SUMMARY
Transcuprein is a high affinity copper carrier in the plasma involved in the initial distribution of copper entering the blood from the digestive tract. To identify and obtain cDNA for this protein, it was purified from rat plasma by size exclusion and copper chelate affinity chromatography, and amino acid sequences were obtained. These revealed a 190 kDa glycosylated protein identified as the macroglobulin, α1inhibitorIII, the main macroglobulin of rodent blood plasma. Albumin (65 kDa) co-purified in variable amounts and was concluded to be a contaminant (although it transiently can bind the macroglobulin). The main macroglobulin in human blood plasma (α2-macroglobulin), homologous to α1inhibitorIII, also bound copper tightly. Expression of α1I3 (transcuprein) mRNA by the liver was examined in rats with and without copper deficiency, using quantitative PCR and Northern analysis. Protein expression was examined by Western blotting. Deficient rats with 40% less ceruloplasmin oxidase activity and liver copper concentrations expressed about twice as much α1I3 mRNA, but circulating levels of transcuprein did not differ. Iron deficiency, which increased liver copper concentrations 3-fold, reduced transcuprein mRNA expression and 7circulating levels of transcuprein relative to what occurred in rats with normal or excess iron. We conclude that transcupreins are specific macroglobulins that not only carry zinc but also transport copper in the blood; and that their expression can be modulated by copper and iron availability.
Keywords: Transcuprein, α1inhibitorIII, α2macroglobulin, copper, regulation, mRNA, liver, iron
INTRODUCTION
Transcuprein was first identified as a copper transport protein in the blood plasma of rats after injection or intragastric administration of trace quantities of high specific activity 67Cu(II) [1]. Immediately after treatment, or upon direct addition of the radioisotope to plasma samples, 67Cu associated with two plasma proteins: albumin, and a 270 kDa component that did not react with antibodies against albumin or ceruloplasmin. The latter was named transcuprein. By following the time course of their 67Cu-labeling in vivo, transcuprein and albumin were shown to participate in the initial distribution of copper to tissues [1–3]. In this initial distribution, most of the copper first deposited in the liver and kidney [1,4]. Transcuprein and albumin appeared to be the main sources of copper for this deposition: Not only were they the first plasma components binding the radioisotope, but radioactive copper bound to them was rapidly lost as it was gained by liver and kidney, with the kinetics of a precursor/product relationship [1,5]. From the liver (and perhaps also the kidney) [3], a major portion of the copper that just entered reemerged in the plasma on ceruloplasmin [1,3] which, in turn, was found to be a major source of copper for other tissues [6–8]. Although there was some copper associated with the amino acid fraction [4,9], repeated studies in rats indicated little or no initial 67Cu labeling of this fraction [1,3]. (The copper with albumin may however be in the form of a histidine/Cu/albumin complex [10].) Thus, transcuprein and albumin appear to be the primary components of the exchangeable copper pool of plasma and interstitial fluid. At physiological pH, radiolabeled copper bound to either protein can exchange with excess ionic Cu(II) in the medium or be removed by high concentrations of chelating agents, including histidine [3,4]. Ceruloplasmin, on the other hand, is not a participant in the exchangeable pool. Although it accounts for two thirds or more of the copper in rat and human blood plasma, its copper is not dialysable and is incorporated during ceruloplasmin synthesis and secretion by the liver [2,4,11].
Both albumin and transcuprein bind copper very tightly. (The same binding characteristics occurred when radioactive copper was added in vivo or in vitro to whole plasma [1,3,4,15].) Most albumins have an N-terminal copper binding site of very high affinity, involving a histidine residue in the third position [6,12–14], with dissociation constants variously reported as from 10−11 [14] to 10−22M [12], similar to values for copper metallothionein (10−17–10−19 [4]). The abundance of albumin provides the blood plasma with a huge potential for binding excess copper: enough to bind up to 40 μg of Cu per ml of plasma. Yet, the total copper in plasma is only about 1 μg/mL, and of that only a small fraction is actually bound to albumin. Although present in much lower amounts than albumin, transcuprein is able to successfully compete for Cu(II) in the blood plasma [1,4,5,9].
Transcuprein and albumin rapidly exchange copper with each other [1,15]: When 67Cu-transcuprein is mixed with non-radioactive albumin, the label instantly re-distributes to both proteins, and vice versa, depending upon their relative concentrations. Since the off-rate for Cu from these proteins is very slow [3,4], the rapidity of exchange indicates that transcuprein and albumin do this by direct interaction. Protein to protein exchange also appears to be the way copper is distributed within cells via so called copper chaperone proteins [16–20]; in fact, it has been calculated that there is less than one free copper atom per cell [21].) This mode of transport may protect against potentially destructive effects of free copper ions and chelates [22,23].
Although albumin is normally involved, it appears that transcuprein alone can target incoming copper to liver and kidney in the initial distribution phase, as shown in analbuminemic rats [5]. This suggests that transcuprein rather than albumin is the actual donor to hepatocytes and kidney cells.
Transcuprein has an apparent molecular weight of 270 k [1]. Upon purification, during which the presence of the protein was followed with 67Cu, it was initially found to be composed of proteins of about 200 and 65 kDa, although traces of material in the range of 100 kDa were sometimes also detected [15,24]. The studies reported here were designed to obtain cDNA for these transcuprein in order to characterize and further study the protein and its copper transport function. The approach taken was to obtain N-terminal and internal amino acid sequence to be used in RT-PCR cloning and screening. As will be shown, the large “subunit” of transcuprein and was identified as a member of the macroglobulin family, one form of which is already recognized as a carrier of the metal ion, zinc [25]. The small “subunit” was due to albumin contamination. In analogy with the iron carrier (transferrin) and iron availability [26,27], copper availability appears to influence expression of transcuprein, as does iron status.
METHODS AND MATERIALS
Animals and treatments
Untreated adult Sprague Dawley rats obtained from Simonson Laboratories (Gilroy, CA) were the source of the blood plasma used to purify transcuprein. To examine effects of copper and iron deficiency, batches of rats (usually 10) were placed on distilled water and a pelleted low copper or low iron diet, designed by E.A. Ulman in accordance with AIN guidelines [28] and produced by Research Diets, Inc. (New Brunswick, NJ). The low copper diet (0.4–0.6 ppm Cu) contained 20% protein (casein, + 0.3% DL-methionine), 66% carbohydrate (45% cornstarch, 10% maltodextrin, 10% sucrose, 5.0% cellulose), 5.0% fat (corn oil), 3.5% salt mix S18101 without added copper, 1.0% vitamin mix V10001 (+ 0.2% choline bitartrate). The low iron diet (about 5 ppm) was identical except that the “salt mix” contained normal amounts of cupric carbonate and no iron salts. Half of each batch of rats was made copper or iron-normal, the former by providing cupric sulfate (7.86 mg/L) in the drinking water, the latter by injecting 25 mg of iron as iron dextran (Iron Dextran injection, Phoenix, Inc., St. Joseph, MO) i.p., after 2–3 weeks on the diet. Rats with excess iron were given an extra 25 mg Fe injection 5 days before sacrifice. Rat tissues and plasma were analyzed after 6–7 weeks on the diets. All protocols were approved by the university IACUC.
Human plasma (IRB HSR# 05-004)
Blood plasma from normal individuals was obtained from volunteers through the Student Health Center. That from subjects being treated for iron overload due to hemochromatosis was obtained from the University of Utah Medical Center in Salt Lake city, courtesy of Dr. Richard Ajioka. Samples were stored frozen at −20° C.
Purification of transcuprein
Purification was by procedures previously described [24], using blood plasma labeled in vitro with 67Cu (II) or 64Cu (II) (10–50 ng actual Cu/ml) added as the 1:1 molar complex of nitrilotriacetate. 67Cu (II) and 64Cu (II) were obtained as chloride salts in 0.1M HCl from the reactor at the University of Missouri (Columbia) and from MIR Radiological Sciences at George Washing University (St. Louis, MO), respectively. In some cases, purification was followed by copper analysis. Size exclusion chromatography was on Sephadex G150 and Sephacryl S300 (Sigma, St. Louis, MO), using 20 mM K phosphate buffer, pH 7.0, with 20 mM ε–amino caproic acid (to inhibit nicking by plasmin) and 0.02% NaN3. Copper chelate affinity chromatography was on Sepharose 6B-bound iminodiacetic acid (Pharmacia Biotech, Piscataway, NJ) precharged with Cu(II), for which the equilibration buffer was the same phosphate buffer (size exclusion chromatography) but with added 0.5 M NaCl. Elution of copper binding proteins was induced by a linear gradient of 10–40 mM imidazole, followed by 40 mM imidazole, in the same buffer. In some cases, a combination of pseudoaffinity, ion exchange, and size exclusion chromatography was applied, as described for the isolation of α1inhibitorIII [29]. Here, 67Cu-labeled plasma was incubated with Cibacron blue Sepharose CL-4B gel (Sigma) for 30 min, with shaking, and the effluent collected by filtration. The latter was applied to DEAE-Sepharose CL-6B chromatography (Sigma) after dialysis into equilibration buffer (50 mM Tris.HCl, pH 7.4, containing 50 mM NaCl). After washing, elution was with a linear gradient of from 0.10–0.40 M NaCl in the same buffer. Purification by the procedures of Lonberg-Holm et al. [30], involving polyethylene glycol, was also carried out.
