Abstract
Background
Intensive training induces two morphological myocardial typologies of athlete's heart. Endurance training (ET) induces eccentric remodeling, bradycardia and better diastolic filling. Strength training (ST) determines concentric chamber remodelling maintaining a normal heart rate (HR). Aim of the study was to compare ET and ST athletes' heart using speckle tracking echocardiography (STE).
Methods
33 professional ET, 36 ST athletes, and 17 healthy controls (CT) were enrolled. All subjects underwent standard transthoracic echocardiography at rest and STE.
Results
In ET group, HR was lower than ST group and CT group (p < 0.001; p < 0.01). ET group had higher E/A ratio than ST group and CT group (p < 0.01; p < 0.001). The left ventricular apical circumferential strain in ET group was lower than ST group and CT group (-21.6 ± 4.1% vs. -26.8 ± 7.7%, p < 0.05; vs. -27.8 ± 5.6%, p < 0.01). ET group had lower left ventricular twist (LVT) and untwisting (UTW) than ST group (6.2 ± 0.1° vs. 12.0 ± 0.1°, p < 0.01; -67.3 ± 22.9°/s vs. -122.5 ± 52.8°/s, p < 0.01) and CT group (10.0 ± 0.1°, p < 0.01; -103.3 ± 29.3°/s, p < 0.01). The univariate analysis showed significant correlation between E/A ratio and HR (r = -0.54; p < 0.001), LVT (r = -0.45; p < 0.01), UTW (r = 0.24; p < 0.05). At the multivariate analysis only HR was confirmed as independent predictor of diastolic function in all groups (Beta -0.52; p < 0.001).
Conclusion
In ET there was a better global systolic and diastolic functional reserve at rest observed with strain analysis and it maybe depended on autonomic modulation.
Keywords: Athlete's heart, Endurance training, Strength training, Speckle tracking echocardiography, Twisting
Introduction
Intense regular physical exercise is often associated with morphologic and physiologic heart modification known as "athlete's heart".1) Morganroth et al.2) were the first to postulate two different morphological forms of athlete's heart: endurance and strength athlete's heart. Indeed, cardiovascular response to exercise largely depends on the type of exercise performed.
The two disciplines, which are typically chosen as the extreme examples of endurance and strength training (ST), are long distance running and weight lifting. The long distance running involves long periods of submaximal-intensity exercise with prolonged elevation in cardiac output and heart rate (HR) and relatively small elevation in blood pressure. In contrast, the weight lifting consists of short bursts of extremely intense exercise accompanied by a lesser rise in cardiac output and marked elevation in blood pressure.2)
A great deal of terms has been used to describe these two types of exercise, including aerobic versus anaerobic, dynamic versus static, and strength versus endurance.3)
Endurance training (ET) (e.g., long-distance running, road cycling, cross-country skiing, rowing) is commonly associated with cardiac remodelling. Several changes in heart structure occur in ET athletes such as mild left atrial enlargement, increase in left ventricular end diastolic diameter and left ventricular end diastolic volume, right atrial and ventricular enlargement.4),5),6),7),8) As reported in literature, in ET athletes a relative wall thickness (RWT) < 0.42 and an increase in left ventricular mass index (LVMi) could be observed, resulting in eccentric myocardial hypertrophy;9),10) these changes of cardiac morphology are related to volume overload during training.
On the other hand, ST (e.g., weight lifting, body building) often determines left ventricular concentric hypertrophy, characterized by a RWT > 0.42 and increase in LVMi.11),12) ST athletes undergo static exercise and may develop a concentric hypertrophy secondary to pressure overload; indeed LV chambers in ST athletes are smaller compared to ET athletes.3),13)
LV diastolic function in ET athletes is improved, compared to ST athletes and to sedentary controls, as revealed by transmitral pulsed wave Doppler and by tissue Doppler imaging (TDI).14)
Speckle tracking echocardiography (STE) is a new method to analyse myocardial deformation properties.
Aim of the study is to compare LV diastolic and systolic function among athletes with ET and ST protocols in highly trained cyclists and weight lifters. Furthermore we explore a possible role of LV function analysing strains deformation and torsional movements during systolic-diastolic coupling to better characterize structural heart modifications due to different protocols of intensive training.
Methods
Study population
Our study population consisted of 69 competitive athletes and 17 sedentary healthy young adults of whom none was engaged in any kind of routine training program or amateur competitive athletics as control group (CT group). All patients were age matched.
Competitive athletes were divided in two groups: ET group, consisting in 33 competitive élite-under 23 cyclists (according to International Cycling Union classification) from team U.S. Fracor Tuscany who trained for 10 months a year for at least 2-3 hours every day (70 to 160 km per session for a total of 20000 to 25000 km/year). They participated into national and international road races from January to October; all cyclists had a training background of at least five years. All cyclists had satisfactory inclusion criteria, such as a strict adherence to training protocols and to competitions, good echocardiographic image quality and they denied doping practices. ST group consisted of 36 highly trained weight lifters who trained every day for 2 hours since at least ten years. These athletes belonged to the same team and attended protocols of weight training consisting of full range-of-motion barbell exercises; they included strength standards for power clean, power snatch, press, bench press, squat, deadlift. We excluded after first selection 3 weight lifters who admitted past use of anabolic steroids; all weight lifters enrolled in the ST group denied using anabolic steroids. None of athletes enrolled had familiar history of ischemic heart disease or sudden cardiac death.
Demographics and standard echocardiographic characteristics of study population are shown in Table 1.
Table 1.
