Abstract
Oxygen (O2) and carbon dioxide (CO2) provoke distinct olfactory behaviors via specialized sensory neurons across metazoa. In the nematode C. elegans, the BAG sensory neurons are specialized to sense changes in both O2 and CO2 levels in the environment. The precise functionality of these neurons is specified by the coexpression of a membrane-bound receptor-type guanylyl cyclase GCY-9 that is required for responses to CO2 upshifts and the soluble guanylyl cyclases GCY-31 and GCY-33 that mediate responses to downshifts in O2. Expression of these gas-sensing molecules in the BAG neurons is partially, although not completely, controlled by ETS-5, an ETS-domain-containing transcription factor, and EGL-13, a Sox transcription factor. We report here the identification of EGL-46, a zinc-finger transcription factor, which regulates BAG gas-sensing fate in partially parallel pathways to ETS-5 and EGL-13. Thereby, three conserved transcription factors collaborate to ensure neuron type-specific identity features of the BAG gas-sensing neurons.
Keywords: gas sensing, neuronal fate specification, transcription factor
THE acquisition of specific neuronal fates is controlled by regulatory transcription factors that activate the expression of neuron type-specific terminal differentiation genes (Hobert 2008, 2011). Specific individual transcription factors may act to control the expression of the entire terminal gene battery of a specific neuron, or alternatively, multiple transcription factors may act in parallel to control distinct aspects of terminal fates (Hobert et al. 2010). The use of combinatorial transcription factors to control aspects of terminal fates may provide flexibility to polymodal neurons that need to rapidly respond to changes in a specific environmental cue. We study the BAG neurons in Caenorhabditis elegans that have a dual function where they are activated by separate mechanisms when carbon dioxide (CO2) levels increase or when oxygen (O2) levels decrease (Zimmer et al. 2009). The membrane-bound receptor-type guanylyl cyclase GCY-9 is expressed specifically in the BAG neurons to mediate CO2 avoidance behavior (Hallem et al. 2011). Whereas the soluble guanylyl cyclases GCY-31 and GCY-33 are expressed in the BAG neurons to mediate behavioral responses when O2 levels decrease (Zimmer et al. 2009). Previous studies have shown that the ETS domain-containing transcription factor ETS-5 is critical for CO2-sensing neuron fate through direct regulation of GCY-9 expression in the BAG neurons (Guillermin et al. 2011; Brandt et al. 2012; Gramstrup Petersen et al. 2013). In addition, the Sox transcription factor EGL-13 is partially required for GCY-9 expression in the BAG neurons (Gramstrup Petersen et al. 2013). Loss of either of these transcription factors causes defects in CO2 avoidance behavior (Guillermin et al. 2011; Brandt et al. 2012; Gramstrup Petersen et al. 2013). The O2-sensing neuron fate of the BAG neurons, as assessed by expression of gcy-31 and gcy-33, is also defective in ets-5 and egl-13 mutant animals (Gramstrup Petersen et al. 2013). However, the expression of these and other BAG terminal fate markers is not fully abrogated by concomitant loss of ets-5 and egl-13 (Gramstrup Petersen et al. 2013). These studies suggest that parallel genetic programs exist that regulate the specification of the O2- and CO2-sensing BAG neuronal fates.
To identify factors that potentially act in parallel to ets-5 and egl-13 in BAG specification, we performed a forward genetic screen, using a fluorescent reporter for gcy-33 that is exclusively expressed in the BAG neurons. Using this approach, we identified two alleles of egl-46, which encodes a TFIIA-like zinc finger transcription factor, a gene previously shown to regulate multiple neuronal fate decisions in C. elegans (Wu et al. 2001; Wang et al. 2010; Feng et al. 2013). We found that egl-46 regulates the expression of a subset of BAG-expressed terminal differentiation genes that include gcy-9, which is required for CO2 avoidance responses, and gcy-31 and gcy-33, which are required for responses to O2 downshifts. In concordance with these molecular deficits, we found that egl-46 mutant animals are defective in the ability to respond to O2 downshifts and upshifts in CO2, which are BAG-mediated behaviors.
We subsequently performed double-mutant analysis to better understand the role of egl-46 in BAG neuron fate specification. We found that egl-46 acts in parallel to ets-5 and egl-13 to regulate gcy-31 and gcy-33 expression, and thereby O2-sensing fate, in the BAG neurons. In addition, we found that egl-46 acts in a partially parallel pathway to that of egl-13 to direct CO2-sensing fate.