Copper and ceruloplasmin determinations
Copper was determined by furnace atomic absorption spectrometry, using a Varian Zeeman 800 instrument (Sugarland TX), as previously described [31,32]. Plasma/serum samples were assayed directly; liver samples were wet ashed in trace element grade nitric acid plus H2O2 prior to analysis. Ceruloplasmin oxidase activity was determined with p-phenylene diamine, as previously described [31].
Ferritin iron determinations
Liver homogenates (10% tissue) were heated to 70° for 10 min to obtain a heat supernatant, which was titrated with antiserum against horse spleen ferritin to maximally precipitate the ferritin, as previously described [33]. Washed immunoprecipitates were assayed for ferritin iron by the bipyridyl method, upon heating with Na bisulfite [33].
SDS-PAGE and immunoblotting
These procedures were performed as previously described [31,32], using 1.5 mm slab gels in the Hoefer Mighty Small electrophoresis system, High Range Protein Standards (BioRad, Hercules, CA), gel staining with Coomassie Brilliant Blue G250 (BioRad), and transfer to Immobilon PSQ membranes (Millipore, Bedford, MA). The primary antibody against rat transcuprein was raised in sheep against two synthetic polypeptides from different portions of the transcuprein large subunit that were linked as haptens to keyhole limpet hemocyanin antigen (prepared for us by Chiron, Clayton, Victoria, Australia). For immunoblotting, the secondary antibody was alkaline phosphatase-conjugated donkey anti-sheep IgG (Sigma). Other primary antibodies employed for immunoblotting were raised in goats against human albumin (Sigma), binding being detected with alkaline phosphatase-conjugated rabbit-anti-goat IgG (Biomeda, Foster City, CA). In some cases, purified transcuprein samples were incubated overnight at 37° in 0.25M Tris-HCl buffer, pH 8.0, containing 0.05M phenanthroline and 5% NP40, with and without 200–250 mU/mL peptide-N-glycosidase F (Sigma) after denaturation in 0.25% SDS/0.05M mercaptoethanol.
Immunoprecipitation and extraction
Rocket immunoelectrophoresis was carried out as previously described [34], using antibodies against human alpha-2-macroglobulin raised in goats (BioRad) or rabbits (Sigma). Samples containing radioactive copper were developed by autoradioagraphy, using a phosphorimager, or stained for protein with Coomassie Brilliant Blue. For analysis of the proteins in the immunoprecipitates, unstained rockets were cut from the gel and extracted by homogenizing with SDS-PAGE sample buffer and heating, prior to SDS-PAGE and immunoblotting. Alternatively, immunoprecipitation was carried out by treating partially purified plasma extracts with protein A immobilized on cross-linked 4% beaded agarose (Sigma, P2545) to remove endogenous immunoglobulins, then incubating with primary antibody, and precipitating the complexes with protein A macrobeads (Sigma, P1925) in 20 mM phosphate buffered saline (pH 7.0; PBS). Washed precipitates (3x with cold PBS) were boiled with SDS-PAGE sample buffer to extract the antigenic proteins for SDS-PAGE separation. Primary antibodies included those raised in rabbits against human ceruloplasmin (Sigma) and horse spleen ferritin (Dako, Carpinteria, CA).
Amino acid sequencing
Protein bands of transcuprein were separated on pre-aged 7.5% acrylamide resolving gels in SDS-PAGE. For N-terminal sequencing, performed at the UCLA Protein Microsequencing Facility, bands were transferred to Immobilon PSQ membranes (Millipore) and lightly stained with Coomassie Brilliant Blue G250 (BioRad). For internal sequencing, subunits in stained SDS-PAGE gels were cut out, minced, and subjected to in-gel digestion with modified trypsin (Promega, Madison, WI) in 200 mM NH4HCO3, overnight at 37°, with shaking, followed by repeated extraction with 1% trifluoroacetic acid in 60% acetonitrile. Concentrated extracts were sent to the California Institute of Technology Protein/Peptide Micro Analytical Laboratory, for separation of peptides and sequencing.
RNA extraction and Northern analysis
Total RNA was extracted from liver tissue with RNAzol B (Tel-Test, Inc., Friendswood, TX). Total RNA was separated in formaldehyde gels containing 1.0 % agarose [32] and transferred to nylon membranes (Zeta-Probe; BioRad) by capillary action. Membranes were hybridized with random-primer labeled cDNA plasmid inserts, using 32P-dCTP and the RapidHyb reagents and protocol of Amersham Pharmacia Biotech (Piscataway, NJ). After high stringency washing (once in 2X SSC, 0.1% SDS, 20 min at room temp, then twice in 0.1 SSC, 0.1% SDS, at 42° for 15 min), membranes were exposed to x-ray film (Kodak X-Omat, Rochester NY) for autoradiography. Loading was assessed by comparing ethidium bromide staining of 28 S and 18 S rRNA bands on the gels and membranes (evaluated by densitometry) and directly by hybridizing the same membranes and/or parallel membranes with cDNA for glyceraldehyde phosphate dehydrogenase. Autoradiographs were recorded by the SpeedLight Photoimaging System (San Diego, CA).
Quantitative PCR of α1inhibitorIII (transcuprein) mRNA
This was performed using the strategy and reagents provided by the Mimic system of Clontech (Palo Alto, CA). A 340 base pair portion of the nucleotide sequence of α1inhibitorIII mRNA was identified (Fig. 1) that had low homology to the other macroglobulin expressed by rat liver (α1 macroglobulin). To amplify this sequence by RT-PCR of rat liver total RNA, oligonucleotide primers were designed and obtained from Ana-Gen Technologies (Palo Alto, CA). The sequences of the α1inhibitorIII-specific primers (distinguishing between it and α1-macroglobulin) were:
Fig. 1.



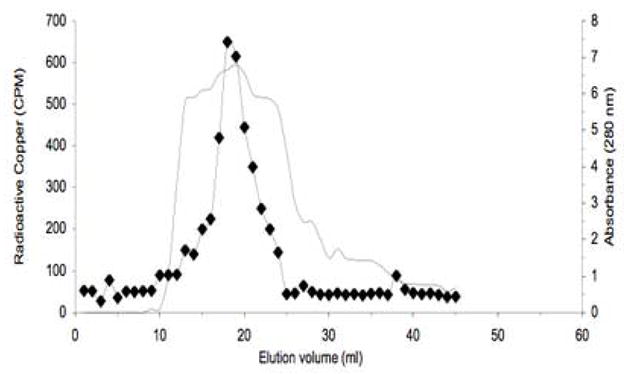
Chromatography of 67Cu-labeled rat plasma extracts during purification of transcuprein by Sephadex G150 (A), Sephacryl S300 (B), and copper chelate affinity chromatography (C), showing absorbance at 280 nm (solid line), and 67Cu radioactivity (black diamonds) or actual Cu concentrations determined by atomic absorption spectrometry (X---X, or black dots in C). (C) Elution of proteins bound to the copper chelate affinity gel, upon application of 20 ml of a 10–40 mM imidazole gradient (up-angled line), followed by an additional 20 ml of 40 mM imidazole.
(+) 5′-ATGAGCAGGTCCTCATCAAAGC (22 nucleotide; Tm 64° C)
(−) 5′-ACAATCTGTTGCAGCACTC (19 nucleotides, Tm 58° C)
Mimic primers were:
(+) 5′-ATGAGCAGGTCCTCATCAAAGCCGCAAGTGAAATCTCCTCCG (42 nucleotides)
(−) 5′-ACAATCTGTTGCAGCACTCATTTGATTGTGGACCATGGC (39 nucleotides)
Underlined portions of sequence are identical to those of the α1inhibitorIII-specific primers.
