Abstract
Identification of families with history of cancer in the municipality of Angra dos Reis, Rio de Janeiro (Brazil), through the Brazilian Unified Primary Health Care System was explored based in the Community Health Agents (CHA) program. This study was divided into two phases: a descriptive one with a cross-sectional epidemiological data of families with history of cancer based on CHA-collected data from home visits in four primary health care units. The second phase consisted in identifying familial clustering of three or more individuals with cancer through construction of a three-generation pedigree and revisited by an itinerant group of medical geneticists. Genetic counseling was carried out with the intent of selecting potential families at risk for hereditary familial cancers. In the first phase of the study, 1,581 families were interviewed by the CHA at their homes. A positive history for cancer was present in 42.3 % of families, comprising 22.3 % with only one case per family, 11.2 % with two cases, and 8.6 % with three or more cases in the family. The informant reported that 15 % of the cases were from the father lineage, 12 % from the mother lineage, and 12.1 % within siblings. In the remaining 60.9 % families, cancer was present in both sides of the family. The types of cancer reported were uterus 8.7 % (n = 137), stomach 7.7 % (n = 122), breast 6.9 % (n = 109), throat 6.8 % (n = 99), prostate 5.4 % (n = 85), lung 5.6 % (n = 88), bowel 3.7 % (n = 59), and unspecified sites in 6.8 % (n = 108) of families. No statistical differences were noted between the data collected on each primary care unit. In the second phase of the study, 136 families (2.9 %) from the total of families interviewed in phase 1 were selected due to the presence of three or more individuals with cancer in the family. Among those, only 73 families attended genetic counseling. Comparison between the data obtained by the CHA and the medical geneticists shows complete agreement in 36 cases (49.3 %), partial agreement in 25 cases (34.2 %) with more detailed information in the CHA sheets, discordance in 4 cases (5.5 %), and not possible to correlate in 8 cases due to identification inconsistency. Risk assessment for cancer was calculated based on the criteria adopted by Scheuner et al. (Genet Med 12(11):726–735, 2010) and revealed that 50.0 % of the families were classified as having a weak risk, 36.1 % a moderate risk, and 13.8 % were considered of high risk. Concerning known hereditary cancer syndromes, we found one family that met the criteria for breast and ovary hereditary cancer (1.4 %) and one family with non-polyposis hereditary colon cancer as revised by Bethesda protocol. Such preliminary results indicated that the Brazilian Primary Health Care system based on the CHA framework can be an effective entrance into the Unified Brazilian Health Care System (SUS-Brazil) for individuals with genetically determined diseases, such as familial cancer. Families with a history of three or more cases of cancer and considered of high risk for familial cancer could be referred to a tertiary health center for proper oncogenetic counseling.
Keywords: Familial clustering of cancer, Primary health care, Community health agents, Community genetics, Genetic counseling
Introduction
Community genetics is one of the interfaces of medical genetics that, by recognizing the role of genetically determined factors as disease burden to people, aims to provide services in this area of medical knowledge through inter- and transdisciplinary actions in the community (Khoury 2000; Ramalho and Silva 2000). One of its field actions is the recognition of genetically determined conditions with two main objectives: the identification of individuals and families at risk of becoming ill and transmitting genetic diseases, such as the hemoglobinopathies or hereditary cancer; and early identification of genetic conditions for tertiary treatment and prevention through genetic counseling (Ramalho and Silva 2000; Llerena 2002; Brasil 2009).
The study of populations, rather than the study of a family, together with the clinical and genetic epidemiology has its interfaces in the public health scenario introducing preventive measures such as genetic counseling. Recently, it has been the main object of interest in the newly founded Brazilian Institute of Population Medical Genetics (INAGEMP) (2009).
The INAGEMP’s collaborative group 5, coordinated by the Medical Genetics Center at the Brazilian Instituto Nacional Fernandes Figueira (IFF/Fiocruz-Rio de Janeiro, Brazil), began a series of interventions in the municipality of Angra dos Reis (Rio de Janeiro, Brazil) at the interface between medical genetics and primary health care aimed initially for individuals with disabilities (Vieira et al. 2011). Such actions prioritize the Family Health Strategy Program with their Community Health Agents (CHA) as the main entrance of patients and families through the primary health care into the Brazilian Unified Health Care System (SUS-Brazil) (Brasil 2006a). Some characteristics of this municipality are relevant for understanding our choice for the implementation of such policies in the area of medical genetics. Among them are the following: (1) the existence in its territory of Brazil’s single nuclear power plant (Xavier et al. 2007), which puts the region often in the news media; (2) the presence of a pediatrician with training in primary care with special interest in genetics and disabilities; and (3) a partnership established in 2004 with the Medical Genetics Department (IFF/Fiocruz) with a common interest in community genetics.
