Abstract
Obesity is an important risk factor for metabolic disease and various cancers. Treatments of obesity include lifestyle intervention, pharmacotherapy, and bariatric surgery. If weight loss with lifestyle intervention is only modest, pharmacotherapy might be needed. Pharmacotherapy agents can be grouped by treatment period as short term or long term use agent. Several sympathomimetic drugs such as benzphetamine, diethylpropion, phendimetrazine and phentermine, are approved for short term treatment due to their safety issues. For long term treatment, orlistat, lorcaserin, and combination of phentermine/topiramate are approved by U.S. Food and Drug Administration (FDA). Orlistat partially blocks intestinal digestion of fat, therefore producing weight loss. Lorcaserin is a serotonin 2C receptor agonist. The combination of phentermine/topiramate produces a mean weight loss of 8-10 kg. Side effects of each drug are quite different. For obesity patient, side effects are important factor when choosing drugs. The goal of this article is to review currently available anti-obesity drugs.
Keywords: Anti-obesity agents, Lorcaserin, Obesity, Phentermine, Topiramte
Introduction
Obesity is defined as excess body fat by positive energy balance. It is associated with metabolic disease such as type 2 diabetes, hypertension, and coronary heart disease. Obesity also increases the risk of several malignancies in breast, colon, pancreas, and endometrium.1,2 Menopause is a well-known risk factor for cardiovascular disease. During menopausal transition, women have a tendency to increase body weight. Consequently, menopause and the increase of body weight lead to an exponential increase of health risk for menopausal women.3 To maintain healthy body weight, treatment of obesity in menopausal women is an important health issue.
Fundamental treatments of obesity include lifestyle intervention such as nutrition, physical activity, and behavior therapy. Pharmacotherapy can be used as an adjunct to lifestyle intervention. However, effects of lifestyle intervention are not always satisfactory in all cases. Pharmacotherapy is used in many patients in conjunction with lifestyle intervention.
There are two groups of approved drugs that can be used to manage weight in patients with obesity: 1) medications approved for obesity per se; 2) medications that affect body weight for obese patients who have complications from their obesity and are receiving these medications for chronic disease management.4 The second group of drugs is for patients with chronic disease such as diabetes, depression, or psychiatric disorders. These drugs have different effects on weight. The best choice is to select drugs that can produce weight loss. In the following sections, we will discuss the use of medications approved for obesity per se, including recently U.S. Food and Drug Administration (FDA) approved novel agents.
Indication for Pharmacotherapy
The goals of obesity treatment are to improve or prevent complication of metabolic diseases, not weight loss itself. So, it is not desirable to prescribe medicine to patient who only wants weight loss. First step of obesity treatment is using non-pharmacologic method such as nutrition, physical activity, and behavior therapy. If the patient does not achieve adequate weight loss by lifestyle intervention for 3-6 months, pharmacotherapy can be considered.5
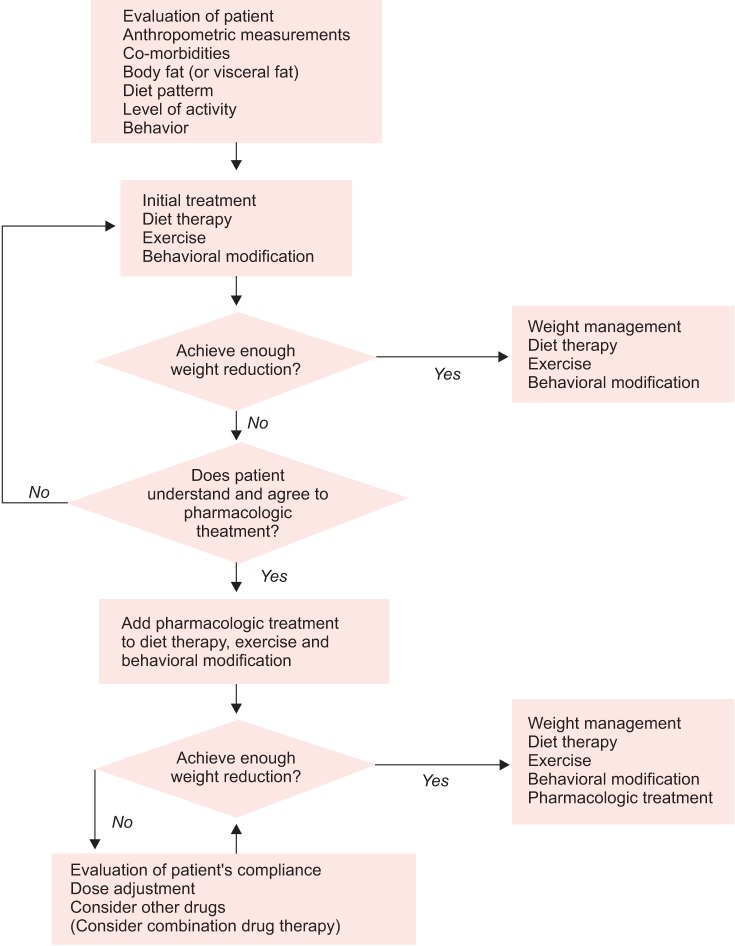
Pharmacotherapy is indicated for individuals with a body mass index (BMI) ≥ 25 kg/m2, or those with a BMI ≥ 23 kg/m2 and diagnosed with comorbidities such as hypertension, dyslipidemia, type 2 diabetes mellitus (T2DM), or sleep apnea.5 The flow of pharmacologic treatment for obesity is shown in Figure 1.5 For drugs approved for long-term use, if 3% mean weight loss is not achieved during 3 months of medication, there is a need to reconsider the treatment modality.