RESULTS
Purification and sequencing of transcuprein
Transcuprein was purified from 1–10 ml batches of 67Cu (II)-labeled rat plasma, by various combinations of procedures, as described in Methods. The most rapid and useful method consisted of taking the void volume fraction obtained by applying the plasma to Sephadex G150 size exclusion chromatography, and fractionating it on the larger pore size exclusion gel, Sephacryl S300, followed by copper-chelate affinity chromatography using an imidazole gradient for elution of bound protein. Examples are given in Fig. 1. As seen previously [1,5], some of the added radioactive Cu(II) associated with a peak in the void volume of Sephadex G150 (Fig. 1A, black dots) and the rest with the second 280 nm-absorbing peak which coincides with albumin elution [1]. Also shown is the analysis of actual copper (by furnace atomic absorption spectroscopy) (X---X). A significant portion of copper eluted in the void volume. The largest portion of the copper was with ceruloplasmin (eluting between the main A280 peaks), which is not labeled by in vitro 64Cu or 67Cu(II) addition; and a small amount was with albumin (seen as a shoulder to the right of the ceruloplasmin peak). (Previous studies had shown that whether added in vitro or in vivo, the radiotracer on rat plasma transcuprein behaved the same with regard to its binding affinity and release of radioactive tracer in response to non-radioactive Cu(II) and other agents [4].)
Following application of the void volume peak to Sephacryl S300 (Fig. 1B), radioactivity eluted in the middle of the column volume (Mr about 270 k), in conjunction with a major peak of protein (A280). When the radiocopper-labeled transcuprein fractions were applied to the copper-chelate gel column, the bound protein eluted with high concentrations of imidazole (35–40 mM; Fig. 1C), upon application of the 10–40 mM gradient (up-angled line). Varying amounts of actual copper (black dots; determined by atomic absorption) eluted roughly in parallel with the protein. It is noteworthy that when pre-labeled, the radioactive copper associated with transcuprein survived the copper-chelate chromatography and was not displaced (data not shown).
The protein eluting from the copper chelate columns was analyzed by SDS-PAGE. Examples from several purifications and different portions of the A280 peak are shown (Figs. 2A). Protein bands of 180–200 and 65–69 kDa were always present, and their combined apparent molecular weights added up to the Mr value of 270 kDa obtained for transcuprein [1]. However, the 65–69 kDa component was present in varying proportions, suggesting it might be a contaminant. In addition, there often were 1–2 diffuse bands in the region of 100 kDa. These accumulated with time as the density of the 190 kDa component diminished (data not shown), indicating that these were degradation products of the larger protein. Occasionally, contaminants of 145 and 45 kDa were also detected (Fig. 2A, 3d and 4th examples). The 190 and 66 kDa components, as well as the 145 kDa contaminant, were processed and sent for N-terminal and internal amino acid sequencing.
Fig. 2.


SDS-PAGE of purified transcuprein: Results of representative purifications (A), separately underlined and labeled 1–4, showing protein components of the transcuprein preparations (TC), and their apparent sizes (k) in kDa, based on protein standards (STD). In the third example, the two TC lanes were of samples collected from earlier and later parts of the copper chelate affinity peak, respectively, eluting with imidazole. In the last example, purification was by Cibacron blue, DEAE-Sepharose 4B, and Sephacryl S300 chromatography. (B) The effect of treatment with (+) and without (−) peptide-N-glycosidase F on the 190 kDa transcuprein band.
Fig. 3.

Full amino acid sequence of the α1inhibitorIII transcuprein component. The first two amino acid sequences underlined are those obtained for the 190 kDa band of transcuprein. Also underlined is the region corresponding to that of the cDNA used for mRNA quantitation (residues 379–492) and for the “bait region” of the macroglobulin (approximately residues 601–750).
Fig. 4.
Elution of transcuprein during purification by published methods used for α1inhibitorIII [29]. The flow-through from Cibacron blue chromatography of radiolabeled rat serum was applied to ion exchange chromatography on DEAE Sepharose and washed with 50 mM NaCl-Tris buffer. What is shown is the elution of proteins (absorbance at 280 nm; solid line) and copper radioactivity (black diamonds) from the ion exchange column, upon application of the NaCl gradient (100–400 mM). Pooled eluate (from 15–24 ml) was then applied to a Sephacryl S300 column (not shown). SDS-PAGE analysis of the resulting preparation is in Fig. 2A, 4th example.
The 190 kDa protein of transcuprein was identified as the major rat macroglobulin, α1inhibitorIII [35]. The full sequence of this protein (in precursor form), as well as the sequences we obtained (underlined) are given in Fig. 3. The N-terminal sequence of the 190 kDa SDS-PAGE band corresponded exactly to residues 25–39 of the precursor protein, suggesting that the first 24 residues constitute its signal sequence for synthesis on the ER-bound polyribosomes. The internal sequence obtained corresponded exactly to residues 154–171 of α1inhibitorIII, the main macroglobulin in rodent plasma [36]. Both sequences also had homology to human α2macroglobulin residues 29–43 (46%) and 157–174 (72%), respectively, the main macroglobulin in human plasma [36]. The apparent molecular weight of the 190 k component of transcuprein was in the range of that reported for α1inhibitorIII (180–210 k) [37], variability being due to 10–23% carbohydrate. Moreover, incubation of this with peptide-N-glycosidase F at 37° for 20 h reduced the apparent molecular weight by 19 k, or 10% (Fig. 2B).
The 145 kDa contaminant of the purified rat transcuprein was identified by sequencing as rat α1macroglobulin: The N-terminal 16 amino acids being identical. α1Macroglobulin has another subunit fragment of 45 kDa [30], and a subunit of this size accompanied the 145 kDa contaminant (Fig. 2A, example 3). The 65–69 kDa component was rat albumin, and this was confirmed several times.
To confirm the macroglobulin identity of transcuprein, it was purified from 67Cu-labeled rat serum by methods used for isolation of α1inhibitorIII [29,30]. Using a combination of Cibacron blue pseudoaffinity chromatography [30] (to remove albumin), fractionation of the flow-through on DEAE Sepharose [30] (Fig. 4), followed by Sephacryl S300 chromatography of the DEAE 67Cu peak, the sample in Fig. 2A (4th example) was obtained. This showed the same components as with the previous methodology: 190 and 66 kDa, as well as the 145 kDa contaminant.
All of this indicated that the major (large) component of transcuprein is α1inhibitorIII, a member of the macroglobulin family. However, this is the major macroglobulin only in rodents, while in humans and most other mammals it is α2macroglobulin. α2Macroglobulin is known to be a major carrier of zinc in human blood [25] and has been shown also to bind copper ions in vitro [38]. All macroglobulins can bind specific proteins and are particularly known for their ability to trap proteases to render them harmless [36] Proteases can cut the 180 kDa subunit in half, resulting in two fragments in the range of 90 kDa [39], such as encountered by us in some preparations (Fig. 2A, 1st and 2nd examples).
α2Macroglobulin has a high homology to α1inhibitorIII, particularly in its histidine and cysteine-rich region (see later). To see whether α2macroglobulin might be the transcuprein of human plasma, we added trace amounts of radiolabeled copper and subjected samples to rocket immuno-electrophoresis (with rabbit anti-human α2macroglobulin), using autoradiography to develop the gel. Consistent with this concept, highly radioactive rockets were obtained (Fig. 5), indicating that this macroglobulin also binds ionic copper tightly.
Fig. 5.
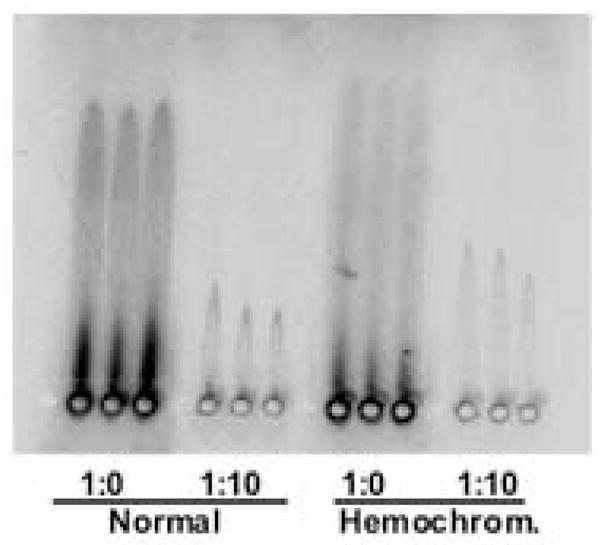
Copper binding to human α2macroglobulin. To demonstrate that α2macroglobulin is the human transcuprein, traces of 64Cu(II) were added to samples of pooled human plasma. Samples on the left half of the gel were from normal subjects; those on the right from two patients with iron overload (hemochromatosis). Portions (6 μL) of undiluted (larger rockets) or ten-fold diluted plasma (smaller rockets) were applied to adjacent wells in triplicate. The resulting gels were developed by autoradiography in a phosphorimager, detecting the 64Cu.