This article describes our preliminary results and experience from a pilot study started in 2010 in the municipality of Angra dos Reis (Rio de Janeiro, Brazil) implementing a community-based program to identify individuals and families with familial clustering of cancer.
Material and methods
This pilot study was part of the project SISVIGEN-Familial Cancer Clustering and Congenital Malformations Surveillance System in the Surroundings of a Nuclear Plant Area as collaborative group 5 of INAGEMP (INAGEMP 2009) and coordinated by the Medical Genetics Center of the Brazilian IFF/Fiocruz. In 1984, a nuclear power plant was officially licensed for commercial operation in the Angra dos Reis Municipality in Rio de Janeiro, Brazil (Xavier et al. 2007). The project was carried out within the Family Health Strategy Program (FHSP) scenario of Angra dos Reis municipality in the state of Rio de Janeiro, Brazil. The city of Angra dos Reis is located in the southern coast of the state of Rio de Janeiro in the Ilha Grande Bay—latitude 23° 00′ 24″ S; longitude 44° 19′ 05″ W (Fig. 1). Its territory was separated into five health districts according to the City Health Plan (Angra dos Reis 2010; Geografos 2014). The city has a network of 34 FHSP teams including 21 Community Health Agents Programs with coverage in year 2010 of 71.4 % of the population comprehending 117,298 people monitored monthly by home visits through the CHA professionals (Angra dos Reis 2010).
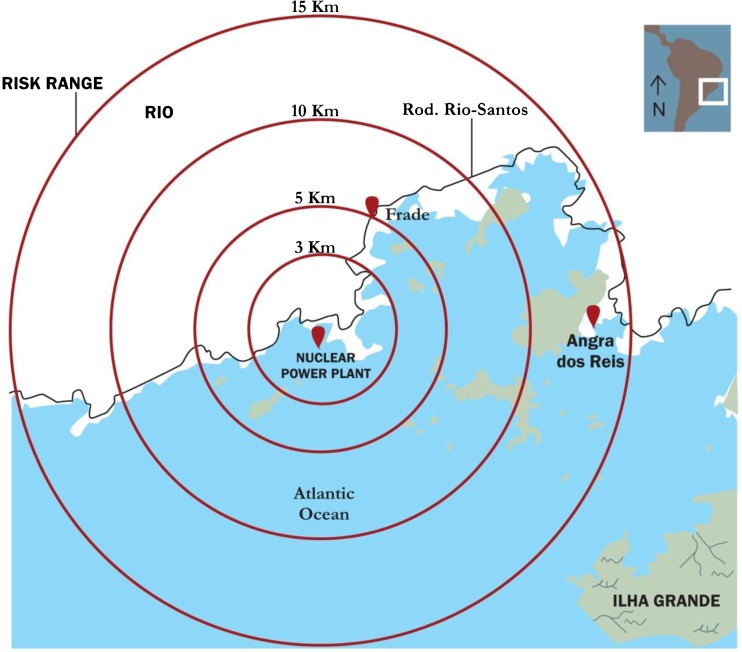
Fig. 1.
Nuclear power plant evacuation emergency plan zones based on risk range scenarios in Angra dos Reis region (Rio de Janeiro, Brazil). Red drops depict the distances separating the Familial Health Strategy Units (Frade I and II and Sapinhatuba I and III) where the study was done and the nuclear power site. More details in Material and methods section
The study was divided into two phases: a descriptive one with a cross-sectional epidemiological data obtained by the CHA through home visits collecting health information including the presence of cancer in four FHSP sites (Frade I and II; Sapinhatuba I and III; Fig. 1). The second phase of the project consisted of families identified by the CHA of clustering three or more individuals with cancer and re-interviewed by an itinerant medical genetic team with the intent of selecting potential families at risk for hereditary cancer. All units of the FHSP that participated in the project were selected for our study based on a criterion where a complete team of health caretakers was present at each unit, comprehending general practitioners, nurses, nurse assistants, dentists, and more importantly, CHA.
