Abstract
Haemoglobinopathies are a leading cause of child mortality worldwide, although with a variable geographical incidence. A reliable estimate of prevalence of the disease is necessary for reducing its burden. However, most studies in India are either hospital based or from certain regions of the country and hence may not realistically reflect the disease burden. The eastern Indian states of Bihar, Chhattisgarh and Jharkhand and eastern region of Uttar Pradesh, which comprise ~25 % population of the country, are poorly studied with respect to haemoglobinopathies. The present study, conducted on 1,642 individuals from this region, shows a frequency of 3.4 % for β-thalassaemia trait (BTT), 3.4 % for sickle cell haemoglobin trait (HbS)/haemoglobin E trait (HbE) and 18 % for α-globin defects. While BTT mutations are distributed rather uniformly across the region, HbS occurs only in Chhattisgarh and Jharkhand, the regions rich in tribal populations. The frequency of α-gene mutation is strikingly high, occurring even in individuals with normal blood count, in tribal as well as non-tribal groups. The mutation spectrum of BTT is also distinct since the common mutations, IVS1-1 (G-T) and 619 bp del, are absent while CD15 (G-A) is the second most frequent. The HbA2 level in the suspected cases is strikingly low. We demonstrate association of the low HbA2 level with vitamin B12 and folate deficiency in this cohort. Thus, the present report besides providing an estimate of the carrier frequency of β-thalassaemia traits also confirms high prevalence of α-gene defects and regional heterogeneity in distribution of HbS in the eastern parts of India.
Keywords: Haemoglobinopathies, Beta-thalassaemia, Alpha-thalassaemia, HbS, Indian population
Introduction
Thalassaemia is one of the prevalent monogenic disorders in the Indian subcontinent. It is estimated that there are 30–40 million carriers, and 8,000 to 10,000 thalassaemics are born every year in India (Mohanty et al. 2013). In contrast to the global frequency of 1.5 %, the average carriers of β-thalassaemia trait (BTT) in India comprise 3.3 % of the population (Edison et al. 2008; Cousens et al. 2010). Though its presence is recorded from different regions of India, the frequency varies widely between 0.5 and 17 % in different geographical regions (Edison et al. 2008). Unlike β-thalassaemia, the distribution of sickle cell anaemia (sickle cell haemoglobin (HbS)) is restricted to specific geographic regions. However, these studies are mostly based on hospital samples covering certain selective geographic regions. Two relatively large studies, one on pregnant women (n > 60,000) and the other on schoolchildren (n > 6,000), from a western Indian city, Mumbai, reveal BTT frequency to be around 2 % with only a marginal occurrence of HbS (Colah et al. 2008; Madan et al. 2010). Another hospital-based study from Mumbai and other districts of Maharashtra and the neighbouring state of Gujarat recorded a frequency of ~3.5 % (Colah et al. 2010; Mohanty et al. 2013). In the northern Indian states of Punjab and Delhi, based on investigations on over 6,000 subjects, largely school children, the frequency is around 4 % (Garewal and Das 2003; Madan et al. 2010), while in the population from the coastal regions of the eastern and south eastern India (Bengal, Odisha and Andhra Pradesh), there is a sharp increase in BTT frequency (>10 %) (Balgir 2006; Munshi et al. 2009; Dolai et al. 2012). Hospital-based studies from these regions, performed mostly on anaemics, show a frequency as high as 23 % (Chandrashekar and Soni 2011). Additional studies on certain restricted/endogamic populations such as those on tribes from Madhya Pradesh, Chhattisgarh (Patra et al. 2011) and other regions show a dramatically high incidence of HbS and α-globin deletions in western and central India and haemoglobin E (HbE) in north-east India (Flint et al. 1998). All these studies also demonstrate that five β-globin mutations, viz. IVS1-5 (G → C), 619 bp del, IVS1-1(G → T), CD41/42 (-TCCT) and CD8/9 (+G), tend to account for more than 85 % of β-thalassaemics, which facilitates the use of a cocktail of primers for these sites as a diagnostic for BTT by amplification-refractory mutation system (ARMS) test (Sinha et al. 2009).
Two features are apparent from the above-noted limited studies on α- and β-globin traits in India: firstly, there are region-wise variations in frequencies of these traits and, secondly, that there is a significant gap in knowledge from regions like Uttar Pradesh, Rajasthan, Bihar, Jharkhand, Tamil Nadu, Kerala, etc. that constitute bulk of the Indian population. This lack of information precludes a realistic estimate of the disease burden in India as a whole as well as development of a comprehensive state policy for management, rehabilitation and counseling of the sufferers. The present study covers a part of eastern India which comprises around 25 % of India’s population to get an estimate of the incidence of haemoglobinopathies in this region.