The question was then whether albumin was truly a component of transcuprein as well, or just a contaminant. The fact that albumin and transcuprein rapidly exchange copper with each other [1,15] indicates they can bind to each other. More consistent with contamination, variable amounts of albumin ended up in the purified transcuprein preparations, and attempts to completely remove it from purified transcuprein with Cibacron blue gel beads were not successful. It was also present in washed transcuprein immunoprecipitates. However, albumin also showed up in immunoprecipitates of other plasma proteins. Results from some of these experiments are presented in Fig. 6. SDS-PAGE of proteins extracted from the rockets obtained by immunoelectrophoresis of human plasma with antibody against human α2macroglobulin (Fig. 6A) showed a major band in the region of 190 kDa, a band of 72 kDa (consistent with albumin), and one about 57 kDa and near the bottom (probably the heavy and light chains of IgG, respectively; unpublished data). However, rockets from immunoelectrophoresis of human plasma (± horse spleen ferritin) with antibodies against not only α2macroglobulin but also human ceruloplasmin or horse spleen ferritin, all also contained albumin, as shown in the sample Western blot in Fig. 6B. Similar results were obtained for immunoprecipitates of rat transcuprein and ceruloplasmin (data not shown; see legend to Fig. 6B). Thus, washed immunoprecipitates of various plasma proteins all showed albumin contamination.
Fig. 6.
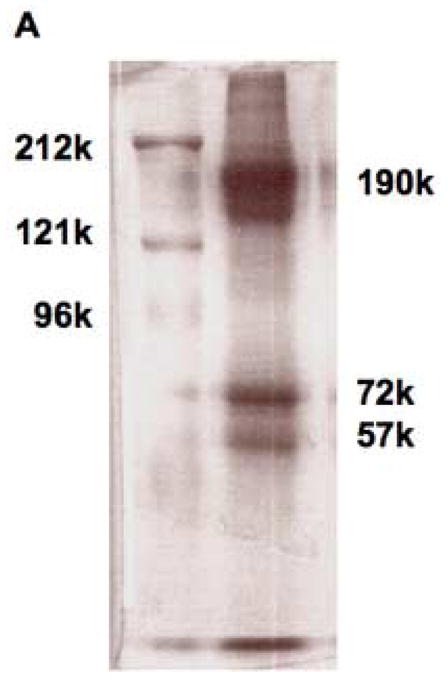

Non-specific co-precipitation of albumin with transcuprein and other proteins. (A) SDS-PAGE of immunoprecipitates extracted from rockets obtained by immunoelectrophoresis of human plasma with antibody against human α2macroglobulin (lane 2) in comparison with prestained high range protein standards (212, 121, and 96 kDa) (lane 1). (B) SDS-PAGE immunoblot with anti human albumin of proteins extracted from immunoelectrophoresis rockets obtained with human plasma and antibodies against horse spleen ferritin (lane 3), α2macroglobulin (lane 4) and ceruloplasmin (lane 5). Lane 2 was whole human plasma, lane 1 prestained protein standards (188, 100 and 45 kDa). In the case of the ferritin rockets, pure horse spleen ferritin had been mixed with the human plasma. The same kinds of results were obtained with immunoprecipitates of rat transcuprein and ceruloplasmin (not shown), using rat plasma prefractionated by size exclusion chromatography on Sephadex G150, and pre-depletion of the transcuprein and ceruloplasmin-containing fractions of rat immunoglobulins with protein A (see Methods). Transcuprein/α1inhibitorIII and ceruloplasmin in the resulting supernatants were immuno-precipitated with primary antibodies and protein A beads.
We had shown previously that copper bound to transcuprein, and copper uptake by liver and other tissues proceeded normally in the absence of albumin (Nagase analbuminemic rats) [5]. This proves that albumin did not have to be part of transcuprein. Taken together with the other data, we thus conclude that it consists of only one kind of protein, namely α1inhibitorIII (in rats) and α2macroglobulin (in humans).
The copper content of some of the purified transcuprein preparations obtained was also examined. Calculated values for 4 purified preparations ranged from 1–13 Cu atoms per molecule of 190 kDa, and there was no correlation between copper content and the degree of albumin contamination. However, we think that measured copper contents do not reflect the actual situation for transcuprein in vivo, and that it picked up additional copper during its purification, since the binding in whole plasma is more like 0.5 copper atoms per 190 kDa. This calculation is based on a ballpark concentration of 1 mg macroglobulin/mL and 120 ng Cu/mL in the transcuprein fraction. The ability to bind additional copper atom, but with complex stoichiometry, was confirmed by in vitro equilibrium dialysis studies (Jeremy Goforth, unpublished data).
Effects of dietary copper and iron availability on expression of transcuprein
Since transcuprein is involved in plasma copper transport, it seemed possible that expression of this protein would vary in relation to the availability of copper to the liver, which produces this protein for the circulation. This is the case for transferrin, the plasma protein involved in iron transport: Its rate of synthesis and blood plasma concentrations are increased by iron deficiency [26,27,40,41]. To begin to test this concept, we examined the expression of transcuprein mRNA by livers of rats on copper deficient and copper sufficient diets. In two different studies, weanling rats were placed on starch-based low copper diets for 6–7 weeks. Half were given deionized water to drink, the other half with added copper. In both studies, copper deficiency was induced, as evidenced by a 40% lower plasma ceruloplasmin oxidase activity and liver copper concentration (Table 1, A). Expression of transcuprein mRNA was assessed in three ways: by competitive and non-competitive PCR and by Northern analysis, based on the known nucleotide sequence of rat α1inhibitorIII mRNA. Total RNA was extracted from the livers of copper normal and copper deficient rats. For PCR, this was reverse transcribed into cDNA and analysed for transcuprein mRNA content.
Table 1.
Effects of dietary copper and iron deprivation on hematocrit, plasma ceruloplasmin, liver copper and ferritin iron. Data are Means ± SD for the N in parentheses.
| Nutritional Copper or Iron Status |
|||
|---|---|---|---|
| Parameter | Deficient | Supplemented | Excess |
| A. Rats on low copper diets | |||
| Hematocrit (%) | 40 ± 6 (5) | 45 ± 6 (5) | -- |
| Plasma ceruloplasmin (p-phenylene diamine oxidase activity) (nmol/min/mL) | 0.072 ± 0.008 (3)* | 0.125 ± 0.010 (3) | -- |
| Liver copper (μg/g) | 2.1 ± 0.4 (4)* | 3.5 ± 0.9 (5) | -- |
| B. Rats on low iron diets | |||
| Hematocrit (%) | 14 ± 5 (6)* | 45 ± 6 (8) | 45 ± 5 (5) |
| Liver ferritin Fe (μg/g) | 4 ± 4 (6)* | 31 ± 7 (8) | 100 ± 37 (5)* |
| Liver copper (μg/g) | 11 ± 5 (4)° | 6 ± 2 (4) | 4 ± 1 (4) |
p < 0.025- <0.001 for difference from supplemented rats by Student’s t-test
p < 0.05 for difference from excess Fe treated rats
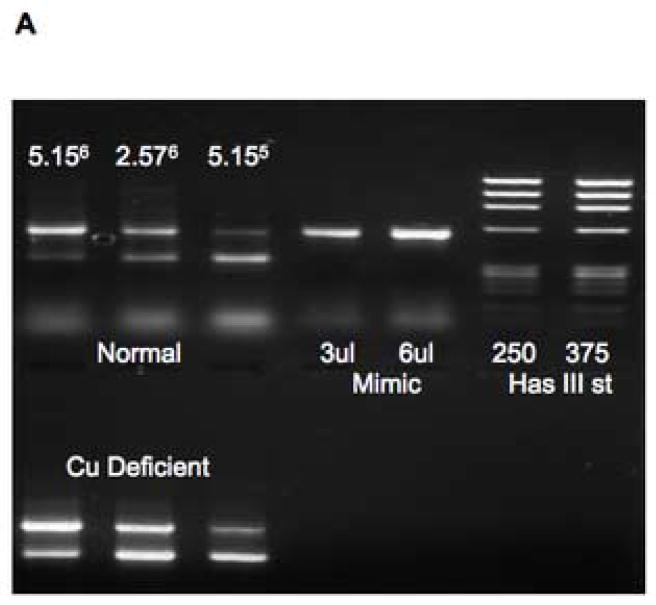
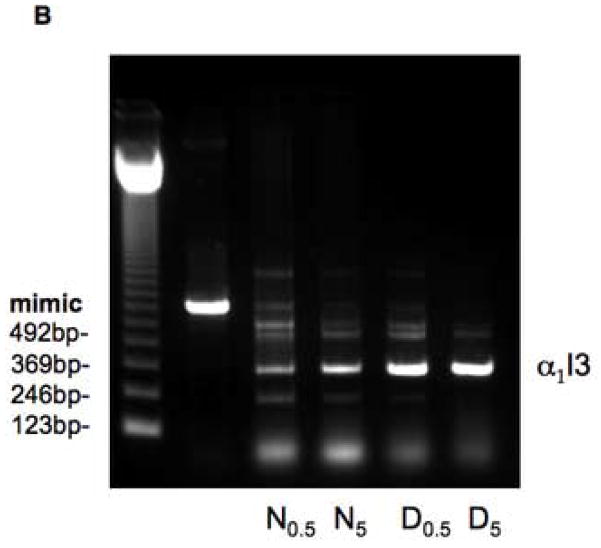
Fig. 7A shows an example of the results of applying the quantitative PCR Mimic system of Clontech (see Methods). As the concentration of competitor (Mimic) cDNA decreased (from left to right), the amount of transcuprein cDNA-amplified product (target) versus Mimic cDNA product increased. In the case of the cDNA transcribed from mRNA of the deficient rats (lower panels), this was clearly more competitive with the Mimic cDNA: At all Mimic cDNA concentrations, the proportion of target (transcuprein) amplification product was greater than with the cDNA from copper-sufficient (normal) livers (upper panels). Calculations of the actual change in two trials indicated that copper deficiency increased transcuprein mRNA concentrations 65 and 83%. Direct, non-competitive PCR of the cDNA from normal and deficient rats (Fig. 7B) gave similar results, with increases of 60 and 106% by densitometry.
