Abstract
Sickle cell disease (SCD) is a genetic disease resulting from the inheritance from both parents of a mutant hemoglobin gene. Its occurrence can, at best, be prevented, and its daily life consequences can, at least, be limited. As the mutant gene is recessive, it should be useful for people living in countries where SCD is endemic to know their own genetic status and that of their actual or potential partner in order to assess the risk of having a baby with SCD. The present study aimed at examining how a convenience sample of 128 lay people and nine physicians in Benin judge the likelihood that a newborn will suffer from SCD as a function of the genetic status of the baby’s mother and father. As expected, several qualitatively different clusters of participants were found. A minority (29 %) made judgments that were largely consistent with the correct rule for determining the likelihood of disease. A larger group (37 %) expressed, however, the view that (a) to know a child’s likelihood of suffering from SCD, information is needed about the genetic statuses of both parents and (b) this likelihood depends additively on these genetic statuses. Finally, another group (34 %) thought that, if one parent is suffering from SCD or is a carrier of a sickle gene, the likelihood that the child will have SCD is high, irrespective of the other parent’s status. Thus, even among a relatively well-educated group of people in Benin, only a minority used the correct judgment rule when assessing the risk of SCD. Work needs to be done to educate the population regarding the proper way to combine information.
Electronic supplementary material
The online version of this article (doi:10.1007/s12687-014-0205-1) contains supplementary material, which is available to authorized users.
Keywords: Sickle cell disease, Benin, Risk assessment
Introduction
Sickle cell disease (SCD) is common in sub-Saharan Africa (World Health Organization 2010). In Benin, the incidence was estimated in 2008 to be 4 per 100 births (Anani et al. 2008). It is a burden on those afflicted with it as well as on their families and on society (Brown et al. 2010; World Health Organization 2011). SCD is a genetic disease, resulting from the inheritance of an S hemoglobin gene from each parent (or, less commonly, an S from one and a C hemoglobin gene from the other). In Benin, the prevalence of hemoglobin S was estimated in 2008 at 24 % and hemoglobin C at 9 % (Anani et al. 2008); thus, one third of the population carried a mutant gene.
SCD cannot be cured, except by bone marrow transplant, which is not a realistic option for most patients. Treatment is important because it can reduce morbidity, but it cannot eliminate all the adverse effects. A major aim of public health authorities, therefore, should be to prevent SCD. Prevention can be done in two ways: (a) preventing, or dissuading, a couple from conceiving a child if they both carry an S or C gene or (b) diagnosing fetuses with SS or SC through prenatal testing and aborting them. The latter is a subject currently of considerable debate (Kagu et al. 2004; Edwin et al. 2011; Animasahun et al. 2012). The former is the focus of this study.
In order to avoid conceiving a baby with someone else who carries the S (or C) gene, carriers and people with SCD must (a) know both their own blood type and the blood type of their potential partners, (b) know what this information means, (c) and act on it. Studies have demonstrated, however, that (a) people’s understanding of SCD, including its genetic causation, is poor, and (b) the majority do not know their own S gene status (Zounon et al. 2012; see also Adewuyi 2000; Alao et al. 2009; Famuyiwa and Aina 2009; Guédéhoussou et al. 2009; Moronkola and Fadairo 2006; Owolabi et al. 2011). This study complements these works by looking specifically at how people would use the information on sickle status if they had it to assess the likelihood of having a baby with SCD.
