Abstract
Lysophosphatidic acid (LPA) is a signaling lipid that binds to six known lysophosphatidic acid receptors (LPARs), named LPA1-LPA6. These receptors initiate signaling cascades relevant to development, maintenance, and healing processes throughout the body. The diversity and specificity of LPA signaling, especially in relation to cancer and autoimmune disorders, makes LPA receptor modulation an attractive target for drug development. Several LPAR-specific analogues and small molecules have been synthesized and are efficacious in attenuating pathology in disease models. To date, at least three compounds have passed phase I and phase II clinical trials for idiopathic pulmonary fibrosis and systemic sclerosis. This review focuses on the promising therapeutic directions emerging in LPA signaling toward ameliorating several diseases, including cancer, fibrosis, arthritis, hydrocephalus, and traumatic injury.
Keywords: Lysophosphatidic acid receptor, Pharmacology, Autotaxin, Cancer, Autoimmune disease, Fibrosis
INTRODUCTION
Lysophosphatidic acid (LPA) is a bioactive lipid that is concentrated in serum and is essential for a variety of cellular and developmental processes (reviewed in (Choi et al., 2010)). While LPA does play a structural role in cell membranes, extracellular LPA is a highly selective and specific activator of a class of G protein-coupled receptors (GPCRs) called LPA receptors (LPARs) (reviewed in (Yung et al., 2014)). There are currently six recognized LPARs, named LPA1–6, with clear homologs between human (LPAR1-6) and mouse (Lpar1-6) genes (reviewed in (Chun et al., 2010)). All six receptors are expressed throughout the body during development and adulthood in unique spatiotemporal patterns. These receptors are involved in a variety of necessary functions, including cell survival, proliferation, migration, differentiation, vascular regulation, and cytokine release (reviewed in (Yung et al., 2014)).
LPA can be produced in several ways through the activity of intracellular or extracellular enzymes. The two most prominent pathways involve the conversion of lysophosphatidyl choline (LPC) to LPA by autotaxin (ATX/Enpp2) (Tokumura et al., 2002; Umezu-Goto et al., 2002) and conversion of phosphatidic acid to LPA by phospholipase A1 or A2 (PLA1/PLA2) (Fourcade et al., 1995; Sonoda et al., 2002). Intriguingly, ATX is highly expressed in blood, brain, kidney, the lymphatic system, and tissue surrounding injury (Bachner et al., 1999; Savaskan et al., 2007; Kanda et al., 2008), suggesting important LPA-mediated mechanisms in these areas. Additionally, LPA is secreted by activated platelets and mature adipocytes (Eichholtz et al., 1993; Valet et al., 1998; Sano et al., 2002). Because of its important roles throughout the body, aberrant LPA signaling has also been implicated in several diseases. This review focuses on the agents that have been developed to modulate LPA signaling and tested in disease models.
LYSOPHOSPHATIDIC ACID RECEPTOR SIGNALING
Interest in LPA as a signaling molecule dates back to the late 1970s when effects on intracellular calcium release, platelet aggregation, and blood pressure were reported (Tokumura et al., 1978; Gerrard et al., 1979). While the involvement of G proteins was postulated (Moolenaar and van Corven, 1990), the mechanism of LPA signaling was not elucidated until 1996 when the first LPA receptor was cloned (Hecht et al., 1996). Since the discovery of LPA1 (originally Vzg-1 or Edg-2), five other LPARs have been validated. LPA2 and LPA3 were elucidated through homology searches by comparing amino acid sequences to that of LPA1 (An et al., 1998; Bandoh et al., 1999). Through efforts aimed at finding ligands for orphan receptors, LPA4 and LPA5 were discovered (Noguchi et al., 2003; Kotarsky et al., 2006; Lee et al., 2006). Most recently, LPA6, a GPCR that is most closely related to LPA4, was added to the ranks of LPA receptors (Pasternack et al., 2008; Yanagida et al., 2009).
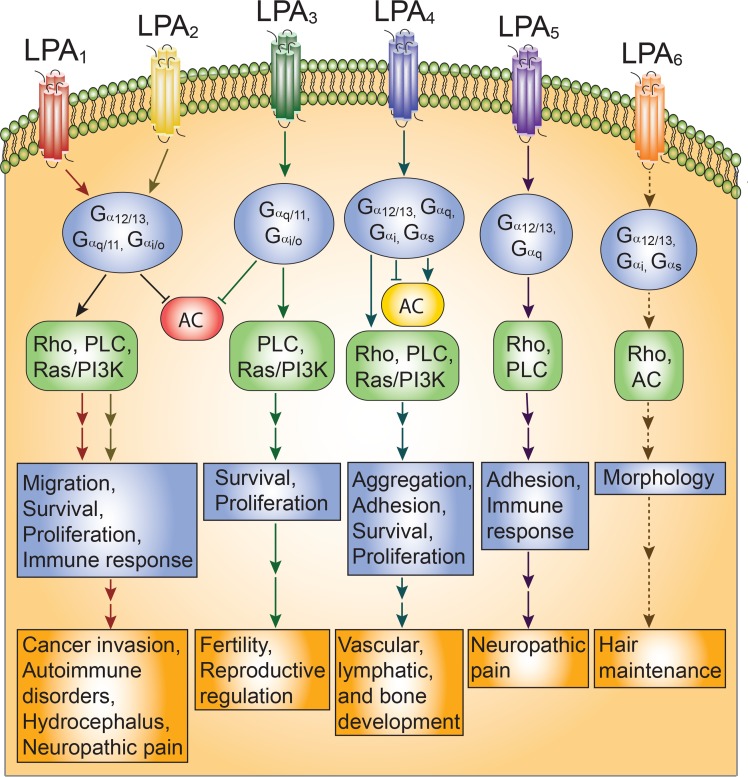
LPAR signaling occurs through a variety of intracellular cascades (reviewed in (Mirendil et al., 2013)) (Fig. 1). The binding of LPA or an LPA analog to its 7-transmembrane GPCR allows the Gα subunit to exchange used GDP for GTP. This results in Gα dissociating from Gβ and Gγ, allowing the Gα and Gβγ complexes to signal through downstream effectors. Several Gα subunits have been implicated in LPAR signaling, including Gα12/13, Gαq/11, Gαs, and Gαi/O. Downstream effectors include activation of several pathways. The Gα12/13-mediated Rho/ROCK and Rho/SRF pathways have been implicated in cell motility, invasion, and cytoskeletal changes (Sotiropoulos et al., 1999; Kim and Adelstein, 2011; Jeong et al., 2012). The Gαq/11 pathway activates phospholipase C (PLC), which induces IP3, and subsequently initiates Ca2+ and diacyl glycerol signaling (Sando and Chertihin, 1996). This cascade can result in vasodilation and a variety of transcriptional changes, including protein kinase C-induced cell growth, immune recruitment, and changes in learning and memory (Lu et al., 1999; Seewald et al., 1999; Cummings et al., 2004; Ruisanchez et al., 2014). Induction of the Gαs pathway leads to adenylyl cyclase (AC) activation and the production of cAMP, preventing cell migration (Jongsma et al., 2011). Activation of Gαi/O is the most versatile, as downstream effectors include PLC, Ras/MAPK-induced morphological changes (Kranenburg and Moolenaar, 2001), PI3K/Rac-mediated migration (Jimenez et al., 2000), modulation of PI3K/Akt survival mechanisms (Kang et al., 2004; Ye et al., 2002), and inhibition of AC.
Fig. 1.
LPAR signaling and functional outcomes. LPAR signaling details are highlighted for each receptor, based on canonical GPCR pathways that have been validated. Dashed lines indicate preliminary data that require further confirmation. Activated downstream effectors are shown in green, inhibited effectors in red, and effectors that are differentially activated or inhibited in yellow. The cellular effects of activating each LPAR are listed beneath the Gα cascades, followed by ultimate phenotypical outcomes as highlighted in this review. Antagonism or functional knockout of each LPAR has been proven to inhibit these disorder phenotypes.