Fig. 7.
Effect of copper deficiency on expression of liver α1inhibitorIII (α1I3) mRNA (transcuprein), determined by competitive and non-competitive PCR. RNA was extracted from livers of rats on a low copper diet, with and without copper supplementation in the drinking water. (A) Results of competitive PCR, based on development and application of the Mimic system of Clontech. The upper and lower pairs of gel bands are examples of results for copper supplemented (normal) and copper deficient (Cu deficient) rats, respectively, in which products were separated in ethidium bromide containing agarose gel electrophoresis. In each set, the 340 and 540 bp “target” and “mimic” PCR products (lower and upper bands, respectively) reflect amplification of the liver transcuprein/α1inhibitorIII cDNA and its Mimic, respectively. Three different (decreasing) amounts of competing (Mimic) cDNA were present from left to right, in this case 5.15 and 2.57 × 106 and 5.15 × 105 molecules, respectively, as indicated. The products obtained with a given amount of Mimic cDNA alone is shown to the right, along with bands for densitometric standards (375 ng of Hae digested DNA (Mimic kit)]. (B) Non-competitive RT-PCR of RNA from normal (supplemented; N) and deficient (D) rat livers (4 lanes at right), using two different amounts of cDNA template (0.5 and 5 ul, shown in subscripts), showing amplification of α1I3 cDNA. The left lane contains the DNA ladder, the next lane the 540 bp Mimic cDNA.
The same result was obtained by Northern analysis, using a 340 bp cDNA fragment of α1inhibitorIII cDNA as the probe (Methods). Fig. 8A shows an example of the RNA loading of a membrane after transfer from an ethidium bromide-containing agarose gel. Fig. 8B shows the corresponding autoradiographic bands obtained with the transcuprein probe (above) and with the control probe (glyceraldehyde-3-phosphate dehydrogenase; below). Clearly, more expression was observed in the case of RNA from the copper deficient livers.
Fig. 8.
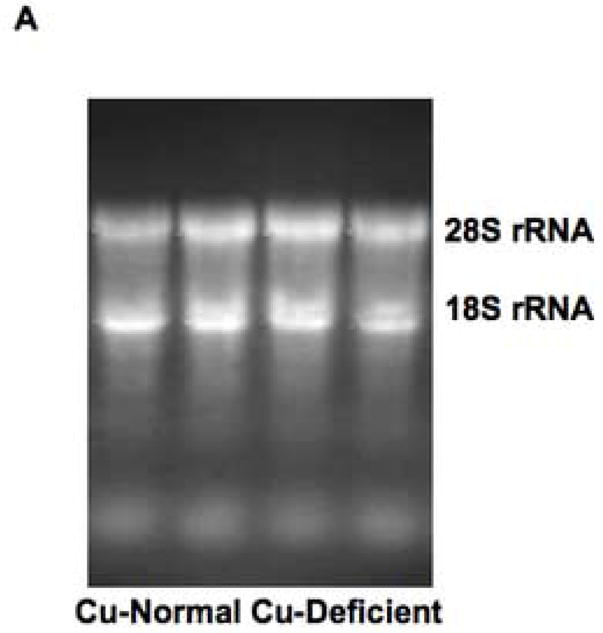
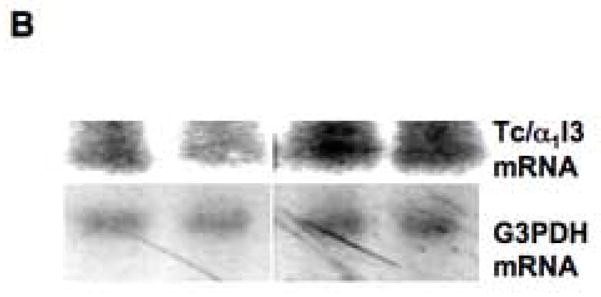
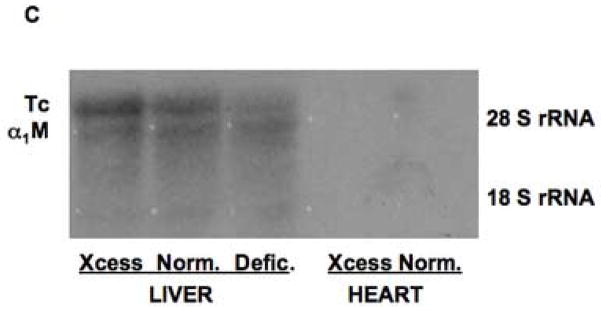
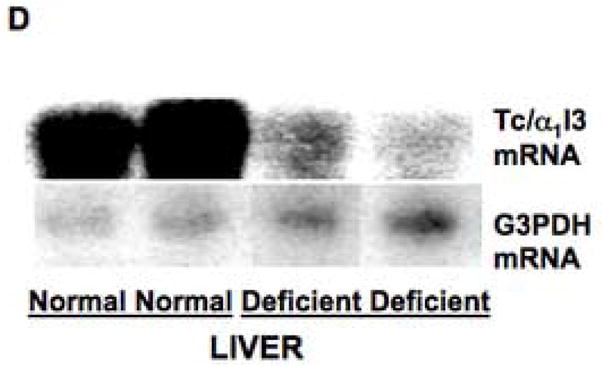
Effects of copper deprivation and iron status on expression of α1inhibitorIII (α1I3) mRNA (transcuprein) (Tc/α1I3), determined by Northern analysis. (A) Representative nylon membrane of total RNA, after capillary transfer from agarose gel containing ethidium bromide, showing the loading of RNA from copper-normal and copper deficient rat livers. (B) Portions of the resulting Northern autoradiograph, showing hybridization with Tc/α1I3 cDNA and that of the glyceraldehyde-3-phosphate dehydrogenase (G3PDH) control, for copper-normal and deficient rats, as indicated. (C) Northern of liver and heart total RNA from rats with normal (Norm.), deficient (Defic.) and excess (Xcess) iron status, showing expression of Tc/α1I3 mRNA above 28 S rRNA migration mark. (D) Northern of total liver RNA from normal and iron deficient rats, showing hybridization with Tc/α1I3 and G3PDH, respectively.
Since iron status can influence copper metabolism, and vice versa [4,42,43], potential effects of iron deficiency were also examined by Northern analyses. As shown in Figures 8C and D, iron availability correlated positively with transcuprein mRNA expression. There was little detectable expression in the RNA from iron deficient rat livers as compared with RNA from normal livers (Fig. 8C, D), and expression was even higher with iron treatment (Fig. 8C). Indeed, a comparison of the data suggested that the availability of iron may be more important in determining the amount of transcuprein mRNA expressed than the availability of copper, in that the magnitude of changes was larger. However, it is noteworthy that the level of copper in the liver was elevated by the induction of iron deficiency (Table 1, B), and this could be a factor.
The expression of transcuprein protein was also examined by immunoblotting, using a polyclonal antibody raised against two synthetic polypeptides of portions of the α1inhibitorIII protein (Methods). As shown in Figure 9A (and other immunoblots, not shown), there was a weak positive relationship between transcuprein levels and iron status when comparing plasma samples from deficient and non-deficient animals, apparent densities of the bands increasing about 35% (from deficiency to normal), from 38 ± 5 to 51 ± 2, respectively (Mean ± SD; n=4). In contrast, we could not detect a clear difference in the samples from copper deficient and supplemented rats (Fig. 9B). However, using standards, we found that the method was insufficient to detect less than a 50% difference (implying that the small difference detected with iron deficiency might be an underestimate). A positive correlation between iron status and human transcuprein appeared to be present in humans as well. Thus, several samples of plasma from subjects with hemochromatosis had more α2macroglobulin than those of normal subjects (Figure 5).