Materials and methods
The study was approved by the Institutional ethical committee. Written informed consent was obtained from all the volunteers. A total of 1,642 (943 males and 699 females) samples were collected from all the six blocks of Varanasi and adjoining areas of the states of Jharkhand, Chhattisgarh and Bihar (Fig. 1), all within 500 km of Varanasi. Sampling was done either through the district Primary Health Centre (PHC) health camps, schools and colleges or through door-to-door visits. Vaccination camps, family planning camps and health camps were preferred for sampling. In case of tribal populations, the samples were collected either by door-to-door sampling with the help of local health workers or through schools reserved for tribal children with the help of the school principal and with permission of volunteers’ parents. Only one member from a family (except for the non-consanguineous spouses) was included in the study. The subjects as well as health service providers were educated to participate in the study by oral and visual presentations along with written information in the form of pamphlets. In some instances, information regarding organisation of the camps was published in advance in local newspapers. More than 95 % of the collected samples were from natives of the region. Individuals with any history of transfusion, TB, cardiovascular disease, renal and other major health problems were excluded from the study. Information regarding the ethnicity, parity, medical and reproductive history, food habits and medication were recorded through a questionnaire from all the volunteers.
Fig. 1.
Map of India showing regions from where the samples were collected (the figure is the same as published in Sukla and Raman (2012) because the same sites were used in that study). The number of samples (n) collected from different regions are indicated in the map
The samples were transported to the laboratory in refrigerated conditions, and haematological studies were performed within 24 h of collection. Complete blood count (CBC) was obtained using an automated blood counter (Abacus Junior, Diatron, Hungary). Haemoglobin was analysed for the presence of any variants by cellulose gel electrophoresis at alkaline pH (Graham and Grunbaun 1963). Quantification of HbA2 was done by anion exchange micro-column chromatography (Galanello et al. 1977). DNA was isolated from all the blood samples (1,642) by the salting-out method (Miller et al. 1988) for analysis of α- and β-thalassaemia (β-thal) mutations. The 18 β-thal mutations, viz. IVS1-5(G-C), IVS1-1(G-T), CD8/9(+G), Cd41/2(-TCTT), 619 bp deletion, HbE (CD26A-C), CD15(TGG-TAG), CD30(AGG-ACG), IVS1-1(G-A), CD55(-A), CD5(-CT), CD121(G-T), CD47/48(+ATCT), CD16(-C), Capsite + 1(A-C), IVS1-130(G-A), HbS CD6(A-T) and -88(C-T), were analysed through ARMS PCR. In a limited number of samples, mutations were cross-checked by automated DNA sequencing (ABI-3130, USA). Presence of α-gene deletion (α-3.7, α-4.2) and triplication (αanti3.7, αanti4.2) was checked by gap PCR.
Results
Table 1 summarises the geographic region-wise distribution of samples, suspected cases and their mutation profile. Out of the 1,642 samples, CBC was obtained for 1,592 (50 samples could not be analysed due to a transient technical snag in the blood cell counter). Of these, 491 samples had low CBC (MCV ≤80 and MCH ≤27). Cellulose gel electrophoresis on all the 1,642 samples yielded 52 samples with HbS (5 of these were out of those 50 whose CBC could not be estimated) and 4 with HbE (total variants 56). The 491 low CBC, 56 Hb variants and the 50 ‘CBC-not-done’ samples were considered as ‘suspected category’ (n = 592, 349 males and 243 females) for estimation of HbA2 by column chromatography and mutational analysis of the selected β (ARMS) and α-gene defects (see ‘Materials and methods’ for the details).
Table 1.
Region-wise distribution of total samples collected and β-gene mutations in the suspected samples (n = 592)
| Samples | Regions | ||||
|---|---|---|---|---|---|
| VNS | CHG | JHD | BHR | Total | |
| No. of samples from various regions | 606 | 261 | 544 | 231 | 1,642 |
| No. of suspected samples (%) | 189 (31.2 %) | 149 (57.0 %) | 202 (37.1 %) | 52 (22.5 %) | 592 (36.1 %) |
| No. of β-gene mutations (%) | 21 | 10 | 18 | 07 | 56 (3.41 %) |
| No. of Hb variants (%) | 02 (E) | 34 (S) | 18 (S) | 02 (E) | 56 (3.41 %) |
VNS Varanasi region, CHG Chhattisgarh region, JHD Jharkhand region, BHR Bihar region, E HbE, S HbS
Mutation analysis
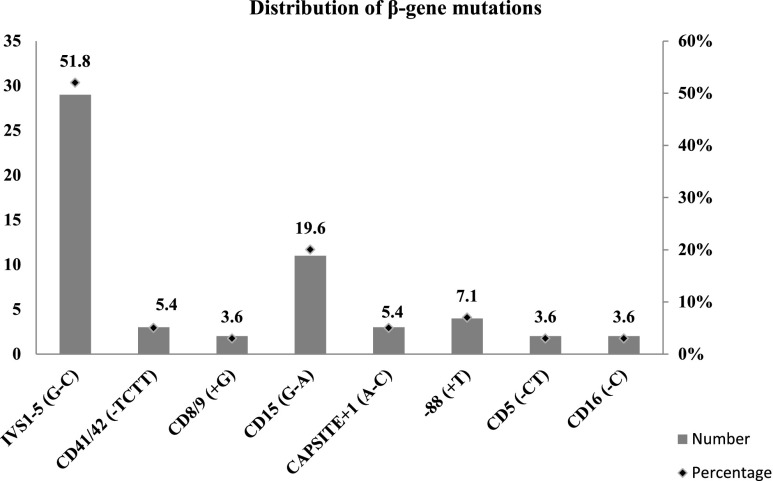
Fifty-six individuals carried β-globin mutations other than HbS and HbE. The rest of the low-CBC samples which did not reveal any mutation by the ARMS test were subjected to sequencing of the β-globin gene. However, no further mutants were detected. Among the 56 individuals, eight mutation types were found, IVS1-5 being the most prevalent (52 %) followed by CD15 (20 %). Interestingly, the IVS1-1 and 619 bp del, considered to be common in India, were completely absent in this cohort (Fig. 2).