Taken together, we have identified the transcription factor EGL-46 as a regulator required for the expression of a subset of O2 and CO2 terminal differentiation genes in C. elegans. The EGL-46 ortholog in Drosophila, Nerfin-1, is also required for CO2-sensing neuron fate specification (Cayirlioglu et al. 2008). In addition, the mouse ortholog Insm1 regulates the differentiation of cells in the olfactory epithelium that harbors CO2-responding neurons (Sun et al. 2009; Rosenbaum et al. 2011). Therefore, our findings suggest a conserved function for this gene family.
Materials and Methods
Mutant and transgenic reporter strains
Worms were grown using standard conditions on NGM agar plates and maintained at 20° (Brenner 1974). A complete list of mutant and transgenic strains used for this study is detailed in Supporting Information, Table S1. Fusion PCR was used to generate egl-46 cDNA and genomic rescue elements (Hobert 2002). For expression analysis, the egl-46 promoter was cloned into a dsRed2::NLS expression vector using the following oligonucleotides: HindIII site oAR39 AAAAAAGCTTtgatttttgccagaaatgttacg and BamHI site oAR38 TTTTGGATCCggccttctgaaatcaaaacg.
Forward genetic screen
In the forward genetic screen, the BAG reporter strain gcy-33prom::gfp; dop-3::rfp was EMS mutagenized according to standard protocols (Flibotte et al. 2010). BAG cell fate mutants were isolated based on decreased expression of gcy-33prom::gfp, using the automated COPAS biosorter platform (Doitsidou et al. 2008).
The genomic lesion of rp13 was identified using the previously described, one-step whole-genome sequencing and SNP mapping strategy (Doitsidou et al. 2010). Subsequent analysis using MAQGene enabled genome-wide analysis of Hawaiian and N2 SNP distribution to identify a region without recombination (Bigelow et al. 2009). A complementation test between rp13 and rp15 revealed that these two mutants are alleles of the same gene and rp15 was Sanger sequenced at the egl-46 locus.
Fluorescence microscopy
Unless stated otherwise, L4/young adult animals were analyzed for BAG cell fate defects by mounting them on a glass slide with a 5% agarose pad, using 50 mM NaN3 as an anesthetic. Expression analysis was conducted using an automated fluorescence microscope [Zeiss (Thornwood, NY) AXIO Imager M2] and images were acquired with the Zen software (Zeiss).
Neuronal scoring
The percentage of animals that expressed GFP in both BAG neurons was scored.
Behavioral assays
Wild-type and egl-46 mutant animals were starved for 1 hr and then transferred to 14-cm NGM plates containing a 56 × 56-mm arena of Whatman filter paper soaked in 20 mM CuCl2. Eighty to 120 animals were used in a single experiment. Each experimental condition was repeated four to six times. A custom-made transparent plexiglass chamber with a flow volume of 60 × 60 × 0.7 mm was placed onto the assay arena and animals were accustomed to a gas flow of 100 ml/min containing 21% (v/v) O2 for 5 min. For O2 experiments animals were stimulated for 6 min with 10% or 17% O2 and 0% CO2. For CO2 experiments animals were stimulated for 6 min with 1% CO2 and 21% O2. In all conditions, the gas compositions were balanced with N2. Gases were mixed by red-y gas mixing units (Vögtlin Instruments) and controlled by LabView software. Recordings were illuminated with flat red LED lights and made at three frames per second on a 4-megapixel CCD camera (Jai), using Streampix software (Norpix).
For movie analysis, MatLab-based image processing and tracking scripts were used as previously described (Ramot et al. 2008; Tsunozaki et al. 2008). The resulting trajectories were used to calculate instantaneous speed during continuous forward movements (1-sec binning). Omega turns were detected based on characteristic changes in object eccentricity and their frequency was calculated in 30-sec bins.
Generation of transgenic worms
Transgenic animals were obtained through microinjection (Mello et al. 1991). Constructs were injected into young adult hermaphrodites as complex arrays, using 1–15 ng⋅μl−1 of PCR product, 150 ng⋅μl−1 of digested bacterial DNA, and elt-2prom::gfp (3–5 ng⋅μl−1) as a co-injection marker.
Statistical analyses
Statistical analyses were performed in Prism 6. egl-46 regulation of BAG terminal differentiation genes and rescue experiments were evaluated using one-way ANOVA with a Newman–Keuls multiple-comparison test. For analysis of behavioral O2 and CO2 responses a two-tailed t-test was used. Differences with a P-value <0.05 were considered significant.