Fig. 9.


Expression of transcuprein protein in serum of rats with varying iron and copper status, determined by Western blotting. (A) Expression of the α1inhibitorIII (190 kDa) component in iron deficient (lanes 1,2), normal (lanes 3,4), and excess iron (lanes 5,6) treated rats. Equal (5 μL) portions of plasma were analyzed. (B) Expression in copper deficient (D) and supplemented (N) rats.
DISCUSSION
As already described, transcuprein was the name given to a large, copper binding protein in the blood plasma of rats that was labeled with tracer 67Cu (II) immediately after injection of ng portions of the radioisotope or upon direct addition of the tracer to aliquots of blood plasma or serum [1,5]. Profiles of the distribution of actual copper among rat [1,4] and human [6,44,45] plasma fractions, separated by size exclusion chromatography, have shown repeatedly that a significant portion of plasma copper is associated with the void volume (transcuprein) fraction. In the case of the rat, and using both tracer 67Cu and actual copper contents as markers, we isolated the protein with which this copper is associated. We found that in rats, transcuprein is the macroglobulin, α1inhibitorIII [35,37], the main macroglobulin found in rodents; that the main macroglobulin found in human plasma (α2macroglobulin) also strongly binds ionic 64Cu tracer; and concluded that the albumin isolated with transcuprein is not essential for its function but due to contamination and the ability to interact with the macroglobulin. We also demonstrated that expression of transcuprein/α1inhibitorIII in rat liver at both the mRNA and proteins levels is influenced by nutritional copper status, as well as by iron status (which can influence copper status). Moreover, preliminary results comparing α2macroglobulin levels in plasma from normal human subjects and those with iron overload (hemochromatosis) was consistent with the findings for the rat.
At first glance, the finding that a copper transport protein belongs to the family of macroglobulins might be surprising. However, the multifunctionality of proteins is becoming more and more apparent. Some examples include the iron regulatory protein, IRP1, which functions both as an aconitase (converting citrate to isocitrate) and as a regulator of the translation and stability of mRNAs for proteins of iron metabolism; albumin itself, which carries many unrelated nutrients and is also an amino acid store; ceruloplasmin, which serves as a ferroxidase, a radical scavenger, and also is the preferred source of copper for placenta, heart and brain [3,7,8]. Macroglobulins are best known for their ability to bind and inactivate proteases [36,38], which are then cleared from the circulation [36] via macroglobulin receptors on hepatocytes [46,47], with a half-life in rats of about 7 minutes [47]. [This might explain the rapid uptake of copper from transcuprein by the liver.] However, macroglobulins also have other, seemingly unrelated properties and functions. Thus, we know that they bind and may clear or modulate the function of several small non proteolytic proteins, including basic polypeptides [48] and some hormones (platelet-derived growth factor [49] and cytokines [50,51]). [Interestingly, there are other connections with hormones: Expression of α1inhibitorIII (and α2macroglobulin) is controlled by the cytokines, IL-6 and TNFα[52], as well as by glucocorticosteroids [53]; and insulin receptor phosphorylates the macroglobulin [48].] α1Microglobulin [46], a lipokalin that transports small hydrophobic substances and may also have a role in the extracellular matrix, also binds to these macroglobulins. This binding prevents α2macroglobulin from acting as an antiprotease. More important with regard to our studies, α2macroglobulin is a major carrier of Zn(II) in the blood plasma of humans [25,38, 54, 55], and perhaps also in milk [56], with 2–5 Zn ions binding per subunit, and a dissociation constant in the range of 10−7 M [55]. Other previous studies showed that human α2macroglobulin also binds copper ions, in vitro [38]; and we have some evidence that transport of Ag(I) in the blood of rats occurs on another macroglobulin, α1macroglobulin [56].
The current work confirms that human α2macroglobulin binds copper tightly, doing so in the presence of the much more abundant, high affinity copper binding protein, albumin, and retaining it during rocket immunoelectrophoresis, which is carried out over many hours (typically overnight, at 4°). [The copper binding was not due to albumin contamination of the rocket immunoprecipitates, since we have confirmed that completely purified α2macroglobulin also binds copper tightly (A. Grana, M. Moriya, and M.C. Linder, unpublished).] These findings and the facts that (a) there is copper associated with proteins in the void volume fraction of Sephadex G150 (where α2macroglobulin elutes) when human plasma or serum is fractionated [4,9,44,45]; and (b) α2macroglobulin (hardly present in rodents)[36,58] and α1inhibitorIII (not present in humans) have a great deal of sequence homology, particularly in histidine and cysteine-rich regions, allows designation of the former as the transcuprein of human plasma. [There is much less homology between α1macroglobulin (present in rodents and non-rodents [36,46,59]) and α1inhibitorIII (or α2macroglobulin).] Both rat α1inhibitorIII [35] and human α2macroglobulin [60] contain a similar, histidine-rich region (between residues 395 and 600) that has 12 and 13 histidines, respectively, 7 of which are conserved. Three of the conserved histidines are part of a conserved motif HXEAHHTAY. This motif also contains the EAH sequence which constitutes the high affinity copper binding site of human albumin (albeit without the N-terminal amino group). The same region extended to about residue 700 also contains 5 conserved cysteines that could be involved in copper binding, one of which is also part of the thiolester group of the “bait” region of these proteins (where proteases bind and get trapped. [Pratt and Pizzo [38] have reported that binding of copper to human α2macroglobulin in vitro eliminates its antiprotease activity (protease trapping).]
In further support of the involvement of transcuprein/macroglobulin in copper transport, we found that liver expression of the mRNA for α1inhibitorIII was enhanced by copper deficiency. The effect was not large, although it was reproducible. The level of liver mRNA about doubled with a copper deficiency that decreased plasma ceruloplasmin oxidase activity and liver copper concentrations about 40%. A similar (and similarly modest) inverse relationship to the availability of the metal it carries has been well documented for transferrin [26,27,40,41], the blood carrier of iron, also produced by the liver. In iron deficiency with a reduction in blood hemoglobin of 25–30%, human transferrin blood levels rise about 50% [40], and at least in some species there is a similar increase in liver mRNA levels for the carrier [41]. Although we were unable to detect a significant change in circulating levels of transcuprein, the immunoblotting method employed would not have detected such a small change in magnitude. In the case of transferrin and iron status, the mechanism involved in liver hepatocytes is still poorly understood (especially for the human) and may involve not just transcriptional but also translational control [26,61]. The transcriptional regulation appears to involve binding of unknown transcription factors to two sites within the first 125 bp of the transferrin gene promoter [26,62] to stimulate expression, and/or other factors that bring a region further upstream into play (−819 to −1000 bp), repressing expression [26,63]. HIF1 (hypoxia inducible factor) elements are also present in the promoter that might mediate alterations of expression related to iron status [26,64].
Surprisingly, we found that iron status also influenced the expression of the transcuprein/macroglobulin, but in the opposite way to copper, i.e. there was a positive relationship. mRNA expression was very low in iron deficiency and increased upon treatment of normal rats with iron (opposite to what occurs with transferrin). The magnitude of the mRNA changes observed with alterations in iron status were greater than those observed with alterations in copper status. Although more tentative, the changes in circulating rat transcuprein/α1inhibitorIII detected were not as large as those in mRNA, particularly with excess iron, suggesting translational as well as transcriptional control. However, our preliminary data for human plasma, from subjects with iron overload as compared to the normal, also showed a substantial difference in levels of α2macroglobulin, consistent with positive regulation by iron status. Thus, iron rather than copper could be responsible for the actual regulation. However, there is a reciprocal relationship between copper and iron in terms of their accumulation within tissues in general and liver in particular [42,43]. (Indeed, in iron overload, plasma ceruloplasmin levels are also lower [65].) Because iron deficiency increased liver copper concentrations 2-fold, it is possible that the iron effect is indirect and mediated by alterations in hepatocyte copper contents. This remains to be further examined. The inverse relationship between liver copper levels and plasma transcuprein levels in iron deficiency versus normal iron status does not in our view contradict it functioning as a supplier of copper to the liver, as there was still plenty of transcuprein present. The reason for the increased copper in liver in iron deficiency is thus likely to be due a reduction in copper excretion.
