Abstract
OBJECTIVES:
There is evidence that outdoor workers exposed to high levels of air pollution exhibit airway inflammation and increased airway symptoms. We hypothesized that these workers would experience increased airway symptoms and decreased nasal mucociliary clearance associated with their exposure to air pollution.
METHODS:
In total, 25 non-smoking commercial motorcyclists, aged 18-44 years, were included in this study. These drivers work 8-12 hours per day, 5 days per week, driving on urban streets. Nasal mucociliary clearance was measured by the saccharine transit test; airway acidification was measured by assessing the pH of exhaled breath condensate; and airway symptoms were measured by the Sino-nasal Outcome Test-20 questionnaire. To assess personal air pollution exposure, the subjects used a passive-diffusion nitrogen dioxide (NO2) concentration-monitoring system during the 14 days before each assessment. The associations between NO2 and the airway outcomes were analyzed using the Mann-Whitney test and the Chi-Square test. Clinicaltrials.gov: NCT01976039.
RESULTS:
Compared with clearance in healthy adult males, mucociliary clearance was decreased in 32% of the motorcyclists. Additionally, 64% of the motorcyclists had airway acidification and 92% experienced airway symptoms. The median personal NO2 exposure level was 75 mg/m3 for these subjects and a significant association was observed between NO2 and impaired mucociliary clearance (p = 0.036).
CONCLUSION:
Non-smoking commercial motorcyclists exhibit increased airway symptoms and airway acidification as well as decreased nasal mucociliary clearance, all of which are significantly associated with the amount of exposure to air pollution.
Keywords: Mucociliary Transport, Airways, Air Pollution, Respiratory Symptoms
INTRODUCTION
The nose and the upper airways are the first barriers encountered by inhaled substances. Impaired mucociliary clearance (MCC) may lead to mucus retention, increased susceptibility to respiratory inflammation and nasal or respiratory symptoms. Epidemiological studies have suggested that the concentration of air pollutants is associated with respiratory symptoms, airway inflammation and hospital admissions (1–4). A higher prevalence of rhinitis and respiratory symptoms as well as decreased nasal MCC have been reported among non-smokers living in urban areas with high concentrations of particulate matter and nitrogen dioxide (NO2) (5,6). Traffic controllers, taxi drivers and bus drivers who work in polluted areas also experience nasal inflammation and decreased nasal MCC (7,8).
The present study aimed to characterize the nasal MCC, airway pH and respiratory symptoms of commercial motorcyclists who drive 8-12 hours per day, 5 days per week, in Belo Horizonte City. We hypothesized that these workers would have increased airway symptoms and decreased nasal MCC associated with their exposure to air pollution.
MATERIALS AND METHODS
Study design
This cross-sectional study was approved by the local ethics committee (CEP 211/11) and was registered at clinicaltrials.gov (number NCT01976039). We recruited non-smoking commercial motorcyclists aged 18-45 years who worked 8-12 hours per day for 5 days each week on the busy urban streets of Belo Horizonte City, Minas Gerais, Brazil. This city has approximately 2.2 million inhabitants (7,200 people per km2) and 1.5 million motor vehicles. The subjects were enrolled in this study after written informed consent was obtained.
The subjects underwent a physical examination between 8 and 11 AM and completed a questionnaire about their job, lifestyle and medical history, including any nasal or pulmonary symptoms. Subjects with a history of nasal surgery, diabetes or hypertension were excluded. To exclude current smokers, we analyzed exhaled carbon monoxide (CO) with a micro-analyzer (Cardinal Health U.K. 232 Rtd., UT, USA) and individuals who exhaled more than 10 ppm of CO were excluded from the study (10).
We performed spirometry (Koko Legend, Inspire Health Inc., Longmont, USA) according to the recommendations of the American Thoracic Society and the European Respiratory Society (11). Data were interpreted based on the methods of Pereira et al., who examined a Brazilian population (12). The absolute values and predicted values of forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) were recorded.
We administered the Sino-Nasal Outcome Test (SNOT-20) questionnaire (13,14) to assess airway symptoms. Each of the 20 questions is associated with a score ranging from zero, for no symptoms, to five points, for the most severe symptoms. The total score, which is calculated by dividing the total number of points (100 maximum) by the number of questions (20), varies between zero and five. The most commonly reported airway symptoms were registered.