Demographic and standard echocardiographic characteristics of study population
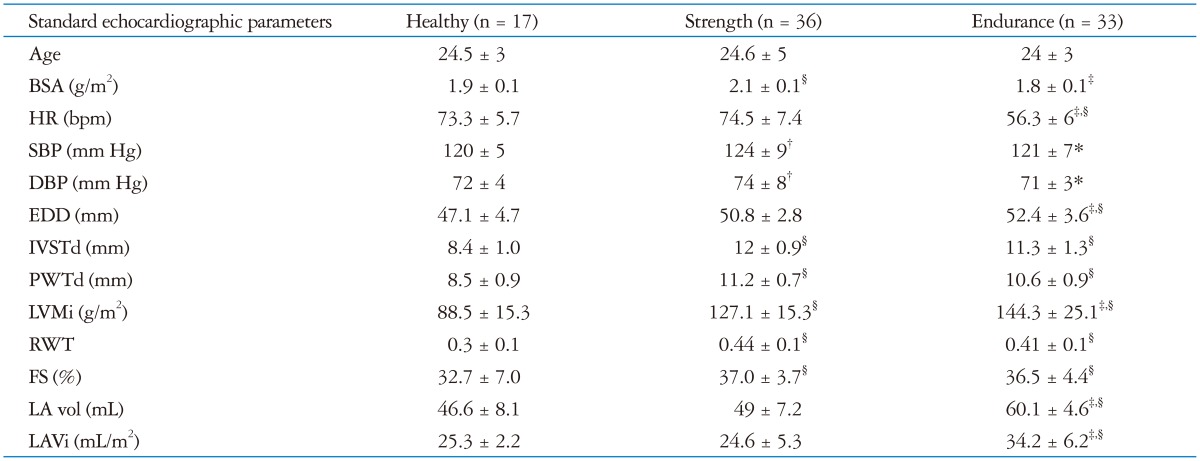
*p < 0.05 vs. strength, †p < 0.05 vs. healthy, ‡p < 0.01 vs. strength, §p < 0.01 vs. healthy. BSA: body surface area, HR: heart rate, SBP: systolic blood pressure, DBP: diastolic blood pressure, EDD: end diastolic diameter, IVSTd: diastolic inter ventricular septum thickness, PWTd: diastolic posterior wall thickness, LVMi: left ventricular mass index, RWT: relative wall thickness, FS: fractional hortening, LA vol: mean of left atrial volume values in 4 and 2 chambers, LAVi: left atrial volume indexed by body surface area
All parameters were obtained at rest. Body surface area was calculate using Mosteller formula.15)
Oscillometric semiautomatic sphygmomanometers was used to blood pressure measures. The device used was Datascope Accutorr Plus (Soma Technology, Bloomfield, CT, USA); it was validated according to standardized protocols and their accuracy was checked periodically through calibration in a technical laboratory.16)
Measurement of blood pressure at the upper arm and cuff and bladder dimensions was adapted to the arm circumference, according to ESC guidelines.17) None of the athletes had abnormal blood pressure values. All subjects of study population underwent resting 12-lead electrocardiogram, performed with Cardioline Delta 1 Plus ECG unit (REMCO ITALIA, Milano, Italy); none of them exhibited pathological electrocardiography abnormalities.
Echocardiographic parameters
Echocardiographic assessment-standard echocardiography: echocardiographic examinations were performed using a high-quality echocardiograph equipped with a 3.5 MHz probe (Vivid 7, GE, Fairfield, CT, USA).
LV measurements were performed by M-mode imaging from parasternal long axis views, in accordance with current American Society of Echocardiography recommendations. LV ejection fraction was obtained using the biplane modified Simpson's method. Concentric left ventricular hypertrophy was defined as the echocardiographic evidences of RWT > 0.42, increased LVMi for body surface area > 115 g/m2 for male calculated according to Devereux formula, and adequate echocardiographic quality; eccentric hypertrophy as RWT < 0.42 and LVMi > 115 g/m2.18) Parietal thickness and its relation to LV chamber size have been recognized as measures of hypertrophy for more than 30 years.19) RWT is measured in clinical studies both as: 2 × posterior wall thickness divided by LV diastolic diameter or, septal wall thickness + posterior wall thickness divided by LV diastolic diameter.
Diastolic function was assessed from apical four chamber view by pulsed-wave Doppler transmitral flow; early peak diastolic filling velocity (E), late peak diastolic filling velocity (A) and their ratio (E/A) were obtained. TDI was used to assess both diastolic and systolic function by measuring wall motion velocities; peak systolic (S'), early diastolic (E'), and late diastolic (A') annular velocities were obtained by averaging values recorded at the interventricular septum and LV free wall positions.20) E' was used as a relatively preload-independent measure of LV relaxation.20),21) The ratio of early to late annular velocity (E'/A') was determined as a parameter of diastolic function, as well as the LV filling index, by the ratio of transmitral flow velocity to annular velocity (E/E').20),21) Frame rate used for TDI measurements was 60-75 frames/s. TDI was performed by transducer frequencies of 3.5 MHz.14),20),21)
Speckle tracking measurements
STE was performed by the acquisition of parasternal short-axis views by conventional 2-dimensional gray-scale echocardiography at the basal and apical levels, at the end of a breathe hold cycle, with the standard and stable electrocardiography recording. Short-axis recordings were obtained from a standard parasternal probe position for the basal plane, and from a more distal anterior or anterolateral position for the apical plane. To standardize acquisitions, the basal plane was acquired at mitral valve plane, whereas the apical plane was identified distally to the papillary muscles as that just proximal to the level where LV cavity end-systolic obliteration occurred.
