Abstract
Prostate cancer is one of the most common neoplasms in men. Transrectal ultrasound (TRUS)-guided systematic biopsy has a crucial role in the diagnosis of prostate cancer. However, it shows limited value with gray-scale ultrasound alone because only a small number of malignancies are visible on TRUS. Recently, new emerging technologies in TRUS-guided prostate biopsy were introduced and showed high potential in the diagnosis of prostate cancer. High echogenicity of ultrasound contrast agent reflect the increased status of angiogenesis in tumor. Molecular imaging for targeting specific biomarker can be also used using ultrasound contrast agent for detecting angiogenesis or surface biomarker of prostate cancer. The combination of TRUS-guided prostate biopsy and ultrasound contrast agents can increase the accuracy of prostate cancer diagnosis. Elastography is an emerging ultrasound technique that can provide the information regarding tissue elasticity and stiffness. Tumors are usually stiffer than the surrounding soft tissue. In two types of elastography techniques, shearwave elastography has many potential in that it can provide quantitative information on tissue elasticity. Multiparametric magnetic resonance imaging (MRI) from high resolution morphologic and functional magnetic resonance (MR) technique enables to detect more prostate cancers. The combination of functional techniques including apparent diffusion coefficient map from diffusion weighted imaging, dynamic contrast enhanced MR and MR spectroscopy are helpful in the localization of the prostate cancer. MR-ultrasound (US) fusion image can enhance the advantages of both two modalities. With MR-US fusion image, targeted biopsy of suspicious areas on MRI is possible and fusion image guided biopsy can provide improved detection rate. In conclusion, with recent advances in multiparametric-MRI, and introduction of new US techniques such as contrast-enhanced US and elastography, TRUS-guided biopsy may evolve toward targeted biopsies rather than systematic biopsy for getting information reflecting the exact status of the prostate.
Keywords: Prostate, Ultrasonography, Contrast media, Elastic imaging techniques, Magnetic resonance imaging
INTRODUCTION
Prostate cancer is the most common neoplasm in Europe and America occupying about 2 or 3 times more than lung and colorectal cancer [1,2]. The incidence is still rising as well as Asian countries including Japan and Korea. Screening, detection and diagnosis of prostate cancer are currently based on serum prostate-specific antigen (PSA) levels, digital rectal examination and transrectal ultrasound (TRUS)-guided systematic biopsies [3].
Only a small number of malignancies are visible on gray-scale TRUS. On grayscale evaluation, prostate cancers are classically described as a hypoechoic lesion; however they may be isoechoic or hyperechoic (Fig. 1) [4,5]. The percentages of prostate cancers known from literature are around 11%–35% [6]. The positive predictive value of the biopsy of a peripheral hypoechoic lesion is 25%–30% [4]. Furthermore, only in 17%–57% of the hypoechoic lesions seen on TRUS is malignancy present [7]. Many prostate cancers are not visible on conventional ultrasound, and any lesion visible on grayscale ultrasound has a high likelihood of having a benign cause. The differential diagnosis in evaluating the low echoic focal lesion includes inflammation, fibrosis, infarction or benign prostate hyperplasia nodules.
Fig. 1.
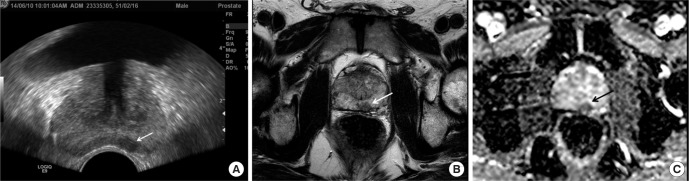
Focal lesion seen on transrectal ultrasound (TRUS) and prostate magnetic resonance imaging in a 55-year-old man. (A) TRUS shows low echoic nodular lesion in left peripheral zone (arrow). TRUS guided biopsy for this lesion confirmed that the lesion was prostate cancer. (B) T2 weighted axial magnetic resonance scan shows relatively well defined nodular lesion in left peripheral zone (arrow). (C) Apparent diffusion coefficient map shows signal drop at the same lesion, which suggest diffusion restriction (arrow).