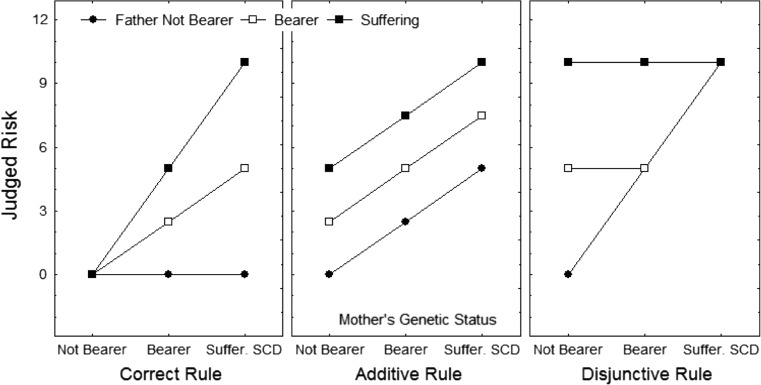
The actual probability of a baby inheriting two S genes (or an S and a C gene) and, therefore, getting SCD can be calculated simply and precisely. It is 0 % if one parent does not have a SCD mutation even if the other is a carrier or has SCD, 25 % if both parents are carriers, 50 % if one is a carrier and the other has SCD, and 100 % if both parents have SCD. The pattern of likelihoods that results from the application of this rule is shown in the left panel of Fig. 1.
Fig. 1.
Theoretical patterns of results that would be observed under three different views: the correct view (left panel), the additive view (second panel), and the disjunctive view (right panel). In each panel, (a) the mean judgments of the likelihood of suffering from SCD are on the y-axis, (b) the three levels of mother’s genetic status are on the x-axis, (c) the points on the graph are the mean judged likelihoods, for each level of mother’s status, of each level of father’s genetic status, and (d) each of the three curves connects the points representing one level of father’s genetic status
It has been repeatedly shown that lay people combine pieces of information to make judgments by using one of several simple integration rules such as additive, disjunctive, or conjunctive rules (Anderson 2013). Application of an additive rule to judge the likelihood of having a baby with SCD would produce the pattern of judgments shown in the center panel of Fig. 1: the likelihood increases as the number of conditions increases. Application of a disjunctive rule to judge this likelihood would produce the pattern of judgments shown in the right panel of Fig. 1: either one or the other of the two conditions is sufficient to produce the effect. Application of a conjunctive (or multiplicative) rule would produce the correct pattern of judgments, shown in the left panel of Fig. 1.
In view of the previous literature, we hypothesized that a majority of people, when given information about two partners’ serological status, would combine the information about the female and male partners in either an additive or a disjunctive fashion. We also tested whether people understand that information from both parents is often needed, not just from one, and that outside influences, such as having a nanny with SCD, do not affect whether a baby will develop SCD.
Methods
Participants
The 137 participants (66 females, 71 males) were unpaid volunteers living in Cotonou—the largest city and economic center of Benin, with a million inhabitants— who were informed about the goals of the study and gave their consent orally. Their mean age was 26 years (SD = 10, range = 18–64). One hundred twenty-eight participants were lay people, and nine were physicians. All participants were nationals.
The lay people were approached by a trained research assistant while they were walking along the main sidewalks of the city or on the campus of the university. Overall, 250 persons were contacted, and after having received a full explanation of the procedure, 51 % of them gave their informed consent and participated. The physicians were general practitioners with offices in the same areas in which the lay people were recruited; they were contacted at these offices. Among the lay people, 35 % had a university degree and 52 % had completed secondary education but did not have university degree; 44 % had been directly confronted with SCD in their family.
Material
The material consisted of 26 cards containing a scenario of a few lines, a question, and a response scale. Eighteen of the scenarios were composed according to a three within-subject factor design: (a) the father’s genetic status (not bearer of the gene, bearer of the gene, and suffering from SCD), (b) the mother’s genetic status, and (c) the child’s sex (a nonrelevant factor added as a check). An example of scenario is the following: “Mr. Do Rego currently has SCD. He suffers from it in daily life. Mrs. Do Rego is simply a carrier of the gene. They just had a son: Adédjouma.” Under each scenario were a question and a response scale. The question was as follows: “What are the chances that [the name of the child] will have sickle cell disease?” The response scale was an 11-point scale (0–10) with a left-hand anchor of “no chance” and a right-hand anchor of “every chance.” The cards were arranged randomly and in a different order for each participant.