Fig. 2.
Distribution of β-gene mutations
All the 592 suspected samples were further screened for α-gene deletions (α-3.7 and α-4.2) and triplications (α3.7anti and α4.2anti). Unlike the uniform distribution of the β-mutations throughout different regions, incidence of α-mutations in Chhattisgarh (40 %), Bihar (30 %) and Jharkhand (25 %), was much greater than in Varanasi (17 %) (Table 2). The frequency of α-gene mutations turned out to be much higher (159/592) than the β-gene mutations. Deletions were far in excess (142) of triplications (17). Thus, a total of 248 out of the 592 suspected cases revealed at least one mutation in β- or α-globin gene. Data revealed that 6 β-thal and 17 HbS individuals coinherited α-mutation.
Table 2.
Distribution of α-globin gene mutation in the regions studied
| Samples | Number of samples having mutation (total number of samples analysed), % | ||||
|---|---|---|---|---|---|
| Regions | Total | ||||
| VNS | CHG | JHD | BHR | ||
| Suspected samples (592) | 32 (189), 16.9 | 61 (149), 40.3 | 50 (202), 24.8 | 16 (52), 30.7 | 159 (592), 26.7 |
| Controls (347) | 8 (111), 7.2 | 18 (81), 22.3 | 14 (98), 14.3 | 6 (57), 10.5 | 46 (347), 13.2 |
| Total (939) | 40 (300)13.3 | 79 (230), 33.9 | 64 (300), 21.3 | 22 (109), 20.2 | 205 (939), 21.8 |
The β-globin gene was also sequenced in 182 individuals, whose blood parameters were optimum, to check the existence of mutations in ‘healthy’ individuals. No mutation was detected in any of the sequenced samples, confirming that the observed frequency of the β-globin mutations in this cohort was reliable. Since the frequency of α-globin mutations turned out to be unexpectedly high in the suspected category, we tested the same deletions/duplications in 347 healthy subjects. This analysis revealed that 46 of them had an α-mutation. Here again, the frequency of these ‘silent carriers’ was much higher in Chhattisgarh (22 %) and Jharkhand (14 %) than in Varanasi (7 %). The distribution of mutations in the controls revealed 34 single deletions and 7 triplications, indicating that the loss or gain of one α-allele was of little consequence (Table 3) since the haematological profile of these 46 individuals carrying α-mutants was not different from those who did not have a mutation (data not shown).
Table 3.
Mutation spectrum for α-gene and its coinheritance with other mutations
| Categories | No. of samples analysed | Single deletions | Double deletions | Triplications | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| α3.7/αα | α4.2/αα | α3.7/α3.7 | α4.2/α4.2 | α3.7/α4.2 | αααanti3.7 | αααanti4.2 | |||
| Suspected samples | 480 | 53 | 24 | 14 | 6 | 23 | 13 | 3 | 136 |
| HbS/E variants | 56 | 7 | 5 | 2 | 2 | 1 | 0 | 0 | 17 |
| β-gene mutants | 56 | 3 | 1 | 0 | 0 | 2 | 0 | 0 | 6 |
| Controls | 347 | 27 | 07 | 01 | 03 | 01 | 06 | 01 | 46 |
| Total | 939 | 90 | 37 | 17 | 11 | 27 | 19 | 4 | 205 |
α3.7/αα and α4.2/αα represent single gene deletion; α3.7/α3.7, α4.2/α4.2 and α3.7/α4.2 represent double gene deletions; and αααanti3.7 and αααanti4.2 represent α-gene triplications
Demographically, maximum number of the suspected cases belonged to Chhattisgarh (57 %) mainly due to larger presence of HbS (34/261). HbS was also detected in Jharkhand (18/544) but was not in Varanasi (U.P.). Only two cases of HbE, but none of HbS, were recorded from Bihar. In contrast to the strictly regional distribution of HbS and HbE, spread of BTT was seen in all the regions, its cumulative frequency being 3.4 % (Table 1). A comparison of the CBC profiles among different mutation groups (α, β-thal and HbS) showed that β-thal carriers had the poorest haematological picture, especially with respect to Hb, MCV and MCH levels (Table 4).