As part of our objectives, each FHSP unit received a 2-day seminar given by an experienced clinical geneticist (JCLlJr) who provided a comprehensive basic medical genetics course containing topics such as polygenic and Mendelian inheritance models and construction of three generation pedigrees (Fig. 2) and topics related to cancer, as proposed by Rose and Lucassen (1999). Other common diseases and endemic disorders were as well reported by the CHA in their routine home visits following the Brazilian Ministry of Health Department Manual for CHA that included tuberculosis, leprosy, Chagas disease, malaria, hypertension, diabetes mellitus, epilepsy, alcoholism, and disabilities (Brasil 2006a).
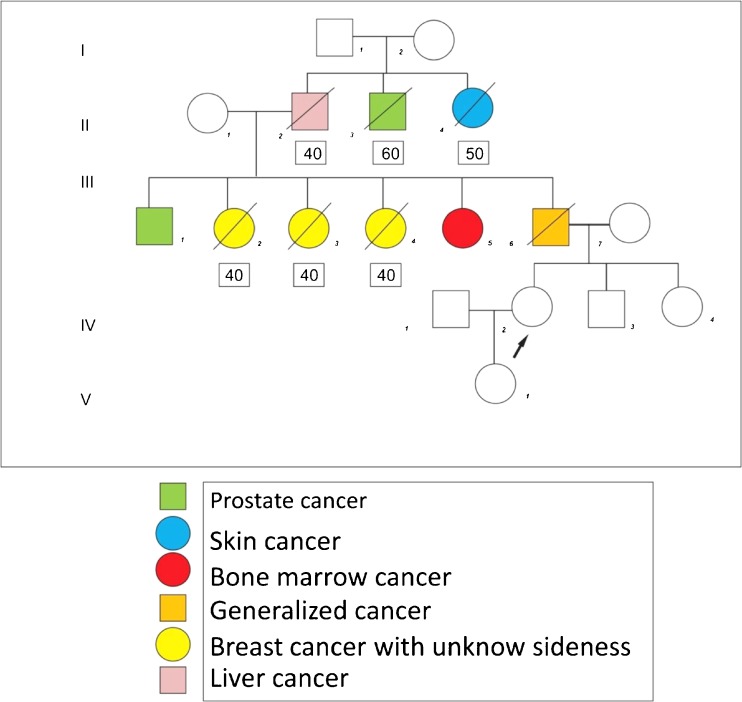
Fig. 2.
Example of a pedigree from one family with clustering of cancer obtained by a community health agent during a home visit. Inside the squares are the ages of onset for each cancer
Cancer is a prevalent disorder in Brazil and is considered the second most common cause of mortality (Brasil 2006b). However, no population-based notification system exists in the Primary Health Care Brazilian database. The main objective of the study was to verify the effectiveness of the primary health care system in identifying familial clustering of cancer.
All families interviewed in this project were already families visited by the CHA inside a well-limited territory previously selected for home visits as part of their routine work following the Brazilian Ministry of Health Department Manual for CHA (Brasil 2006a). The very first home visits by the CHA after the training seminar was accompanied by one of the authors (DKRV) that supervised the work on field especially regarding pedigree construction. All information obtained by the CHA where those obtained directly from one family member based on oral information. Very few medical reports were available. If the response was positive for the presence of cancer, a validated version of a hereditary cancer questionnaire was applied following Ashton-Prolla et al. (2009). The variables evaluated in this phase of the study were presence of cancer, family history of cancer, total of relatives with cancer, and type of cancer. Only one person per household was interviewed.
In the second phase of the project, families selected through the CHA as having a history of clustering three or more cases of cancer in the family were re-interviewed by a group of medical geneticists. All clinical appointments took place at the FHSP unit near the home address of the family. The clinical genetics team was composed of seniors and medical residents in genetics from the Brazilian IFF/Fiocruz, Rio de Janeiro, Brazil.
To carry out the genetic assessments, a three-generation pedigree for each family was accessed and applied a questionnaire that included the following variables: proband age, personal history of cancer, and if so, the age at diagnosis and tumor location. The informant interviewed in phase 2 was the same who first responded to the questionnaire of the CHA in phase 1 of the study. Reviewed questionnaires and pedigrees of each family were discussed within the medical team in order to maintain the quality of data. An analysis and assessment of family risk for cancer was based on the criteria outlined by Scheuner et al. (2010). In summary, each cancer type was assigned for each individual as a weak, moderate, or strong familial risk. Assignment to the familial risk groups was based on rules derived from empirical data, and when such data were unavailable, general principles of familial risk assessment were followed that consider degree of relatedness of affected relatives, maternal or paternal lineage, and their age at cancer diagnosis. The rules for familial risk stratification considered the presence or absence of each respective cancer and associated cancers diagnosed before the age of 50 years or at the age of 50 years and older in all first- and second-degree relatives.