Results
egl-46 mutants display specific defects in BAG neuronal fate determination
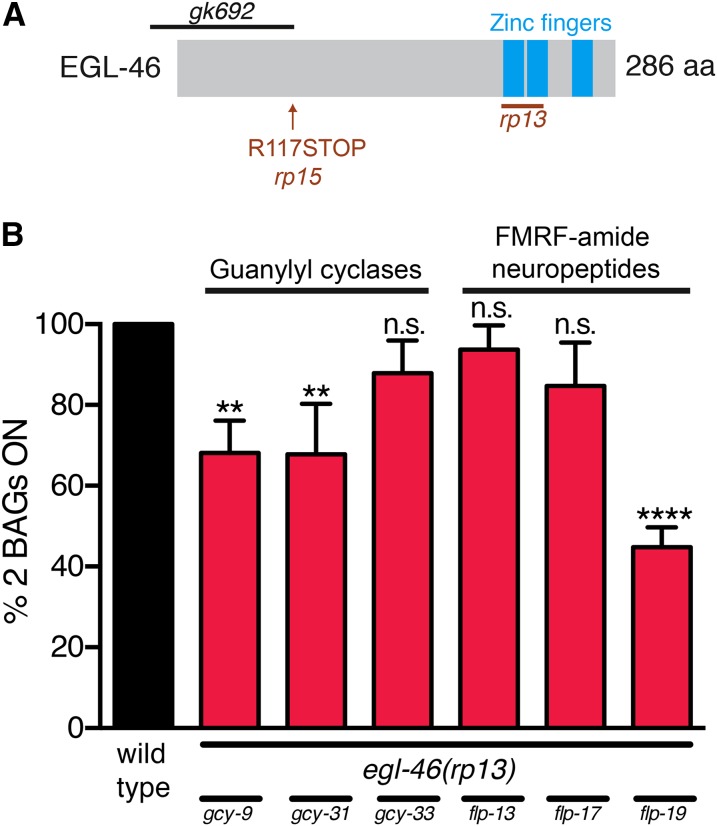
We previously showed that the conserved transcription factors ETS-5 and EGL-13 are important for the specification of O2- and CO2-sensing fate in the BAG neurons (Guillermin et al. 2011; Brandt et al. 2012; Gramstrup Petersen et al. 2013). However, expression of the O2-sensing fate markers, GCY-31 and GCY-33, is not fully abrogated in ets-5egl-13 double-mutant animals, and the CO2-sensing fate marker, GCY-9, is only partially affected in egl-13 mutant animals (Gramstrup Petersen et al. 2013). This suggests that other molecules contribute to the regulation of O2- and CO2-sensing fate in the BAG neurons. To identify such factors, we performed a forward genetic screen, using a COPAS Biosorter (Doitsidou et al. 2008). As a starting strain we used an integrated reporter (gcy-33prom::gfp), which is exclusively expressed in the BAG neurons (Figure S1). We isolated two alleles (rp13 and rp15) that were both egg-laying defective (Egl) and exhibited partial loss of gcy-33prom::gfp expression (Figure S1). Whole-genome sequencing of the rp13 allele identified a 517-bp deletion in the egl-46 gene (Figure 1A). Complementation tests between rp13 and rp15 indicated that they were allelic and subsequent Sanger sequencing of rp15 revealed a point mutation in exon 2 of egl-46 that introduces a premature STOP codon (Figure 1A). Animals carrying the previously isolated egl-46(gk692) deletion allele display a similar BAG phenotype to rp13 (Figure S2), further corroborating a role for egl-46 in BAG development.
Figure 1.
The identification of egl-46 as a BAG neuronal fate specification gene. (A) Molecular identity of the egl-46 alleles obtained from the forward genetic screen. egl-46 alleles first described in this article are shown in red (rp13 and rp15) and the previously described gk692 allele is shown in black. The nature of the molecular lesions we isolated is as follows: rp13 is a 517-bp out-of-frame deletion that removes the first two zinc fingers, and rp15 is a C to T transition that converts an arginine to an opal stop codon early in the protein sequence. (B) Quantification of rp13-induced BAG defects in reporters for BAG neuronal fate. Loss of egl-46 affects fluorescent reporter expression of the neuropeptide flp-19 and the guanylyl cyclases, gcy-9 and gcy-31. Black bar, BAG reporters in wild-type animals; red bars, reporters in egl-46(rp13) mutant animals. n > 50. *P < 0.05, **P < 0.01, ****P < 0.0001.
To better understand the role egl-46 plays in BAG specification we used fluorescent reporter strains to monitor expression of a battery of terminal differentiation genes expressed in the BAG neurons (Figure 1B). We analyzed the expression of guanylyl cyclases (gcy-9, gcy-31, and gcy-33) and Phe-Met-Arg-Phe-NH2 (FMRF-amide)-related peptides (flp-13, flp-17, and flp-19) (Figure 1B). Using the rp13 allele, we found that loss of egl-46 affected the expression of gcy-9, gcy-31, and flp-19, indicating that egl-46 expression is required for both O2 and CO2 aspects of BAG cell fate (Figure 1B).