Six additional scenarios were composed according to a two-within subject factor design: (a) the mother’s genetic status and (b) the child’s sex. The father’s status was unknown. In two further scenarios, neither the father nor the mother was a bearer of the gene, but the nanny was.
Procedure
The site was a vacant room in the university or in the participant’s home. Each person was tested individually. In the familiarization phase, the experimenter explained to each participant what was expected and presented him or her with 13 scenarios taken from the complete set. The participant then provided a likelihood rating for each story. After completing the 13 ratings, the participant was allowed to compare responses and change them. In the experimental phase, the whole set of 26 scenarios was presented. Each participant provided ratings at his or her own pace but was no longer allowed to compare responses nor to go back and make changes as in the familiarization phase. In both phases, the experimenters routinely made certain that each participant, regardless of age or educational level, was able to grasp all the necessary information before making a rating. The participants took 20–30 min to complete both phases. The ethics and work laboratory of the Institute of Advanced Studies, Paris, France, approved the research.
Analyses
Cluster analyses were performed on the raw data—using the procedure advocated by Hofmans and Mullet (2013)—to determine if there were groups of participants who combined information in ways that were similar within each group and different from those used in the other groups. Analyses of variance (ANOVAs) were then performed on the data from each cluster. Owing to the multiple comparisons done, the significance threshold was set at .001.
Results
Three clusters were identified; they are shown in Fig. 2. The results of the ANOVAs on each cluster are shown in Table 1.
Fig. 2.
Patterns of results for each of the three observed clusters. In each panel, (a) the mean judgments of the likelihood of suffering from SCD are on the y-axis, (b) the three levels of mother’s genetic status are on the x-axis, (c) the points on the graph are the mean judged likelihoods, for each level of mother’s status, of each level of father’s genetic status, and (d) each of the four curves connects the points representing one level of father’s genetic status, including the unknown status
Table 1.
Results of the ANOVAs performed on the data from each cluster
| Effect | Error | ||||||
|---|---|---|---|---|---|---|---|
| Cluster and factor | df | MS | df | MS | F | p | η 2 p |
| I. Correct rule | |||||||
| Gender (G) | 1 | 0.57 | 39 | 0.61 | 0.93 | .34 | .02 |
| Mother’s status (M) | 2 | 1 507.59 | 78 | 3.28 | 459.78 | .001 | .92 |
| Father’s status (P) | 2 | 1 605.71 | 78 | 2.19 | 731.92 | .001 | .95 |
| G × M | 2 | 0.77 | 78 | 1.00 | 0.77 | .47 | .02 |
| G × P | 2 | 2.06 | 78 | 0.69 | 3.00 | .06 | .07 |
| M × P | 4 | 371.20 | 156 | 3.07 | 120.99 | .001 | .75 |
| Bilinear | 1 | 1453.51 | 39 | 2.25 | 646.84 | .001 | .94 |
| G × M × P | 4 | 0.91 | 156 | 1.19 | 0.76 | .55 | .02 |
| II. Additive rule | |||||||
| Gender (G) | 1 | 6.35 | 50 | 5.97 | 1.06 | .31 | .02 |
| Mother’s status (M) | 2 | 1 437.72 | 100 | 8.56 | 167.88 | .001 | .77 |
| Father’s status (P) | 2 | 949.13 | 100 | 7.94 | 119.53 | .001 | .71 |
| G × M | 2 | 10.41 | 100 | 5.02 | 2.08 | .13 | .04 |
| G × P | 2 | 25.33 | 100 | 7.02 | 3.61 | .03 | .07 |
| M × P | 4 | 10.81 | 200 | 5.78 | 1.87 | .12 | .04 |
| G × M × P | 4 | 3.60 | 200 | 5.74 | 0.63 | .64 | .01 |
| III. Disjunctive rule | |||||||
| Gender (G) | 1 | 0.07 | 45 | 3.25 | 0.02 | .88 | .00 |
| Mother’s status (M) | 2 | 1 365.77 | 90 | 5.27 | 259.15 | .001 | .85 |
| Father’s status (P) | 2 | 1 288.06 | 90 | 6.40 | 201.15 | .001 | .82 |
| G × M | 2 | 4.07 | 90 | 2.78 | 1.46 | .24 | .03 |
| G × P | 2 | 4.14 | 90 | 2.61 | 1.59 | .21 | .03 |
| M × P | 4 | 112.44 | 180 | 5.08 | 22.13 | .001 | .33 |
| Bilinear | 1 | 311.50 | 45 | 7.08 | 44.21 | .001 | .49 |