Three consecutive cardiac cycles for each short-axis view image were obtained during end-expiratory breath. After manual demarcation of LV endocardium by a point-and-click approach, epicardial tracking was automatically generated by the system, thus delineating the strain region of interest throughout the entire myocardial circumference. The software algorithm then automatically segmented the LV circumference into six myocardial segments and tracked the motion of LV myocardial speckles throughout the cardiac cycle, generating rotation-time curves for each segment. For STE analysis, the timing of mitral and aortic valve opening and closure were defined by pulsed wave Doppler tracking of LV inflow and outflow, and superimposed to LV twist (LVT) curves, accordingly to previous studies.22),23)
Frame rate was kept between 65 and 90 Hz to obtain the best spatial resolution. Afterwards, the software algorithm automatically segmented the LV circumference into six myocardial segments and tracked the motion of LV myocardial speckles throughout the cardiac cycle, generating rotation-time curves for each segment. In normal subjects, LV apical motion assessed by STE shows a clockwise rotation during early systole and a more evident counterclockwise rotation during ejection, whereas an opposite pattern is detectable at the basal level. The global basal and apical rotations were estimated as the average angular displacement of 6 myocardial segments during systole. By convention, counterclockwise rotation, as viewed from LV apex, was marked as a positive value, whereas clockwise rotation was expressed as a negative value. Rotation angles were expressed in degrees (°). LVT curve was automatically generated as the net difference between mean apical and basal rotation at isochronal points. Peak twist angle during ejection phase was assumed as LVT. The degree of untwisting (UTW) was defined as the directional reversal of systolic counterclockwise twist during diastole. LV longitudinal deformation in apical four chamber view and apical two chamber view, defined as peak ventricular longitudinal strain, LV basal circumferential deformation and LV apical circumferential deformation (AVCS) in short axis view, were also obtained.
Analyses were performed off-line using a dedicated software package (EchoPac, PC version 3.0, GE Medical Systems, Fairfield, CT, USA) by 2 experienced investigators who were not involved in image acquisition and who were unaware of the results of standard echocardiographic examination.
Statistical analysis
Continuous data were expressed as means ± standard deviation. Statistical comparison among the groups was performed using one-way analysis of variance. Pearson's correlation coefficients were calculated to assess the relationships between continuous variables. A p value < 0.05 was considered statistically significant, Spearman test was used to determine non linear correlations. A multiple stepwise linear regression analysis was performed to explore the independent determinants of diastolic function expressed as E/A ratio in all population. Beta value (β) was the regression coefficient for stepwise multiple linear regression. Analyses were performed using SPSS 16 for Windows (SPSS Inc., Chicago, IL, USA).
Reproducibility of STE measurements was evaluated in a subset of 30 randomly selected subjects.
For assessment of inter-observer variability, images were independently analyzed by a second experienced investigator, blinded to the results of standard echocardiography and not involved in image acquisition. Coefficients of variation and intraclass correlation coefficients for intra-observer analysis was of 5.9%, R = 0.92; p < 0.0001. For inter-observer variability, coefficients of variation, and intraclass correlation coefficients was of 6.6%, R = 0.89; p < 0.0001.
Results
In ET group, HR was lower than ST group and CT group (56.3 ± 6.0 bpm vs. 74.5 ± 7.4 bpm, p < 0.01 and vs. 73.3 ± 5.7 bpm, p < 0.01) while there were no difference of HR between ST group and CT group. ET group had a higher QRS amplitude respect to CT and ST groups, but it was no significant. There was no correlation between typology training and the Sokolow index. All athletes had significant increased mass and ET and ST groups differed in RWT: ET athletes had an eccentric hypertrophy and ST athletes had a concentric hypertrophy.
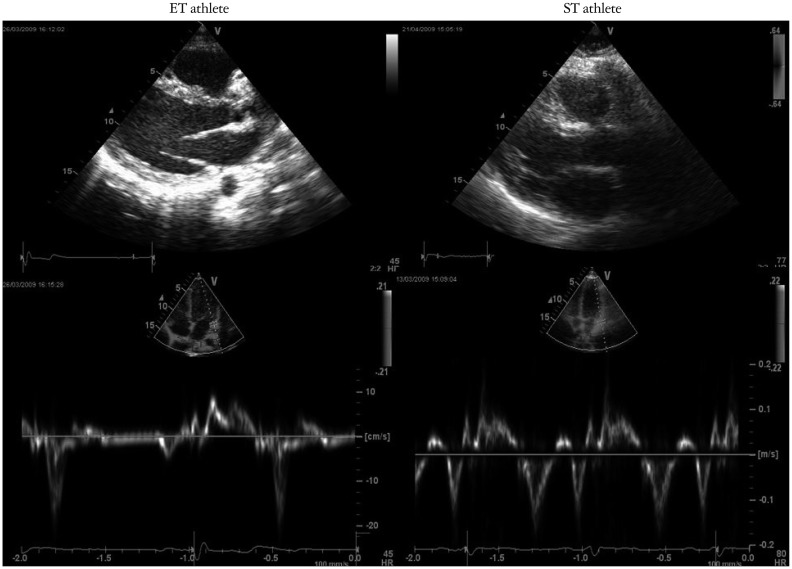
Doppler parameters are summarized in Table 2. ET group had similar peak E, but lower peak A, resulting in higher E/A ratio than CT group and ST group (1.9 ± 0.3 vs. 1.3 ± 0.3, p < 0.01 and vs. 1.4 ± 0.2, p < 0.01); Doppler TDI images are shown in Fig. 1.
Table 2.