Each LPAR has multiple important regulatory functions throughout the body (reviewed in (Yung et al., 2014)). Many of these have been elucidated through the use of knockout animals, pharmacological LPAR agonists or antagonists, and gene association studies. The first discovered LPAR, LPA1, appears to be responsible for several developmental, physiological, and pathological processes. These include cell survival, proliferation, adhesion, migration, immune function, and myelination (reviewed in (Fukushima et al., 2001)). LPA2 signaling has also been implicated in cell survival, migration, immune function, and myelination (reviewed in (Ishii et al., 2004)), often appearing to contribute to complementary LPA1 mechanisms (Contos et al., 2002). LPA3, while expressed in many different tissues, is most heavily characterized as being involved in reproduction; it mediates fertility, embryo spacing, and embryo implantation (Ye et al., 2005). LPA4 influences cell aggregation, cell adhesion, vascular development, and osteogenesis regulation (reviewed in (Mirendil et al., 2013)). Additionally, LPA4-mediated adhesion appears to counteract LPA1/LPA2-stimulated migration processes (Lee et al., 2008). LPA5 also negatively regulates cell motility and is involved in chemokine release (Jongsma et al., 2011; Lundequist and Boyce, 2011). Although LPA6 is the most recently discovered LPAR, several genome screening studies have been published linking mutations in LPA6 to genetic hair loss and autosomal recessive hypotrichosis, or “wooly hair” syndrome (Azeem et al., 2008; Pasternack et al., 2008; Petukhova et al., 2008). LPA6 is also under investigation for further functionality. The effects of LPAR signaling are outlined in Figure 1.
PHARMACOLOGICAL ADVANCES MODULATING LPA SIGNALING
As LPAR signaling has been strongly implicated in many disease states, great interest has been expressed in developing specific LPAR inhibitors. Currently, no LPA or LPAR-targeting drugs have been FDA approved, though several are in development or undergoing clinical trials (Yung et al., 2014) (Table 1). Furthermore, the ability to develop safe and efficacious drugs targeting lysophospholipid signaling has already been proven; fingolimod (FTY720), an analog of sphingosine 1-phosphate (S1P) and inhibitor of S1P receptors, has been FDA-approved for the treatment of multiple sclerosis (Brinkmann et al., 2002; Chun and Hartung, 2010; Calabresi et al., 2014).
Table 1.
