3.3.168. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Cohen NJ. Processing and short-term retention of relational information in amnesia. Neuropsychologia. 2004;42:497–511. doi: 10.1016/j.neuropsychologia.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. Journal of Neuroscience. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Levy DA, Hopkins RO, Squire LR. Working memory and the organization of brain systems. Journal of Neuroscience. 2008;28:4818–4822. doi: 10.1523/JNEUROSCI.0710-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. Journal of Cognitive Neuroscience. 2008;20:1478–1489. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Mind the gap: binding experiences across space and time in the human hippocampus. Neuron. 2009;63:267–276. doi: 10.1016/j.neuron.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stark CEL, Bayley PJ, Squire LR. Recognition memory for single items and for associations is similarly impaired following damage to the hippocampal region. Learning & Memory. 2002;9:238–242. doi: 10.1101/lm.51802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CEL, Squire LR. Hippocampal damage equally impairs memory for single items and memory for conjunctions. Hippocampus. 2003;13:281–292. doi: 10.1002/hipo.10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus. 2001;11:337–346. doi: 10.1002/hipo.1048. [DOI] [PubMed] [Google Scholar]
- Turriziani P, Fadda L, Caltagirone C, Carlesimo GA. Recognition memory for single items and for associations in amnesic patients. Neuropsychologia. 2004;42:426–433. doi: 10.1016/j.neuropsychologia.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Voss JL, Warren DE, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Spontaneous revisitation during visual exploration as a link among strategic behavior, learning, and the hippocampus. Proc Natl Acad Sci. 2011;108:E402–E409. doi: 10.1073/pnas.1100225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. Journal of Neuroscience. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Tranel D, Cohen NJ. Observing degradation of visual representations over short intervals when medial temporal lobe is damage. J Cogn Neurosci. 2011;23:3862–3873. doi: 10.1162/jocn_a_00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Jensen U, Tranel D, Cohen NJ. Hiding in plain view: lesions of the medial temporal lobe impair online representation. Hippocampus, epub ahead of print. 2012 doi: 10.1002/hipo.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Magnotta V, Capizzano AA, Cassell MD, Tranel D. Long-term neuropsychological, neuroanatomical, and life outcome in hippocampal amnesia. Clinical Neuropsychology. 2012;26:335–369. doi: 10.1080/13854046.2012.655781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PD, Voss JL, Warren DE, Tranel D, Cohen NJ. Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus. 2013;23:570–580. doi: 10.1002/hipo.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. 2004 doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee LT, Hannula DE, Tranel D, Cohen NJ. Short-term retention of relational memory in amnesia revisited: Accurate performance depends on hippocampal integrity. Frontiers in Human Neuroscience. 2014a;8 doi: 10.3389/fnhum.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee LT, Warren DE, Voss JL, Duff MC, Tranel D, Cohen NJ. The hippocampus uses information just encountered to guide efficient ongoing behavior. Hippocampus. 2014b;24:154–164. doi: 10.1002/hipo.22211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The Nature of Recollection and Familiarity: A review of 30 Years of Research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP. The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behavioral Brain Research. 2013;254:34–44. doi: 10.1016/j.bbr.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Aly M, Wang WC, Koen JD. Recollection and familiarity: examining controversial assumptions and new directions. Hippocampus. 2010;20:1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.