Abstract
Skin is an emerging target tissue in pharmaceutical and cosmetic science. Safety assessment for dermal toxicity is a critical step for development of topically applicable pharmaceutical agents and ingredients in cosmetics. Urgent needs exist to set up toxicity testing methods for dermal safety, and identification of novel biomarkers for pathological cutaneous alteration is highly required. Here we will discuss if vascular endothelial growth factor (VEGF) has a potential as a biomarker for dermal impairment. Experimental and clinical evidences for induction of keratinocytic VEGF under pathological conditions will be reviewed.
Keywords: Vascular endothelial growth factor (VEGF), Biomarker, Keratinocytic damage, Dermal toxicity
INTRODUCTION
The interest on skin has been increased both in pharmaceutical and cosmetic industries. Epidermal tissue composes of a physical barrier structure, maintaining homeostasis by preserving water and protecting inner organs against various external stresses including chemicals, microbial products, and UV irradiation. Keratinocytes are the main components of epidermis, and play active roles in skin function. In many skin problems such as allergic contact dermatitis, atopic dermatitis, psoriasis and photo-toxicity, serious damage and functional impairment such as epidermal barrier dysfunction, impaired differentiation/proliferation and dysregulated intercellular communication in keratinocytes are observed (Kubo et al., 2012; Hänel et al., 2013). There have been intensive efforts to elucidate the pathogenic alteration of keratinocytes and identify new biomarkers for keratinocytic damage during skin diseases (Enerbäck, 2011; Bernard et al., 2012). Here we will discuss the potential of vascular endothelial growth factor (VEGF) as a novel biomarker for keratinocyte damage, by examining experimental and clinical evidences for the role of VEGF in skin disorders.
BASIC CHARACTERISTICS OF VEGF AND VEGFR
VEGF, a dimeric heparin-binding glycoprotein of approximately 40 kDa in its active form, is known to be a main regulator of physiological and/or pathological angiogenesis. Since it was first described as vascular permeability factor (VPF) (Senger et al., 1983; Keck et al., 1989), several VEGF sub-family members and isoforms have been reported. Although the existence of VEGF-E and -F is newly suggested (Suto et al., 2005; Takahashi and Shibuya, 2005), it is generally accepted that VEGF sub-family consists of five members, VEGF-A, B, C, D and placenta growth factor (PLGF) in mammals (Olsson et al., 2006). Due to the alternative splicing of the original mRNA transcript, VEGF is occurring in isoforms with different biological activities. In case of VEGF-A, at least four isoforms of VEGF-A121, 165, 189 and 206 exist (Tischer et al., 1991; Gille et al., 2000). The bioactivity of VEGF sub-family members is also affected by proteolytic processing which enables specific interactions with different receptor types (Lee et al., 2005).
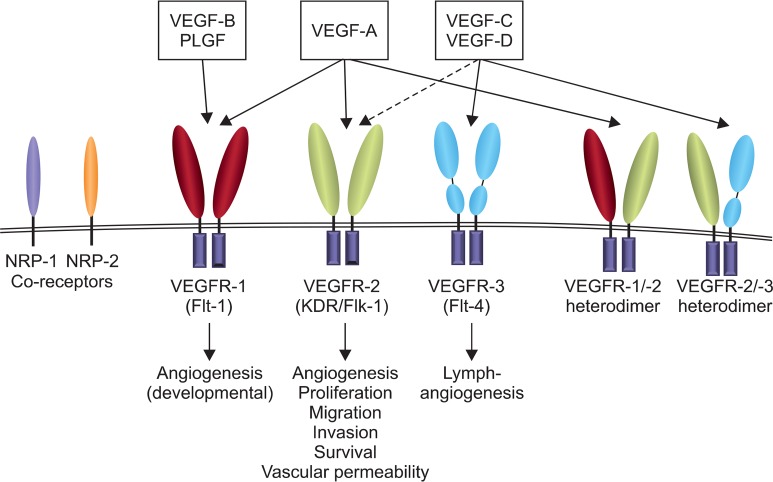
VEGF selectively binds to high affinity tyrosine kinase receptors (RTKs) that are predominantly expressed on endothelial cells (ECs) (de Vries et al., 1992; Olsson et al., 2006), resulting in receptor activation and intracellular signal transduction. There are three types of VEGF receptors, which are VEGFR-1 (fms-like tyrosine kinase-1 or Flt-1), VEGFR-2 (kinase insert-domain containing receptor (KDR) or fetal liver kinase (Flk-1)), and VEGFR-3 (Flt-4) (Skobe et al., 1999; Carmeliet, 2000). VEGF receptors usually form homodimers, but heterodimeric complexs of VEGF receptor are also expressed. With different affinities and selectivities, each of VEGF sub-family members binds to distinct VEGFRs (Ruiz de Almodovar et al., 2009). Specific interaction between VEGF sub-family members and VEGFR is shown in Fig. 1. The neuropilin receptors (NRP-1 and 2) are known to enhance VEGF signaling by modulating VEGF-VEGFR interaction as co-receptors (Geretti et al., 2008).