Fig. 1.
Flow chart for pharmacologic treatment of obesity. [Reprinted from "Pharmacotherapy for obesity", by Kim KK, 2011, J Korean Med Assoc, 54, pp.409-18. Copyright 2011 by the Korean Medical Association. Reprinted with permission].
Drugs Approved by the U.S. FDA
Sibutramine, a highly selective inhibitor of reuptake of noradrenaline and serotonin at nerve endings, was the most frequently prescribed drug. However, after significantly more cardiovascular events were reported after using it compared to placebo group, it was withdrawn from the market.6,7 Drugs approved by U.S. FDA for obesity treatment are summarized in Table 1.8 Orlistat, lorcaserin, and combination of phentermine and topiramate are approved for long-term use. Benzphetamine, diethylpropion, phendimetrazine, and phentermine are approved for short-term use. Lorcaserin and combination of phentermine and topiramate are recently approved by U.S. FDA.
Table 1.
Drug approved by the U.S. Food and Drug Administration (FDA) that produce weight loss. [Reprinted from "Update on obesity pharmacotherapy", by Bray GA, Ryan DH, 2014, Ann N Y Acad Sci, 1311, pp.1-13. Copyright 2014 by by John Wiley & Sons, Inc. Reprinted with permission]
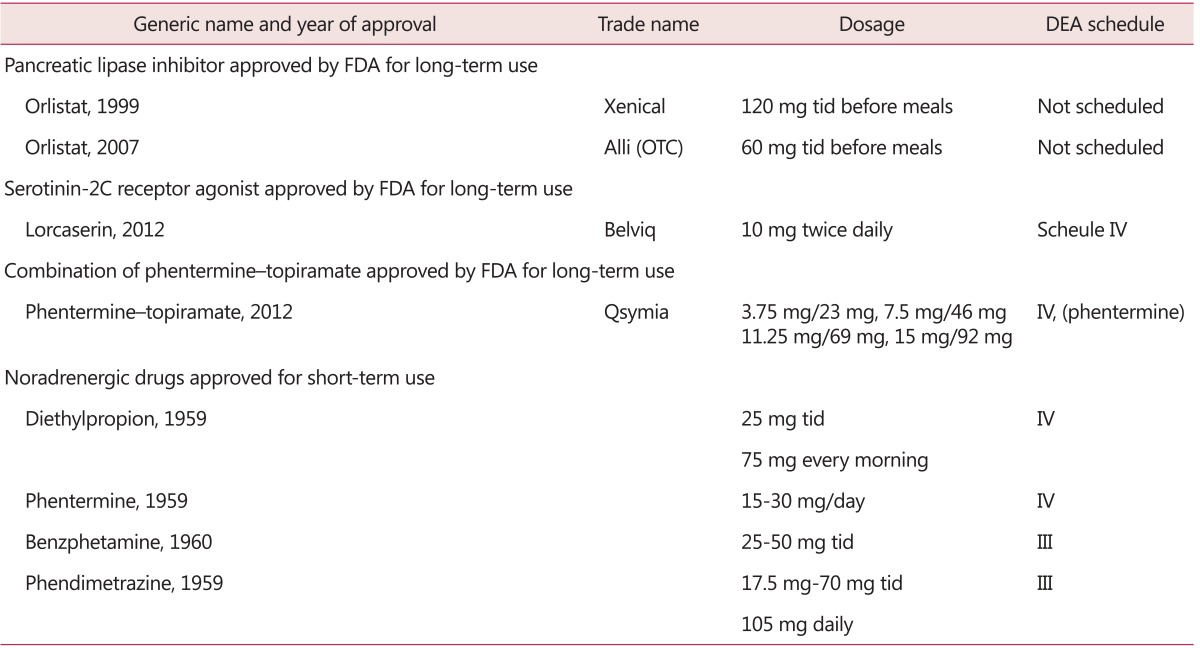
FDA: Food and Drug Administration, DEA: data envelopment analysis, OTC: over-the-counter
Orlistat (Xenical)
Orlistat is a potent and selective inhibitor of pancreatic lipase required for hydrolysis of dietary fat in gastrointestinal tract into fatty acids and monoacylglycerol.9 It was approved by the U.S. FDA in 1999 as a long-term obesity management in conjunction with reduced-calorie diet. Because orlistat is not absorbed to any significant degree, there is hardly any systemic reaction.10 A 4-year double-blind, randomized, placebo-controlled trial with orlistat in 3304 overweight patients revealed that 21% of patients with impaired glucose tolerance achieved mean weight loss more than 11% below baseline in the orlistat-treated group compared to 6% below baseline in the placebo-treated group during the first year of use.11 Compared to lifestyle changes alone, orlistat plus lifestyle changes resulted in a greater reduction in the incidence of type 2 diabetes over 4 years.11 In addition, orlistat can improve cardiovascular risk factors. Systolic and diastolic blood pressure, serum levels of total cholesterol, low-density lipoproteins (LDL)-cholesterol, and glucose were reported to be decreased in orlistat-treated group.12
Orlistat can cause small but significant decreases in fat-soluble vitamins such as vitamin A, D, E, and K. Usually, vitamin levels would remain within normal range. It is clinically challenging to discriminate which patient needs vitamins. Therefore, it is recommended to provide a multivitamin routinely before bedtime. Gastrointestinal symptoms are common initially, including oily spotting, flatus with discharge, fecal urgency, fatty oily stool, oily evacuation, and increased defecation. However, these events are generally diminished when patients control their dietary fat intake. Such events rarely cause patients to withdraw from clinical trials.4 Rare cases of severe liver injury and development of breast cancer have been reported with the use of orlistat, but the causal relationship has not been established.13