Nasal MCC was measured using the saccharine transit time (STT) test and was recorded in minutes (9). The subjects sat in a chair with their chin elevated 30 degrees and were asked to maintain normal ventilation and to swallow freely but to avoid deep breaths, talking, coughing, sneezing, sniffing or moving. Saccharine powder (25 mg) was deposited 2 cm inside the free-airflow nostril and a timer was displayed when they returned their chin to the horizontal position. When the subjects reported the first perception of a sweet taste after saccharine deposition, the time was noted (15).
Exhaled breath condensate (EBC) was collected as previously described (7). Briefly, the subjects breathed through a glass collector device surrounded by dry ice (-76°C) for 15 minutes, which resulted in 1.5-2.5 mL EBC. The EBC sample was degassed with ultrapure (99.9%) argon gas (Gama Gases Ltd., São Paulo, Brazil) for 15 minutes and the pH was determined with the aid of a microelectrode and a pH meter (827 pH Lab, Metrohm Ltd., Herisau, Switzerland). The pH meter was calibrated before each measurement using solutions with pH values of 4, 7 and 9.
To investigate whether air pollution could affect nasal MCC, airway inflammation and symptoms, the subjects used an individual diffusive monitoring system for nitrogen dioxide (NO2) assessment (16,17). This system was used for 14 consecutive days before assessment by all subjects who entered into the study. Cellulose filters (37 mm in diameter; Energética, Rio de Janeiro, Brazil) were impregnated with 2 µL triethanolamine absorbent solution and dried at 37°C for 24 hours. This process converted the gas into nitrite, which was extracted in methanol by sonication. The concentration of NO2 was determined by calorimetry with the aid of a spectrophotometer (Ultrospec 4000 UV/Visible, Pharmacia Biotech, Allerod, Denmark). NO2 data are reported in μg/m3 (18). The pollution fixed monitoring station in Belo Horizonte City provided an estimate of NO2 exposure over the 14 days before the clinical assessment. We used SPSS (version 18) and Minitab (version 16) for statistical analysis. We evaluated the normality of variable distribution in the study using normal probability plots. Blood pressure, heart rate, pulse oximetry, exhaled CO, total SNOT-20 score, STT, and EBC pH values are reported as the mean ± standard deviation (SD) or as the median and interquartile range (IQR) when appropriate. The Kruskal-Wallis test was applied to assess the association between quantitative variables and dichotomous variables. To test the association between two qualitative variables, the Chi-Square test was applied. The level of significance was set at 0.05.
RESULTS
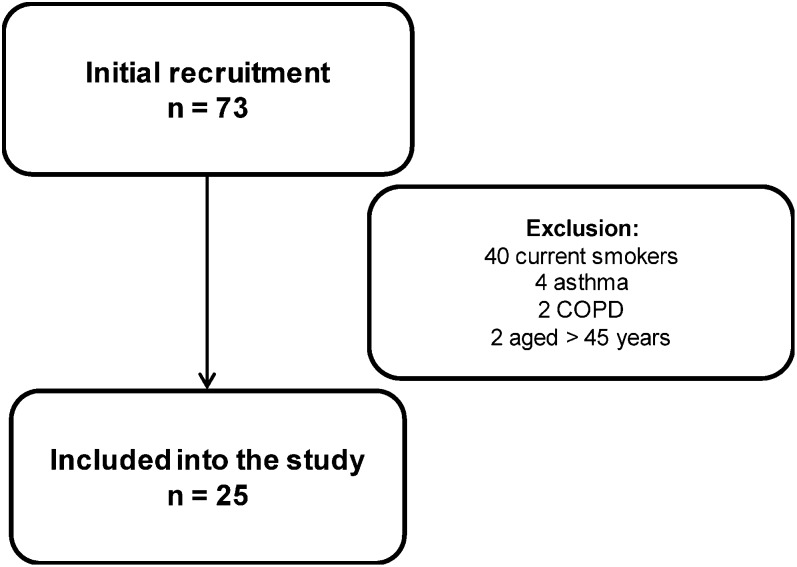
In total, 25 subjects were enrolled in the study (Figure 1). The median in motorcycle delivery was 12 years (IQR: 11.5), with a median daily working time of 10 hours (IQR: 4) for 5 days each week. Demographic and clinical data are shown in Table 1. Exhaled CO was within the normal range for non-smoking subjects. Spirometry showed normal lung function in all subjects.