Standard Doppler echocardiographic analysis and pulsed tissue Doppler imaging analysis of the studied population
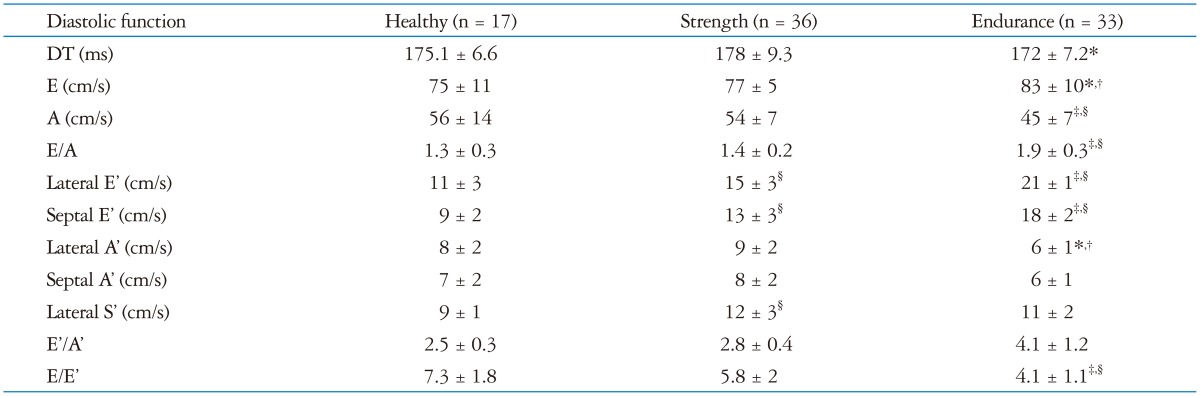
*p < 0.05 vs. strength, †p < 0.05 vs. healthy, ‡p < 0.01 vs. strength, §p < 0.01 vs. healthy. DT: deceleration time, E: transmitral early diastolic filling velocity, A: transmitral late diastolic filling velocity, E': tissue Doppler early diastolic velocity, A': tissue Doppler late diastolic velocity, S': tissue Doppler systolic velocity
Fig. 1.
ET and ST athletes tissue Doppler imaging pattern. ET: endurance training, ST: strength training.
All STE parameters are summarized in Table 3. Circumferential and longitudinal left ventricular strain curves values are reported in Table 2. There were no differences in peak ventricular longitudinal strain and basal circumferential deformation among the three groups.
Table 3.
Speckle tracking measurements of studied population
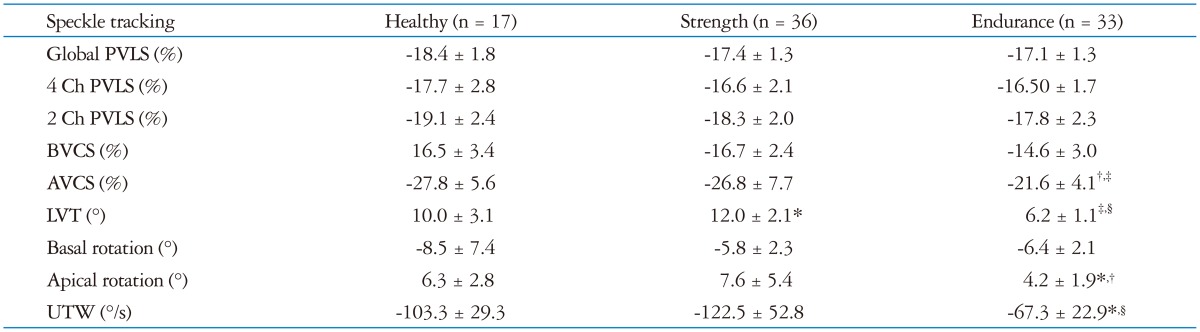
*p < 0.05 vs. healthy, †p < 0.05 vs. strength, ‡p < 0.01 vs. healthy, §p < 0.01 vs. strength. PVLS: peak ventricular longitudinal strain, BVCS: left ventricular basal circumferential strain, AVCS: left ventricular apical circumferential strain, LVT: left ventricular twisting, UTW: untwisting rate
The AVCS in ET group was lower than ST group (-21.6 ± 4.1% vs. -26.8 ± 7.7%, p < 0.05) and CT group (-21.6 ± 4.1% vs. -27.8 ± 5.6%, p < 0.01). There was no difference for AVCS between ST and CT group.
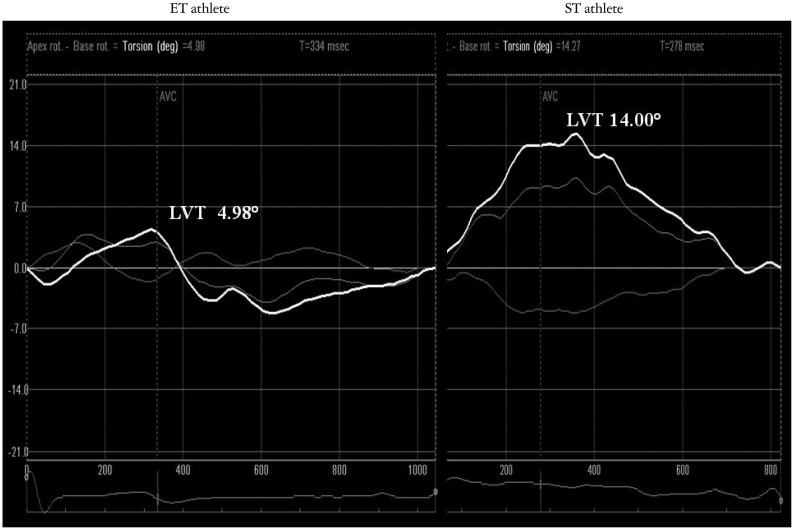
ET group had lower LVT (as showed in Fig. 2) and UTW than ST group (6.2 ± 0.1° vs. 12.0 ± 0.1°, p < 0.01; -67.3 ± 22.9°/s vs. -122.5 ± 52.8°/s, p < 0.01) and CT group (10.0 ± 0.1°, p < 0.01; -103.3 ± 29.3°/s, p < 0.01).
Fig. 2.
ET and ST athletes speckle tracking echocardiography pattern. ET: endurance training, LVT: left ventricular twist, ST: strength training.
Univariate correlation was reported in Table 4. E/A ratio showed significant correlation with HR (r = -0.54; p < 0.001), LVT (r = -0.45; p < 0.01), UTW (r = 0.24; p < 0.05).
Table 4.