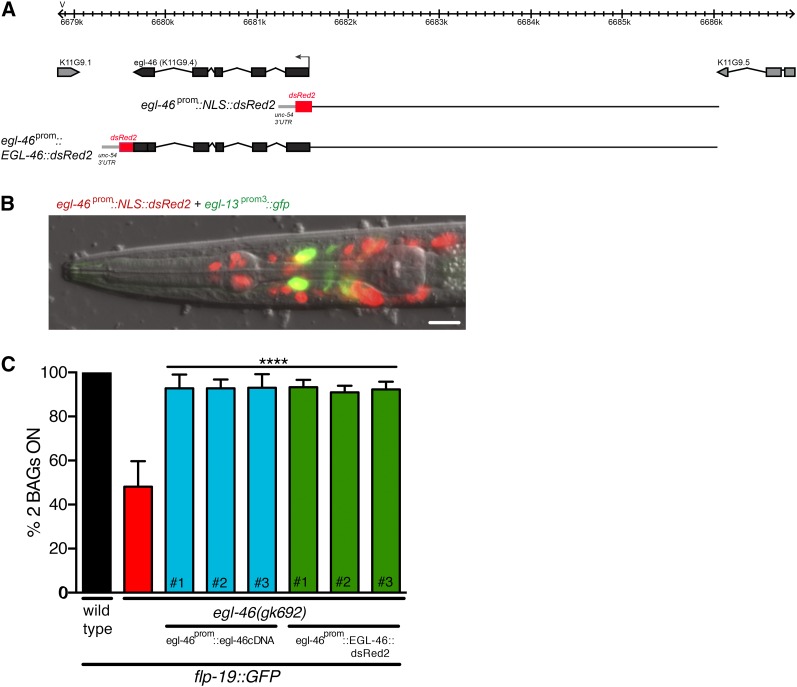
Previous expression analysis of egl-46 did not report expression in the BAG neurons (Wu et al. 2001). We therefore generated a transcriptional reporter line in which nuclear localized dsRed2 protein was driven by the full-length 4.5-kb egl-46 promoter to help with cellular identification (Figure 2A). The reporter confirmed the previously published expression pattern for egl-46 in the FLP, HSN, and PVD neurons and other neurons in the head (data not shown). We used an egl-13::gfp reporter (expressed in BAG, URX, AQR, and PQR) to examine colocalization in the BAG neurons. We observed colocalization events in the BAG neurons at the L2 and L3 stages of larval development, suggesting that egl-46 is expressed during a short temporal window in the BAG neurons, as has been observed in other neurons previously (Figure 2B) (Wu et al. 2001). We next tested whether egl-46 coding sequences could rescue defects in BAG neuron expression by using the most strongly affected terminal fate marker, flp-19prom::gfp. We found that both transgenic expression of egl-46 cDNA under the control of the egl-46 4.5-kb promoter and an egl-46prom::EGL-46::dsRed2 translational fusion rescued the flp-19prom::gfp loss of expression in egl-46(gk692) mutant animals (Figure 2C). These data, along with the presence of multiple independent egl-46 mutant alleles, indicate that egl-46 is required for BAG terminal fate specification.
Figure 2.
egl-46 rescue of BAG neuronal fate defects. (A) Schematic representation of the egl-46 genomic locus. The ATG codon is marked with an arrow and the exons are represented as black blocks. The egl-46 translational reporter was constructed by driving egl-46 genomic DNA with dsRed2 coding sequence under the control of the 4.5-kb egl-46 promoter. The transcriptional reporter was constructed using the 4.5-kb promoter to drive nuclear-localized dsRed2 (NLS::dsRed2). (B) The 4.5-kb egl-46 promoter drives NLS::dsRed2 expression in multiple nuclei in the head. Colocalization (yellow) of NLS::dsRed2 was observed with cytoplasmic gfp driven by an egl-13 promoter in the BAG neurons. Note that we only rarely observed colocalization with the BAG marker, suggesting that the egl-46 promoter drives expression in the BAG neurons in a transient manner. Ventral view, anterior is to the left. Bar, 20 μm. (C) Transgenic expression of the egl-46 cDNA or egl-46 genomic sequence fused to dsRed2 under the control of the egl-46 promoter rescues the egl-46(gk692) mutant loss of flp-19prom::gfp expression. n > 50. ****P < 0.0001. # refers to independent transgenic lines.