Weak risk was assigned if there was no family history, if there was late-onset cancer in only one second-degree relative from one or both sides of the family, or if there was a first-degree relative with an associated cancer (e.g., endometrial cancer in a mother corresponding to weak familial risk of colorectal cancer).
Moderate risk was generally assigned if there was only one first-degree relative with late-onset cancer, two second-degree relatives from the same lineage with late-onset cancer, or one second-degree relative with early-onset cancer and other second-degree relatives with an associated cancer (e.g., one second-degree relative with ovarian cancer and another from the same lineage with late-onset breast cancer corresponding to a moderate familial risk of ovarian cancer).
Strong familial risk was generally assigned if there was a first-degree relative with early-onset cancer, when multiple relatives were affected, or when a hereditary syndrome was suspected.
It was performed a descriptive statistical analysis with determination of simple frequencies as contained in the program EpiInfo 6.0 (Centers for Disease Control and Prevention, Atlanta, GA, USA).
The project was registered and approved by the Brazilian Ethical Committee Board (CAEE 10–5566.0.000.008).
Results
Fifty-six health professionals, including 25 CHA from all four FHSP teams, attended the phase 1 seminar and participated in the project. A total of 1,581 families were visited and interviewed in phase 1 by the CHA. The history of cancer was positive in 42.3 % of families interviewed distributed along the four areas covered in the project according to Table 1. Only 1.3 % of families reported a history of childhood cancer. In relation to the number of cancer cases in the total of families interviewed, 22.3 % had only one case in the family, 11.2 % two cases, 4.3 % three cases, and 4.3 % four or more cases (Table 1).
Table 1.
Number of cancer per family in four primary care units that participated in the study
| Sapinhatuba 1 | Percentage | Sapinhatuba 3 | Percentage | Frade 1 | Percentage | Frade 2 | Percentage | Total families | Percentage | |
|---|---|---|---|---|---|---|---|---|---|---|
| No history of cancer | 177 | 67.8 | 348 | 77.3 | 181 | 45.0 | 184 | 46.3 | 890 | 58.9 |
| One case | 58 | 22.2 | 62 | 13.8 | 100 | 24.9 | 112 | 28.2 | 332 | 22.0 |
| Two cases | 19 | 7.3 | 28 | 6.2 | 58 | 14.4 | 67 | 16.9 | 172 | 11.4 |
| Three or more cases | 7 | 2.7 | 12 | 2.7 | 63 | 15.7 | 34 | 8.6 | 116 | 7.7 |
| Total of families registered | 261 | 100.0 | 450 | 100.0 | 402 | 100.0 | 397 | 100.0 | 1510 | 100.0 |
In determining which parent lineage contributed to the history of cancer, it was reported by the informant that the father was affected in 15 % of the cases, mother in 12 %, and among siblings in 12.1 % of families.
The remaining 60.9 % of cancer was present in other members of the family. The sites of cancer reported were uterus 8.7 % (n = 137), stomach 7.7 % (n = 122), breast 6.9 % (n = 109), throat 6.8 % (n = 99), unspecified site 6.8 % (n = 108), prostate 5.4 % (n = 85), lung 5.6 % (n = 88), and bowel 3.7 % (n = 59) (Table 2).
Table 2.