Univariate correlation
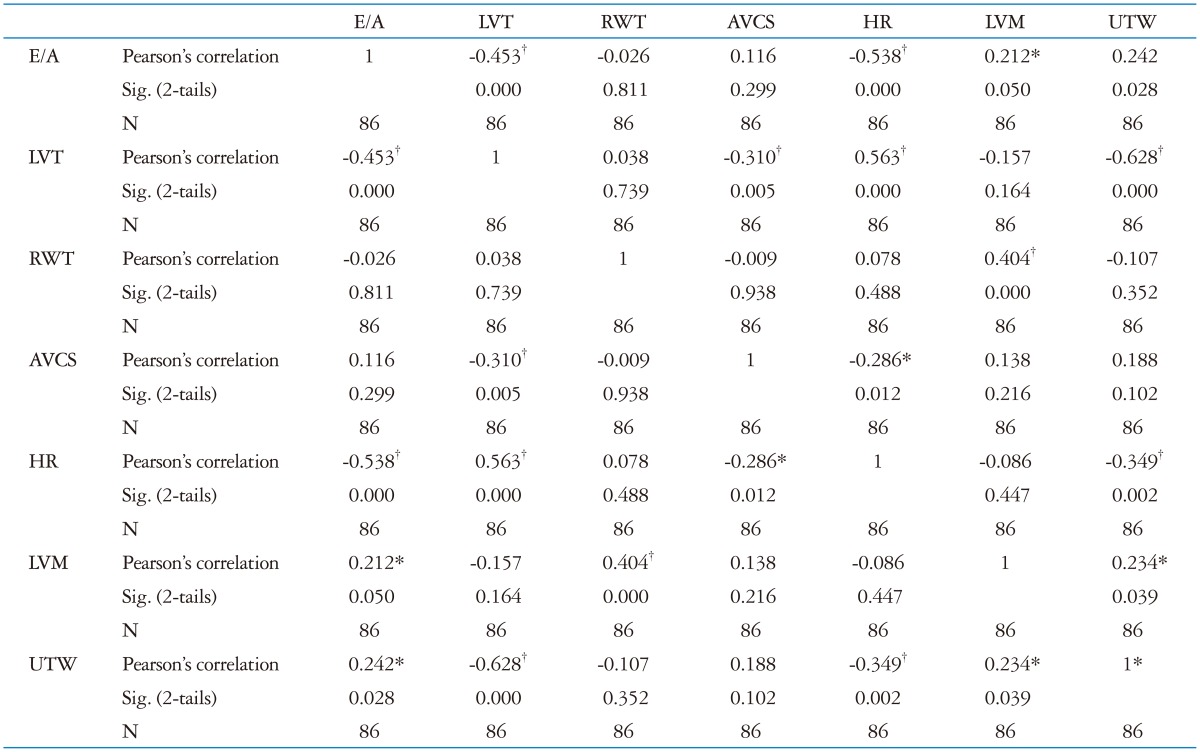
*The correlation is significant at 0.05 (2-tails), †The correlation is significant at 0.01 (2-tails). LVT: left ventricular twist, RWT: relative wall thickness, AVCS: apical ventricular circumferential strain, HR: heart rate, LVM: left ventricular mass, UTW: untwisting rate
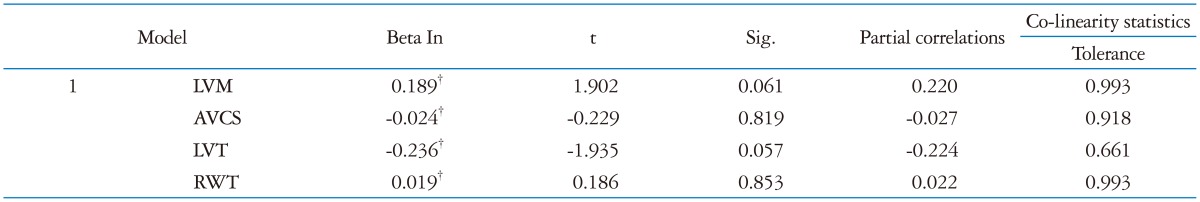
At multivariate analysis only HR was confirmed as independent predictor of diastolic function (Beta -0.52; p < 0.001) as reported in Table 5.
Table 5-1.
Multiple stepwise linear regression analysis: Model's summary

*Dependent variable: E/A, †Predictor: (Constant), HR. LVT: left ventricular twist, RWT: relative wall thickness, AVCS: apical ventricular circumferential strain, HR: heart rate, LVM: left ventricular mass, UTW: untwisting rate
Discussion
In our study two different typologies of athlete's heart have been observed: ST heart and ET hearts. There were no differences between systolic function of ET and ST. ET athletes had better global diastolic function than ST athletes, as we confirmed. The greater E/A ratio in ET athletes suggested less dependence in trained hearts on the atrial contribution to global diastolic filling at rest, because of 'supernormal' early diastolic relaxation or ventricular suction as reported in previous studies.24) In our study ET athletes showed a lower HR than others groups; this phenomenon probably represented an exaggerated parasympathetic tone developed cardiac adaptation to ET. According to literature, ET athletes showed larger endocardial diameters than other groups, slightly less thickness than ST athletes, which frame the eccentric left ventricular hypertrophy pattern in this category of athletes. Eccentric hypertrophy of ET athletes is often associated with an increased LA volume, increased E/A ratio, lower E/E' ratio, higher E'/A' and then a better diastolic function defined supernormal pattern Doppler of athletes heart, as found in our study.24),25) These cardiac characteristics are due to chronic volume overload that develops as a result of an increase of cardiac output from 5-6 L/min at rest to 40 L/min during submaximal exercise of ET sessions.26) The ET athlete's Doppler pattern was determined by an improvement of diastolic passive properties of myocardium and by an increased volume of LV refill, which increased the contribution of early diastolic phase.24),25) This supernormal pattern could be referred to as an increase of stroke volume in ET athletes at rest and especially during exercise.3) Indeed, volume overload is the prevalent stimulus that determines improvement in diastolic function.11) In our study ST athletes had resting HR values and end-diastolic diameter similar to those of CT group; LV wall thickness in ST group was of a higher degree than ET group, defining a frame of concentric hypertrophy. In ST athletes, during aortic valve closure, the high levels of afterload and intraventricular pressure determine an increase in myocardial wall stress, which is the main stimulus for cardiac concentric hypertrophy in the pressure-overloaded heart.27) In our study ST group had similar diastolic parameters of CT group. Adaptation of the heart to ST could be summarized by blood pressure response during weight-lifting that determines a concentric hypertrophy and do not influence diastolic function.9),12) In literature the athlete's heart hypertrophy is considered a physiologic adaptation to training and it is associated with increased ventricular mass and normal organization of cardiac structure, without increase in extracellular collagen content, and not impaired diastolic function.3),27) Conversely, the cardiac hypertrophy observed in patients with hypertensive cardiopathy is characterized by high mass value, concentric remodeling, collagen accumulation and diastolic dysfunction, as proven by Doppler waves and systolic impairment in advanced stages of cardiopathy.24),25) However, in ST and ET athletes the hypertrophy pattern was respectively associated with normal and supernormal E/A ratio, excluding pathological pattern of hypertrophy.28),29) In our study STE analysis showed that ET athletes had low resting LVT and UTW compared with ST and CT groups, expression of a contractile reserve mechanism to obtain a better cardiovascular performance during effort. In fact, as reported in previous studies, LVT and UTW increased progressively during exercise to ensure adequate stroke volume to perform a competitive exercise, whereas systolic longitudinal strain remained unchanged.30) This mechanism of torsional reserve in systolic-diastolic matching was driven mainly by early apical rotation.30) In ET athletes during exercise the high preload induces an increased protodiastolic relaxation