Figure 1.
Motorcyclist recruitment.
Table 1.
Mean (± SD) for demographic data, heart rate (HR), pulse oximetry (SpO2), exhaled carbon monoxide (CO) and spirometry data for 25 commercial motorcyclists.
| Age (years) | 36±5 |
| Body mass index (kg/m2) | 25.6±3.5 |
| Vital signs | |
| HR (bpm) | 69±10 |
| SpO2 (%) | 98±0.7 |
| Exhaled CO (ppm) | 1.2±1.3 |
| Lung function | |
| FEV1 (L) | 3.65±0.44 |
| FEV1 pred (%) | 92.2±11.0 |
| FVC (L) | 4.46±0.52 |
| FVC pred (%) | 94.4±11.3 |
| FEV1/FVC | 0.82±0.05 |
| FEV1/FVC pred (%) | 97.8±4.8 |
Abbreviations: FEV1, forced expiratory volume in the first second; FEV1 pred, percent predicted FEV1; FVC, forced vital capacity; FVC pred, percent predicted FVC; FEV1/FVC, FEV1/FVC ratio.
The median NO2 concentration from the fixed monitoring station was 35 µg/m3 (IQR: 31). The median personal diffusive NO2 exposure was 75 µg/m3 (IQR: 6) for each subject. The subjects had an EBC pH below the normal values for healthy subjects and an STT greater than 12 minutes, which is abnormal (9) (Table 2). Higher personal exposure to NO2 was associated with a greater decrease in nasal MCC (p = 0.036), as determined by the Kruskal-Wallis test. Most of the subjects reported experiencing airway symptoms (Table 2). The most commonly reported symptoms were sneezing, rhinitis and coughing. Only two subjects did not report any airway symptoms.
Table 2.
Nasal mucociliary clearance (MCC), exhaled breath condensate (EBC) pH and reports of airway symptoms based on the Sino-Nasal Outcome Test (SNOT-20) questionnaire (columns: frequencies of subjects, median values of EBC pH and personal nitrogen dioxide (NO2) exposure).
| n° subjects (% total) | Median EBC pH | Median NO2 μg/m3 | ||
| Nasal MCC | <12 minutes | 17 (68) | 7.85 | 73.5 |
| ≥12 minutes | 8 (32) | 7.64 | 76.3 | |
| EBC pH | ≥7.90 | 9 (36) | 8.08 | 75.5 |
| <7.90 | 16 (64) | 7.52 | 75.2 | |
| SNOT-20 | No symptoms | 2 (8) | 8.16 | 64.8 |
| Symptomatic | 23 (92) | 7.67 | 74.7 |
DISCUSSION
All 25 motorcyclists who participated in this study had an NO2 exposure greater than 50 µg/m3, which is consistent with reported traffic-related air pollution exposure (19–22). Of these subjects, 92% reported airway symptoms on the SNOT-20 questionnaire, 64% had a decreased EBC pH and 32% had nasal MCC lasting longer than 12 minutes. Greater MCC impairment was associated with greater NO2 exposure. Exposure to air pollution from road traffic is associated with greater odds of suffering from rhinitis in non-smoking adults (19) and increased coughing and wheezing in infants (31).
The nasal mucosa is the first barrier encountered by inhaled particulates and gases. Noxious agents often acutely stimulate MCC, which serves as a protective response, in healthy volunteers (6) and this is thought to be due to increased mucus production and increased ciliary beating (23). However, chronic exposure to pollution airway inflammation with rhinitis, sneezing and mucus hypersecretion.