According to Onur et al. [8], prostate cancer was reported results of biopsy study of 3,912 consecutive patients revealed that prostate cancer was detected in 25.5% with a hypoechoic lesion, and in 25.4% without a hypoechoic lesion. The percentage of core detection was 9.3% for hypoechoic and 10.4% for iso-echoic areas. Over the past decade, there has been a trend to obtain larger numbers of biopsy specimens, with most clinicians taking 8- to 12-biopsy cores, most current studies are recommending a 12-core biopsy scheme [9]. Autopsy studies have demonstrated that sextant prostate biopsy sensitivities at 30%, with increasing sensitivity with increasing numbers of biopsy cores, 36%–58% for 12-core biopsies, and 53%–58% for 18-core biopsies [10].
In other attempts were tried. Lee et al. [11] tried to classify the focal lesions seen on TRUS with parameters of shape, margin irregularity, vascularity, the location of the lesion. They concluded that the positive predictive value was up to 80% when the focal lesion located in peripheral portion showed nodular, irregular, and increased vascularity.
In spite of these results, many research reports showed that the focal lesion shows low sensitivity and specificity of gray-scale ultrasound for the detection of prostate cancer. And there is limited value for gray-scale-targeted biopsies. However, due to the high-quality images and the inexpensive and simple procedure, it is still the most optimal technique for guiding prostate biopsies [3].
Besides, recently, new emerging technologies in TRUS-guided prostate biopsy were introduced and showed high potential in the diagnosis of prostate cancer. Ultrasound contrast agent studies can provide the information regarding vascularity of the lesion. New novel technologies for the synthesis of new microbubbles (MBs) with specific ligand and visualize the portion with specific marker. The advent of ultrasonic molecular imaging may provide a new diagnostic method for the early diagnosis of prostate cancer. Elastography is an emerging ultrasound technique that can provide the information regarding tissue elasticity and stiffness. The hybrid imaging, which can show the information both from multiparametric (MP) magnetic resonance imaging (MRI) and TRUS imaging, will have a potential modality in performing targeted biopsy. In this review article, the new upcoming technology which can be used in TRUS-guided biopsy will be introduced.
DYNAMIC CONTRAST ENHANCED TRANSRECTAL PROSTATE ULTRASOUND
Sonographic contrast agents made up of MBs are composed of an outer shell and inner gas core, ranging in size from 1 to 7 μm in diameter [12]. The thickness of outer shell is denatured albumin or phospholipids ranging from 10 to 200 nm [13]. The inner space is filled with gases having a high molecular weight and low solubility such as perfluorocarbon or sulfur hexafluoride which has characteristics of prolong the agents’ existence in the blood pool [14].
Ultrasound contrast agents are mainly used as intravascular contrast media although they can be instilled into urinary bladder to evaluate ureteric reflux or into the uterus to look out tubal patency [15,16]. The size of MB is equal to that of red blood cells and they behave as intravascular blood pool agents (Fig. 2).
Fig. 2.
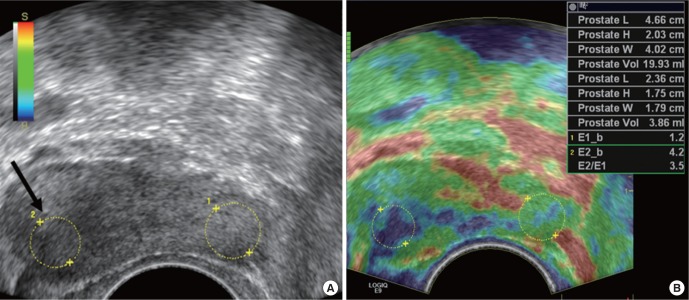
Contrast enhanced transrectal ultrasound (TRUS) findings of prostate cancer in a 62-year-old man. Contrast enhanced TRUS image shows increase vascularity and contrast agent signals from left peripheral zone suggesting increased vascularity (arrows). Note that the focal lesion shows low echogenicity in gray-scale TRUS, which is one of common findings of prostate cancer. This lesion was confirmed as prostate cancer after TRUS guided targeted biopsy.
The tumor growth and metastasis require angiogenesis, the growth of new blood vessels. The measure of tumor angiogenesis correlates with the microvessel density (MVD) and metastasis in various malignancies [17]. Hence, MBs have considerable potential for imaging of tumor angiogenesis in preclinical studies using small animal models. According to our preclinical study in xenograft prostate cancer model using PC-3 prostate tumor cells, maximum intensity was positively correlated with the MVD with statistical significance [18]. Weidner et al. [17] reported that microvessel counts increased with increasing Gleason score in prostatectomy specimen. In 2001, Sedelaar et al. [19] showed that ultrasound contrast enhanced areas had a 1.93 times higher MVD as compared to the nonenhanced areas.