related to increased myocardial stretch necessary to improve stroke volume and cardiac performance.28),31),32) A reduction in sympathetic activity might be involved in the low AVCS, LVT and UTW values, as observed in our ET athletes population, as well as a significant difference of diastolic function and HR.33),34) The reason for these regional differences might be related to local differences in the sequence of depolarization, or anatomical reasons (i.e., the LV base is fixed and tethered by the ascending aorta, the mitral ring, and the base of right ventricular, whereas the apex is free from surrounding structure); as reported in previous studies LVT is mainly determined by sympatho-vagal modulation and this is considered a LV function that store additional potential energy that is released to increase diastolic suction.28) In this study ET athletes showed a lower values of LVT compared to ST athletes and controls as well as a significant difference in diastolic function. Thus, a physiological interaction between LVT during ejection and LV relaxation could modulate LV diastolic performance, even in normal subjects.28) Previous studies observed that inotropic stimulation (i.e., high dose dobutamine infusion) increased contractility with a prevalent effect on apical rotation; LVT and autonomic balance are related.31) In our study, HR emerged as only independent predictor of E/A ratio. Thus, a physiological interaction between LVT during ejection and LV relaxation could modulate LV diastolic performance. In ET athletes the magnitude of training volume overload alters cardiac and vascular autonomic modulation by increasing parasympathetic and reducing sympathetic activity that rapidly determines an increased E/A ratio at rest with HR reduction. These two mechanisms influence LV deformation dynamics and diastolic filling.35) This was confirmed in our study, where LVT, UTW and AVCS at the STE decreased. Conversely, a higher LV afterload and HR, as observed in ST athletes, resulted in a higher LVT value as well as a lower E/A ratio, more similar to diastolic filling pattern of sedentary people rather than ET athletes. Other authors recently reported similar pattern of eccentric hypertrophy in endurance-trained athletes but no evidence of supernormal diastolic function; it can be attributable to the older age of this population and to less training volume than our élite cyclists. Furthermore, unlike our findings, they observed worse LV deformational properties in strength trained athletes respect to endurance-trained athletes.36) Our investigation pointed out the entire LV deformation properties including longitudinal and circumferential strains, torsional reserve mechanism, given by LVT and UTW, and their relationship with diastolic function in two highly selected cohort of top-level athletes. STE has the advantage of angle independency and could be a tool to better characterize the athletes' heart in patients with different typologies of physiological and pathological cardiac hypertrophy associated with adequate ejection fraction and diastolic filling respect to a standard echocardiographic exam.
In conclusion, ET athletes had a better diastolic function compared with ST athletes. In ET athletes population there was a better diastolic function at rest and low LVT, UTW and AVCS values associated with normal longitudinal function necessary to guarantee an adequate reserve mechanisms to increase the systolic function during exercise. These mechanisms are not present in ST athletes.
A limitation of this study was the relatively small sample size; this can be attributed to a strict selection whereas all the cyclists and weight lifters belonged to the same team and followed similar training protocols and competitions. All athletes denied using performance-enhancing drugs; it is impossible quantify these athletes affirmations and we must consider this parameter a limit of our study. E/A ratio is not the only parameter to study diastolic function but in patients that explain elites athletics performances and after secure exclusion of restrictive and constrictive heart pathology with electrocardiography and standard echocardiographic measurements, STE and particularly LVT could be a good tool to characterize the typology of athletes heart and his ET status. A lot of study and more extensive study population are necessary to examine the utility and reproducibility of this method.
Table 5-2.
Multiple stepwise linear regression analysis: Anova*
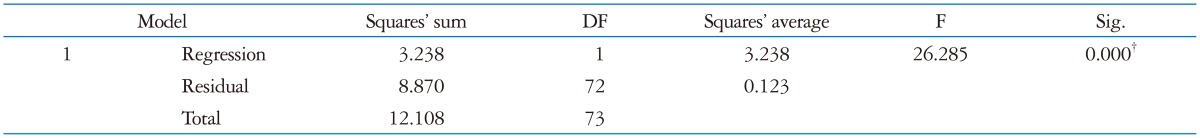
*Dependent variable: E/A, †Predictor: (Constant), HR. LVT: left ventricular twist, RWT: relative wall thickness, AVCS: apical ventricular circumferential strain, HR: heart rate, LVM: left ventricular mass, UTW: untwisting rate
Table 5-3.