Summary of compounds that target LPA signaling. The name, target, structure and development stage for each LPA signaling antagonist discussed in the article are outlined, along with their therapeutic
| Drug | Target | Structure | Phase | Indication | Reference |
|---|---|---|---|---|---|
| FTY720 | S1P1, S1P3–5 |
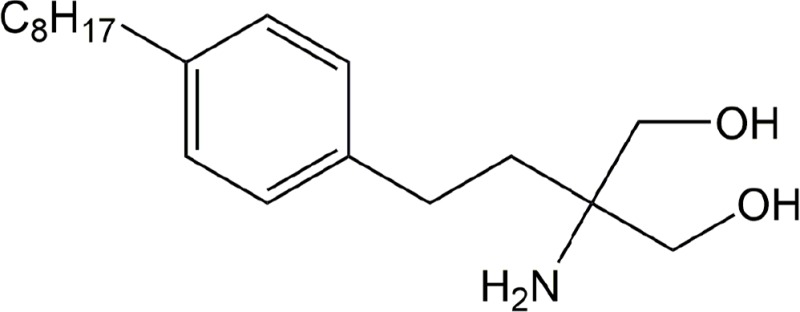
|
FDA approved | Multiple sclerosis | (Brinkmann et al., 2002; Chun and Hartung, 2010) |
| BMS-986202/AM152 | LPA1 | See patent WO/2012/162592 A1 for more information | Phase I complete | Idiopathic pulmonary fibrosis | (BMS, 2011; Bradford, 2012) |
| BMS-986020 | LPA1 | See patent WO/2012/162592 A1 for more information | Phase II complete | Idiopathic pulmonary fibrosis | (BMS, 2014; Bradford, 2012) |
| VPC 12249 | LPA1 |
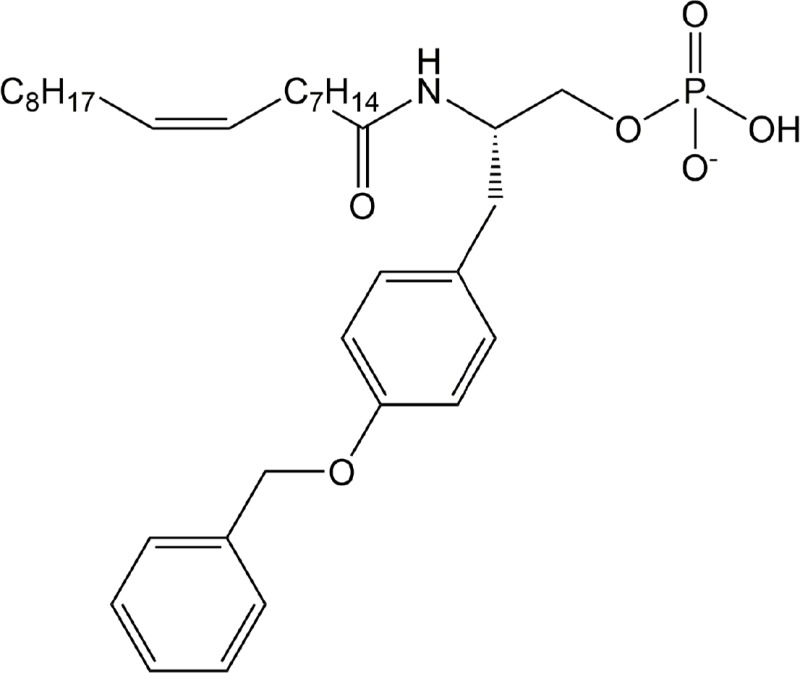
|
Preclinical | Idiopathic pulmonary fibrosis | (Okusa et al., 2003) |
| AM966 | LPA1 |
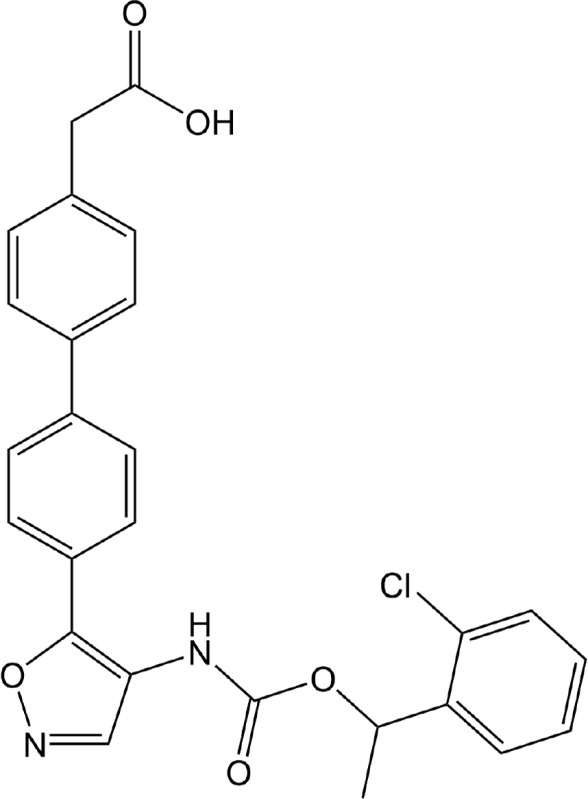
|
Preclinical | Idiopathic pulmonary fibrosis | (Swaney et al., 2010) |
| AM095 | LPA1 |
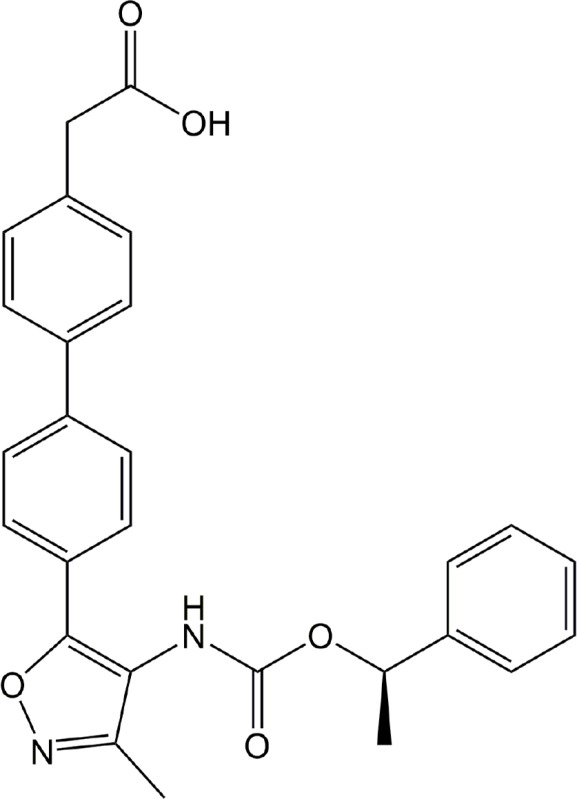
|
Preclinical | Dermal fibrosis, kidney fibrosis | (Castelino et al., 2011; Swaney et al., 2011) |
| BMS patent | LPA1 | See patent WO/2013/070879 A1 for more information | Preclinical | Spinal injury, neuropathic pain | (Nogueira and Vales, 2013) |
| SAR 100842 | LPA1, LPA3 | See patent WO/2012/162592 A1 for more information | Phase II complete | Systemic sclerosis | (Bradford, 2012; Sanofi, 2014) |
| Ki16425 | LPA1, LPA3 |
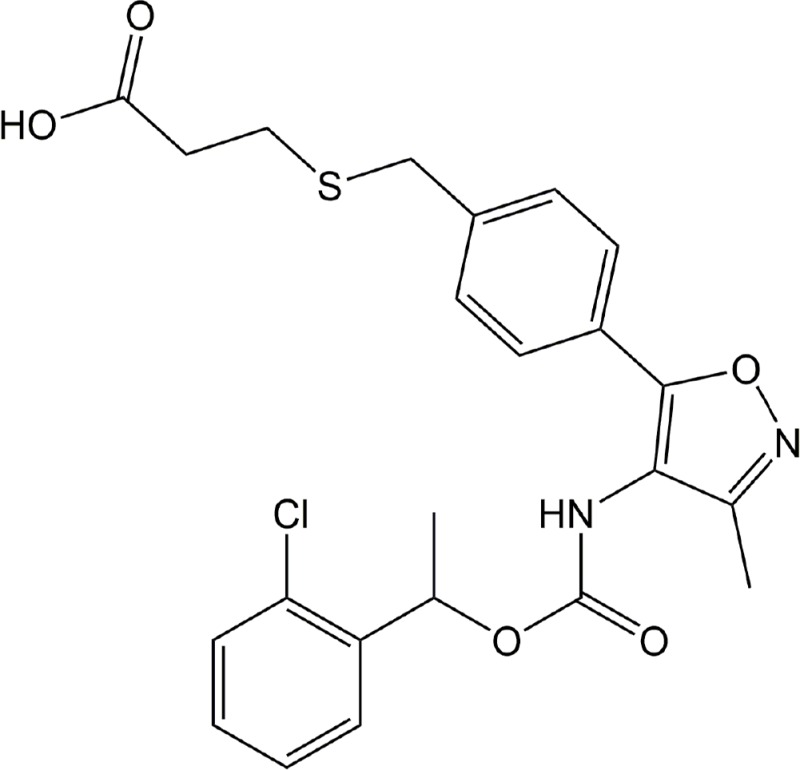
|
Preclinical | Cancer, rheumatoid arthritis, hydrocephalus | (Hama et al., 2004; Liao et al., 2013; Orosa et al., 2014; Su et al., 2013; Yung et al., 2011) |
| Debio 0719 | LPA1, LPA3 | R-stereoisomer of Ki16425 | Preclinical | Cancer | (Marshall et al., 2012) |
| Ki16198 | LPA1–3 |
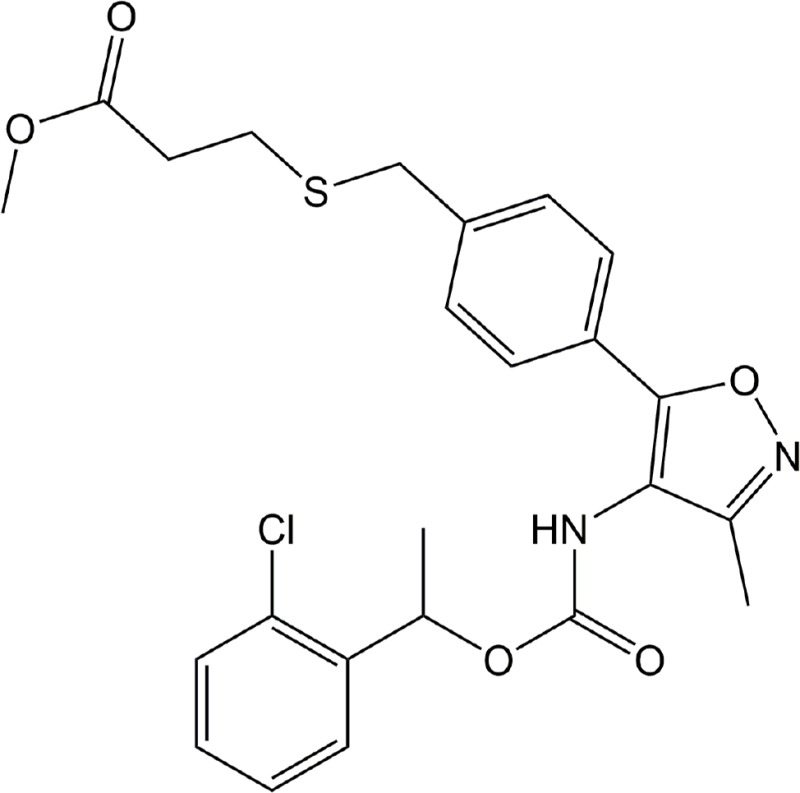
|
Preclinical | Cancer | (Komachi et al., 2012) |
| Cmpd. 35 | LPA2 |
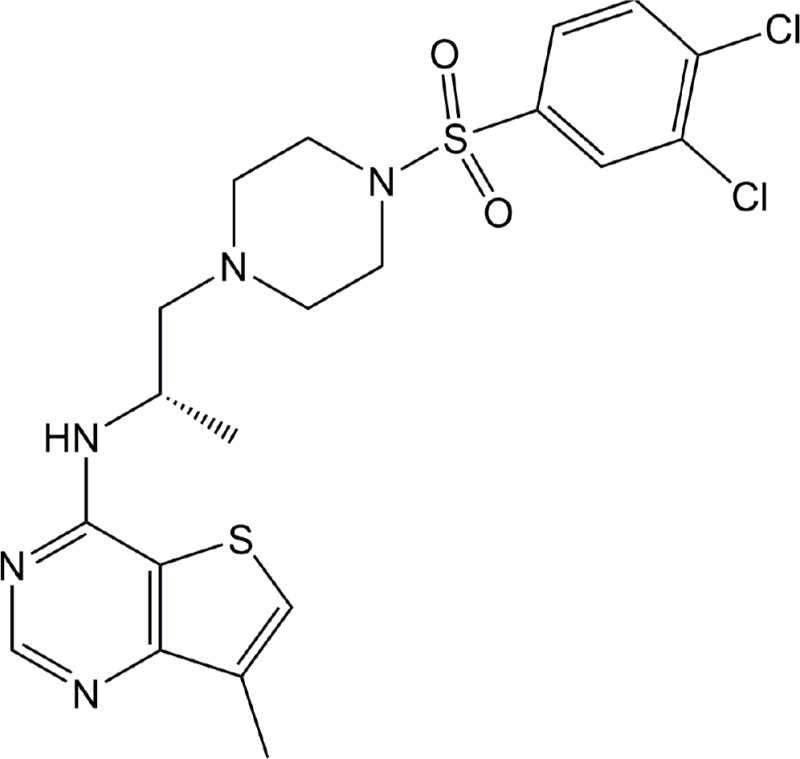
|
Preclinical | Cancer | (Beck et al., 2008) |
| Anti-LPA | All LPAR signaling | Antibody | Preclinical | Traumatic brain injury | (Crack et al., 2014; Goldshmit et al., 2012) |
| HLZ-56 | All LPARs | Unavailable | Preclinical | Kidney fibrosis | (Geng et al., 2012) |
| BrP-LPA | ATX, all LPARs |
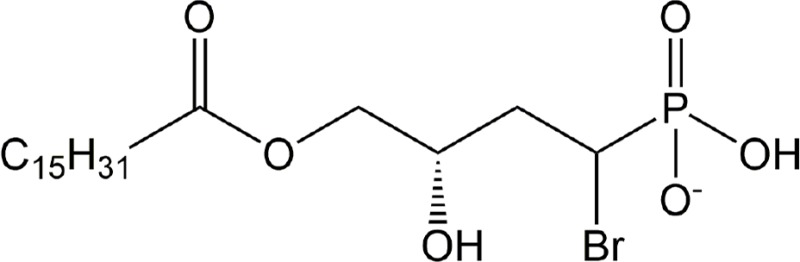
|
Preclinical | Cancer, rheumatoid arthritis | (Nikitopoulou et al., 2013; Schleicher et al., 2011; Xu and Prestwich, 2010; Zhang et al., 2009) |
| ONO-8430506 | ATX |
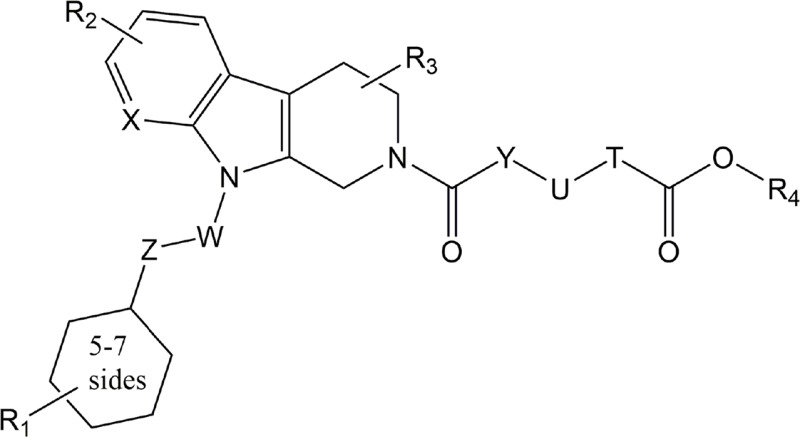 Backbone only, see patent WO/2012/005227 A1 Backbone only, see patent WO/2012/005227 A1 |