Types of cancer identified by the community health agents (CHA) (phase 1) and medical geneticists (phase 2)
| Types of cancer identified by the CHA in phase 1 (total of families 1,581) | Types of cancer identified by medical geneticists in phase 2 (total of families 73) | Type of cancer in Scheuner et al. 2010 | Number of new cases per type of cancer expected for the year 2012, Brazil (190,732,694 hab) | |||
|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | % | ||||
| N | % | N | % | |||
| Uterus | 137 | 8.7 | 27 | 37.5 | – | 17 new cases per 100.000 women |
| Stomach | 122 | 7.7 | 24 | 33.0 | – | 13 new cases per 100.000 men and 7 per 100.000 women |
| Breast | 109 | 6.9 | 22 | 30.5 | 47.9 | 52 new cases per 100.000 women |
| Unspecified site | 108 | 6.8 | 10 | 13.9 | – | – |
| Throat | 99 | 6.3 | 20 | 27.7 | – | 6 new cases per 100.000 men |
| Lung | 88 | 5.6 | 18 | 25.0 | – | 18 new cases per 100.000 men and 10 per 100.000 women |
| Prostate | 85 | 5.4 | 22 | 30.5 | 14.9 | 62 new cases per 100.000 men |
| Bowel | 59 | 3.7 | 9 | 12.5 | 13.8 | 15 new cases per 100.000 men and 16 per 100.000 women |
Phase 1 comprehended oral information obtained by community health agents through home visits. Phase 2 comprehended re-interview by medical geneticists with families clustering three or more cancers
One hundred and thirty-six (n = 136) families were selected for phase 2 based in their history of three or more cancers in the family (8.6 %). Among those, only 73 families attended the phase 2 genetic counseling sessions, representing 46.3 % absenteeism. A family history of cancer under 50 years was positive in 61.1 % of the families. The evaluation of pedigrees allowed the identification of the parental lineage in which there was a history of cancer, such as the offspring in 2 cases (2.7 %), maternal lineage in 22 cases (30.1 %), paternal lineage in 14 cases (19.2 %), and from both sides in 35 cases (47.9 %). Only in two families consanguinity was present (2.7 %).
The cancer sites most frequently reported in phase 2 were uterus (37.5 %), stomach (33,0 %), prostate (30.5 %), breast (30.5 %), throat (27.7 %), lung (25.0 %), and intestine (25.0 %) (Table 2).
Cancer risks were assessed using the criteria proposed by Scheuner et al. (2010). In 50.0 % of families, a weak risk was considered; 36.1 %, a moderate risk was attributed; and 13.8 %, a high risk was established. Concerning known hereditary cancer syndromes, we found only one family that met the criteria for breast and ovary hereditary cancer (1.4 %) and one family that met the criteria for non-polyposis hereditary colon cancer, as revised by Bethesda protocol.
When performing a comparison between the data obtained by the health care agents and the team of geneticists, there was partial agreement in 25 cases (34.2 %) with better-detailed information in the health care agent sheet and complete agreement in 36 cases (49.3 %). It was not possible to perform such evaluation in eight cases due to identification problems of the families (11 %). Complete discordance was found in four cases (5.5 %). All data showed statistical significance for a 95 % confidence interval.
Discussion
Common diseases and endemic disorders are relevant in Brazil as a public health problem and supervised with compulsory notification by the CHA in the primary health system, such as the following: tuberculosis, leprosy, Chagas disease, malaria, hypertension, diabetes mellitus, epilepsy, alcoholism, and disabilities (Brasil 1998). Cancer is a prevalent disorder in Brazil and is considered the second most common cause of mortality (Brasil 2008). However, no population-based notification exists in the primary health care system.
The proposition of a population-based intervention to identify families with a genetic predisposition for cancer and hereditary forms of cancer has been debated and applied by few experts in the field (Acton et al. 2000; Ho 2004; Ashton-Prolla et al. 2009). In Brazil, Ashton-Prolla et al. (2009) developed and validated a questionnaire for the identification of hereditary breast cancer through the primary care set, which was able to identify women and families at risk for this condition, allowing the implementation of follow-up protocols and early treatment. In the same sample, it was analyzed the consistency of information collected by primary care teams and it was compared to the assessment of clinical geneticists involved in the research, pointing that primary care agents were able to properly identify individuals and families at risk (Roth et al. 2009).
Among the families who should be considered at risk for hereditary cancer, those with certain specific information in their family history would serve as a sentinel alert, such as age less than 50 years for cases of breast/ovarian cancer, bilateral tumor, involvement of cancer in successive generations, different types of primary cancers in one patient, and the occurrence of more than a case of rare cancer in the same family (Bancroft et al. 2006; Scheuner et al. 2010). These families must be followed up by specific protocols and, if needed, referred to specialist services in oncogenetic and offered adequate genetic counseling (Bancroft et al. 2006).
The training of physicians and nurses in primary health care has been done successfully in programs for early detection of diseases in several countries (Bancroft et al. 2006; Ho 2004; Vieira et al. 2011). The training of primary care physicians, through newsletters and discussion of sensitive cases, has proven effective in the identification and appropriate follow-up of cases of breast and ovarian hereditary cancer (Watson et al. 2001).