Table 4.
Median values of different haematological parameters among different mutational groups and controls
| Blood count parameters | Median values (IQR) in different mutant groups of suspected category (n = 542) and controls (n = 1,050) | ||||
|---|---|---|---|---|---|
| β (n = 47) | HbS/E (n = 51) | α (n = 131) | None (n = 313) | Controls (n = 1,050) | |
| Hb | 11.6 (9.8–12.8) | 12.2 (11.1–13.1) | 11.4 (9.3–12.6) | 11.6 (10.1–13) | 12.3 (11.1–13.3) |
| Hct | 36.6 (31.2–39.8) | 36.8( 11.6–39.4) | 36.2 (31.9–39.5) | 37.0 (32.3–40.1) | 38.2 (34.6–41) |
| RBC | 5.23 (4.35–5.83) | 5.1 (4.39–5.5) | 4.92 (4.53–5.3) | 4.88 (4.38–5.34) | 4.37 (3.94–4.71) |
| MCV | 69 (63–79) | 73 (68–80) | 73 (67–79) | 76 (70–79) | 86 (83–92) |
| MCH | 21.8 (19.6–25.5) | 24.0 (21.3–25.8) | 23.1 (21.1–24.7) | 24 (21.3–25.4) | 28.3 (27.7–29.8) |
| MCHC | 32.1 (30.7–33.6) | 32.4 (31.5–33.4) | 31.3 (29.8–32.4) | 31.8 (30.1–33.1) | 32.6 (31.3–33.8) |
| RDW | 16.4 (15.3–18.2) | 16.1(15.4–17.7) | 16.3 (15.4–17.8) | 16.3 (15.5–17.5) | 16.1 (15.4–17.2) |
The first value represents the first quartile and the second value represents the third quartile
IQR interquartile range
HbA2 level and its association with globin gene mutations
HbA2 (α2δ2) was measured in 552 out of 592 suspected cases (measurements on 40 samples failed). More than 3.5 % HbA2 level is considered a characteristic feature of β-thal condition. However, in the present study, only 27 samples showed HbA2 > 3.5, and merely 13 of these had a globin mutation (6 in β- and 7 in α-globin genes). Since in this cohort, 428 (77.5 %) samples had lower than 2.5 % HbA2 we took 2.5 % HbA2 as the cut-off value for BTT. As seen from the data in Table 5, 60 % of the124 individuals with HbA2 ≥ 2.5 had one or the other β-thal, HbS or α-mutations, with the highest frequency, 37/124 (30 %), for BTT. However, even in those having <2.5 % HbA2 (428), 31 % (n = 133) individuals had one of these mutations, dominant one being the α-deletion (80 %, 106/133) while β-thal contributing only 10 % (13/133) to this pool. The low HbA2 level in the suspected group was rather intriguing. Since micronutrient deficiency is known to affect HbA2 levels (Mosca et al. 2009; Denic et al. 2013), and since we had earlier measured vitamin B12 and folic acid levels in these individuals (Sukla and Raman 2012), we checked their levels in the suspected (n = 489) and control (780) samples (those having normal CBC, presumably optimum HbA2). Table 6 shows clearly that the median value of vitamin B12 and folates in the suspected cases is significantly lower than in the controls, confirming their contribution to the low HbA2,
Table 5.
Distribution of samples on the basis of HbA2value and mutational profile (n = 552)
| Type of mutation | Number of mutants | HbA2 ≥ 2.5, n = 124 (%) | HbA2 < 2.5, n = 428 (%) |
|---|---|---|---|
| Only β | 50 | 37 (74) | 13 (26) |
| Only α | 136 | 30 (22) | 106 (78) |
| α + β | 6 | 03 (50) | 3 (50) |
| Only HbS | 10 | 01 (10) | 9 (90) |
| HbS + α | 6 | 04 (67) | 2 (33) |
| Total | 208 | 75 (60.5) | 133 (31.1) |
Percent values in column 3 and 4 are calculated from column 2
Table 6.
Association of levels of HbA2 with folate and vitamin B12
| Categories | Median value (IQR) of folate (3–24 ng/mL) | p value (Kruskal-Wallis test) | Median value (IQR) of B12 (150–1,200 pg/mL) | p value (Kruskal-Wallis test) |
|---|---|---|---|---|
| Suspected samples (n = 458) | 4.6 (3.8–5.6) | 0.0009a | 219.5 (180–267) | 0.0009a |
| Controls (n = 780) | 5.4 (3.6–5.8) | 233 (189–264) |
IQR interquartile range
a p value significant after Bonferroni correction
Ethnicity and globin gene mutations
Out of the 1,642 samples analysed, information on the ethnicity of 1,512 could be collected. It was stratified broadly into three major ethnic groups: scheduled tribes (ST), scheduled castes (SC) and general category (GC, represented mostly by Brahmins, Vaishyas, Rajputs, Kayasthas and other backward classes). These three categories formed 19.7 % (n = 298), 21.5 % (n = 325) and 58.8 % (n = 889), respectively, of the studied samples. As shown in Table 7, 13 % of the tribals (ST) had a β-mutation as against 5.5 % in SC and GC. The tribals in the present cohort belonged almost exclusively to the states of Jharkhand and Chhattisgarh, with HbS being the most common mutation in the tribals. With the sample size being relatively small, we did not further subcategorise within the ethnic groups.