Preclinical | Cancer | (Benesch et al., 2014; Morimoto, 2012) |
| PF-8380 | ATX |
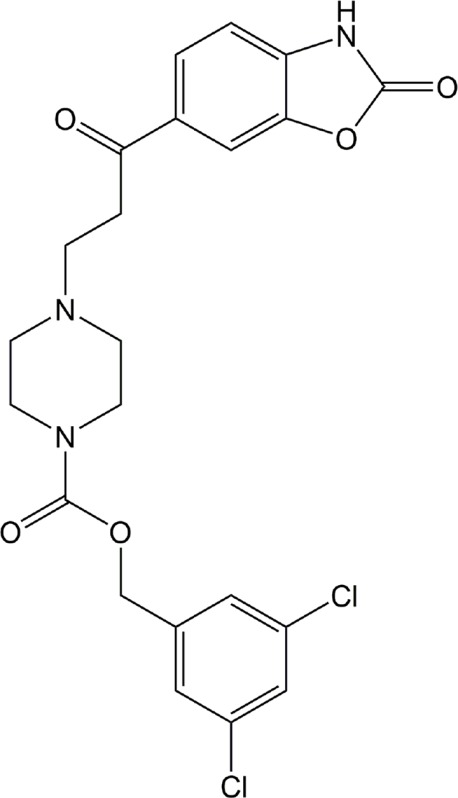
|
Preclinical | Cancer, inflammation | (Bhave et al., 2013; Gierse et al., 2010; St-Coeur et al., 2013) |
| 4PBPA | ATX |
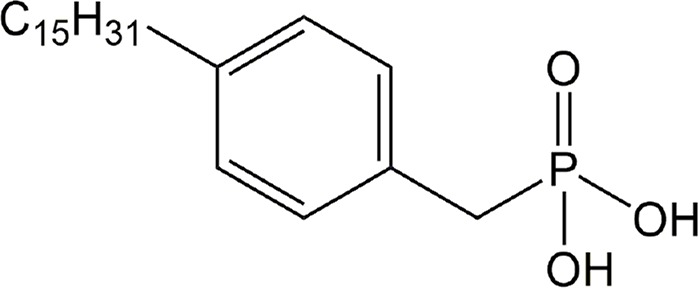
|
Preclinical | Cancer | (Gupte et al., 2011) |
| Gintonin | ATX | Glycolipoprotein, structure not available | Preclinical | Cancer | (Hwang et al., 2013) |
| GWJ-A-23 | ATX |
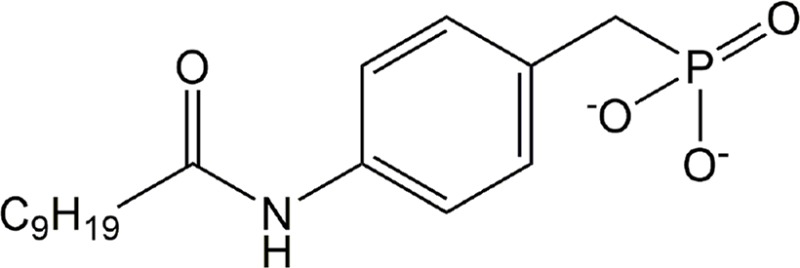
|
Preclinical | Asthma, idiopathic pulmonary fibrosis | (Oikonomou et al., 2012; Park et al., 2013) |
| S32826 | ATX |
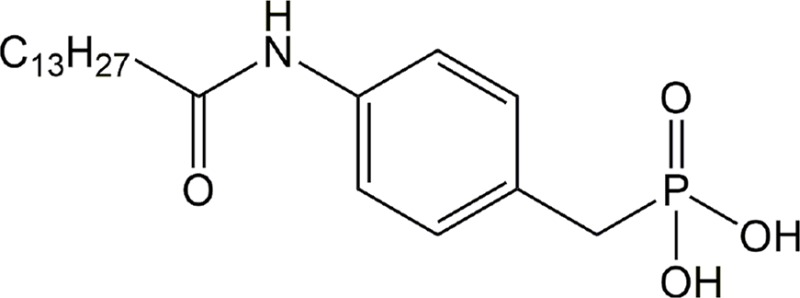
|
Preclinical | Glaucoma | (Iyer et al., 2012) |
LPA signaling has long been implicated in immune reactions (reviewed in (Lin and Boyce, 2006)). To this end, several therapeutic advances have been made concerning autoimmune disorders. In fact, an LPA1/3 inhibitor, SAR100842, has completed phase II clinical trials to protect against systemic sclerosis (Sanofi, 2014), an autoimmune disorder characterized by accumulated collagen in connective tissue, leading to scarring of the skin and vasculature (Lafyatis, 2014). LPA1 inhibitors are also of great interest in fibrosis, with BMS-986202 (previously AM152) having successfully completed phase I and BMS-986020 beginning phase II clinical trials for idiopathic pulmonary fibrosis (IPF) (2011, Amira Pharmaceuticals Announces Completion of Phase 1 Clnical Study for AM152, a Novel LPA1 Receptor Antagonist. In PR Newswire, PRNewswire.com. http://www.prnewswire.com/news-releases/amira-pharmaceuticals-announces-completion-of-phase-1-clinical-study-for-am152-a-novel-lpa1-receptor-antagonist-121087874.html, Access Date: 2014/09/15; BMS, 2011, 2014). The LPA1 inhibitor AM966 and the LPA1/3 antagonist VPC12249 have also shown efficacy in murine IPF studies (Okusa et al., 2003; Swaney et al., 2010). Concurrently, an LPA3 agonist, oleoyl-methoxy phosphothionate (OMPT), enhanced IPF injury and reduced the therapeutic effects of VPC12249, suggesting that LPA3 signaling may also be relevant in fibrotic disease. The pan-LPAR antagonist HLZ-56 and LPA1 inhibitor AM095 attenuated kidney and dermal fibrosis in mouse models by preventing Smad2 phosphorylation, which reduced TGFβ signaling and subsequent CTGF release (Castelino et al., 2011; Swaney et al., 2011; Geng et al., 2012), a mechanism that may be central to LPAR inhibitor effectiveness in other fibrotic disorders.