egl-46 mutants are defective in O2 and CO2 sensing
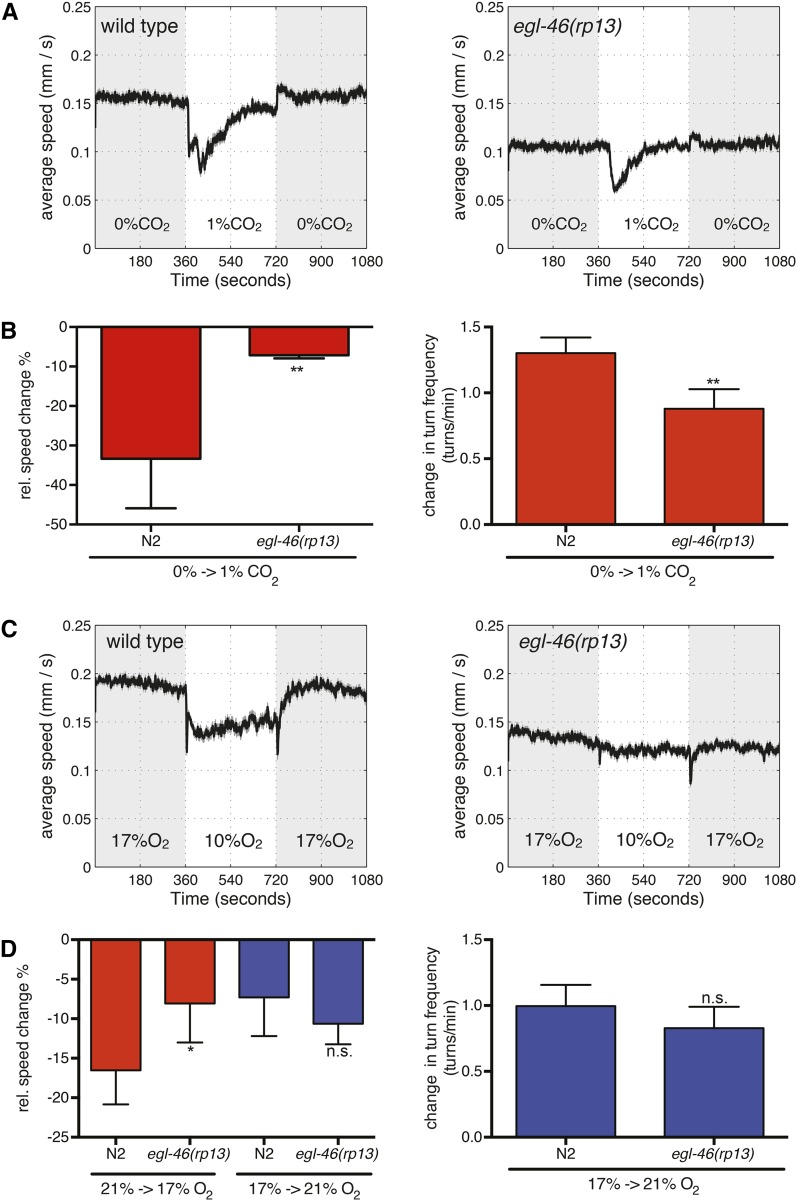
egl-46 mutant animals exhibit reduction of reporter expression of gcy-9, gcy-31, and flp-19 (Figure 1). Previous work has shown that loss-of-function mutations in gcy-9 cause a defective CO2 avoidance response (Hallem et al. 2011) and that loss of gcy-31 or gcy-33 causes a defect in the BAG-mediated response to O2 downshifts (Zimmer et al. 2009). Therefore, we asked whether the effect on reporter expression for these BAG terminal differentiation genes in egl-46 mutant animals has a consequence on these worm behaviors (Figure 3). We performed well-established behavioral paradigms to examine the importance of egl-46 function on gas sensing. First, we tested whether egl-46 mutant animals have defects in a CO2-mediated behavior. Wild-type animals respond to increases in CO2 concentrations by slowing their forward locomotion and changing direction by using an ω-turn (Bretscher et al. 2008). We found that egl-46 mutant animals show a reduced response to 1% CO2 with regard to change of speed and ω-turns (Figure 3, A and B).