In clinical practice, contrast enhanced sonography has many advantages in that it can be performed on patients safely, easily, and repeatedly without any radiation. However, contrast enhanced sonography has disadvantages in its lack of objectivity in determining the extent of enhancement because the image qualities are affected by many factors, and they are sometimes operator dependent. Even though the several software for semiquantitative analysis about dynamic contrast study were developed and embedded in ultrasound machine, the disadvantages of lack of objectiveness still exists.
Many clinical researches were performed for the evaluation of the TRUS-guided biopsy results using ultrasound contrast agents. According to Mitterberger et al. [20], the detection rate of prostate cancer was higher when they use contrast enhanced color Doppler targeted biopsy comparing ten systemic biopsies in 690 men (26% vs. 20%). The Gleason score was also higher in contrast enhanced color Doppler targeted biopsy than that of systemic biopsy (mean: 6.8 vs. 5.4). In recent report published by Jiang, the peak intensity on contrast enhanced ultrasound correlated with Gleason score and MVD in 147 prostate cancer patients [21].
Li et al. [22] reported meta-analysis reports regarding the diagnostic performance of contrast enhanced ultrasound in patients with prostate cancer. The pooled sensitivity and specificity were 0.7 and 0.74 from 2,624 patients who were included in their meta-analysis. They concluded that contrast enhanced ultrasound is a promising tool in the detection of prostate cancer, but it cannot completely replace systematic biopsy under the present circumstances.
TARGETED ULTRASOUND CONTRAST AGENT SPECIFIC TO PROSTATE CANCER
Targeted MBs are new generation of ultrasound contrast agent. These bubbles have additional ligand molecules that bind to the specific sites. Possible receptor targets for prostate cancer are those that are up-regulated during the process of angiogenesis. Most research has been focusing on the vascular endothelial growth factor (VEGF) receptors [23]. Exploiting the high expression of VEGF receptor 2 (VEGFR2) in tumor neovasculature, Fischer et al. [24] developed VEGFR2 receptor-loaded targeted micrometer-scale MBs based on the conventional MB and compared the contrast enhancement of conventional MB and VEGFR2 receptor-loaded MB in prostate cancer and normal prostate tissue.
There are other novel technologies for targeting prostate cancer cells using nanoscale ultrasound contrast agents. As well-known tissue marker of prostate cancer, prostate-specific membrane antigen (PSMA) is considered to be the most important protein target in diagnostic specific immunolocalization imaging and immune-directed therapy [25,26]. Current studies have demonstrated that PSMA is a type II transmembrane glycoprotein in the prostate cell membrane. The levels of PSMA expression are different in normal prostate tissue, benign prostatic hyperplasia and prostate cancer epithelial tissue. And it is also known that PSMA positive expression rate in hormone refractory prostate cancer and metastases are significantly higher than that of the normal or benign tissue [25].
Loading nanoscale MBs with prostate cancer-targeted specific ligands or antibodies is critical for specific ultrasound imaging in prostate cancer. Wang et al. [26] reported in vitro and in vivo results of PSMA-targeted nanoscale MBs in prostate cancer. They synthesized stable PSMA monoclonal antibody-loaded MBs using biotin-avidin complex technology and investigate their in vitro target binding capability with the selected prostate cancer cells. In addition, targeted contrast enhancement and specificity were also examined with a xenograft prostate tumor models. The results showed that targeted nanoscale MBs can significantly increase peak intensity and duration of contrast enhancement than blank nanoscale MBs in transplanted prostate tumors. Increased peak intensity and prolonged duration of enhanced contrast are the main characteristics of targeted nanoscale MB enhanced imaging [26].
Even though these targeted ultrasound contrast agents are on the stage of clinical trial and preclinical study, it would be very potential methodology for targeted ultrasound guided prostate biopsy.