Table 7.
Distribution of suspected cases and detected mutations in the different ethnic groups
| Ethnic groups | VNS | BHR | JHD | CHG | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Suspa | Mutb | Total | Susp | Mut | Total | Susp | Mut | Total | Susp | Mut | Total | Susp (%) | Mut (%) | |
| ST | 6 | 2 | 2 | 5 | 0 | 0 | 184 | 91 | 29 | 103 | 66 | 58 | 298 | 159 (53) | 89 (30) |
| SC | 119 | 36 | 13 | 67 | 17 | 9 | 117 | 47 | 7 | 22 | 9 | 9 | 325 | 109 (34) | 38 (12) |
| Others | 418 | 145 | 38 | 159 | 35 | 16 | 210 | 53 | 17 | 102 | 51 | 30 | 889 | 284 (32) | 101 (11) |
| Total | 543 | 183 | 53 | 231 | 52 | 25 | 511 | 191 | 53 | 227 | 126 | 97 | 1,512 | 552 (37) | 228 (15) |
a‘Suspected cases’ in whom mutations were analysed
bMutants (β, α or HbS/E) in each ethnic group from an area
In summary, carrier frequency of β-thal and HbS is 3.4 % each while that of α-globin is 18 %. Demographically, 29 % of the tribals (ST) are carriers while in SCs and the general category (GC), the frequency is 11 %. Geographically, the population of the Chhattisgarh region has the highest frequency of mutations, as expected, because they also have the highest frequency of tribals.
Discussion
Demographic and mutational diversity in BTT
In a comprehensive, meta-analytical format, Sinha et al. (2009) have brought into focus the abundance of haemoglobinopathies in the Indian subcontinent. That analysis also highlights the complexity of the Indian population structures and the limitations of the studies hitherto carried out. In a country of over 1.25 billion people, living in 29 states and stratified through highly diverse geographical and environmental conditions, numerous languages and dialects, religions, castes, tribes and their endogamous nature make India a complex conglomerate of various biological populations. The major studies done so far on haemoglobinopathies are confined to only a few regions of the country, and, as noted in ‘Introduction’, they reveal different frequencies of BTT and other Hb variants in different regions and in different ethnicities. Among the few studies done from and around the present area of investigation, Tamhankar et al. (2009) have shown BTT frequency of 2.9 % in western Uttar Pradesh, and the hospital-based pilot study of Sinha et al. (2004) on patients and their families confirms a fair presence of β-thalassaemia in Varanasi and in nearby regions. All these limited sample studies underscore the heterogeneous distribution of haemoglobinopathies in India, but they fall short of providing a representative picture of the genetic diversity prevailing in this land mass.
The present study is an initiative to explore the most populous regions of India to arrive at a reasonable estimate of the prevalence of globin gene defects. The states of Bihar, Chhattisgarh, Jharkhand and eastern Uttar Pradesh are generally rated poorly on health indices with more than 60 % people suffering from anaemia, and incidences of preterm delivery, low birth weight and child mortality are very high (Ministry of Health and Family Welfare and, Government of India 2007; James 2011). Varanasi is the largest city in eastern Uttar Pradesh. Its proximity with western Bihar, Jharkhand and Chhattisgarh and the fact that its university hospital is the biggest referral centre for serious health problems in these regions make it an ideal place to conduct this kind of study. The present observations, based on data from more than 1,600 persons, show that with respect to the frequency of BTT, the studied populations are genetically homogeneous. The collective BTT carrier frequency of 3.4 % is similar to those reported in the main land states of western and northern India, although much lower than in the coastal regions of Bengal, Odisha and Andhra Pradesh. In agreement with a previous report (Balgir 2007), we also find the HbS trait to be restricted to the states of Chhattisgarh and Jharkhand, both of which are rich in tribal populations. However, Chhattisgarh has much higher proportion of HbS (13 %) than in Jharkhand (3 %). In relation to the relatively lower incidence of HbS trait in the latter, it is noteworthy that our Jharkhand samples are drawn from different regions of the state, some that are adjacent to Chhattisgarh and Odisha and others close to Bihar and north-eastern states (Fig. 1). Although all these regions are rich in tribal populations and most of our samples have indeed been drawn from tribals only, HbS trait in Jharkhand is present only in the region closer to Chhattisgarh and Odisha, but is absent from Sahibganj (Fig. 1). Compared to the earlier reported incidence of HbS in Odisha and adjacent region of Chhattisgarh (Balgir 2005; 2007), the frequency of HbS (13 %) in the present studied cohort in Chhattisgarh is much lower. Whereas it seems to reflect genetic diversity in the populations of Chhattisgarh and Jharkhand, it also raises the possibility that there was a founder population for HbS near the Odisha-Chhattisgarh border which got progressively diluted as it moved towards the central and northern regions due to their limited movement and/or lesser selective advantage. A better understanding of the diversity can be obtained through studies on larger sample size with expanded areas of collection.