Noradrenergic Drugs
Sympathomimetic drugs are inhibitors for reuptake of noradrenalin in the synapse. As a result, adrenergic stimulus is increased. U.S. FDA approved several sympathomimetic drugs such as benzphetamine, diethylpropion, phendimetrazine, and phentermine for only a few weeks, which is usually interpreted as up to 12 weeks. Phentermine is the most often prescribed drug in this group. According to meta-analysis, patients treated with phentermine 15-30 mg daily lost on average additional 3.6 kg of weight at 6 months compared to placebo.14 In the newly developed formulation of phentermine diffusion controlled release (DCR), it showed significant reductions in body weight (-8.1 ± 3.9 vs. -1.7 ± 2.9 kg, P < 0.001) and waist circumference (7.2 ± 0.5 vs. 2.1 ± 0.6 cm, P < 0.001) compared to placebo group.15 It is very effective and relatively safe drug. However, there is little data on its long-term efficacy and safety. Therefore, it is only approved for short-term use. Frequently reported side effects include dry mouth, headache, and insomnia. To prevent insomnia, it is usually used in the morning. It may increase heart rate and blood pressure. Therefore, it is not recommended for individuals with history of cardiovascular disease.8
Lorcaserin (Belviq®)
Lorcaserin (1R-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-2-benzazepine; Belviq®, Arena Pharmaceuticals, Inc., San Diego, CA, USA) is approved by the U.S. FDA for long-term weight management in June, 2012. Lorcaserin is a serotonin type 2C receptor agonist. Serotonin has been shown pharmacologic effects on weight loss.16 Fenfluramine and dexfenfluramine as serotonin type 2B receptor agonists were also used for weight loss. However, they were removed from market due to damage to heart valves through serotonin 2B receptor.17 When serotonin-2C receptor is activated in the hypothalamus, food intake is reduced.16
Lorcaserin is prescribed at 10 mg twice daily. Three clinical studies have provided evidence for the approval of lorcaserin,18,19,20 all of which have demonstrated effective weight loss compared to placebo group, along with a favorable safety profile. In a Behavioral Modification and Lorcaserin for Obesity and Overweight Management in Diabetes Mellitus (BLOOM-DM) study, mean glycated hemoglobin (HbA1c) was decreased 0.9 ± 0.06 with twice-daily lorcaserin, compared to 0.4 ± 0.06 with placebo group (P < 0.001), and mean fasting glucose was decreased 27.4 ± 2.5 mg/dL compared to a decrease of 11.9 ± 2.5 mg/dL for placebo (P < 0.001; Fig. 2).20
Fig. 2.