| G × M × P | 4 | 1.79 | 180 | 1.97 | 0.91 | .46 | .02 |
The dependent variable in the ANOVAs was the participants’ responses (about degree of risk). The independent variables were the child’s gender, the mother’s genetic status, and the father’s status. The other terms were interactions between the independent variables
The main results from the first cluster (N = 40) are shown in the left panel of Fig. 2. For participants in this cluster, the likelihood that the child will suffer from SCD was determined by the interaction of the mother’s and father’s statuses; that is, it was only when both parents suffered from SCD that the likelihood that the child will suffer from SCD was judged very high. When one or both parents did not carry the gene, the likelihood was judged as near zero. Given the similarity between this observed pattern of judgments and the one shown in Fig. 1 (left panel), this cluster was termed correct rule. It was composed of 29 % of the lay people and 33 % of the physicians. As also shown in the figure, the curve that corresponds to the additional cases (father’s status unknown) integrates itself very well into the diverging fan formed by the three other curves.
The second cluster (N = 51) was termed additive rule since, as shown in the center panel of Fig. 2, for participants in this cluster, the likelihood that the child will suffer from SCD appears to be an additive function of the mother’s status and of the father’s status. The cluster was composed of 37 % of the lay people and 44 % of the physicians. The curve that corresponds to the additional cases (father’s status unknown) is much less steep than the three other curves and located in the middle of the response scale.
The third cluster (N = 46) was termed disjunctive rule since, as shown in the right panel of Fig. 2, for participants in this cluster, the likelihood that the child will suffer from SCD was judged low only when both parents were not bearers of the gene. The cluster was composed of 34 % of the lay people and 22 % of the physicians. As in the previous cluster, the curve that corresponds to the additional cases is much less steep than the three other curves and located in the middle of the response scale.
Finally, an ANOVA conducted on the two remaining scenarios (nanny affected) showed that, in every cluster, probability judgments were not affected by the nanny variable.
Discussion
Our study of the assessment by people in Benin of the risk that a couple will have a baby with SCD has several important findings. First, a large majority of lay participants believed that knowledge of the genetic status of both parents was needed to assess the risk that a couple would have a baby with SCD. When only the mother’s sickle status was known, they showed indecision, although knowing that she was not a carrier would have allowed them to predict that the child will not be ill.
Second, all lay participants were aware that the newborn’s sex did not matter. This is important both methodologically—it showed that participants distinguished among the different types of information when integrating them—and culturally, i.e., they did not, at least in this domain, consider females as more or less stigmatized than males.
Third, most lay participants were aware that SCD is not contagious, in particular, that a nanny who has SCD cannot pass it to the babies under her care.
Fourth, most of the lay participants (66 %) did quite well in integrating the information pertinent to the risk of SCD. Twenty-nine percent of them made judgments quite consistent with the scientific model. They understood that there is no risk if one parent is not a carrier, no matter what the status of the other parent. In addition, 37 % used an additive rule. Although this rule was not quite correct, these participants appreciated that both parents contributed to risk. One may hope that at least some of them might, therefore, be inclined to seek knowledge of their own sickle gene status and to avoid sexual unions that would increase the risk of having a baby with SCD.