So far, 52 different BTT mutations have been recorded in India (Sinha et al. 2009), of which only 18 have been reported in Uttar Pradesh (Agrawal et al. 2000). In the present study, however, we found only eight BTT mutations in all the regions put together. Of the five most common mutations, believed to account for 82.5 % of all mutations in India (Sinha et al. 2009), IVS1-5 is the most common mutation (52 %) in this cohort as in all other populations. However, IVS1-1(G-T) and 619 bp del, considered to be the most common after IVS1-5, are absent in the present group. The next common CD15 (20 %) mutation in this collection is not among the common five mutations. The negligible presence of IVS1-1(G-T) and 619 bp del in eastern and southern India also (Sinha et al. 2009) confirms their restricted presence in particular ethnic groups (Punjabi and Sindhis belonging to the northern part of Indian subcontinent) (Garewal and Das 2003).
Predominance of α-globin deletions in suspected cases and controls
Unlike the β-globin mutations, studies on α-globin are rather few. Balgir (2000) in his review of the status of mutations in haemoglobinopathies has noted that in certain tribal communities in the Gujarat, Odisha and Nilgiri ranges in southern India, the frequency of one or more copies of α-deletion ranges between 42 and 95 %. Other reports from populations of the north-eastern (Sen et al. 2005) and northern regions of India have recorded the frequency of α-trait to be 5 % or less (Choubsia et al. 2000; Dastidar and Talukder 2007). Several of these studies have been carried out in clinical set-up on cord blood samples to check suspected Hb Bart’s (γ4). Unlike these, our population-based analysis of α-gene deletions and duplications shows the α-mutation frequency (26.8 %) to be much higher than in the β-gene not only in the suspected group but also in the general control population (13 %) in which no β-gene mutation was detected. Compared with the mutation frequencies in other parts of India, the frequency in the present case is higher than in north-east and north India but lower than in Odisha (south-eastern), Gujarat (western) and southern India, suggesting an interesting incremental gradient in its frequency from the south to north with our samples roughly falling in between the two groups, both geographically as well as with respect to the frequency of α-mutations. This possibility further strengthens the likelihood of the previously suggested spread and dilution of a founder population from the south to the north.
Our results also show that individuals with deletion of two α-alleles are most frequent in the suspected group, whereas in the general population, it is the deletion or addition of a single allele which is by far the most common (Table 2). Apparently, loss or gain of a single α-allele is asymptomatic and thus would go undetected. In agreement with the earlier reports that populations with higher HbS trait also have higher α-mutations (Balgir 2000), we also find a similar trend, except in Bihar where HbS is absent but 31.7 % individuals of the suspected group have α-mutation. Whether the loss or gain of one or two alleles provides a selective advantage, especially to HbS carriers, or is neutral needs to be ascertained.
Low HbA2 level in suspected cases
Curiously, the HbA2 level was rather low in the suspected cases. Similar observation is also reported by Dolai et al. (2012) from West Bengal. A large-scale study by Colah et al. (2010) also shows β-thal traits in individuals with low HbA2 (≤3.5 %). Therefore, the cut-off HbA2 value for mutation analysis in this study has been reduced to ≥2.5 %, of which 60 % show α- or β-mutations. Even in those having <2.5 % value, 31 % harbour a mutation albeit more than 80 % of these are single allele α-mutations which would apparently be neutral in effect. We now show that vitamin B12 and folate deficiency in these cases is a contributory factor to low HbA2 in the suspected category. Though we have not evaluated iron deficiency in this cohort, it is inevitably an additional and important contributor to low HbA2 borne out by the fact that in this cohort, vitamin B12 and folate deficiency is associated with normo and microcytic rather than with macrocytic anaemia which is generally be the situation if anaemia was caused due to vitamin B12 deficiency alone (Sukla et al. 2014). We should also consider the role of α-deletion in low HbA2 values, since their deletion/point mutation could lead to lesser availability of α-globin, reducing its chance of combining with δ-globin and causing lowered HbA2 level. Since most α-deletions in the suspected group involve two α-globin genes, their effect may indeed be quite pronounced (Tables 2 and 3).