Figure 3.
egl-46 gas-sensing behavior analysis. (A) Locomotion speed of wild type (left) and egl-46(rp13) mutants (right) during CO2 concentration shifts. Data presented are averages of multiple assays (four or more repetitions). CO2 concentrations were switched between 0% and 1%. (B) Quantification of changes in relative speed (left) and ω-turn frequency (right) in response to a BAG-mediated upshift in CO2. egl-46(rp13) mutant animals exhibit a reduced response. (C) Locomotion speed of wild type (left) and egl-46(rp13) mutants (right) during O2 concentration shifts. Data presented are averages of multiple assays (four or more repetitions). O2 concentrations were switched between 21% and 17%. (D) Quantification of changes in relative speed (left) and ω-turn frequency (right) in response to BAG-mediated downshift in O2 (red) and URX-mediated O2 upshift (blue). egl-46(rp13) mutant animals exhibit a decreased response to O2 downshifts (BAG-mediated behavior) but exhibit a similar response to that of wild-type animals to O2 upshifts (URX-mediated behavior). Each experimental condition was repeated a minimum four independent times with 80–120 animals in each assay. Statistical significance between wild-type and egl-46(rp13) animals was evaluated with a two-tailed t-test. P-values <0.05 were considered significant; *P < 0.05; **P < 0.01; n.s., not significantly different from wild-type controls.
Next we assayed the ability of egl-46 mutant animals to respond to an O2 downshift from 21% O2 to 10% O2 (BAG-mediated behavior) and O2 upshift from 10% O2 to 21% O2 (URX-mediated behavior) (Zimmer et al. 2009) (Figure S3). Both of these O2-triggered responses normally result in a reduction of locomotion speed and the performance of an ω-turn in response to an O2 upshift (Zimmer et al. 2009). egl-46 mutant animals exhibit a similar response compared to wild-type animals with regard to change of speed and ω-turns (Figure S3). We hypothesized that the partially penetrant defects in BAG terminal fate marker expression may cause defects in O2 sensing to a less acute O2 downshift. We therefore retested egl-46 mutant animals in their responses to an O2 downshift from 21% O2 to 17% O2 and an O2 upshift from 17% O2 to 21% O2. Using these parameters we observed a defect in the ability of egl-46 mutant animals to respond to an O2 downshift but not to an O2 upshift (Figure 3). Taken together, these data indicate that loss of egl-46 causes defects in the response of C. elegans to CO2 upshifts and O2 downshifts, two BAG-controlled behaviors.
egl-46 acts in parallel to egl-13 and ets-5 to regulate O2- and CO2-sensing fate in the BAG neurons
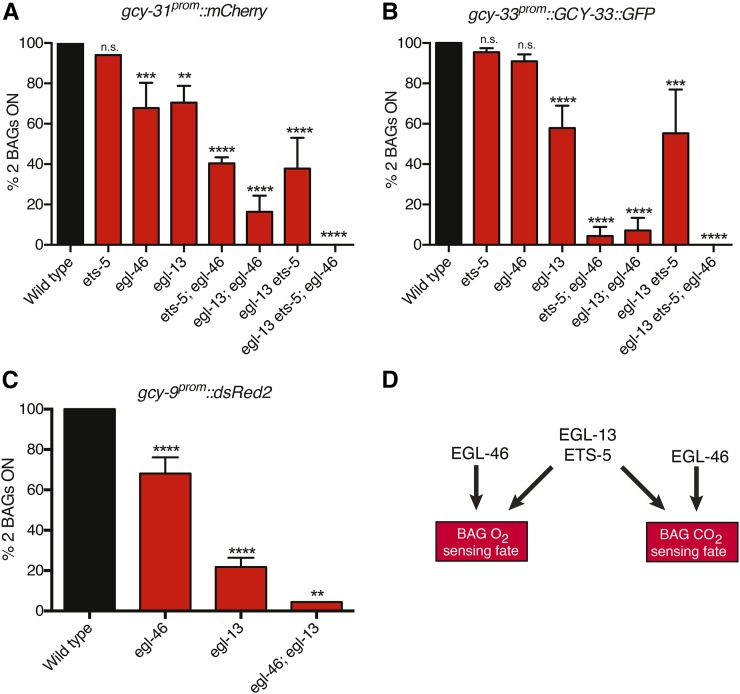
Previous work has shown that ets-5 and egl-13 regulate specification of the BAG neurons. However, the expression of some BAG terminal fate markers, especially GCY-31 and GCY-33 that report O2-sensing fate, is only partially affected by simultaneous loss of ets-5 and egl-13 (Gramstrup Petersen et al. 2013). In addition, the fully penetrant loss of GCY-9, a reporter for CO2-sensing fate, in ets-5 mutants is not observed in egl-13 mutant animals (Gramstrup Petersen et al. 2013). We found that egl-46 does not affect the expression of ets-5 or egl-13 reporters, suggesting that it acts in a parallel manner to these transcription factors in specifying BAG neuronal fate (data not shown). To examine how egl-46 may regulate BAG O2- and CO2-sensing fate specification in parallel to ets-5 and egl-13, we analyzed the expression of gcy-31 and gcy-33 (O2 fate) and gcy-9 (CO2 fate) transgenic reporters. As previously reported, gcy-31 and gcy-33 reporter transgenes are partially affected in ets-5egl-13 double-mutant animals (Figure 4, A and B) (Gramstrup Petersen et al. 2013). Therefore, we constructed double- and triple-mutant combinations to decipher the role of egl-46 (Figure 4, A and B). We found that egl-46 acts in parallel to ets-5 and egl-13 to drive gcy-31 and gcy-33 expression (Figure 4, A and B). Loss of egl-46 reduces the number of animals that express gcy-31prom::mCherry and gcy-33prom::GCY-33::gfp in both BAG neurons when combined with ets-5 or egl-13 mutations and when all three transcription factors are removed expression of these markers is abrogated (Figure 4, A and B). Next, we asked whether egl-46 may act in parallel to egl-13 to specify the CO2 fate of the BAG neurons (we did not perform this analysis with ets-5 as mutant animals are fully penetrant for loss of gcy-9 expression) (Guillermin et al. 2011). We found that loss of egl-46 reduces the number of animals that express gcy-9prom::dsRed2 when compared to the egl-13 mutant, indicating that these genes act in parallel pathways to direct CO2 fate of the BAG neurons (Figure 4C). Taken together, egl-46 acts in parallel pathways to those of ets-5 and egl-13 to regulate the O2- and CO2-sensing fate of the BAG neurons (Figure 4D).