Acknowledgments
This work was supported in part by two Faculty Minigrants to M.C. Linder from California State University, Fullerton, two instrumentation grants from the National Science Foundation (BIR 9220666 and DUE 9352396), U.S. Public Health Service Grants RO1 HD46949 (M.C. Linder, PI), and IR24CA86307 (MIR, Washington University, St. Louis).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiss KC, Linder MC. Copper transport in rats involving a new plasma protein. Am J Physiol. 1985;249:E77–E88. doi: 10.1152/ajpendo.1985.249.1.E77. [DOI] [PubMed] [Google Scholar]
- 2.Linder MC, Hazegh-Azam M. The biochemistry and molecular biology of copper. Am J Clin Nutr. 1996;63:797S–811S. doi: 10.1093/ajcn/63.5.797. [DOI] [PubMed] [Google Scholar]
- 3.Linder MC, Wooten L, Cerveza P, Cotton S, Shulze R, Lomeli N. Copper transport. Am J Clin Nutr. 1998;67:965S–971S. doi: 10.1093/ajcn/67.5.965S. [DOI] [PubMed] [Google Scholar]
- 4.Linder MC. The Biochemistry of Copper. Plenum; New York: 1991. [Google Scholar]
- 5.Vargas EJ, Shoho AR, Linder MC. Copper transport in the Nagase analbuminemic rat. Am J Physiol. 1994;267:G259–G269. doi: 10.1152/ajpgi.1994.267.2.G259. [DOI] [PubMed] [Google Scholar]
- 6.Linder MC, Lomeli N, Donley S, Mehrbod F, Cerveza P, Cotton S, Wooten L. Copper transport in mammals. Copper Transport and its Disorders: Molecular and Cellular Aspects. In: Leone A, Mercer J, editors. Adv Exp Med Biol. Vol. 448. 1999. pp. 1–16. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Lancey R, Montaser A, Madani N, Linder MC. Ceruloplasmin and copper transport during the latter part of gestation in the rat. Proc Soc Exp Biol Med. 1993;203:428–439. doi: 10.3181/00379727-203-43619. [DOI] [PubMed] [Google Scholar]
- 8.Campbell CH, Brown R, Linder MC. Circulating ceruloplasmin is an important source of copper for normal and malignant cells. Biochim Biophys Acta. 1981;678:27–38. doi: 10.1016/0304-4165(81)90044-1. [DOI] [PubMed] [Google Scholar]
- 9.Wirth PL, Linder MC. Distribution of copper among components of human serum. J Nat Cancer Inst. 1985;75:277–284. [PubMed] [Google Scholar]
- 10.Lau S, Sarkar B. Ternary coordination complex between human serum albumin, copper(II) and L-histidine. J Biol Chem. 1971;246:5938–5943. [PubMed] [Google Scholar]
- 11.Yukitoshi Y, Heiny ME, Shimizu N, Aoki T, Matsumoto K. Inhibition of the copper incorporation into ceruloplasmin leads to the deficiency in serum ceruloplasmin activity in Long-Evans Cinnamon mutant rat. J Biol Chem. 1993;268:8965–8971. [PubMed] [Google Scholar]
- 12.Laussac JP, Sarkar B. Characterization of the copper(II)- and nickel(II)-transport site of human serum albumin. Biochem. 1984;23:2832–2838. doi: 10.1021/bi00307a046. [DOI] [PubMed] [Google Scholar]
- 13.Breslow E. Comparison of cupric ion-binding sites in myoglobin derivatives and serum albumin. J Biol Chem. 1964;239:3252–3259. [PubMed] [Google Scholar]
- 14.Masuoka J, Hegenauer J, Van Dyke BR, Saltman P. Intrinsic stoichiometric equilibrium constants for the binding of zinc(II) and copper(II) to the high affinity site for albumin. J Biol Chem. 1993;268:21533–21537. [PubMed] [Google Scholar]
- 15.Tsai MT, Dinh CT, Linder MC. Interactions of transcuprein and albumin in copper transport. FASEB J. 1992;6 Abstract #922. [Google Scholar]
- 16.Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD. The copper chaperone for superoxide dismutase. J Biol Chem. 1999;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- 17.Pena MMO, Lee J, Thiele DJ. A delicate balance: Homeostatic control of copper uptake and distribution. J Nutr. 1999;129:1251–1260. doi: 10.1093/jn/129.7.1251. [DOI] [PubMed] [Google Scholar]
- 18.Casareno RL, Waggoner D, Gitlin JD. The copper chaperone CCS directly interacts with copper/zinc superoxide dismutase. J Biol Chem. 1998;273:23625–23628. doi: 10.1074/jbc.273.37.23625. [DOI] [PubMed] [Google Scholar]
- 19.Klomp LW, Lin SJ, Yuan DS, Klausner RD, Culotta VC, Gitlin JD. Identification and functional expression of HAH1: a novel human gene involved in copper homeostasis. J Biol Chem. 1997;272:9221–9226. doi: 10.1074/jbc.272.14.9221. [DOI] [PubMed] [Google Scholar]
- 20.Glerum DM, Shtanko A, Tzagoloff A. SCO1 and SCO2 act as high copy suppressors of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae. J Biol Chem. 1996;271:20531–20535. doi: 10.1074/jbc.271.34.20531. [DOI] [PubMed] [Google Scholar]
- 21.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 22.Linder MC. Copper and genomic stability. Mutation Res. 2000;475:141–152. doi: 10.1016/s0027-5107(01)00076-8. [DOI] [PubMed] [Google Scholar]
- 23.Koppenol WH. Chemistry of iron and copper in radical reactions. In: Rice-Evans CA, Burdon RH, editors. Free Radical Damage and its Control. Elsevier Science B.V.; Amsterdam: 1994. pp. 3–24. [Google Scholar]
- 24.Goode CA, Dinh CT, Linder MC. Mechanism of copper transport and delivery in mammals: Review and recent findings. Adv Exp Med Biol. 1990;258:131–144. doi: 10.1007/978-1-4613-0537-8_11. [DOI] [PubMed] [Google Scholar]
- 25.Adham NF, Song MK, Rinderknecht H. Binding of zinc to α2macroglobulin and its role in enzyme binding activity. Biochim Biophys Acta. 1977;495:212–219. doi: 10.1016/0005-2795(77)90378-6. [DOI] [PubMed] [Google Scholar]
- 26.Ponka P, Beaumont C, Richardson DR. Function and regulation of transferrin and ferritin. Sem Hematol. 1998;35:35–54. [PubMed] [Google Scholar]
- 27.Barnum-Huckins K, Adrian GS. Iron regulation of transferrin synthesis in the human hepatoma cell line HepG2. Cell Biol Internat. 2000;24:71–77. doi: 10.1006/cbir.1999.0456. [DOI] [PubMed] [Google Scholar]
- 28.Report of the American Institute of Nutrition Ad Hoc Committee for Nutritional Studies. J Nutr. 1977;107:1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 29.Esnard F, Gauthier F. Purification and physicochemical characterization of a new rat plasma proteinase inhibitor, alpha 1-inhibitor III. Biochim Biophys Acta. 1980;614:553–563. doi: 10.1016/0005-2744(80)90244-2. [DOI] [PubMed] [Google Scholar]
- 30.Lonberg-Holm K, Reed DL, Roberts RC, Damato-McCabe D. Three high molecular weight protease inhibitors of rat plasma. J Biol Chem. 1987;262:4844–4853. [PubMed] [Google Scholar]
- 31.Wooten L, Shulze R, Lancey R, Lietzow M, Linder MC. Ceruloplasmin is found in milk and amniotic fluid and may have a nutritional role. J Nutr Biochem. 1996;7:632–639. [Google Scholar]
- 32.Cerveza PJ, Cotton SJ, Mehrbod F, Hazegh-Azam M, Williams P, Fonda EG, Wickler SJ. Ceruloplasmin expression in pig mammary gland, milk and serum during lactation. Arch Biochem Biophys. 2000;373:451–461. doi: 10.1006/abbi.1999.1572. [DOI] [PubMed] [Google Scholar]
- 33.Linder MC, Munro HN. Assay of tissue ferritin. Analyt Bioch. 1972;48:266–278. doi: 10.1016/0003-2697(72)90189-3. [DOI] [PubMed] [Google Scholar]
- 34.Truty JW, Malpe R, Linder MC. Iron prevents ferritin turnover in hepatic cells. J Biol Chem. 2001;276:48775–48780. doi: 10.1074/jbc.M105392200. [DOI] [PubMed] [Google Scholar]
- 35.Braciak TA, Northemann W, Hudson GO, Shiels BR, Gehring MR, Fey GH. Sequence and acute phase regulation of rat alpha 1-inhibitor III messenger RNA. J Biol Chem. 1988;263:3999–4012. [PubMed] [Google Scholar]
- 36.Sottrup-Jensen L. αMacroglobulins: Structure, shape, and mechanism of proteinase complex formation. J Biol Chem. 1989;264:11539–11542. [PubMed] [Google Scholar]