In Brazil, the main structure of the primary health care team through the FHSP included a new professional category, CHA, historically evolved in expanding access to health in Brazil, starting in the early 1970s (Marques and Padilha 2004). The focus of these professionals begins in 1991 and characterized by a shift in the curative axis nature of medicine towards prevention and health promotion. The selection of these agents is made from their own community, and a specific knowledge in health care is not mandatory. The conjunction between popular wisdom and biomedical knowledge, as well as the proximity of their own community, enables such professionals once properly trained to identify health needs for families in a more informal way (Marques and Padilha 2004; Nunes et al. 2002; Silva and Dalmaso 2002; Filgueiras and Silva 2011; Vieira et al. 2011, 2012).
This project in the municipality of Angra dos Reis has enabled the CHA to identify 42.3 % of families with positive history of cancer. Furthermore, 8.6 % of families reported clustering of three or more cases of cancer.
It was not our intention to collect data from the community to establish cancer incidence or prevalence among several types of cancer. Even though, there is considerable epidemiological data available from the Brazilian National Institute of Cancer (Table 2) (Guerra et al. 2005; Brasil 2006b). The main proposal in our study was to train and enable the CHA to identify presumptive hereditary cancer based on a simple approach using the pedigree analysis (Vieira et al. 2013).
There is a complex understanding of lay knowledge relating nuclear plant sites and health hazards (Silva 2009, 2010). Since 2003, our group has been working with the primary health care units in Angra dos Reis municipality identifying handicapped children through their disabilities (Vieira et al. 2011) and/or congenital malformations/genetic syndromes (Vieira et al. 2012) using similar methodology with the HCA. As a consequence, a considerable increase of notifications through the local and national health system database occurred especially regarding disabilities. At the time, such expected notification increase of disabilities was negatively exploited by one particular Brazilian newspaper suggesting that such increase in Angra dos Reis municipality was in some way due as a consequence of a “hidden” chronic irradiation leak from the nuclear plant or at least for the presence in site Official statements from the Brazilian Ministry of Health Department and from the Angra dos Reis local government responded promptly and swiftly pointing to the inconsistency of the newspaper’s arguments. Apart from the general interest in such subject, it was not our intention to investigate the relationship between cancer and the presence of a nuclear plant. Even though, there is a considerable robust data from the World Health Organization discussing health hazards and nuclear radiation (WHO 2001).
A similar study as ours conducted in another region of Brazil and with no relation to a nuclear power plant, Porto Alegre (Rio Grande do Sul, Brazil) found a higher notification for cancer than our study, ranging from 34 % from the mother side, 29 % from the father side, 22 % a sister, and 11.8 % the brother (Roth et al. 2009).. However, the study referred only to breast and ovarian cancer. The cancer sites reported in our study included uterus, stomach, breast, throat, unspecified sites, prostate, lung, and bowel-colorectal. It is worth noting that the reported type of cancers in our study is in accordance with the annual report from the Brazilian National Cancer Institute such as for men, prostate, lung, stomach, colon, rectum, and esophagus; and, for women, breast, cervix, colon and rectum, lung, and stomach (Guerra et al. 2005; Brasil 2006b).
Strategies for continuing health education can impact the results of entries in the FHSP and SUS (Brazil), held by the CHA, in particular because of the relationship of trust established between the families and the CHA personnel in the territory under their responsibility in obtaining health information (Sala et al. 2004; Nascimento and Correa 2008).
In our study, all families selected as high risk for familial hereditary cancer based on the criteria of Scheuner et al. 2010 were referred to the Brazilian National Institute of Cancer for further oncogenetic counseling. Clinical follow-up feedback from these families was not available at the time of this report.
Conclusion
Our approach for identification of families with positive family history for cancer can be considered in the scope of community genetics in the Brazilian National Health System (SUS). Moreover, an approach through the FHSP was well accepted and conducted by the CHA. The contribution of this study strengthens the proposition of the introduction of medical genetics in primary care, taking into account local specificities.
The results of this study indicate that primary care health system, through the CHA properly trained, can be an effective entrance and recognition for individuals with genetically determined diseases.
One of the limitations of our study was the lack of medical reports or documents confirming the positive history of cancer. However, identification of families with a positive history of cancer through the health community programs brings into attention the recurrence risk for familial cancer. As employed by Scheuner et al. (2010), through such methodology, a low, moderate, or high risk based in population surveys could be achieved.