Normal nasal MCC can be measured using the STT and lasts 12 minutes or less in healthy adult non-smokers (9). Slower nasal MCC is found in 19% of healthy individuals. In the present study, 8 of the 25 subjects (32%) had a slow STT, indicating impaired nasal MCC. MCC dysfunction was significantly associated with higher concentrations of NO2. These nasal MCC results are consistent with morphological studies ciliary loss and goblet cell hyperplasia are associated with long-term exposure to urban pollution (24,25).
We also measured EBC pH, as a lower pH has been reported to be associated with exposure to air pollution (7). The normal EBC pH in healthy young subjects is between 7.90 and 8.20 (26). Although chemical characterization of acidic pH yields values lower than 7.0, EBC pH values lower than 7.8 have been associated with airway inflammation, bronchoconstriction and coughing (27), decreased MCC (28), epithelial damage and epithelial sloughing (29). We have previously reported that traffic controllers working in polluted areas have EBC with a lower pH (30). In the present study, 64% of the motorcyclists exhibited airway acidification.
This study has certain limitations. Although there was no control group in this study, identifying a control group for commercial motorcyclists would be very difficult because of the nature of their work. Another aspect is that the air pollution data were reported by the only fixed monitoring station in the city. It is known that fixed monitoring stations can underestimate real values of air pollutant concentrations; thus, it is not surprising that the median personal nitrogen dioxide exposure level of our subjects was approximately 70 µg/m3, or two-fold higher than the NO2 concentration determined from the fixed monitoring station (35 µg/m3). It could be argued that the small number of commercial motorcyclists (n = 25) could have limited our ability to statistically adjust the outcome variables (nasal MCC, airway symptoms and pH) for work duration and age. In the present study, the work duration varied between 4 and 13 hours per day. However, most of the motorcyclists experienced airway symptoms and dysfunction. Additionally, the confounding variable “age” was controlled at study inclusion. Aging is independently associated with decreased nasal MCC and is markedly demonstrated in elderly people (>65 years old) (9).
This study showed that non-smoking commercial motorcyclists working on heavily polluted urban roads have increased airway symptoms and airway acidification as well as decreased nasal MCC.
Conflict-of-interest statement for each author: Conflict of interest is a financial relationship or other set of circumstances that might affect, or might reasonably be thought by others to affect, an author's judgment, conduct or manuscript. A conflict of interest exists based on the author's circumstances. The author's behavior, subjective beliefs, and outcomes are irrelevant. For purposes of CLINICS, all the authors disclose a conflict of interest, even if the circumstances do not actually influence the author's actions or manuscript and even if the author believes that the circumstances cannot or will not affect the author's actions or manuscript.
CURRENT KNOWLEDGE
Traffic controllers, taxi drivers and bus drivers are exposed to traffic-related air pollution, putting them at increased risk of airway inflammation and impairment.
WHAT THIS PAPER CONTRIBUTES TO OUR KNOWLEDGE
We show that commercial motorcyclists working in an urban environment with heavy air pollution experience airway symptoms, inflammation and decreased mucociliary clearance. Future studies should investigate interventions with the aim to reduce or prevent these alterations in the respiratory defense mechanisms of these outdoor workers and to improve their quality of life.
ACKNOWLEDGMENTS
The authors would like to thank Luci Fuscaldi Teixeira-Salmel and Frederico Moreira for initial discussions of the results. This study was partially supported by FAPESP 09/51605-9 and by CNPq 134839/2012-9.