Much of the enthusiasm for LPAR therapies is directed at cancer, as LPAR signaling has been shown in numerous studies to promote motility and invasion of several cancer types, including breast, ovarian, colon, and brain tumors (Mills et al., 2002; Hama et al., 2004; Hoelzinger et al., 2008; Hayashi et al., 2012). In vitro studies utilizing the pan LPAR/ATX antagonist α-bromomethylene phosphonate LPA (BrP-LPA) and LPA1/3 antagonists Ki16425, Ki16198, and Debio 0719 have been shown to decrease tumor aggressiveness and increase radiosensitivity through varied mechanisms, including inhibited Rho/ROCK and MEK/ERK signaling, prevention of FAK/paxillin localization to focal adhesions, and reduced matrix metalloproteinase accumulation (Hama et al., 2004; Zhang et al., 2009; Komachi et al., 2012; Marshall et al., 2012; Schleicher et al., 2011; Liao et al., 2013; Su et al., 2013). While many studies focus on the migratory effects of LPA1 signaling, use of the LPA2 inhibitor “compound 35” attenuated Erk phosphorylation and reduced proliferation of colorectal cancer cells (Beck et al., 2008). LPA itself has been proposed as a screening molecule for ovarian cancer, as increased levels of LPA have been repeatedly observed in the blood of patients with malignant ovarian tumors and may have prognostic value in lung cancer patients as well (Sedlakova et al., 2011; Bai et al., 2014; NCI, 2014). Although no LPAR-targeting cancer drugs have reached clinical trial stages thus far, pharmaceutical inquiry is progressing rapidly and the initiation of cancerfocused clinical trials is projected to follow.
In addition to cancer and fibrosis, LPAR inhibitors have been utilized as potential therapeutics in other areas of study. For instance, Ki16425 and BrP-LPA have been shown to decrease the clinical score of murine arthritis (Nikitopoulou et al., 2013; Orosa et al., 2014). The development of an LPA-induced neonatal model of post-hemorrhagic hydrocephalus was also abrogated utilizing Ki16425 (Yung et al., 2011). While LPA signaling is reported to be involved in wound-healing processes (Lee et al., 2000), it may exacerbate severe trauma. In fact, anti-LPA antibodies that diminish LPAR binding and activation have shown some efficacy in modulating murine brain lesion severity and recovery (Goldshmit et al., 2012; Crack et al., 2014), although the actual mechanism of these immunological agents remains to be determined. Additionally, Bristol- Myers Squibb has patented LPAR inhibitors for spinal cord injury and neuropathic pain indications (Nogueira and Vales, 2013), since there is a substantial body of evidence implicating LPA1 and LPA5 signaling in the initiation and maintenance of neuropathic pain (reviewed in (Ueda et al., 2013)).
The most common output for screening drug efficacy against an LPAR is determining the status of Ca2+ influx within the tested cell types. Generally, LPAR agonists will increase intracellular Ca2+ mobilization while LPAR antagonists will inhibit Ca2+ release. Using this method, several studies have been published on the synthesis and relative efficacy of potential therapeutics against LPA1–3, LPA1–5, and more recently LPA1–6 (reviewed in (Im, 2010)). While this article only discusses pharmacological modulators with functional, disease-related readouts, a more comprehensive list of LPAR agonists and antagonists can be found in a previous review (Yung et al., 2014).
COMPOUNDS TARGETING ATX INHIBITION
In addition to direct pharmacological modulation of LPARs, several research groups have targeted the upstream enzyme ATX for discovery of potential therapeutics (Table 1). ATX inhibitors prevent the enzymatic conversion of LPC to LPA. As ATX expression can account for at least half of plasma LPA levels (Tanaka et al., 2006; van Meeteren et al., 2006), these drugs ultimately attenuate LPA signaling. Although this pathway lies upstream of LPAR signaling, targeting ATX allows for structure-based drug design (Fells et al., 2013; Kawaguchi et al., 2013; Norman et al., 2013), a process that is limited in LPAR drug discovery because of the lack of receptor crystal structures; work in progress should rectify this deficiency.
In particular, oncology researchers are interested in developing these agents. Several ATX inhibitors have been synthesized and tested in tumor migration, metastasis, survival, and radiosensitivity studies. These inhibitors include the small molecules ONO-8430506 (Benesch et al., 2014) and PF-8380 (Bhave et al., 2013; St-Coeur et al., 2013), lipid analogs 4PBPA (Gupte et al., 2011) and pan-ATX/LPAR antagonist BrP-LPA (Xu and Prestwich, 2010; Schleicher et al., 2011), and gintonin - a plant-derived LPA/ginseng glycolipoprotein complex that results in feedback inhibition of ATX through LPAR signaling (Hwang et al., 2013). These compounds ultimately reduced survival and invasive behaviors of in vitro cancer cells and tumor xenografts. As ATX and LPARs are often upregulated in cancer (reviewed in (Gotoh et al., 2012)), the success of these compounds in research may spur therapeutic development.
ATX antagonism is also being investigated as a solution to inflammatory disease. PF-8380 has been shown to drastically reduce plasma LPA concentrations during inflammation (Gierse et al., 2010), suggesting that targeting ATX may be useful to reduce chronic inflammation. As mentioned above, BrP-LPA has been utilized to ameliorate arthritis in mice (Nikitopoulou et al., 2013). Furthermore, GWJ-A-23 showed efficacy in attenuating allergen-induced asthmatic attacks and bleomycin-induced IPF (Oikonomou et al., 2012; Park et al., 2013). The effects of reduced LPA signaling stretch even further, as the potent ATX inhibitor S32826 has been utilized to decrease intraocular pressure in a rabbit model of glaucoma (Iyer et al., 2012).
CONCLUSION
Over the past four decades, interest in the signaling lipid LPA has grown from understanding its synthesis to encompassing several key processes in development and disease. To this end, several compounds have been fine-tuned by researchers and pharmaceutical companies to inhibit LPARs and ATX in order to mitigate the destructive pathologies related to cancer, autoimmune diseases, and other afflictions. The LPA1-targeting inhibitors SAR100842, BMS-986202, and BMS-986020 have passed phase I or phase II clinical trials with the potential of advancing toward FDA approval. The increasing availability of chemical tool compounds will enhance our understanding of LPAR signaling mechanisms in disease towards the development of new disease-modifying therapeutics.
Acknowledgments
This work was supported by NIH NS082092 and MH051699 (JC), and NIH T32 GM007752 (NS). We thank Ms. Danielle Jones, Dr. Hope Mirendil, and Dr. Yun Yung for assistance and manuscript edits.
Footnotes
CONFLICT OF INTEREST
Jerold Chun declares the following industry relationships which include consultancies and research fundung: Amira Pharmaceuticals, Celgene, Mitsubishi Tanabe, Novartis, and Ono Pharmaceuticals.