Figure 4.
egl-46 acts in parallel to ets-5 and egl-13 to direct gas-sensing fate of the BAG neurons. (A and B) Double-mutant analysis shows that EGL-46 acts in parallel to EGL-13 and ETS-5 to drive the gcy-31prom::mCherry and gcy-33prom::GCY-33::GFP transgenes in the BAG neurons. Expression of the gcy-31prom::mCherry (A) and gcy-33prom::GCY-33::GFP (B) fate markers in the BAG neurons is partially, if at all, affected in egl-46(rp13), ets-5(tm1734), or egl-13(ku194) single-mutant animals. A synergistic interaction is observed, however, when egl-46 is removed in either egl-13 or ets-5 mutants and when all three genes are deleted, expression of gcy-31 and gcy-33 in the BAG neurons is abrogated. n > 50. **P < 0.01, ***P < 0.001, ****P < 0.0001. (C) Double-mutant analysis shows that EGL-46 acts in parallel to EGL-13 to drive the gcy-9prom::dsRed2 transgenes in the BAG neurons. n > 50. **P < 0.01, ****P < 0.0001. (D) Schematic showing the factors known to regulate BAG neuronal fate specification in C. elegans. We have shown that egl-46 acts in parallel to egl-13 and ets-5 to control O2 and CO2 sensory fate of the BAG neurons through the control of gcy-9, gcy-31, and gcy-33 expression. Arrows denote regulatory activities.
Discussion
In this study, we show that the zinc-finger transcription factor EGL-46 is required for the correct expression of specific terminal differentiation genes in the gas-sensing BAG neurons. We found that loss of egl-46 causes defects in the ability of worms to mount BAG-mediated behavioral responses to O2 and CO2. In addition, EGL-46 acts in a partially parallel pathway to that of EGL-13 and ETS-5 for the regulation of BAG terminal differentiation genes gcy-9, gcy-31, and gcy-33. In the absence of ets-5 and egl-13, two previously identified genes required for expression of the terminal differentiation genes in the BAG neurons, gcy-31 and gcy-33 expression is only partially affected (Guillermin et al. 2011; Brandt et al. 2012; Gramstrup Petersen et al. 2013). Additionally, gcy-9 is partially affected in egl-13 mutant animals. We found that when egl-46 is removed in ets-5egl-13 double-mutant animals, gcy-31 and gcy-33 expression in the BAG neurons is abrogated. Therefore, egl-46 acts in a parallel pathway to that of ets-5 and egl-13 to regulate expression of O2 cell fate markers in the BAG neurons. In addition, we found that egl-46 acts in a parallel pathway to that of egl-13 to drive gcy-9 expression, a reporter of CO2-sensing fate in the BAG neurons.
The reason for an EGL-46-driven parallel pathway to ensure gas-sensing neuronal fate in the BAG neurons is unclear. Interestingly, however, in Drosophila, the egl-46 ortholog Nerfin-1 is downregulated in maxillary palps by the mir-279 microRNA to prevent CO2 neuronal fate (Cayirlioglu et al. 2008; Hartl et al. 2011). In fact, overexpression of Nerfin-1 together with a second zinc-finger protein Escargot is sufficient to induce CO2 neurons on the maxillary palps (Hartl et al. 2011). Therefore, the requirement for this family of zinc-finger transcription factors in the regulation of gas-sensing neuron specification is evolutionarily conserved.