ELASTOGRAPHY
Elastography is an emerging ultrasound technique that can visualize tissue elasticity and stiffness [27]. It is based on the assumptions that if force is applied to the unit area (stress), relative displacement of points (strain) will be proportional to the applied force and is represented by well-described Young’s modulus. Tumors are usually stiffer than normal tissue because of its increased cellular density. Prostatic cancer is normally 5–28 times stiffer than the surrounding soft tissue [28]. This change of local stiffness is the background of digital rectal exam of prostate gland. However, digital rectal exam is subjective to the examiner and only part of prostate is palpable.
There are two types of elastography; using strain and shear wave. Strain forces are generated by manual compression by transducers, while shear wave is a technique that uses a sonographic push pulse to generate a shear wave in the tissues [29]. A strain profile in a direction perpendicular to the tissue surface in response to an externally applied force is calculated in compression elastography. Tissue deformation is estimated from the relative difference in tissue movement from one to another frame. The deformation measurements are mapped on elastogram, stiffer areas as dark and more-elastic area as brighter color (Fig. 3). Elastography permits depiction of the cancer of isoechogenecity on gray scale ultrasound (US), otherwise can be missed by conventional TRUS.
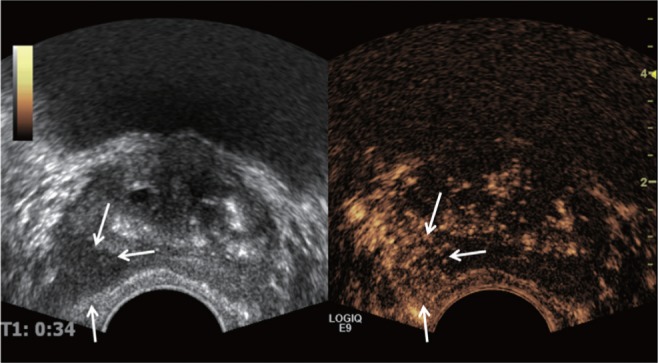
Fig. 3.
Typical prostate cancer seen on transrectal ultrasound (TRUS) and elastography in a 60-year-old man. (A) Gray-scale TRUS shows low echoic focal lesion in right lobe of prostate gland (arrow). (B) Elastography shows bluish color on right lobe, suggesting more rigidity comparing surrounding prostate tissue. Stiffness ratio of this focal lesion to contralateral normal area was 3.5, which means that 3.5 times stiffer than contralateral area by measurement of circular region of interest.
A metaanalysis study of US elastography using strain reported sensitivity in the range of 71%–82%, a specificity of 60%–95% with reference standard of radical prostatectomy specimen [30]. Elastography guided prostate biopsies in patients with cancer were 2.9 folds more likely to detect prostate cancer than systemic biopsy, while requiring fewer core samples [31].
Another prospective study of elastography by Brock et al. [32] concluded that overall prostate cancer detection rate was significantly higher in patients who underwent biopsy with the elastography guided approach compared to the gray scale ultrasound guided biopsy (51.1% vs. 39.4%). However the sensitivity of elastography did not reach levels to omit a systematic biopsy approach.
Even comparison of elastography with T2 weighted conventional MRI was reported by Aigner et al. [33]. Overall sensitivities and specificities were similar between elastography and T2 weighted MRI. Negative predictive values of both studies are over 80%, these findings are both examinations may be useful to obviate the need for prostate biopsy.
The drawback of strain elastography is that quantification of tissue elasticity is not achievable. Semiquantitative stiffness evaluation using a strain index (strain ratio of tissue over normal tissue) is introduced to overcome this limitation and reported to be useful in the evaluation of prostate cancer [34]. At the cutoff value of 17.44, elastography yielded sensitivity of 74.5% and specificity of 83.3% for discriminating prostate cancer from benign lesions. However, these studies are all based on region of interest drawing, which have some difficulties in reproducibility and standardization.
Shear wave elastography (SWE) is another type of elastography that can provide quantitative information on tissue elasticity. Another advantage of SWE over strain elastography is that SWE does not require compression by the transducer, which means that measurement is operator independent. A few initial reports showed very promising results for SWE, including high sensitivities and specificities over 90% for prostate cancer [29,35]. However, the sensitivity and specificity were decreased to be 50% to 60% in another recent study by Woo et al. [36]. Nevertheless, SWE parameters of mean stiffness and mean stiffness ratio are significantly different between prostate cancer and benign tissue and correlate with Gleason score.
Therefore, more validation studies beyond the initial hype will be required for the clinical implication of SWE through more objective measurement of SWE parameters, prospective trials and radical prostatectomy specimen basis.