REFERENCES
- An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem. 1998;273:7906–7910. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- Azeem Z, Jelani M, Naz G, Tariq M, Wasif N, Kamran-Ul-Hassan Naqvi S, Ayub M, Yasinzai M, Amin-Ud-Din M, Wali A, Ali G, Chishti MS, Ahmad W. Novel mutations in G proteincoupled receptor gene (P2RY5) in families with autosomal recessive hypotrichosis (LAH3) Hum Genet. 2008;123:515–519. doi: 10.1007/s00439-008-0507-7. [DOI] [PubMed] [Google Scholar]
- Bachner D, Ahrens M, Betat N, Schroder D, Gross G. Developmental expression analysis of murine autotaxin (ATX) Mech Dev. 1999;84:121–125. doi: 10.1016/S0925-4773(99)00048-9. [DOI] [PubMed] [Google Scholar]
- Bai CQ, Yao YW, Liu CH, Zhang H, Xu XB, Zeng JL, Liang WJ, Yang W, Song Y. Diagnostic and prognostic significance of lysophosphatidic acid in malignant pleural effusions. J Thorac Dis. 2014;6:483–490. doi: 10.3978/j.issn.2072-1439.2014.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K, Tsujimoto M, Arai H, Inoue K. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem. 1999;274:27776–27785. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- Beck HP, Kohn T, Rubenstein S, Hedberg C, Schwandner R, Hasslinger K, Dai K, Li C, Liang L, Wesche H, Frank B, An S, Wickramasinghe D, Jaen J, Medina J, Hungate R, Shen W. Discovery of potent LPA2 (EDG4) antagonists as potential anticancer agents. Bioorg Med Chem Lett. 2008;18:1037–1041. doi: 10.1016/j.bmcl.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Benesch MG, Tang X, Maeda T, Ohhata A, Zhao YY, Kok BP, Dewald J, Hitt M, Curtis JM, McMullen TP, Brindley DN. Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. FASEB J. 2014;28:2655–2666. doi: 10.1096/fj.13-248641. [DOI] [PubMed] [Google Scholar]
- Bhave SR, Dadey DY, Karvas RM, Ferraro DJ, Kotipatruni RP, Jaboin JJ, Hallahan AN, Dewees TA, Linkous AG, Hallahan DE, Thotala D. Autotaxin inhibition with PF- 8380 enhances the radiosensitivity of human and murine glioblastoma cell lines. Front Oncol. 2013;3:236. doi: 10.3389/fonc.2013.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BMS Bristol-myers squibb to acquire amira pharmaceuticals. Bristol-Myers Squibb, Online. 2011. http://news.bms.com/press-release/partnering-news/bristol-myers-squibb-acquire-amira-pharmaceuticals, Access Date: 2014/09/15.
- BMS Safety and efficacy of a lysophosphatidic acid receptor antagonist in idiopathic pulmonary fibrosis. 2014. https://clinicaltrials.gov/ct2/show/NCT01766817, Access Date: 2014/09/15.
- Bradford WZ. Intermune, Inc; 2012. Pirfenidone and anti-fibrotic therapy in selected patients, International Patent: WO/2012/162592 A1. International http://www.google.im/patents/WO2012162592A1, Access Date: 2014/09/15. [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, Vollmer T, Agius MA, Kappos L, Stites T, Li B, Cappiello L, von Rosenstiel P, Lublin FD. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:545–556. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- Castelino FV, Seiders J, Bain G, Brooks SF, King CD, Swaney JS, Lorrain DS, Chun J, Luster AD, Tager AM. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 2011;63:1405–1415. doi: 10.1002/art.30262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33:91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2010;62:579–587. doi: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2) Mol Cell Biol. 2002;22:6921–6929. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack PJ, Zhang M, Morganti-Kossmann MC, Morris AJ, Wojciak JM, Fleming JK, Karve I, Wright D, Sashindranath M, Goldshmit Y, Conquest A, Daglas M, Johnston LA, Medcalf RL, Sabbadini RA, Pebay A. Anti-lysophosphatidic acid antibodies improve traumatic brain injury outcomes. J. Neuroinflammation. 2014;11:37. doi: 10.1186/1742-2094-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R, Zhao Y, Jacoby D, Spannhake EW, Ohba M, Garcia JG, Watkins T, He D, Saatian B, Natarajan V. Protein kinase Cdelta mediates lysophosphatidic acid-induced NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem. 2004;279:41085–41094. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291(Pt 3):677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fells JI, Lee SC, Fujiwara Y, Norman DD, Lim KG, Tsukahara R, Liu J, Patil R, Miller DD, Kirby RJ, Nelson S, Seibel W, Papoian R, Parrill AL, Baker DL, Bittman R, Tigyi G. Hits of a high-throughput screen identify the hydrophobic pocket of autotaxin/lysophospholipase D as an inhibitory surface. Mol Pharmacol. 2013;84:415–424. doi: 10.1124/mol.113.087080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade O, Simon MF, Viode C, Rugani N, Leballe F, Ragab A, Fournie B, Sarda L, Chap H. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 1995;80:919–927. doi: 10.1016/0092-8674(95)90295-3. [DOI] [PubMed] [Google Scholar]
- Fukushima N, Ishii I, Contos JJ, Weiner JA, Chun J. Lysophospholipid receptors. Annu Rev Pharmacol Toxicol. 2001;41:507–534. doi: 10.1146/annurev.pharmtox.41.1.507. [DOI] [PubMed] [Google Scholar]
- Geng H, Lan R, Singha PK, Gilchrist A, Weinreb PH, Violette SM, Weinberg JM, Saikumar P, Venkatachalam MA. Lysophosphatidic acid increases proximal tubule cell secretion of profibrotic cytokines PDGF-B and CTGF through LPA2- and Galphaq-mediated Rho and alphavbeta6 integrin-dependent activation of TGF-beta. Am J Pathol. 2012;181:1236–1249. doi: 10.1016/j.ajpath.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard JM, Kindom SE, Peterson DA, Peller J, Krantz KE, White JG. Lysophosphatidic acids. Influence on platelet aggregation and intracellular calcium flux. Am J Pathol. 1979;96:423–438. [PMC free article] [PubMed] [Google Scholar]
- Gierse J, Thorarensen A, Beltey K, Bradshaw-Pierce E, Cortes-Burgos L, Hall T, Johnston A, Murphy M, Nemirovskiy O, Ogawa S, Pegg L, Pelc M, Prinsen M, Schnute M, Wendling J, Wene S, Weinberg R, Wittwer A, Zweifel B, Masferrer J. A novel autotaxin inhibitor reduces lysophosphatidic acid levels in plasma and the site of inflammation. J Pharmacol Exp Ther. 2010;334:310–317. doi: 10.1124/jpet.110.165845. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Matteo R, Sztal T, Ellett F, Frisca F, Moreno K, Crombie D, Lieschke GJ, Currie PD, Sabbadini RA, Pebay A. Blockage of lysophosphatidic acid signaling improves spinal cord injury outcomes. Am J Pathol. 2012;181:978–992. doi: 10.1016/j.ajpath.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh M, Fujiwara Y, Yue J, Liu J, Lee S, Fells J, Uchiyama A, Murakami-Murofushi K, Kennel S, Wall J, Patil R, Gupte R, Balazs L, Miller DD, Tigyi GJ. Controlling cancer through the autotaxin-lysophosphatidic acid receptor axis. Biochem Soc Trans. 2012;40:31–36. doi: 10.1042/BST20110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte R, Patil R, Liu J, Wang Y, Lee SC, Fujiwara Y, Fells J, Bolen AL, Emmons-Thompson K, Yates CR, Siddam A, Panupinthu N, Pham TC, Baker DL, Parrill AL, Mills GB, Tigyi G, Miller DD. Benzyl and naphthalene methylphosphonic acid inhibitors of autotaxin with anti-invasive and anti-metastatic activity. ChemMedChem. 2011;6:922–935. doi: 10.1002/cmdc.201000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama K, Aoki J, Fukaya M, Kishi Y, Sakai T, Suzuki R, Ohta H, Yamori T, Watanabe M, Chun J, Arai H. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem. 2004;279:17634–17639. doi: 10.1074/jbc.M313927200. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Okabe K, Kato K, Okumura M, Fukui R, Fukushima N, Tsujiuchi T. Differential function of lysophosphatidic acid receptors in cell proliferation and migration of neuroblastoma cells. Cancer Lett. 2012;316:91–96. doi: 10.1016/j.canlet.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzinger DB, Nakada M, Demuth T, Rosensteel T, Reavie LB, Berens ME. Autotaxin: a secreted autocrine/paracrine factor that promotes glioma invasion. J Neurooncol. 2008;86:297–309. doi: 10.1007/s11060-007-9480-6. [DOI] [PubMed] [Google Scholar]