- 37.Esnard F, Gutman N, Moujahed A, Gauthier F. Rat plasma alpha 1-inhibitor III: a member of the alpha macroglobulin family. FEBS Lett. 1985;182:125–129. doi: 10.1016/0014-5793(85)81168-6. [DOI] [PubMed] [Google Scholar]
- 38.Pratt CW, Pizzo SV. The effect of zinc and other divalent cations on the structure and function of human α2macroglobulin. Biochim Biophys Acta. 1984;791:123–130. doi: 10.1016/0167-4838(84)90002-5. [DOI] [PubMed] [Google Scholar]
- 39.Gonias SL, Roche PA, Pizzo SV. Purification and characterization of human a2-macroglobulin conformational variants by non-ideal high performance size-exclusion chromatography. Biochem J. 1986;235:559–567. doi: 10.1042/bj2350559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritzmann SE, Daniels JC. Diagnostic and Clinical Aspects. Alan R. Liss, Inc.; New York: 1982. Serum Protein Abnormalities. [Google Scholar]
- 41.Idzerda RL, Huebers H, Finch CA, McKnight GS. Rat transferrin gene expression: Tissue specific regulation by iron deficiency. Proc Natl Acad Sci USA. 1986;83:3723–3727. doi: 10.1073/pnas.83.11.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Owen CA., Jr Effects of iron on copper metabolism and copper on iron metabolism in rats. Am J Physiol. 1973;224:514–518. doi: 10.1152/ajplegacy.1973.224.3.514. [DOI] [PubMed] [Google Scholar]
- 43.Linder MC. Interactions between copper and iron in mammalian metabolism. In: Elsenhans BE, Forth W, Schumann K, editors. Metal - Metal Interactions. Bertelsheim Foundation; Gutersloh, Germany: 1993. pp. 11–41. [Google Scholar]
- 44.Barrow L, Tanner MS. Copper distribution among serum proteins in paediatric liver disorders and malignancies. Eur J Clin Invest. 1988;18:555–560. doi: 10.1111/j.1365-2362.1988.tb01267.x. [DOI] [PubMed] [Google Scholar]
- 45.Evans DJ, Fritze K. The identification of metal-protein complexes by gel chromatography and neutron activation analysis. Anal Chim Acta. 1969;44:117–123. doi: 10.1016/S0003-2670(01)81729-3. [DOI] [PubMed] [Google Scholar]
- 46.Falkenberg C, Allhorn M, Thogersen IB, Valnickova Z, Pizzo SV, Salvesen G, Akerstrom B, Enghild JJ. α1Microglobulin destroys the proteinase inhibitory activity of α1inhibitor3 by complex formation. J Biol Chem. 1995;270:4478–4483. doi: 10.1074/jbc.270.9.4478. [DOI] [PubMed] [Google Scholar]
- 47.Gliemann J, Sottrup-Jensen L. Rat plasma α1inhibitor3 binds to receptors for α2macroglobulin. FEBS Lett. 1987;221:55–60. doi: 10.1016/0014-5793(87)80351-4. [DOI] [PubMed] [Google Scholar]
- 48.Komori K, Robinson KA, Block NE, Roberts RC, Buse MG. Phosphorylation of the rodent negative acute-phase protein α1inhibitor-III by the insulin receptor tyrosine kinase. Endocrinol. 1992;131:1288–1296. doi: 10.1210/endo.131.3.1380438. [DOI] [PubMed] [Google Scholar]
- 49.Braciak TA, Northemann W, Hudson GO, Shiels BE, Gehring MR, Fey GH. Sequence and acute phase regulation of rat α1inhibitor-III. J Biol Chem. 1988;263:3999–4012. [PubMed] [Google Scholar]
- 50.Kurdowska A, Carr FK, Stevens MD, Baughman RP, Martin TR. Studies on the interaction of IL08 with human plasma alpha 2-macroglobulin: Evidence for the presence of IL-8 complexed to alpha 2-macroglobulin in lng fluids of patients with adult repiratory distress syndrome. J Immunol. 1997;158:1430–1440. [PubMed] [Google Scholar]
- 51.Lysiak JJ, Hussaini IM, Webb DJ, Glass WF, II, Allietta M, Gonias SL. Alpha 2-macroglobulin functions as a cytokine carrier to induce nitric oxide synthesis and cause nitric oxide-dependent cytotoxicity in the RAW 264.7 macrophage cell line. J Biol Chem. 1995;270:21919–21927. doi: 10.1074/jbc.270.37.21919. [DOI] [PubMed] [Google Scholar]
- 52.Saad B, Frei K, Scholl FA, Fontana A, Maier P. Hepatocyte-derived interleukin-6 and tumor-necrosis factor α mediate the lipopolysaccharide-induced actue-phase response and nitric oxide release by cultured rat hepatocytes. Eur J Biochem. 1995;229:349–355. doi: 10.1111/j.1432-1033.1995.0349k.x. [DOI] [PubMed] [Google Scholar]
- 53.Abraham L, Bradshaw AD, Northemann W, Frei GH. Identification of a glucocorticoid response element contributing to the constitutive expression of the rat liver α1inhibitor-III gene. J Biol Chem. 1991;266:18268–18275. [PubMed] [Google Scholar]
- 54.Sampson B, Kovar IZ, Beattie JH, McArdle HJ, Rauscher A. Investigations in a child with hyperzincemia: partial characterization of an abnornal zinc binding protein, kinetics and studies of liver pathology. In: Fisher PWF, editor. Trace Elements in Man and Animals-9. National Research Council Canada; Ottawa: 1997. pp. 484–486. [Google Scholar]
- 55.Osterbergand B, Malmensten R. Methylamine-induced conformational change of α2macroglobulin and its zinc(II) binding capacity. An E-ray scattering study. Eur J Biochem. 1984;143:541–544. doi: 10.1111/j.1432-1033.1984.tb08403.x. [DOI] [PubMed] [Google Scholar]
- 56.Bashgetoor D, Lonnerdal B. Identification of an alpha 2 macroglobulin receptor in human mammary epithelial cells. J Nutr. 1999;129:152–157. doi: 10.1093/jn/129.1.152. [DOI] [PubMed] [Google Scholar]
- 57.Hanson S, Donley S, Linder MC. Transport of silver and its potential interactions with copper metabolism in the rat. Tenth International Symposium on Trace Elements in Man and Animal (TEMA 10); Evian, France. May 2–7, 1999. [Google Scholar]
- 58.Stevenson FT, Greene S, Kaysen GA. Serum α2-macroglobulin and α1-inhibitor 3 concentrations are increased in hypoalbuminemia by post-transcriptional mechanisms. Kidney Internat. 1998;53:67–75. doi: 10.1046/j.1523-1755.1998.00734.x. [DOI] [PubMed] [Google Scholar]
- 59.Falkenberg C, Enghild JJ, Thogersen IB, Salvesen G, Akerstrom B. Isolation and characterization of fibronectin-α1microglobulin-complex in rat plasma. Biochem J. 1994;301:745–751. doi: 10.1042/bj3010745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matthijs G, Devriendt K, Cassiman JJ, Van den Berghe H, Marynen P. Structure of the human alpha-2 macroglobulin gene and its promoter. Bioch Biophys Res Comm. 1992;184:596–603. doi: 10.1016/0006-291x(92)90631-t. [DOI] [PubMed] [Google Scholar]
- 61.Cox LA, Kennedy MC, Adrian GS. The 5′ untranslated region of human transferrin mRNA, which contains a putative iron-regulatory element, is bound by purified iron-regulatory protein in a sequence-specific manner. Biochem Biophys Res Comm. 1995;212:925–932. doi: 10.1006/bbrc.1995.2058. [DOI] [PubMed] [Google Scholar]
- 62.Theisen M, Behringer RR, Cadd GG, Brinster RL, Stanley GS. A C/EBP-binding site in the transferrin promoter is essential for expression in the liver but not the brain of transgenic mice. Mol Cell Biol. 1993;13:7666–7676. doi: 10.1128/mcb.13.12.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sawaya BE, Aunis D, Schaeffer E. Distinct positive and negative regulatory elements control neuronal and hepatic transcription of the human transferrin gene. J Neurosci Res. 1996;43:261–272. doi: 10.1002/(SICI)1097-4547(19960201)43:3<261::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 64.Rolfs A, Kvietikova I, Gassman M, Wenger RH. Oxygen regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem. 1997;272:20055–20062. doi: 10.1074/jbc.272.32.20055. [DOI] [PubMed] [Google Scholar]
- 65.Cairo G, Conte D, Bianchi L, Franquelli M, Recalcati S. Reduced serum ceruoplasmin levels in hereditary haemochromatosis. Br J Haematol. 2001;114:226–229. doi: 10.1046/j.1365-2141.2001.02917.x. [DOI] [PubMed] [Google Scholar]