Acknowledgments
The authors wish to acknowledge Guilherme de Oliveira Macedo for reviewing the manuscript and INAGEMP—National Institute of Population Medical Genetics (grant CNPq 573993/2008-4) for the financial support provided to this project.
Compliance with ethics guidelines
This project complied with the current laws of Brazil and was registered and approved by the Brazilian Ethical Committee Board (CAEE 10–5566.0.000.008).
References
- Acton RT, Burst NM, Casebeer L, et al. Knowledge, attitudes and behaviors of Alabama’s primary care physicians regarding cancer genetics. Acad Med. 2000;75(8):850–852. doi: 10.1097/00001888-200008000-00021. [DOI] [PubMed] [Google Scholar]
- Angra dos Reis (2010) Fundação Municipal de Saúde. Plano Municipal de Saúde - 2010–2013. http:www//angra.rj.gov.br, Accessed 20 January 2014.
- Ashton-Prolla P, Giacomazzi J, Schmidt AV, et al. Development and validation of a simple questionnaire for the identification of hereditary breast cancer in primary care. BMC Cancer. 2009;9:283–290. doi: 10.1186/1471-2407-9-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft E, Arden-Jones A, Lynch E (2006) Cancer genetics: the importance of obtaining a family history. 102(40): 28–29 [PubMed]
- Brasil (1998) Ministério da Saúde: Secretaria de Assistência à Saúde. Coordenação de Saúde da Comunidade. SIAB: Manual do sistema de informação de atenção básica; Brasília, 98p. http://dtr2001.saude.gov.br/editora/produtos/livros/ pdf/03_1543_M.pdf. Accessed 30 Apr 2014.
- Brasil (2006a) PORTARIA N° 648, DE 28 DE MARÇO DE 2006. http://dtr2001.saude.gov.br/sas/PORTARIAS/Port2006/GM/GM-648.htm. Accessed 30 Apr 2014
- Brasil (2006b) Ministério da Saúde. Secretaria de Atenção à Saúde. Instituto Nacional de Câncer. Coordenação de Prevenção e Vigilância. A situação do câncer no Brasil / Ministério da Saúde, Secretaria de Atenção à Saúde, Instituto Nacional de Câncer. Coordenação de Prevenção e Vigilância. Rio de Janeiro. http://bvsms.saude.gov.br/bvs/publicacoes/situacao cancer_brasil.pdf. Accessed 30 Apr 2014
- Brasil (2008) Ministério da Saúde, Departamento de Informação e Informática do SUS -DATASUS, Sistema de Informações de Saúde. Pesquisa Nacional por Amostra de Domicílios – Acesso e Utilização de Serviços de Saúde. Rio de Janeiro. http://tabnet.datasus.gov.br/cgi/dh.exe?pnad2008/pnad.def. Accessed 30 Apr 2014
- Brasil (2009) Ministério da Saúde. Instituto Nacional do Câncer. Rede nacional de câncer familial: manual operacional / Instituto Nacional de Câncer – Rio de Janeiro: INCA. 229p.