Change in glycemic parameters by study week from Behavioral Modification and Lorcaserin for Obesity and Overweight Management in Diabetes Mellitus (BLOOM-DM) trial. Values are mean ± standard error of the mean (SEM); *P ≤ 0.001; **P < 0.05 compared to placebo. BID: twice daily, HbA1c: glycated hemoglobin, LS: least square, QD: once daily. [Reprinted from "Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study", by O'Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, et al., 2012, Obesity (Silver Spring), 20, pp.1426-36. Copyright 2012 by John Wiley & Sons, Inc. Reprinted with permission].
Lorcaserin was carefully investigated for potential effects on heart valves during phase III studies where echocardiograms were done on more than 5200 subjects. There was no statistically significant increase in FDA-defined valvulopathy with drug treatment as compared to placebo.18,19,20 Therefore, U.S. FDA has not recommended routine echocardiography for prescription of this medication. Lorcaserin is relatively well tolerated. The most common adverse events in clinical trials include headache, nausea, dizziness, fatigue, dry mouth, and constipation. These were mild that could be resolved quickly. Because of the risk of serotonin syndrome, it should not be used with selective serotonin reuptake inhibitors (SSRIs) or with monoamine oxidase inhibitors (MAOIs).
Phentermine/Topiramate Extended-release (ER) (Qsymia®)
Qsymia® is the combination of phentermine and topiramate as an ER medication (Vivus Inc. Mountain View, CA, USA). As previous described, phentermine, a sympathomimetic drug, has been widely used as short-term appetite suppressant. Topiramate is an anticonvulsant drug. Following anecdotal reports of weight loss occurring in patients with epilepsy, it was evaluated as a potential antiobesity drug in clinical trials.21 In terms of mechanism of action, topiramate is not thoroughly understood, although it might work through its effect on γ-aminobutyric acid (GABA) receptors. This combination uses lower doses of phentermine (starting dose at 3.75 mg, recommended dose at 7.5 mg, full dose at 15 mg) than as a single agent. Topiramate is an ER formulation. Its dose in the combination (starting dose at 23 mg, recommended dose at 46 mg, full dose at 92 mg) is lower than that when it is used for migraine prophylaxis or to control seizures. Two clinical studies provided efficacy and safety data for the approval of this medication: EQUIP and CONQUER.22,23 The CONQUER study was extended for a second year of observation with patients keeping their treatment assignment. This was called the SEQUEL study.24 The combined weight loss results are shown in Figure 3. At the end of the second year of treatment, patients completing the trial taking the recommended dose (7.5 mg/46 mg) maintained a mean weight loss of 9.3% below baseline. In the CONQUER study, there were clinically and statistically significant improvements in blood pressure, glycemic measures, high-density lipoproteins (HDL) cholesterol, and triglycerides after taking the medication at both recommended dose and full dose.
Fig. 3.
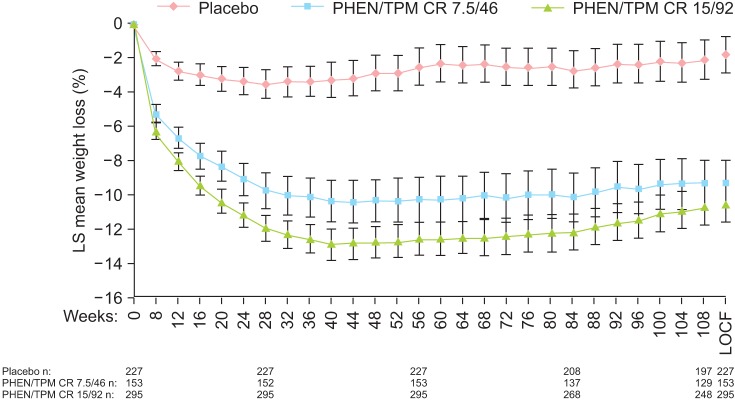
Mean (95% confidence interval [CI]) percentage weight loss from baseline to week 108 from SEQUEL trial. LOCF: last observation carried forward, LS: least-squares, PHEN/TPM CR: controlled-release phentermine/topiramate. [Reprinted from "Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study", by Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al., 2012, Am J Clin Nutr, 95, pp.297-308. Copyright 2012 by the American Society for Nutrition. Reprinted with permission].