Fifth, a minority of the lay participants (34 %), however, used a disjunctive rule in judging risk, so that carriage of the S gene by either parent would make it “risky” to have a baby. For them, the only situation with low risk is when neither patient carries the S gene. They did not understand that since SCD requires two S genes (or an S and a C gene), a person who is negative can have a child who is a carrier but can never have a child with SCD. They might themselves, therefore, unnecessarily avoid intimate relations with people with one or two S genes. They might not, furthermore, distinguish between people who carry the S gene and those who have SCD. It is not uncommon for people to use a disjunctive rule in estimating the overall risk contributed by more than one risk factor. Hermand et al. (1997), for example, showed that people in France judged the risk of cancer to be maximal when either one of two risk factors, heavy drinking or heavy smoking, were present. It is this group that most needs to be educated about the true risks of SCD.
Sixth, the physicians were not greatly different from the lay people in the way they combined genetic information: 33 % used a conjunctive and 44 % an additive rule, while 22 % used the incorrect disjunctive rule. Incomplete understanding among health professionals and medical students has also been found in Nigeria (Animasahun et al. 2009).
The sample size, especially of physicians, was small, and the participants were better educated than the average residents of Benin. Our findings must, therefore, be generalized with care to the general population and to the medical community of Benin.
Implications
Before discussing the main implications of the study, we must recall that many people do not know their sickle gene status. In a survey in 2004 of residents of Lomé, Togo, only 22.4 % had had a hemoglobin electrophoresis (to test for the S or C gene) (Guédéhoussou et al. 2009). In contrast, among a more highly educated group in Cotonou, Benin, in 2011, 76 % knew whether or not they carried a mutant gene (Zounon et al. 2012). To increase knowledge, testing will need to be more widespread, i.e., available and cheap, with the support of national and community leaders. The model may be vaccination, e.g., the recent campaign in Burkina Faso that vaccinated almost the whole population against meningococcus A (Center for Disease Control 2013).
The first implication of the present study involves health communication. When discussing with patients about transmission of SCD, physicians and health professionals may expect to find two types of people: (a) people who are able to integrate the information in a way that allows them to make a reasonable assessment of the risk of having a sick child (about two thirds in this study) and (b) people who integrate the information in a way that leads them systematically to overestimate the risk (about one third in this study). Awareness of people’s level of knowledge a priori may help health professionals to adjust quickly their communication style.
The second implication of the present study involves a cognitive-educational goal. The minority who uses a disjunctive rule can be taught how to make use of the genetic information. They can be taught not only that two factors confer risk (in this case, the sickle gene status of each partner), but how to apply this knowledge, i.e., how to combine these risks correctly. It is possible for them to learn this; Bonnin-Scaon and colleagues (2002)—using the same methodology employed in the present study—were able to teach such people, through feedback on a series of concrete cases, to use a conjunctive rule to combine the risks of smoking and drinking when estimating the likelihood of developing esophageal cancer.
The third implication relates to ethics (Edwin et al. 2011; Deans et al. 2013). If someone finds out that the person whom he or she has come to love and wants to marry carries an S or C gene, is it acceptable, on an individual level, to break off relations with that person and is it acceptable, on a social level, not to break off relations? These ethical questions are in turn related to the empirical issues of the psychological (Lewis et al. 2011; Metcalfe 2012; Riedijk et al. 2012) and behavioral impacts of learning one’s sickle gene status. Screening of potential carriers of genes for β-thalassemia and SCD has variable effects on subsequent marital and reproductive behavior in different populations (Alswaidi and O’Brien 2009; Alswaidi et al. 2012; Amato et al. 2013; Al-Allawi et al. 2013). We are studying these issues in Benin.
Electronic supplementary material
(DOCX 20 kb)
Compliance with ethical standards
Conflict of interest
Ornheilia Zounon, Paul Clay Sorum, and Etienne Mullet declare that they have no conflict of interest.