The present study from a region of India re-emphasises the need for more and larger studies from all over the country and the need to pay greater attention to α-globin gene. In community terms, two significant points emerge from the present study: firstly, even with a low frequency, in terms of absolute numbers, the size of affected and carriers of haemoglobinopathies in densely populated India is very large and, secondly, more extensive studies on selected regional and/or communal groups are necessary to devise diagnostic and therapeutic strategies for reducing the societal burden.
Acknowledgments
The help extended by the medical teams of respective primary health centres in sample collection, both at the PHC and in the field, is gratefully acknowledged. Thanks are due to Krishna Kishore Sukla, Cytogenetics Laboratory, Department of Zoology, BHU for his help in sample collection, micronutrient estimation and statistical analysis. We heartily thank our colleagues, Profs. S. C. Lakhotia and Mercy J Raman, Department of Zoology, BHU for reading the text and tables of the manuscript and making critical suggestions for improving the language and content of the paper. We also record our appreciation of all the voluntary blood donors for their help and support. This work was supported by a research grant from the Council of Scientific and Industrial Research, New Delhi which is gratefully acknowledged.
Ethical standards
The work is approved by the Institutional ethical committee that complies with the current laws of the country.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
This work was done at Cytogenetics Laboratory, Department of Zoology, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
References
- Agrawal S, Pradhan M, Gupta UR, Sarwai S, Agrawal SS. Geographic and ethnic distribution of beta thalssemia mutations in Uttar Pradesh, India. Hemoglobin. 2000;24:89–97. doi: 10.3109/03630260009003427. [DOI] [PubMed] [Google Scholar]
- Balgir RS. The burden of hemoglobinopathies in India and the challenges ahead. Curr Sci. 2000;79:1536–1547. [Google Scholar]
- Balgir RS. Spectrum of hemoglobinopathies in the state of Orissa, India, A ten years cohort Study. J Assoc Physicians India. 2005;53:1021–1026. [PubMed] [Google Scholar]
- Balgir RS. Scenario of haemoglobin variants in Central-East coast of India. Curr Sci. 2006;90:1651–1657. [Google Scholar]
- Balgir RS. Aberrant heterosis in hemoglobinopathies with special reference to β-thalasssemia and structurally abnormal hemoglobins E and S in Orissa, India. J Clin Diagn Res. 2007;1:122–130. doi: 10.1111/j.1752-699X.2007.00027.x. [DOI] [Google Scholar]
- Chandrashekar V, Soni M. Hemoglobin disorders in south India. ISRN Hematol. 2011;2011:1–6. doi: 10.5402/2011/748939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubsia SL, Choubsia DK, Khare S. α-Thalassemia (Hb-Bart’s) in Rajasthan (India) Haematologia. 2000;30:209–213. doi: 10.1163/156855900300109215. [DOI] [PubMed] [Google Scholar]
- Colah R, Surve R, Wadia M, Solanki P, Mayekar P, Thomas M, Gorakshakar A, Dastur A, Mohanty D. Carrier screening for β-thalassemia during pregnancy in India, a 7 year evaluation. Genet Test. 2008;12:181–185. doi: 10.1089/gte.2007.0066. [DOI] [PubMed] [Google Scholar]
- Colah RB, Gorakshakar A, Phanasgaonkar S, D’Souza E, Nadkarni A, Surve R, Sawant P, Master D, Patel R, Ghosh K, Mohanty D. Epidemiology of beta-thalassaemia in Western India, mapping the frequencies and mutations in sub-regions of Maharashtra and Gujarat. Br J Haematol. 2010;149:739–747. doi: 10.1111/j.1365-2141.2010.08131.x. [DOI] [PubMed] [Google Scholar]
- Cousens NE, Gaff CL, Metcalfe SA, Delatycki MB. Carrier screening for beta-thalassemia, a rview of international practice. Eur J Hum Genet. 2010;18:1077–1083. doi: 10.1038/ejhg.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastidar R, Talukder G. The molecular basis of alpha thalassemias in India: a review. In: Bhasin VBMK, editor. Anthropology today: trends, scope and applications. Kamla Raj Enterprises: New Delhi; 2007. pp. 349–354. [Google Scholar]
- Denic S, Agarwal MM, Al Dabbagh B, El Essa A, Takala M, Showqi S, Yassin J. Hemoglobin A2 lowered by iron deficiency and α-thalassemia: should screening recommendation for β-thalassemia change? ISRN Hematol. 2013;2013:858294. doi: 10.1155/2013/858294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolai TK, Dutta S, Bhattacharya M, Ghosh MK. Prevalence of hemoglobinopathies in rural Bengal, India. Hemoglobin. 2012;36:57–63. doi: 10.3109/03630269.2011.621007. [DOI] [PubMed] [Google Scholar]