Supplementary Material
Acknowledgments
We thank members of the Pocock Laboratory for comments on the manuscript. Some strains were provided by the Caenorhabditis Genetics Center (University of Minnesota), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This work was supported by grants from the European Research Council (ERC Starting Grant number 260807 to R.P.), the Lundbeck Foundation (Project numbers R140-2013-13330 and R93-A8391 to R.P) and the Carlsberg Foundation (Grant number 2013_01_0047 to R.P.).
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.172049/-/DC1.
Communicating editor: D. I. Greenstein
Literature Cited
- Bigelow H., Doitsidou M., Sarin S., Hobert O., 2009. MAQGene: software to facilitate C. elegans mutant genome sequence analysis. Nat. Methods 6: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. P., Aziz-Zaman S., Juozaityte V., Martinez-Velazquez L. A., Petersen J. G., et al. , 2012. A single gene target of an ETS-family transcription factor determines neuronal CO(2)-chemosensitivity. PLoS ONE 7: e34014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. J., Busch K. E., de Bono M., 2008. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 105: 8044–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayirlioglu P., Kadow I. G., Zhan X., Okamura K., Suh G. S., et al. , 2008. Hybrid neurons in a microRNA mutant are putative evolutionary intermediates in insect CO2 sensory systems. Science 319: 1256–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsidou M., Flames N., Lee A. C., Boyanov A., Hobert O., 2008. Automated screening for mutants affecting dopaminergic-neuron specification in C. elegans. Nat. Methods 5: 869–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsidou M., Poole R. J., Sarin S., Bigelow H., Hobert O., 2010. C. elegans mutant identification with a one-step whole-genome-sequencing and SNP mapping strategy. PLoS ONE 5: e15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G., Yi P., Yang Y., Chai Y., Tian D., et al. , 2013. Developmental stage-dependent transcriptional regulatory pathways control neuroblast lineage progression. Development 140: 3838–3847. [DOI] [PubMed] [Google Scholar]
- Flibotte S., Edgley M. L., Chaudhry I., Taylor J., Neil S. E., et al. , 2010. Whole-genome profiling of mutagenesis in Caenorhabditis elegans. Genetics 185: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramstrup Petersen J., Rojo Romanos T., Juozaityte V., Redo Riveiro A., Hums I., et al. , 2013. EGL-13/SoxD specifies distinct O2 and CO2 sensory neuron fates in Caenorhabditis elegans. PLoS Genet. 9: e1003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillermin M. L., Castelletto M. L., Hallem E. A., 2011. Differentiation of carbon dioxide-sensing neurons in Caenorhabditis elegans requires the ETS-5 transcription factor. Genetics 189: 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E. A., Spencer W. C., McWhirter R. D., Zeller G., Henz S. R., et al. , 2011. Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 108: 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl M., Loschek L. F., Stephan D., Siju K. P., Knappmeyer C., et al. , 2011. A new Prospero and microRNA-279 pathway restricts CO2 receptor neuron formation. J. Neurosci. 31: 15660–15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O., 2002. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32: 728–730. [DOI] [PubMed] [Google Scholar]
- Hobert O., 2008. Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc. Natl. Acad. Sci. USA 105: 20067–20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O., 2011. Regulation of terminal differentiation programs in the nervous system. Annu. Rev. Cell Dev. Biol. 27: 681–696. [DOI] [PubMed] [Google Scholar]
- Hobert O., Carrera I., Stefanakis N., 2010. The molecular and gene regulatory signature of a neuron. Trends Neurosci. 33: 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot D., Johnson B. E., Berry T. L., Jr, Carnell L., Goodman M. B., 2008. The Parallel Worm Tracker: a platform for measuring average speed and drug-induced paralysis in nematodes. PLoS ONE 3: e2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. N., Duggan A., Garcia-Anoveros J., 2011. Insm1 promotes the transition of olfactory progenitors from apical and proliferative to basal, terminally dividing and neuronogenic. Neural Dev. 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Wang H., Hu J., Han J., Matsunami H., et al. , 2009. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc. Natl. Acad. Sci. USA 106: 2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunozaki M., Chalasani S. H., Bargmann C. I., 2008. A behavioral switch: cGMP and PKC signaling in olfactory neurons reverses odor preference in C. elegans. Neuron 59: 959–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Schwartz H. T., Barr M. M., 2010. Functional specialization of sensory cilia by an RFX transcription factor isoform. Genetics 186: 1295–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Duggan A., Chalfie M., 2001. Inhibition of touch cell fate by egl-44 and egl-46 in C. elegans. Genes Dev. 15: 789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M., Gray J. M., Pokala N., Chang A. J., Karow D. S., et al. , 2009. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron 61: 865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.