The most common side effects reported in the clinical trials include paraesthesia, dizziness, dysgeusia (altered taste), insomnia, constipation, and dry mouth. Topiramate is associated with oral clefts if used during pregnancy. Combination of phentermine and topiramate is contraindicated during pregnancy. It requires a negative pregnancy test before treatment and monthly thereafter.8
Conclusion
After menopause, serum estradiol level is decreased, the protective effect of sex steroid for cardiovascular disease is also gradually disappeared. Weight gain during menopausal transition is an additional risk factor for cardiovascular disease. Consequently, weight gain and menopause simultaneously increase cardiovascular disease risk. Therefore, with routine check-up and education for health problem, intervention to weight management is a very important issue for menopausal women. Basic treatment modalities of obesity include lifestyle intervention, pharmacotherapy, and bariatric surgery. In pharmacotherapy, many drugs have been withdrawn form market due to unacceptable side effects. In the past, only one medicine (orlistat) was available for long-term treatment of obesity. However, two novel agents were approved by U.S. FDA for long-term usage. Although further studies such as clinical outcome for longer period or for a specific age group are needed, it is obvious that the progression of pharmacotherapy for obesity treatment gives us chance to manage weight problem more effectively.
Acknowledgement
This work was supported by Pusan National University Hospital Research Grant 2014.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Ryan JG. Cost and policy implications from the increasing prevalence of obesity and diabetes mellitus. Gend Med. 2009;6(Suppl 1):86–108. doi: 10.1016/j.genm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Greenway FL. Pharmacological treatment of the overweight patient. Pharmacol Rev. 2007;59:151–184. doi: 10.1124/pr.59.2.2. [DOI] [PubMed] [Google Scholar]
- 5.Kim KK. Pharmacotherapy for obesity. J Korean Med Assoc. 2011;54:409–418. [Google Scholar]
- 6.Luque CA, Rey JA. The discovery and status of sibutramine as an anti-obesity drug. Eur J Pharmacol. 2002;440:119–128. doi: 10.1016/s0014-2999(02)01423-1. [DOI] [PubMed] [Google Scholar]
- 7.James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 8.Bray GA, Ryan DH. Update on obesity pharmacotherapy. Ann N Y Acad Sci. 2014;1311:1–13. doi: 10.1111/nyas.12328. [DOI] [PubMed] [Google Scholar]
- 9.Lucas KH, Kaplan-Machlis B. Orlistat-a novel weight loss therapy. Ann Pharmacother. 2001;35:314–328. doi: 10.1345/aph.19412. [DOI] [PubMed] [Google Scholar]
- 10.Zhi J, Melia AT, Eggers H, Joly R, Patel IH. Review of limited systemic absorption of orlistat, a lipase inhibitor, in healthy human volunteers. J Clin Pharmacol. 1995;35:1103–1108. doi: 10.1002/j.1552-4604.1995.tb04034.x. [DOI] [PubMed] [Google Scholar]
- 11.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 12.Broom I, Wilding J, Stott P, Myers N. Randomised trial of the effect of orlistat on body weight and cardiovascular disease risk profile in obese patients: UK Multimorbidity Study. Int J Clin Pract. 2002;56:494–499. [PubMed] [Google Scholar]
- 13.Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, Elisaf MS. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Saf. 2008;31:53–65. doi: 10.2165/00002018-200831010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Shekelle PG, Morton SC, Maglione M, Suttorp M, Tu W, Li Z, et al. Pharmacological and surgical treatment of obesity. Evid Rep Technol Assess (Summ) 2004:1–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Kang JG, Park CY, Kang JH, Park YW, Park SW. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes Obes Metab. 2010;12:876–882. doi: 10.1111/j.1463-1326.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- 16.Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs : effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Rothman RB, Baumann MH. Serotonergic drugs and valvular heart disease. Expert Opin Drug Saf. 2009;8:317–329. doi: 10.1517/14740330902931524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 19.Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067–3077. doi: 10.1210/jc.2011-1256. [DOI] [PubMed] [Google Scholar]
- 20.O'Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012;20:1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- 21.Astrup A, Toubro S. Topiramate: a new potential pharmacological treatment for obesity. Obes Res. 2004;12:167s–173s. doi: 10.1038/oby.2004.284. [DOI] [PubMed] [Google Scholar]
- 22.Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring) 2012;20:330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 24.Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]