- Edison ES, Shaji RV, Devi SG, Moses A, Viswabandhya A, Mathews V, George B, Srivastava A, Chandy M. Analysis of beta globin mutations in the Indian population, presence of rare and novel mutations and region-wise heterogeneity. Clin Genet. 2008;73:331–337. doi: 10.1111/j.1399-0004.2008.00973.x. [DOI] [PubMed] [Google Scholar]
- Flint J, Harding RM, Boyce AJ, Clegg JB. The population Genetics of haemoglobinopathies. Bailliere Clin Hematol. 1998;11:1–50. doi: 10.1016/S0950-3536(98)80069-3. [DOI] [PubMed] [Google Scholar]
- Galanello R, Melis MA, Muroni P, Cao A. Quantification of HbA2 with DE 52 microchromatography in whole blood as screenming test for beta thalassemia heterozygous. Acta Hematol. 1977;57:32–36. doi: 10.1159/000207857. [DOI] [PubMed] [Google Scholar]
- Garewal G, Das R. Spectrum of β-thalassemia mutations in Punjabis. J Med. 2003;3:217–219. [Google Scholar]
- Graham JL, Grunbaun BW. A rapid method for microelectrophoresis and quantitation of Hb on cellulose acetate. Am J Clin Pathol. 1963;39:567. doi: 10.1093/ajcp/39.6.567. [DOI] [PubMed] [Google Scholar]
- James KS. India’s demographic change, opportunities and challenges. Science. 2011;333:576–580. doi: 10.1126/science.1207969. [DOI] [PubMed] [Google Scholar]
- Madan N, Sharma S, Sood SK, Colah R, Bhatia LHM. Frequency of β-thalassemia trait and other hemoglobinopathies in northern and western India. Indian J Hum Genet. 2010;16:16–21. doi: 10.4103/0971-6866.64941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. Simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:55404. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health and Family Welfare, Government of India (2007) National Family Health Survey (NFHS-3), 2005-06. http,//www.measuredhs.com/pubs/pdf/. Accessed 25 Aug 2013
- Mohanty D, Colah RB, Gorakshakar AC, Patel RZ, Master DC, Mahanta J, Sharma SK, Chaudhari U, Ghosh M, Das S, Britt RP, Singh S, Ross C, Jagannathan L, Kaul R, Shukla DK, Muthuswamy V. Prevalence of β-thalassemia and other haemoglobinopathies in six cities in India: a multicentric study. J Community Genet. 2013;4:33–42. doi: 10.1007/s12687-012-0114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca A, Paleari R, Ivaldi G, Galanello R, Giordano PC. the role of hemoglobin A2 testing in the diagnosis of thalassemias and related haemoglobinopathies. J Clin Pathol. 2009;62:13–17. doi: 10.1136/jcp.2008.056945. [DOI] [PubMed] [Google Scholar]
- Munshi A, Anandraj MPJS, Joseph J, Shafi G, Anila AN, Jyothy A. Inherited hemoglobin disorders in Andhra Pradesh, India, A population study. Clin Chim Acta. 2009;400:117–119. doi: 10.1016/j.cca.2008.10.025. [DOI] [PubMed] [Google Scholar]
- Patra PK, Chauhan VS, Khodiar PK, Dalla AR, Serjeant GR. Screening for the sickle cell gene in Chhattisgarh state, India: an approach to a major public health problem. J Community Genet. 2011;2:147–151. doi: 10.1007/s12687-011-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R, Chakrabarti S, Sengupta B, De M, Haldar A, Poddar S, Gajra B, Talukder G, Sengupta S. Alpha-thalassemia among tribal populations of Eastern India. Hemoglobin. 2005;29:277–280. doi: 10.1080/03630260500310711. [DOI] [PubMed] [Google Scholar]
- Sinha S, Kumar A, Gupta V, Kumar S, Singh VP, Raman R. Haemoglobinopathies-thalassemias and abnormal haemoglobins in eastern Uttar Pradesh and adjoining districts of neighboring states. Curr Sci. 2004;87:775–780. [Google Scholar]
- Sinha S, Black ML, Agarwal S, Colah R, Das R, Ryan K, Bellgard M, Bittles AH. Profiling β-thalassaemia mutations in India at state and regional levels, implications for genetic education, screening and counselling programmes. HUGO J. 2009;3:51–62. doi: 10.1007/s11568-010-9132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukla KK, Raman R. Association of MTHFR and RFC1 gene polymorphism with hyperhomocysteinemia and its modulation by vitamin B12 and folic acid in an Indian population. Eur J Clin Nutr. 2012;66:111–118. doi: 10.1038/ejcn.2011.152. [DOI] [PubMed] [Google Scholar]
- Sukla KK, Nagar R, Raman R. Vitamin B12 and folate deficiency, major contributing factors for anemia: A population based study. e-SPEN. 2014;9:e45–e48. doi: 10.1016/j.clnme.2013.11.003. [DOI] [Google Scholar]
- Tamhankar PM, Agarwal S, Arya V, Kumar R, Gupta UR, Agarwal SS. Prevention of homozygous beta thalassemia by premarital screening and prenatal diagnosis in India. Prenat Diagn. 2009;29:83–88. doi: 10.1002/pd.2176. [DOI] [PubMed] [Google Scholar]