REFERENCES
- 1.Brunekreef B, Sunyer J. Asthma, rhinitis and air pollution: is traffic to blame. Eur Respir J. 2003;21(6):913–5. doi: 10.1183/09031936.03.00014903. [DOI] [PubMed] [Google Scholar]
- 2.Cesaroni G, Badaloni C, Porta D, Forastiere F, Perucci CA. Comparison between various indices of exposure to traffic-related air pollution and their impact on respiratory health in adults. J Occup Environ Med. 2008;65(10):683–90. doi: 10.1136/oem.2007.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Amato G, Cecchi L, D'Amato M, Liccardi G. Urban air pollution and climate change as environmental risk factors of respiratory allergy: an update. J Investig Allergol Clin Immunol. 2010;20(2):95–102. [PubMed] [Google Scholar]
- 4.Cançado JED, Braga ALF, Pereira LAA, Arbex MA, Saldiva PHN, Santos UP. Clinical repercussions of exposure to atmospheric pollution. J Bras Pneumol. 2006;32(2):5–11. doi: 10.1590/s1806-37132006000800003. [DOI] [PubMed] [Google Scholar]
- 5.Norbäck D, Walinder R, Wieslander G, Smedje G, Erwall C, Venge P. Indoor air pollutants in schools: nasal patency and biomarkers in nasal lavage. Allergy. 2000;55(2):163–70. doi: 10.1034/j.1398-9995.2000.00353.x. [DOI] [PubMed] [Google Scholar]
- 6.Riechelmann H, Rettinger G, Weschta M, Keck T, Deutschle T. Effects of low-toxicity particulate matter on human nasal function. J Occup Environ Med. 2003;45(1):54–60. doi: 10.1097/00043764-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Lima TM, Kazama CM, Koczulla AR, Hiemstra RS, Macchione M, Fernandes ALG, et al. pH in exhaled breath condensate and nasal lavage as a biomarker of air pollution-related inflammation in street traffic-controllers and office-workers. Clinics. 2013;68(12):1488–94. doi: 10.6061/clinics/2013(12)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones AYM, Lam PKW, Dean E. Respiratory health of bus drivers in Hong Kong. Int Arch Occup Environ Health. 2006;79(5):414–8. doi: 10.1007/s00420-005-0061-8. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira-Maul JP, de Carvalho HB, Miyuki Goto D, Mendonça Maia R, Fló C, Barnabé V, et al. Aging, diabetes and hypertension are associated with decreased nasal mucociliary clearance. Chest. 2013;143(4):1091–7. doi: 10.1378/chest.12-1183. [DOI] [PubMed] [Google Scholar]
- 10.Sato S, Nishimura K, Koyama H, Tsukino M, Oga T, Hajiro T, et al. Optimal Cutoff Level of Breath Carbon Monoxide for Assessing Smoking Status in Patients With Asthma and COPD. Chest. 2003;124(5):1749–54. doi: 10.1378/chest.124.5.1749. [DOI] [PubMed] [Google Scholar]
- 11.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force: Standardization of lung function testing. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 12.Pereira CAC, Sato T, Rodrigues SC. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol. 2007;33(4):397–406. doi: 10.1590/s1806-37132007000400008. [DOI] [PubMed] [Google Scholar]
- 13.Piccirillo JF, Merritt MG, Jr, Richards ML. Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20) Otolaryngol Head Neck Surg. 2002;126(1):41–7. doi: 10.1067/mhn.2002.121022. [DOI] [PubMed] [Google Scholar]
- 14.Bezerra TF, Piccirillo JF, Fornazieri MA, de M Pilan RR, Abdo TR, de Rezende Pinna F, et al. Cross-Cultural Adaptation and Validation of SNOT-20 in Portuguese. Int J Otolaryngol. 2011:306529. doi: 10.1155/2011/306529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa NK, Franchini ML, Driusso P, de Oliveira LR, Saldiva PH, Lorenzi-Filho G. Mucociliary clearance is impaired in acutely ill patients. Chest. 2005;128(4):2772–7. doi: 10.1378/chest.128.4.2772. [DOI] [PubMed] [Google Scholar]