- Filgueiras AS, Silva ALA (2011) Agente Comunitário de Saúde: um novo ator no cenário da saúde do Brasil. Phys Rev Saúde Coletiva Rio de Janeiro, 21 [3]: 899–915
- Geografos, Angra dos Reis. http://physicsweb.org/articles/news/11/6/16/1. Accessed 30 Apr 2014
- Guerra MR, Gallo CVM, Mendonça GAS. Risco de câncer no Brasil: tendências e estudos epidemiológicos mais recentes. Rev Bras Cancerologia. 2005;51(3):227–234. [Google Scholar]
- Ho C (2004) How to develop and implement a cancer genetics risk assessment program. Clinical and economic considerations. Oncology Issues: 22–26
- INAGEMP – Instituto Nacional de Genética Medica Populacional. http://www.inagemp.bio.br/overview. Accessed 30 Apr 2014
- Khoury MJ (2000) Genetics and public health in the 21st century. In: Khoury MJ, Burke W, Thomson EJ (eds) Using genetic information to improve health and prevent disease. p. 5 – Oxford Press
- Llerena JC., Jr Genética Médica, Sistema único de Saúde Brasileiro (SUS) e integralidade na atenção e no cuidado à saúde. Rev Ciênc Saúde Coletiva Rio de Janeiro. 2002;7(1):21–25. [Google Scholar]
- Marques CMS, Padilha EM. Contexto e Perspectivas da formação profissional do Agente Comunitário de Saúde. Trab Educ Saúde. 2004;2(2):345–352. doi: 10.1590/S1981-77462004000200008. [DOI] [Google Scholar]
- Nascimento EPL, Correa CRS. O agente comunitário de saúde: formação, inserção e práticas. Cad Saúde Pública Rio de Janeiro. 2008;24(6):1304–1313. doi: 10.1590/S0102-311X2008000600011. [DOI] [PubMed] [Google Scholar]
- Nunes MO, Trad LB, Almeida BA, Homem CR, Melo MCIC. O agente comunitário de saúde: construção da identidade deste personagem híbrido e polifônico. Cad Saúde Pública Rio de Janeiro. 2002;18(6):1639–1646. doi: 10.1590/S0102-311X2002000600018. [DOI] [PubMed] [Google Scholar]
- Ramalho AS, Silva RBP. Genética Comunitária: uma nova disciplina e sua aplicação no Brasil. Cad Saúde Pública. 2000;16(1):22–29. doi: 10.1590/S0102-311X2000000100029. [DOI] [Google Scholar]
- Rose, Lucassen (1999) Practical genetics for primary care. Oxford Medical Publication. Ed. Peter W Rose PW, Lucassen A. Oxford University Press
- Roth FL, Camey SA, Caleffi M, et al. Consistency of self-reported first-degree family history of cancer in a population-based study. Familial Cancer. 2009;8:195–202. doi: 10.1007/s10689-008-9228-2. [DOI] [PubMed] [Google Scholar]
- Sala A, Simões O, Luppi CG, Mazziero MC. Cadastro ampliado em saúde da família como instrumento gerencial para diagnóstico de condições de vida e saúde. Cad Saúde Pública. 2004;20(6):1556–1564. doi: 10.1590/S0102-311X2004000600013. [DOI] [PubMed] [Google Scholar]
- Scheuner MT, McNeel TS, Freedman AN. Population prevalence of familial cancer and common hereditary cancer syndromes. The 2005 California health interview survey. Genet Med. 2010;12(11):726–735. doi: 10.1097/GIM.0b013e3181f30e9e. [DOI] [PubMed] [Google Scholar]
- Silva G. Expertise e participação da população em contexto de risco nuclear: democracia e licenciamento ambiental de Angra 3. DADOS Rev Ciênc Sociais Rio de Janeiro. 2009;52(3):771–805. [Google Scholar]
- Silva G. Imaginário coletivo: estudos do sensível na teoria do jornalismo. FAMECOS. 2010;17(3):244–252. [Google Scholar]
- Silva JA, Dalmaso ASW. Agente comunitário de Saúde: o ser, o saber, o fazer. Rio de Janeiro: Fiocruz; 2002. [Google Scholar]
- Vieira DKR, Duarte NO, Attianezi M, Llerena JC., Jr Registro de adolescentes com deficiência no Sistema de Informação da Atenção Básica: experiência do município do município de Angra dos Reis – Rio de Janeiro, Brasil. Adolescência Saúde. 2011;8(3):10–17. [Google Scholar]
- Vieira DKR, Horovitz DDG, Llerena JC., Jr Avaliação genética itinerante de crianças e adolescentes com deficiência vinculadas à Estratégia Saúde da Família. Rev Bras Med Fam Comunidade. 2012 [Google Scholar]
- Vieira DKR, Attianezi M, Horovitz DDG, Llerena JC., Jr Atenção em genética médica no SUS: a experiência de um município de médio porte. Phys Rev Saúde Coletiva. 2013;23(1):243–261. doi: 10.1590/S0103-73312013000100014. [DOI] [Google Scholar]
- Watson E, Clements A, Yudkin P, et al. Evaluation of the impact of two educational interventions on GP management of familial breast/ovarian cancer cases: a cluster randomized controlled trial. Br J Gen Pract. 2001;51:817–821. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2001) UNSCEAR REPORT - Report of the United Nations Scientific Committee on the effects of atomic radiation to the General Assembly, 2001
- Xavier AM, Lima AG, Vigna CRM, et al. Marcos da História da Radioatividade e Tendências Atuais. Quim Nova. 2007;30(1):83–91. doi: 10.1590/S0100-40422007000100019. [DOI] [Google Scholar]