MR-US FUSION PROSTATE BIOPSY
TRUS plays a crucial role in the screening imaging study and guidance of the biopsy of the prostate glands. However, overall detection rate of TRUS for prostate cancer remains approximately 50%, and biopsies yield at least 1 positive biopsy in only 25% of the patients [37,38]. Increasing number of biopsy cores is reported to improve cancer detection rates [39]. However, outnumbered core biopsy jeopardizes patients by increased complication rates. Moreover, over detection of the clinically insignificant cancer is another important issue, which leads to overtreatment. Nearly 50% of currently detected prostate cancer cases may be insignificant [40]. Therefore, detection of highest grade or representative cancer tissue in the prostate gland is required to decide optimal treatment plan.
Application of stronger magnet and MP MRI from morphologic and functional MRI technique enables to detect more cancers. T2 weighted MRI is excellent in the evaluation of anatomy and detection of peripherally located cancer. However, T2 weighted image has limited value in the detection of central gland cancer. In addition, T2 weighted images are very susceptible to post biopsy change, which shows low signal intensity and hampers tumor detection [41].
Introduction of 3.0 Tesla MRI in clinical field impacts improved image quality from increased signal to noise ratio. Still there remain some controversies, but the use of fearsome endorectal coil is not obligatory for the prostate MRI because of its increased signal to noise ratio of 3.0 Tesla MRI [42].
Functional techniques including apparent diffusion coefficient (ADC) map from diffusion weighted imaging, dynamic contrast enhanced MRI with fast imaging and magnetic resonance (MR) spectroscopy are very helpful in the diagnosis of prostate cancer. The detection rates of prostate cancer are increased with these techniques, and even centrally located cancer can be more easily and confidently diagnosed [43]. For the staging of prostate cancer, MP MRI is superior to detect extracapsular extension and seminal vesicle invasion. To monitor treatment effect, MP MRI significantly improves the assessment of patients with suspected recurrence after treatment [44].
ADC map can discriminate cancers with Gleason score over 7 (4+3) from cancer with lower Gleason score [43]. Because of this superior detectability of cancer with highest Gleason score with MRI, MRI guided prostate biopsy is introduced. Although, it has great advantage of reducing the number of biopsy core, the increased procedure time and costs make the approach impractical [45].
MR-US fusion image can be another powerful option for guidance of prostate biopsy (Fig. 4). Reduction of time and cost of direct MRI guidance without sacrificing diagnostic accuracy can be achievable. Sonn et al. [45] reported that targeted biopsy with MR-US fusion was 3 times more likely to identify cancer than a systematic biopsy (27% vs. 7%). Of the men with Gleason score 7 or greater cancer 38% had disease detected only on targeted biopsies. Fusion biopsy can provide improved detection of prostate cancer in men with prior negative biopsies and elevated PSA values [46].
Fig. 4.
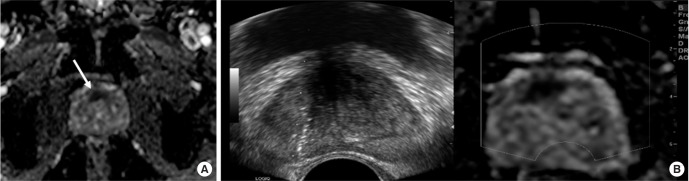
Magnetic resonance-ultrasound (MR-US) fusion image guided biopsy proven prostate cancer in 67-year-old man. (A) Apparent diffusion coefficient (ADC) map image shows decreased ADC area in anterior prostate gland (arrow), suggesting diffusion restriction in this area. (B) Real time MR-US fusion image guided biopsy revealed prostate cancer with Gleason score 7 in this area.
There is still some technical issue to be solved in MR-US fusion. Precise registration of MR and US is the key for the successful image fusion. MRI can be performed with either pelvic array surface coil or endorectal coil. The prostate gland inevitably deformed during TRUS by introducing ultrasound transducer. Nonrigid registration of the prostate gland for this elastic deformation is needed, but still many fusion techniques are based on rigid registration which cannot reflect elastic deformation by transducer. However, this issue can be overcome by development of fusion technique [47].