Multiple stepwise linear regression analysis: Coefficients*
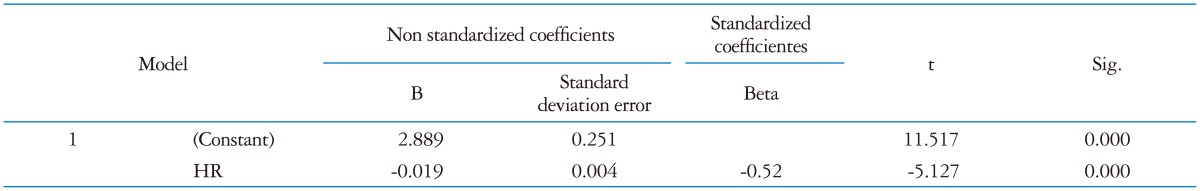
*Dependent variable: E/A, †Predictor: (Constant), HR. LVT: left ventricular twist, RWT: relative wall thickness, AVCS: apical ventricular circumferential strain, HR: heart rate, LVM: left ventricular mass, UTW: untwisting rate
Table 5-4.
Multiple stepwise linear regression analysis: Excluded Variables*
*Dependent variable: E/A, †Predictor: (Constant), HR. LVT: left ventricular twist, RWT: relative wall thickness, AVCS: apical ventricular circumferential strain, HR: heart rate, LVM: left ventricular mass, UTW: untwisting rate
Acknowledgements
The authors wish to thank all managers, athletes, coaching staff of U.S. Fracor team Tuscany for their support in our study.
References
- 1.Maron BJ. Structural features of the athlete heart as defined by echocardiography. J Am Coll Cardiol. 1986;7:190–203. doi: 10.1016/s0735-1097(86)80282-0. [DOI] [PubMed] [Google Scholar]
- 2.Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Intern Med. 1975;82:521–524. doi: 10.7326/0003-4819-82-4-521. [DOI] [PubMed] [Google Scholar]
- 3.D'Andrea A, Limongelli G, Caso P, Sarubbi B, Della Pietra A, Brancaccio P, Cice G, Scherillo M, Limongelli F, Calabrò R. Association between left ventricular structure and cardiac performance during effort in two morphological forms of athletes heart. Int J Cardiol. 2002;86:177–184. doi: 10.1016/s0167-5273(02)00194-8. [DOI] [PubMed] [Google Scholar]
- 4.D'Andrea A, Riegler L, Cocchia R, Scarafile R, Salerno G, Gravino R, Golia E, Vriz O, Citro R, Limongelli G, Calabrò P, Di Salvo G, Caso P, Russo MG, Bossone E, Calabrò R. Left atrial volume index in highly trained athletes. Am Heart J. 2010;159:1155–1161. doi: 10.1016/j.ahj.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Pelliccia A, Culasso F, Di Paolo FM, Maron BJ. Physiologic left ventricular cavity dilatation in elite athletes. Ann Intern Med. 1999;130:23–31. doi: 10.7326/0003-4819-130-1-199901050-00005. [DOI] [PubMed] [Google Scholar]
- 6.Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Picard MH, Hutter AM, Jr, Wood MJ. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol (1985) 2008;104:1121–1128. doi: 10.1152/japplphysiol.01170.2007. [DOI] [PubMed] [Google Scholar]
- 7.Kuchynka P, Palecek T, Vilikus Z, Havranek S, Taborska K, Louch WE, Linhart A. Cardiac structural and functional changes in competitive amateur cyclists. Echocardiography. 2010;27:11–16. doi: 10.1111/j.1540-8175.2009.00965.x. [DOI] [PubMed] [Google Scholar]
- 8.Teske AJ, Prakken NH, De Boeck BW, Velthuis BK, Martens EP, Doevendans PA, Cramer MJ. Echocardiographic tissue deformation imaging of right ventricular systolic function in endurance athletes. Eur Heart J. 2009;30:969–977. doi: 10.1093/eurheartj/ehp040. [DOI] [PubMed] [Google Scholar]
- 9.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete's heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101:336–344. doi: 10.1161/01.cir.101.3.336. [DOI] [PubMed] [Google Scholar]
- 10.Weiner RB, Hutter AM, Jr, Wang F, Kim J, Weyman AE, Wood MJ, Picard MH, Baggish AL. The impact of endurance exercise training on left ventricular torsion. JACC Cardiovasc Imaging. 2010;3:1001–1009. doi: 10.1016/j.jcmg.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 11.D'Andrea A, Caso P, Scarafile R, Salerno G, De Corato G, Mita C, Di Salvo G, Allocca F, Colonna D, Caprile M, Ascione L, Cuomo S, Calabrò R. Biventricular myocardial adaptation to different training protocols in competitive master athletes. Int J Cardiol. 2007;115:342–349. doi: 10.1016/j.ijcard.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 12.D'Andrea A, Caso P, Severino S, Galderisi M, Sarubbi B, Limongelli G, Cice G, D'Andrea L, Scherillo M, Mininni N, Calabrò R. Effects of different training protocols on left ventricular myocardial function in competitive athletes: a Doppler tissue imaging study. Ital Heart J. 2002;3:34–40. [PubMed] [Google Scholar]
- 13.Spence AL, Naylor LH, Carter HH, Buck CL, Dembo L, Murray CP, Watson P, Oxborough D, George KP, Green DJ. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J Physiol. 2011;589(Pt 22):5443–5452. doi: 10.1113/jphysiol.2011.217125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Andrea A, Cocchia R, Riegler L, Scarafile R, Salerno G, Gravino R, Golia E, Pezzullo E, Citro R, Limongelli G, Pacileo G, Cuomo S, Caso P, Russo MG, Bossone E, Calabrò R. Left ventricular myocardial velocities and deformation indexes in top-level athletes. J Am Soc Echocardiogr. 2010;23:1281–1288. doi: 10.1016/j.echo.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien E, Waeber B, Parati G, Staessen J, Myers MG. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322:531–536. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31:1925–1938. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr Echocardiography. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Sjögren AL. Left ventricular wall thickness determined by ultrasound in 100 subjects without heart disease. Chest. 1971;60:341–346. doi: 10.1378/chest.60.4.341. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 21.Kang SJ, Lim HS, Choi BJ, Choi SY, Hwang GS, Yoon MH, Tahk SJ, Shin JH. Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr. 2008;21:907–911. doi: 10.1016/j.echo.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Park SJ, Miyazaki C, Bruce CJ, Ommen S, Miller FA, Oh JK. Left ventricular torsion by two-dimensional speckle tracking echocardiography in patients with diastolic dysfunction and normal ejection fraction. J Am Soc Echocardiogr. 2008;21:1129–1137. doi: 10.1016/j.echo.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. quiz 453-5. [DOI] [PubMed] [Google Scholar]
- 24.Vinereanu D, Florescu N, Sculthorpe N, Tweddel AC, Stephens MR, Fraser AG. Left ventricular long-axis diastolic function is augmented in the hearts of endurance-trained compared with strength-trained athletes. Clin Sci (Lond) 2002;103:249–257. doi: 10.1042/cs1030249. [DOI] [PubMed] [Google Scholar]
- 25.Störk T, Möckel M, Müller R, Eichstädt H, Hochrein H. Left ventricular filling behaviour in ultra endurance and amateur athletes: a stress Doppler-echo study. Int J Sports Med. 1992;13:600–604. doi: 10.1055/s-2007-1024573. [DOI] [PubMed] [Google Scholar]
- 26.Ekblom B, Hermansen L. Cardiac output in athletes. J Appl Physiol. 1968;25:619–625. doi: 10.1152/jappl.1968.25.5.619. [DOI] [PubMed] [Google Scholar]
- 27.Ballo P, Mondillo S, Guerrini F, Barbati R, Picchi A, Focardi M. Midwall mechanics in physiologic and hypertensive concentric hypertrophy. J Am Soc Echocardiogr. 2004;17:418–427. doi: 10.1016/j.echo.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Santoro A, Caputo M, Antonelli G, Lisi M, Padeletti M, D'Ascenzi F, Cameli M, Giacomin E, Mondillo S. Left ventricular twisting as determinant of diastolic function: a speckle tracking study in patients with cardiac hypertrophy. Echocardiography. 2011;28:892–898. doi: 10.1111/j.1540-8175.2011.01441.x. [DOI] [PubMed] [Google Scholar]
- 29.Roy A, Doyon M, Dumesnil JG, Jobin J, Landry F. Endurance vs. strength training: comparison of cardiac structures using normal predicted values. J Appl Physiol (1985) 1988;64:2552–2557. doi: 10.1152/jappl.1988.64.6.2552. [DOI] [PubMed] [Google Scholar]
- 30.Doucende G, Schuster I, Rupp T, Startun A, Dauzat M, Obert P, Nottin S. Kinetics of left ventricular strains and torsion during incremental exercise in healthy subjects: the key role of torsional mechanics for systolic-diastolic coupling. Circ Cardiovasc Imaging. 2010;3:586–594. doi: 10.1161/CIRCIMAGING.110.943522. [DOI] [PubMed] [Google Scholar]
- 31.Esch BT, Scott JM, Warburton DE, Thompson R, Taylor D, Cheng Baron J, Paterson I, Haykowsky MJ. Left ventricular torsion and untwisting during exercise in heart transplant recipients. J Physiol. 2009;587(Pt 10):2375–2386. doi: 10.1113/jphysiol.2009.170100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caso P, D'Andrea A, Galderisi M, Liccardo B, Severino S, De Simone L, Izzo A, D'Andrea L, Mininni N. Pulsed Doppler tissue imaging in endurance athletes: relation between left ventricular preload and myocardial regional diastolic function. Am J Cardiol. 2000;85:1131–1136. doi: 10.1016/s0002-9149(00)00709-8. [DOI] [PubMed] [Google Scholar]
- 33.Nottin S, Doucende G, Schuster-Beck I, Dauzat M, Obert P. Alteration in left ventricular normal and shear strains evaluated by 2D-strain echocardiography in the athlete's heart. J Physiol. 2008;586(Pt 19):4721–4733. doi: 10.1113/jphysiol.2008.156323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akagawa E, Murata K, Tanaka N, Yamada H, Miura T, Kunichika H, Wada Y, Hadano Y, Tanaka T, Nose Y, Yasumoto K, Kono M, Matsuzaki M. Augmentation of left ventricular apical endocardial rotation with inotropic stimulation contributes to increased left ventricular torsion and radial strain in normal subjects: quantitative assessment utilizing a novel automated tissue tracking technique. Circ J. 2007;71:661–668. doi: 10.1253/circj.71.661. [DOI] [PubMed] [Google Scholar]
- 35.Baldesberger S, Bauersfeld U, Candinas R, Seifert B, Zuber M, Ritter M, Jenni R, Oechslin E, Luthi P, Scharf C, Marti B, Attenhofer Jost CH. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J. 2008;29:71–78. doi: 10.1093/eurheartj/ehm555. [DOI] [PubMed] [Google Scholar]
- 36.Monte IP, Mangiafico S, Buccheri S, Bottari VE, Lavanco V, Arcidiacono AA, Leggio S, Deste W, Tamburino C. Myocardial deformational adaptations to different forms of training: a real-time three-dimensional speckle tracking echocardiographic study. Heart Vessels. 2014 doi: 10.1007/s00380-014-0520-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]