Ethical approval
All procedures were in accordance with the ethical standards of the French Psychological Association and the Institute of Advanced Studies, with the laws of France and Benin, and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- Adewuyi JO. Knowledge of and attitudes to sickle cell disease and sickle cell carrier screening among new graduates of Nigerian tertiary educational institutions. Niger Postgrad Med J. 2000;7:120–123. [PubMed] [Google Scholar]
- Al-Allawi NAS, Jalal SD, Ahmed NH, Faraj AH, Shalli A, Hamamy H. The first five years of a preventive programme for haemoglobinopathies in Northeastern Iraq. J Med Screen. 2013;20:171–176. doi: 10.1177/0969141313508105. [DOI] [PubMed] [Google Scholar]
- Alao OO, Aroaye M, Ojabo C. Knowledge of sickle cell disease and haemoglobin electrophoresis: a survey of students of a tertiary institution. Niger J Med. 2009;18:326–329. doi: 10.4314/njm.v18i3.51208. [DOI] [PubMed] [Google Scholar]
- Alswaidi FM, O'Brien SJ (2009) Premarital screening programmes for haemoglobinopathies, HIV and hepatitis viruses: review and factors affecting their success. J Med Screen 16:22–28 [DOI] [PubMed]
- Alswaidi FM, Memish ZA, O’Brien SJ, Al-Hamdan NA, Al-Enzy FM, Alhayani OA, Al-Wadey AM. At-risk marriages after compulsory premarital testing and counseling for β-thalassemia and sickle cell disease in Saudi Arabia, 2005–2006. J Genet Counsel. 2012;21:245–255. doi: 10.1007/s10897-011-9395-4. [DOI] [PubMed] [Google Scholar]
- Amato A, Cappabianca MP, Lerone M, Colosimo A, Grisanti P, Ponzini D (2013) Carrier screening for inherited haemoglobin disorders among secondary school students and young adults in Latium, Italy. J Commun Genet 5(3):265–268. doi:10.1007/s12687-013-0171-z. [DOI] [PMC free article] [PubMed]
- Anani L, Latoundji S, Saghoban V, Dehoumon J, Bigot A, Zuhoun I. Sickle cell disease: the Benin experience. Haematol Meet Rep. 2008;2:5–6. [Google Scholar]
- Anderson NH. Unified psychology based on three laws of information integration. Rev Gen Psychol. 2013;17:125–132. doi: 10.1037/a0032921. [DOI] [Google Scholar]
- Animasahun BA, Akitove CO, Njokamma OF. Sickle cell anaemia: awareness among health professionals and medical students at the Lagos University Teaching Hospital, Lagos. Nig Q J Hosp Med. 2009;19:195–199. doi: 10.4314/nqjhm.v19i4.54524. [DOI] [PubMed] [Google Scholar]
- Animasahun BA, Nwodo U, Njokanma OF. Prenatal screening for sickle cell anemia: awareness among health professionals and medical students at the Lagos University Teaching Hospital and the concept of prevention by termination. J Pediatr Hematol Oncol. 2012;34:252–256. doi: 10.1097/MPH.0b013e31824e3109. [DOI] [PubMed] [Google Scholar]
- Bonnin-Scaon S, Lafon P, Chasseigne G, Mullet E, Sorum PC. Learning the relationship between smoking, drinking alcohol, and the risk of esophageal cancer. Health Educ Res. 2002;17:415–424. doi: 10.1093/her/17.4.415. [DOI] [PubMed] [Google Scholar]
- Brown BJ, Okereke JO, Lagunju IA, Orimadegun AE, Ohaeri JU, Akinyinka OO. Burden of health-care of carers of children with sickle cell disease in Nigeria. Health Soc Care Commun. 2010;18:289–295. doi: 10.1111/j.1365-2524.2009.00903.x. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control (2013) Serogroup A meningococcal conjugate vaccine coverage after the first national immunization campaign—Burkina Faso, 2011. MMRW 50:1022–1024. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6150a2.htm. Accessed 30 June 2013 [PubMed]