CONCLUSIONS
Gray-scale TRUS is the gold standard for prostate imaging and is essential tool for TRUS guided prostate biopsy. With current trends in demanding more tissue and more cores to constitute a satisfactory sampling of the prostate, many solutions to increase sensitivity and to decrease the number of cores are suggested. MBs, which have inner gas and outer biocompatible shells composed of phospholipids or denatured albumin, are good ultrasound contrast agents for the visualization of the vascular morphology and perfusion in the malignant lesions. Using MBs, microvascular abnormalities related to tumor angiogenesis in prostate cancer can be identified and represent an ideal biopsy target representing whole status of prostate.
Elastography reflects the tissue elasticity and stiffness in prostate. Although not yet established for routine clinical use, US elastography is a promising adjunctive modality for evaluating prostate lesions. Between two types of elastography, shearwave elastography has several advantages in that it can provide quantitative information on tissue elasticity and does not need manual compression. Therefore, more validation studies will be needed for the evaluation about the role of elastography in the diagnosis of prostate cancer.
MP MRI, which includes T2 weighted image, diffusion weighted image, and dynamic contrast enhanced image, gives us information regarding prostate cancer. The MRI images can be used to guide TRUS-guided biopsy via image registration and fusion. With MR-US image fusion, targeted biopsy of suspicious areas on MRI is possible.
In conclusion, with recent advances in MP MRI, and introduction of new US techniques such as contrast-enhanced US and elastography, TRUS-guided biopsy may evolve toward targeted biopsies rather than systematic biopsy for getting information about exact status of the prostate.
ACKNOWLEDGMENTS
This work was funded by the National Research Foundation of Korea (the Basic Science Research Program 2010-0009271).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe H, Igari D, Tanahasi Y, Harada K, Saito M. Development and application of new equipment for transrectal ultrasonography. J Clin Ultrasound. 1974;2:91–8. doi: 10.1002/jcu.1870020203. [DOI] [PubMed] [Google Scholar]
- 3.Smeenge M, de la Rosette JJ, Wijkstra H. Current status of transrectal ultrasound techniques in prostate cancer. Curr Opin Urol. 2012;22:297–302. doi: 10.1097/MOU.0b013e3283548154. [DOI] [PubMed] [Google Scholar]
- 4.Rifkin MD, Dahnert W, Kurtz AB. State of the art: endorectal sonography of the prostate gland. AJR Am J Roentgenol. 1990;154:691–700. doi: 10.2214/ajr.154.4.1690499. [DOI] [PubMed] [Google Scholar]
- 5.Dähnert WF, Hamper UM, Eggleston JC, Walsh PC, Sanders RC. Prostatic evaluation by transrectal sonography with histopathologic correlation: the echopenic appearance of early carcinoma. Radiology. 1986;158:97–102. doi: 10.1148/radiology.158.1.3510032. [DOI] [PubMed] [Google Scholar]
- 6.Beemsterboer PM, Kranse R, de Koning HJ, Habbema JD, Schroder FH. Changing role of 3 screening modalities in the European randomized study of screening for prostate cancer (Rotterdam) Int J Cancer. 1999;84:437–41. doi: 10.1002/(sici)1097-0215(19990820)84:4<437::aid-ijc19>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Engelbrecht MR, Barentsz JO, Jager GJ, van der Graaf M, Heerschap A, Sedelaar JP, et al. Prostate cancer staging using imaging. BJU Int. 2000;86(Suppl 1):123–34. doi: 10.1046/j.1464-410x.2000.00592.x. [DOI] [PubMed] [Google Scholar]
- 8.Onur R, Littrup PJ, Pontes JE, Bianco FJ., Jr Contemporary impact of transrectal ultrasound lesions for prostate cancer detection. J Urol. 2004;172:512–4. doi: 10.1097/01.ju.0000131621.61732.6b. [DOI] [PubMed] [Google Scholar]
- 9.Eichler K, Hempel S, Wilby J, Myers L, Bachmann LM, Kleijnen J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175:1605–12. doi: 10.1016/S0022-5347(05)00957-2. [DOI] [PubMed] [Google Scholar]