- 16.Novaes P, Saldiva PHN, Kara-José N, Macchione M, Matsuda M, Racca L, et al. Ambient levels of air pollution induce goblet-cell hyperplasia in human conjuctival epithelium. Environ Health Perspect. 2007;115(12):1753–6. doi: 10.1289/ehp.10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krupa SV, Legge AH. Passive sampling of ambient, gaseous air pollutants: an assessment from an ecological perspective. Environ Pollut. 2000;107(1):31–45. doi: 10.1016/s0269-7491(99)00154-2. [DOI] [PubMed] [Google Scholar]
- 18.Studinicka M, Hackl E, Pischinger J, Fangmeyer C, Haschke N, Kuhr J, et al. Traffic-related NO2 and the prevalence of asthma and respiratory symptoms in seven year olds. Eur Respir J. 1997;10(10):2275–8. doi: 10.1183/09031936.97.10102275. [DOI] [PubMed] [Google Scholar]
- 19.Cesaroni G, Badaloni C, Porta D, Forastiere F, Perucci CA. Comparison between various índices of exposure to traffic-related air pollution and their impact on respiratory health in adults. J Occup Environm Med. 2008;65(10):683–90. doi: 10.1136/oem.2007.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caballero S, Esclapez R, Galind N, Mantilla E, Cresp J. Use of a passive sampling network for the determination of urban NO2 spatiotemporal variations. Atmos Environ. 2012;63:148–55. [Google Scholar]
- 21.Gaga EO, Dogeroglu T, Ozden O, Ari A, Yay OD, Altug H, et al. Evaluation of air quality by passive and sampling in an urban city in Turkey: current status and spatial analysis of air pollution exposure. Environ Sci Pollut Res Int. 2012;19(8):3579–96. doi: 10.1007/s11356-012-0924-y. [DOI] [PubMed] [Google Scholar]
- 22.Stranger M, Krata A, Kontozova-Deutsch V, Bencs L, Deutsch F, Worobiec A, et al. Monitoring of NO2 in the ambient air with passive samplers before and after a road reconstruction event. Microchem J. 2008;90:93–98. [Google Scholar]
- 23.Riechelmann H, Rettinger G, Lautebach D, Schmittinger D, Deutschle T. Short-term exposure to urban dust alters the mediator release of human nasal mucosa. J Occup Environ Med. 2004;46(1):316–22. doi: 10.1097/01.jom.0000121125.05741.7b. [DOI] [PubMed] [Google Scholar]
- 24.Lemos M, Lichtenfels AJ, Amaro Junior E, Macchione M, Martins MA, King M, et al. Quantitative pathology of nasal passages in rats exposed to urban levels of air pollution. Environ Res. 1994;66(1):87–95. doi: 10.1006/enrs.1994.1046. [DOI] [PubMed] [Google Scholar]
- 25.Calderon-Garciduenas L, Rodriguez-Alcaraz A, Villarreal-Calderón A, Lyght O, Janszen D, Morgan KT. Nasal epithelium as a sentinel for airborne environmental pollution. Toxicol Sci. 1998;46:352–64. doi: 10.1006/toxs.1998.2549. [DOI] [PubMed] [Google Scholar]
- 26.Nicola ML, Carvalho HB, Yoshida CT, Anjos FD, Nakao M, de Paula Santos U, et al. Young "healthy" smokers have functional and inflammatory changes in the nasal and the lower airways. Chest. 2014;145(5):998–1005. doi: 10.1378/chest.13-1355. [DOI] [PubMed] [Google Scholar]
- 27.Ricciardolo FL, Rado V, Fabbri LM, Sterk PJ, Di Maria GU, Geppetti P. Bronchoconstriction induced by citric acid inhalation in guinea pigs: role of tachykinins, bradykinin, and nitric oxide. Am J Respir Crit Care Med. 1999;159(2):557–62. doi: 10.1164/ajrccm.159.2.9804022. [DOI] [PubMed] [Google Scholar]
- 28.Holma B. Effects of inhaled acids on airway mucus and its consequences for health. Environ Health Perspect. 1989;79:109–13. doi: 10.1289/ehp.8979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol. 2002;110(1):28–34. doi: 10.1067/mai.2002.124966. [DOI] [PubMed] [Google Scholar]
- 30.Brucker N, Moro AM, Charao MF, Durgante J, Freitas F, Baierle M, et al. Biomarkers of occupational exposure to air pollution, inflammation and oxidative stress damage in taxi-drivers. Sci Total Env. 2013;463-464:884–93. doi: 10.1016/j.scitotenv.2013.06.098. [DOI] [PubMed] [Google Scholar]
- 31.Studnicka M, Hackl E, Pischinger J, Fangmeyer C, Haschke N, Kuhr J, Urbanek R, et al. Traffic-related NO2 and the prevalence of asthma and respiratory symptoms in seven year olds. Eur Respir J. 1997;10(10):2275–8. doi: 10.1183/09031936.97.10102275. [DOI] [PubMed] [Google Scholar]