- Deans Z, Hill M, Chitty LS, Lewis C. Non-invasive prenatal testing for single gene disorders: exploring the ethics. Eur J Hum Genet. 2013;21:713–718. doi: 10.1038/ejhg.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwin AK, Edwin F, Etwire V. Controlling sickle cell disease in Ghana—ethics and options. Pan Afr Med J. 2011;10:14. doi: 10.4314/pamj.v10i0.72223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famuyiwa OO, Aina OF. Mothers’ knowledge of sickle cell anemia in Nigeria. Int Q Commun Health Educ. 2009;30:69–80. doi: 10.2190/IQ.30.1.f. [DOI] [PubMed] [Google Scholar]
- Guédéhoussou T, Gbadoé AD, Lawson-Evi K, Atadouma DY, Ayikoé AK, Vovor A, Tatagan-Agbi K, Assimadi JK. Connaissance de la drépanocytose et pratiques de prevention dans la population d’un district urbain de Lomé, Togo. Bul Soc Pathol Exot. 2009;102:247–251. [PubMed] [Google Scholar]
- Hermand D, Mullet E, Lavieville S. Estimation of the combined effect of tobacco and alcohol on cancer risks. J Health Psychol. 1997;2:481–491. doi: 10.1177/135910539700200405. [DOI] [PubMed] [Google Scholar]
- Hofmans J, Mullet E (2013) Towards unveiling individual differences in different stages of information processing: A clustering-based approach. Qual Quant 47:555–564
- Kagu MB, Abjah UA, Ahmed SG. Awareness and acceptability of prenatal diagnosis of sickle cell anaemia among health professionals and students in North Eastern Nigeria. Niger J Med. 2004;13:48–51. [PubMed] [Google Scholar]
- Lewis C, Skirton H, Jones R. Can we make assumptions about the psychosocial impact of living as a carrier, based on studies assessing the effects of carrier testing? J Genet Counsel. 2011;20:80–97. doi: 10.1007/s10897-010-9327-8. [DOI] [PubMed] [Google Scholar]
- Metcalfe SA. Carrier screening in preconception consultation in primary care. J Commun Genet. 2012;3:193–203. doi: 10.1007/s12687-011-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moronkola OA, Fadairo RA. University students in Nigeria: knowledge, attitudes towards sickle cell disease, and genetic counseling before marriage. Int Q Commun Health Educ. 2006;26:85–93. doi: 10.2190/JN25-4353-75PK-3733. [DOI] [PubMed] [Google Scholar]
- Owolabi RS, Alabi P, Olusoji D, Ajavi S, Out T, Ogunidiran A. Knowledge and attitudes of secondary school students in Federal Capital Territory (FCT), Abuja, Nigeria towards sickle cell disease. Niger J Med. 2011;20:479–485. [PubMed] [Google Scholar]
- Riedijk S, Oudesluijs G, Tibben A. Psychosocial aspects of preconception consultation in primary care: lessons from our experience in clinical genetics. J Commun Genet. 2012;3:213–219. doi: 10.1007/s12687-012-0095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2011) Sickle cell disease and other haemoglobin disorders. Available at http://www.who.int/mediacentre/factsheets/fs308/en/. Accessed 22 Jan 2012
- World Health Organization. Regional Committee for Africa 2010. Sickle-cell disease: a strategy for the WHO African region. Available at http://www.who.int/mediacentre/factsheets/fs308/en/. Accessed 22 Jan 2012
- Zounon O, Anani L, Latoundji S, Sorum PC, Mullet E. Misconceptions about sickle cell disease (SCD) among lay people in Benin. Prev Med. 2012;55:251–253. doi: 10.1016/j.ypmed.2012.06.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 20 kb)