- 10.Delongchamps NB, de la Roza G, Jones R, Jumbelic M, Haas GP. Saturation biopsies on autopsied prostates for detecting and characterizing prostate cancer. BJU Int. 2009;103:49–54. doi: 10.1111/j.1464-410X.2008.07900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HY, Lee HJ, Byun SS, Lee SE, Hong SK, Kim SH. Classification of focal prostatic lesions on transrectal ultrasound (TRUS) and the accuracy of TRUS to diagnose prostate cancer. Korean J Radiol. 2009;10:244–51. doi: 10.3348/kjr.2009.10.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosgrove D. Ultrasound contrast agents: an overview. Eur J Radiol. 2006;60:324–30. doi: 10.1016/j.ejrad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol. 2007;17:1995–2008. doi: 10.1007/s00330-007-0623-0. [DOI] [PubMed] [Google Scholar]
- 14.Burns PN, Wilson SR. Microbubble contrast for radiological imaging: 1. Principles. Ultrasound Q. 2006;22:5–13. [PubMed] [Google Scholar]
- 15.Darge K, Troeger J, Duetting T, Zieger B, Rohrschneider W, Moehring K, et al. Reflux in young patients: comparison of voiding US of the bladder and retrovesical space with echo enhancement versus voiding cystourethrography for diagnosis. Radiology. 1999;210:201–7. doi: 10.1148/radiology.210.1.r99ja40201. [DOI] [PubMed] [Google Scholar]
- 16.Degenhardt F. Contrast sonography in gynaecology. Stuttgart, DE: Thieme; 1996. [Google Scholar]
- 17.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HJ, Hwang SI, Chung JH, Jeon JJ, Choi JH, Jung HS. Evaluation of tumor angiogenesis in a mouse PC-3 prostate cancer model using dynamic contrast-enhanced sonography. J Ul trasound Med. 2012;31:1223–31. doi: 10.7863/jum.2012.31.8.1223. [DOI] [PubMed] [Google Scholar]
- 19.Sedelaar JP, van Leenders GJ, Hulsbergen-van de Kaa CA, van der Poel HG, van der Laak JA, Debruyne FM, et al. Microvessel density: correlation between contrast ultrasonography and histology of prostate cancer. Eur Urol. 2001;40:285–93. doi: 10.1159/000049788. [DOI] [PubMed] [Google Scholar]
- 20.Mitterberger M, Pinggera GM, Horninger W, Bartsch G, Strasser H, Schafer G, et al. Comparison of contrast enhanced color Doppler targeted biopsy to conventional systematic biopsy: impact on Gleason score. J Urol. 2007;178:464–8. doi: 10.1016/j.juro.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Chen Y, Zhu Y, Yao X, Qi J. Contrast-enhanced ultrasonography for the detection and characterization of prostate cancer: correlation with microvessel density and Gleason score. Clin Radiol. 2011;66:732–7. doi: 10.1016/j.crad.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Tang J, Fei X, Gao Y. Diagnostic performance of contrast enhanced ultrasound in patients with prostate cancer: a meta-analysis. Acad Radiol. 2013;20:156–64. doi: 10.1016/j.acra.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Smeenge M, Mischi M, Laguna Pes MP, de la Rosette JJ, Wijkstra H. Novel contrast-enhanced ultrasound imaging in prostate cancer. World J Urol. 2011;29:581–7. doi: 10.1007/s00345-011-0747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer T, Thomas A, Tardy I, Schneider M, Hunigen H, Custodis P, et al. Vascular endothelial growth factor receptor 2-specific microbubbles for molecular ultrasound detection of prostate cancer in a rat model. Invest Radiol. 2010;45:675–84. doi: 10.1097/RLI.0b013e3181efd6b2. [DOI] [PubMed] [Google Scholar]
- 25.Perner S, Hofer MD, Kim R, Shah RB, Li H, Moller P, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38:696–701. doi: 10.1016/j.humpath.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Li L, Guo Y, Tong H, Fan X, Ding J, et al. Construction and in vitro/in vivo targeting of PSMA-targeted nanoscale microbubbles in prostate cancer. Prostate. 2013;73:1147–58. doi: 10.1002/pros.22663. [DOI] [PubMed] [Google Scholar]
- 27.Ginat DT, Destounis SV, Barr RG, Castaneda B, Strang JG, Rubens DJ. US elastography of breast and prostate lesions. Radiographics. 2009;29:2007–16. doi: 10.1148/rg.297095058. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Nigwekar P, Castaneda B, Hoyt K, Joseph JV, di Sant’Agnese A, et al. Quantitative characterization of viscoelastic properties of human prostate correlated with histology. Ultrasound Med Biol. 2008;34:1033–42. doi: 10.1016/j.ultrasmedbio.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 29.Barr RG, Memo R, Schaub CR. Shear wave ultrasound elastography of the prostate: initial results. Ultrasound Q. 2012;28:13–20. doi: 10.1097/RUQ.0b013e318249f594. [DOI] [PubMed] [Google Scholar]