- Hwang SH, Lee BH, Kim HJ, Cho HJ, Shin HC, Im KS, Choi SH, Shin TJ, Lee SM, Nam SW, Kim HC, Rhim H, Nah SY. Suppression of metastasis of intravenouslyinoculated B16/F10 melanoma cells by the novel ginseng-derived ingredient, gintonin: involvement of autotaxin inhibition. Int J Oncol. 2013;42:317–326. doi: 10.3892/ijo.2012.1709. [DOI] [PubMed] [Google Scholar]
- Im DS. Pharmacological tools for lysophospholipid GPCRs: development of agonists and antagonists for LPA and S1P receptors. Acta Pharmacol Sin. 2010;31:1213–1222. doi: 10.1038/aps.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- Iyer P, Lalane R, 3rd, Morris C, Challa P, Vann R, Rao PV. Autotaxin-lysophosphatidic acid axis is a novel molecular target for lowering intraocular pressure. PloS ONE. 2012;7:e42627. doi: 10.1371/journal.pone.0042627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong KJ, Park SY, Cho KH, Sohn JS, Lee J, Kim YK, Kang J, Park CG, Han JW, Lee HY. The Rho/ROCK pathway for lysophosphatidic acid-induced proteolytic enzyme expression and ovarian cancer cell invasion. Oncogene. 2012;31:4279–4289. doi: 10.1038/onc.2011.595. [DOI] [PubMed] [Google Scholar]
- Jimenez C, Portela RA, Mellado M, Rodriguez-Frade JM, Collard J, Serrano A, Martinez AC, Avila J, Carrera AC. Role of the PI3K regulatory subunit in the control of actin organization and cell migration. J Cell Biol. 2000;151:249–262. doi: 10.1083/jcb.151.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma M, Matas-Rico E, Rzadkowski A, Jalink K, Moolenaar WH. LPA is a chemorepellent for B16 melanoma cells: action through the cAMP-elevating LPA5 receptor. PloS ONE. 2011;6:e29260. doi: 10.1371/journal.pone.0029260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol. 2008;9:415–423. doi: 10.1038/ni1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YC, Kim KM, Lee KS, Namkoong S, Lee SJ, Han JA, Jeoung D, Ha KS, Kwon YG, Kim YM. Serum bioactive lysophospholipids prevent TRAIL-induced apoptosis via PI3K/Akt-dependent cFLIP expression and Bad phosphorylation. Cell Death Differ. 2004;11:1287–1298. doi: 10.1038/sj.cdd.4401489. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Okabe T, Okudaira S, Nishimasu H, Ishitani R, Kojima H, Nureki O, Aoki J, Nagano T. Screening and X-ray crystal structure-based optimization of autotaxin (ENPP2) inhibitors, using a newly developed fluorescence probe. ACS Chem Biol. 2013;8:1713–1721. doi: 10.1021/cb400150c. [DOI] [PubMed] [Google Scholar]
- Kim JH, Adelstein RS. LPA(1) -induced migration requires nonmuscle myosin II light chain phosphorylation in breast cancer cells. J Cell Physiol. 2011;226:2881–2893. doi: 10.1002/jcp.22631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komachi M, Sato K, Tobo M, Mogi C, Yamada T, Ohta H, Tomura H, Kimura T, Im DS, Yanagida K, Ishii S, Takeyoshi I, Okajima F. Orally active lysophosphatidic acid receptor antagonist attenuates pancreatic cancer invasion and metastasis in vivo. Cancer Sci. 2012;103:1099–1104. doi: 10.1111/j.1349-7006.2012.02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- Kranenburg O, Moolenaar WH. Ras-MAP kinase signaling by lysophosphatidic acid and other G protein-coupled receptor agonists. Oncogene. 2001;20:1540–1546. doi: 10.1038/sj.onc.1204187. [DOI] [PubMed] [Google Scholar]
- Lafyatis R. Transforming growth factor beta-at the centre of systemic sclerosis. Nat Rev Rheumatol. 2014;10:706–719. doi: 10.1038/nrrheum.2014.137. [DOI] [PubMed] [Google Scholar]
- Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- Lee H, Goetzl EJ, An S. Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. American journal of physiology. Am J Physiol Cell Physiol. 2000;278:C612–618. doi: 10.1152/ajpcell.2000.278.3.C612. [DOI] [PubMed] [Google Scholar]
- Lee Z, Cheng CT, Zhang H, Subler MA, Wu J, Mukherjee A, Windle JJ, Chen CK, Fang X. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol. Biol. Cell. 2008;19:5435–5445. doi: 10.1091/mbc.E08-03-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Mu G, Zhang L, Zhou W, Zhang J, Yu H. Lysophosphatidic acid stimulates activation of focal adhesion kinase and paxillin and promotes cell motility, via LPA1-3, in human pancreatic cancer. Dig Dis Sci. 2013;58:3524–3533. doi: 10.1007/s10620-013-2878-4. [DOI] [PubMed] [Google Scholar]
- Lin DA, Boyce JA. Lysophospholipids as mediators of immunity. Ad Immunol. 2006;89:141–167. doi: 10.1016/S0065-2776(05)89004-2. [DOI] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- Lundequist A, Boyce JA. LPA5 is abundantly expressed by human mast cells and important for lysophosphatidic acid induced MIP-1beta release. PloS ONE. 2011;6:e18192. doi: 10.1371/journal.pone.0018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JC, Collins JW, Nakayama J, Horak CE, Liewehr DJ, Steinberg SM, Albaugh M, Vidal-Vanaclocha F, Palmieri D, Barbier M, Murone M, Steeg PS. Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J Natl Cancer Inst. 2012;104:1306–1319. doi: 10.1093/jnci/djs319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills GB, Eder A, Fang X, Hasegawa Y, Mao M, Lu Y, Tanyi J, Tabassam FH, Wiener J, Lapushin R, Yu S, Parrott JA, Compton T, Tribley W, Fishman D, Stack MS, Gaudette D, Jaffe R, Furui T, Aoki J, Erickson JR. Critical role of lysophospholipids in the pathophysiology, diagnosis, and management of ovarian cancer. Cancer Treat Res. 2002;107:259–283. doi: 10.1007/978-1-4757-3587-1_12. [DOI] [PubMed] [Google Scholar]
- Mirendil H, Lin ME, Chun J. Lysophospholipid receptors: signaling and biochemistry. In: Chun J, Hla T, Spiegel S, Moolenaar WH, editors. In lysophospholipid receptors: signaling and biochemistry. John Wiley & Sons, Inc; Hoboken, NJ: 2013. [Google Scholar]
- Moolenaar WH, van Corven EJ. Growth factor-like action of lysophosphatidic acid: mitogenic signalling mediated by G proteins. Ciba Found Symp. 1990;150:99–106. doi: 10.1002/9780470513927.ch7. [DOI] [PubMed] [Google Scholar]
- Morimoto T. Ono Pharmaceutical Co., Ltd; 2012. Tetrahydrocarboline derivative, International Patent: WO/2012/005227 A1. International. http://www.google.com/patents/WO2012005227A1, Access Date: 2014/09/15. [Google Scholar]
- NCI A pilot study of a protein profile test in ovarian cancer patients in remission to see if protein changes can predict relapse. 2014. https://clinicaltrials.gov/ct2/show/NCT00001938, Access Date: 2014/09/15.