- 30.Aboumarzouk OM, Ogston S, Huang Z, Evans A, Melzer A, Stolzenberg JU, et al. Diagnostic accuracy of transrectal elastosonography (TRES) imaging for the diagnosis of prostate cancer: a systematic review and meta-analysis. BJU Int. 2012;110:1414–23. doi: 10.1111/j.1464-410X.2012.11106.x. [DOI] [PubMed] [Google Scholar]
- 31.Pallwein L, Mitterberger M, Struve P, Horninger W, Aigner F, Bartsch G, et al. Comparison of sonoelastography guided biopsy with systematic biopsy: impact on prostate cancer detection. Eur Radiol. 2007;17:2278–85. doi: 10.1007/s00330-007-0606-1. [DOI] [PubMed] [Google Scholar]
- 32.Brock M, von Bodman C, Palisaar RJ, Loppenberg B, Sommerer F, Deix T, et al. The impact of real-time elastography guiding a systematic prostate biopsy to improve cancer detection rate: a prospective study of 353 patients. J Urol. 2012;187:2039–43. doi: 10.1016/j.juro.2012.01.063. [DOI] [PubMed] [Google Scholar]
- 33.Aigner F, Pallwein L, Schocke M, Lebovici A, Junker D, Schafer G, et al. Comparison of real-time sonoelastography with T2-weighted endorectal magnetic resonance imaging for prostate cancer detection. J Ultrasound Med. 2011;30:643–9. doi: 10.7863/jum.2011.30.5.643. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Tang J, Li YM, Fei X, Lv FQ, He EH, et al. Differentiation of prostate cancer from benign lesions using strain index of transrectal real-time tissue elastography. Eur J Radiol. 2012;81:857–62. doi: 10.1016/j.ejrad.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad S, Cao R, Varghese T, Bidaut L, Nabi G. Transrectal quantitative shear wave elastography in the detection and characterisation of prostate cancer. Surg Endosc. 2013;27:3280–7. doi: 10.1007/s00464-013-2906-7. [DOI] [PubMed] [Google Scholar]
- 36.Woo S, Kim SY, Cho JY, Kim SH. Shear wave elastography for detection of prostate cancer: a preliminary study. Korean J Radiol. 2014;15:346–55. doi: 10.3348/kjr.2014.15.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stroumbakis N, Cookson MS, Reuter VE, Fair WR. Clinical significance of repeat sextant biopsies in prostate cancer patients. Urology. 1997;49(3A Suppl):113–8. doi: 10.1016/s0090-4295(97)00178-7. [DOI] [PubMed] [Google Scholar]
- 38.Halpern EJ, Strup SE. Using gray-scale and color and power Doppler sonography to detect prostatic cancer. AJR Am J Roentgenol. 2000;174:623–7. doi: 10.2214/ajr.174.3.1740623. [DOI] [PubMed] [Google Scholar]
- 39.Babaian RJ, Toi A, Kamoi K, Troncoso P, Sweet J, Evans R, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000;163:152–7. [PubMed] [Google Scholar]
- 40.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Con temporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178(3 Pt 2):S14–9. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007;243:28–53. doi: 10.1148/radiol.2431030580. [DOI] [PubMed] [Google Scholar]
- 42.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–57. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Futterer JJ, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011;59:477–94. doi: 10.1016/j.eururo.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Westphalen AC, Reed GD, Vinh PP, Sotto C, Vigneron DB, Kurhanewicz J. Multiparametric 3T endorectal mri after external beam radiation therapy for prostate cancer. J Magn Reson Imaging. 2012;36:430–7. doi: 10.1002/jmri.23672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, Huang J, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189:86–91. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonn GA, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–15. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ukimura O, Desai MM, Palmer S, Valencerina S, Gross M, Abreu AL, et al. 3-Dimensional elastic registration system of prostate biopsy location by real-time 3-dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. J Urol. 2012;187:1080–6. doi: 10.1016/j.juro.2011.10.124. [DOI] [PubMed] [Google Scholar]