- Nikitopoulou I, Kaffe E, Sevastou I, Sirioti I, Samiotaki M, Madan D, Prestwich GD, Aidinis V. A metabolicallystabilized phosphonate analog of lysophosphatidic acid attenuates collagen-induced arthritis. PloS ONE. 2013;8:e70941. doi: 10.1371/journal.pone.0070941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- Nogueira ES, Vales RL. Bristol-Myers Squibb; 2013. Methods for treating spinal cord injury with lpa receptor antagonists, International Patent: WO/2013/070879 A1. International. http://www.google.com/patents/WO2013070879A, Access Date: 2014/09/15. [Google Scholar]
- Norman DD, Ibezim A, Scott WE, White S, Parrill AL, Baker DL. Autotaxin inhibition: development and application of computational tools to identify site-selective lead compounds. Bioorg Med Chem. 2013;21:5548–5560. doi: 10.1016/j.bmc.2013.05.061. [DOI] [PubMed] [Google Scholar]
- Oikonomou N, Mouratis MA, Tzouvelekis A, Kaffe E, Valavanis C, Vilaras G, Karameris A, Prestwich GD, Bouros D, Aidinis V. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;47:566–574. doi: 10.1165/rcmb.2012-0004OC. [DOI] [PubMed] [Google Scholar]
- Okusa MD, Ye H, Huang L, Sigismund L, Macdonald T, Lynch KR. Selective blockade of lysophosphatidic acid LPA3 receptors reduces murine renal ischemia-reperfusion injury. American journal of physiology. Am J Physol Renal Physiol. 2003;285:F565–574. doi: 10.1152/ajprenal.00023.2003. [DOI] [PubMed] [Google Scholar]
- Orosa B, Garcia S, Martinez P, Gonzalez A, Gomez-Reino JJ, Conde C. Lysophosphatidic acid receptor inhibition as a new multipronged treatment for rheumatoid arthritis. Am Rheum Dis. 2014;73:298–305. doi: 10.1136/annrheumdis-2012-202832. [DOI] [PubMed] [Google Scholar]
- Park GY, Lee YG, Berdyshev E, Nyenhuis S, Du J, Fu P, Gorshkova IA, Li Y, Chung S, Karpurapu M, Deng J, Ranjan R, Xiao L, Jaffe HA, Corbridge SJ, Kelly EA, Jarjour NN, Chun J, Prestwich GD, Kaffe E, Ninou I, Aidinis V, Morris AJ, Smyth SS, Ackerman SJ, Natarajan V, Christman JW. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am J Respir Crit Care Med. 2013;188:928–940. doi: 10.1164/rccm.201306-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack SM, von Kugelgen I, Al Aboud K, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A, Nurnberg P, Nothen MM, Betz RC. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008;40:329–334. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- Petukhova L, Sousa EC, Jr, Martinez-Mir A, Vitebsky A, Dos Santos LG, Shapiro L, Haynes C, Gordon D, Shimomura Y, Christiano AM. Genome-wide linkage analysis of an autosomal recessive hypotrichosis identifies a novel P2RY5 mutation. Genomics. 2008;92:273–278. doi: 10.1016/j.ygeno.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruisanchez E, Dancs P, Kerek M, Nemeth T, Farago B, Balogh A, Patil R, Jennings BL, Liliom K, Malik KU, Smrcka AV, Tigyi G, Benyo Z. Lysophosphatidic acid induces vasodilation mediated by LPA1 receptors, phospholipase C, and endothelial nitric oxide synthase. FASEB J. 2014;28:880–890. doi: 10.1096/fj.13-234997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando JJ, Chertihin OI. Activation of protein kinase C by lysophosphatidic acid: dependence on composition of phospholipid vesicles. Biochem J. 1996;317(Pt 2):583–588. doi: 10.1042/bj3170583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, Igarashi Y, Tigyi G. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- Sanofi Proof of Biological Activity of SAR100842 in Systemic Sclerosis. 2014. https://clinicaltrials.gov/ct2/show/NCT01651143, Access Date: 2014/09/15.
- Savaskan NE, Rocha L, Kotter MR, Baer A, Lubec G, van Meeteren LA, Kishi Y, Aoki J, Moolenaar WH, Nitsch R, Brauer AU. Autotaxin (NPP-2) in the brain: cell typespecific expression and regulation during development and after neurotrauma. Cell Mol Life Sci. 2007;64:230–243. doi: 10.1007/s00018-006-6412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher SM, Thotala DK, Linkous AG, Hu R, Leahy KM, Yazlovitskaya EM, Hallahan DE. Autotaxin and LPA receptors represent potential molecular targets for the radiosensitization of murine glioma through effects on tumor vasculature. PloS ONE. 2011;6:e22182. doi: 10.1371/journal.pone.0022182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlakova I, Vavrova J, Tosner J, Hanousek L. Lysophosphatidic acid (LPA)-a perspective marker in ovarian cancer. Tumor Biol. 2011;32:311–316. doi: 10.1007/s13277-010-0123-8. [DOI] [PubMed] [Google Scholar]
- Seewald S, Schmitz U, Seul C, Ko Y, Sachinidis A, Vetter H. Lysophosphatidic acid stimulates protein kinase C isoforms alpha, beta, epsilon, and zeta in a pertussis toxin sensitive pathway in vascular smooth muscle cells. Am J Hypertens. 1999;12:532–537. doi: 10.1016/S0895-7061(98)00269-6. [DOI] [PubMed] [Google Scholar]
- Sonoda H, Aoki J, Hiramatsu T, Ishida M, Bandoh K, Nagai Y, Taguchi R, Inoue K, Arai H. A novel phosphatidic acid- selective phospholipase A1 that produces lysophosphatidic acid. J Biol Chem. 2002;277:34254–34263. doi: 10.1074/jbc.M201659200. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/S0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- St-Coeur PD, Ferguson D, Morin P, Jr, Touaibia M. PF-8380 and closely related analogs: synthesis and structure-activity relationship towards autotaxin inhibition and glioma cell viability. Arch Pharm. 2013;346:91–97. doi: 10.1002/ardp.201200395. [DOI] [PubMed] [Google Scholar]
- Su SC, Hu X, Kenney PA, Merrill MM, Babaian KN, Zhang XY, Maity T, Yang SF, Lin X, Wood CG. Autotaxin- lysophosphatidic acid signaling axis mediates tumorigenesis and development of acquired resistance to sunitinib in renal cell carcinoma. Clin Cancer Res. 2013;19:6461–6472. doi: 10.1158/1078-0432.CCR-13-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney JS, Chapman C, Correa LD, Stebbins KJ, Broadhead AR, Bain G, Santini AM, Darlington J, King CD, Baccei CS, Lee C, Parr TA, Roppe JR, Seiders TJ, Ziff J, Prasit P, Hutchinson JH, Evans JF, Lorrain DS. Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist. J Pharmacol Exp Ther. 2011;336:693–700. doi: 10.1124/jpet.110.175901. [DOI] [PubMed] [Google Scholar]
- Swaney JS, Chapman C, Correa LD, Stebbins KJ, Bundey RA, Prodanovich PC, Fagan P, Baccei CS, Santini AM, Hutchinson JH, Seiders TJ, Parr TA, Prasit P, Evans JF, Lorrain DS. A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol. 2010;160:1699–1713. doi: 10.1111/j.1476-5381.2010.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–25830. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Fukuzawa K, Akamatsu Y, Yamada S, Suzuki T, Tsukatani H. Identification of vasopressor phospholipid in crude soybean lecithin. Lipids. 1978;13:468–472. doi: 10.1007/BF02533615. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- Ueda H, Matsunaga H, Olaposi OI, Nagai J. Lysophosphatidic acid: chemical signature of neuropathic pain. Biochim. Biophys. Acta. 2013;1831:61–73. doi: 10.1016/j.bbalip.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet P, Pages C, Jeanneton O, Daviaud D, Barbe P, Record M, Saulnier-Blache JS, Lafontan M. Alpha2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. A paracrine signal for preadipocyte growth. J Clin Invest. 1998;101:1431–1438. doi: 10.1172/JCI806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Prestwich GD. Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in an engineered three-dimensional lung cancer xenograft model. Cancer. 2010;116:1739–1750. doi: 10.1002/cncr.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y, Taguchi R, Shimizu T, Ishii S. Identification and Characterization of a Novel Lysophosphatidic Acid Receptor, p2y5/LPA6. J Biol Chem. 2009;284:17731–17741. doi: 10.1074/jbc.M808506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Ishii I, Kingsbury MA, Chun J. Lysophosphatidic acid as a novel cell survival/apoptotic factor. Biochim. Biophys. Acta. 2002;1585:108–113. doi: 10.1016/S1388-1981(02)00330-X. [DOI] [PubMed] [Google Scholar]
- Yung YC, Mutoh T, Lin ME, Noguchi K, Rivera RR, Choi JW, Kingsbury MA, Chun J. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med. 2011;3:99ra87. doi: 10.1126/scitranslmed.3002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res. 2014;55:1192–1214. doi: 10.1194/jlr.R046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xu X, Gajewiak J, Tsukahara R, Fujiwara Y, Liu J, Fells JI, Perygin D, Parrill AL, Tigyi G, Prestwich GD. Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res. 2009;69:5441–5449. doi: 10.1158/0008-5472.CAN-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]