Fig. 1.
Interactions between VEGF and VEGF receptors, and their biological functions. Flt, fms-like tyrosine kinase; Flk, fetal liver kinase; NRP, neuropilin; KDR, kinase insert-domain containing receptor; PLGF, placenta growth factor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
The overall regulatory mechanism of VEGF receptor signaling is similar to typical RTK signaling of other growth factors, such as cellular signaling in cell migration, proliferation and survival (Leung et al., 1989; Ruiz de Almodovar et al., 2009). Besides these typical roles, the unique bioactivity of VEGF receptor is to transduce signals for angiogenesis and lymphangiogenesis, and to regulate permeability (Ferrara et al., 2003; Olsson et al., 2006). VEGFR-1 mainly binds to VEGF-A and B, and plays key roles in developmental/embryonic angiogenesis. The majority of angiogenic activities of VEGF, such as EC proliferation, migration, and microvascular permeability, are mediated by VEGFR-2 following binding with VEGF-A. Meantime, VEGFR-3 is predominantly found in lymphatic ECs, and promotes lymphangiogenesis by binding with VEGF-C and D (Jeltsch et al., 1997; Hicklin and Ellis, 2005; Olsson et al., 2006; Zgraggen et al., 2013).
VEGF SYNTHESIS IN KERATINOCYTES
Following the initial in vivo observation of expression of VEGF mRNA in the newly generated epithelium (Brown et al., 1992), the source of cutaneous VEGF was extensively studied. It is possible that VEGF during wound repair can be induced by macrophages or fibroblast (Nissen et al., 1998, Trompezinski et al., 2004), but keratino-cytes are found to be one of the main sources of cutaneous VEGF. In cultured human keratinocytes, significant up-regulation of VEGF was observed after stimulation with serum, epidermal growth factor (EGF), transforming growth factor-β1 (TGF-β1), tumor necrosis factor-α (TNF-α), insulin-like growth factor-2, or keratinocyte growth factor (Frank et al., 1995; Kim and Kim, 2005). Three major spliced forms of VEGF can be synthesized in human keratinocytes that are the secreted 121 and 165 isoforms and cell-associated 189 isoform (Ballaun et al., 1995). Acting as a key mediator for cutaneous angiogenesis and vascular permeability, keratinocytic VEGF is a potent and selective mitogen for dermal microvascular ECs during physiological processes such as wound repair (Wilgus et al., 2005) and hair growth (Yano et al., 2001), and pathological conditions including cutaneous inflammation, skin cancer (Brown et al., 1992) and psoriasis (Detmar et al., 1994).
SIGNALING PATHWAYS IN VEGF INDUCTION IN KERATINOCYTES
Generally, the expression of VEGF is tightly regulated at the transcriptional level and also at the post-transcriptional level (Ruiz de Almodovar et al., 2009). Various growth factors and cytokines are known to induce VEGF expression in keratinocytes, including interleukins (ILs), TGF-α, TGF-β, TNF-α, EGF, and platelet-derived growth factor (PDGF) (Frank et al., 1995; Cohen et al., 1996; Gille et al., 1997; Kozlowska et al., 1998; Ma et al., 2014). Contribution of signaling kinases including phosphatidylinositol 3-kinase (PI3K) or mitogen-activated protein kinase (MAPK; p42/p44 MAPK) to VEGF synthesis in keratinocytes are well-established (Nakai et al., 2009; Yu et al., 2010). Transcription factors of activation protein (AP)-1, AP-2, hypoxia-induced factors (HIFs), specificity protein (SP)-1 and nuclear factor κB (NFκB) are found to mediate transcriptional regulation of VEGF in keratinocytes (Forsythe et al., 1996; Finkenzeller et al., 1997; Gille et al., 2000, Brenneisen et al., 2003; Scortegagna et al., 2008). Several pathological conditions such as hypoxia or UV irradiation induce up-regulation of keratinocytic VEGF (Detmar et al., 1997; Gille et al., 2000; Brenneisen et al., 2003; Weir et al., 2011), either by direct activation of transcriptional pathways or by secretion of cytokines.
UP-REGULATION OF VEGF IN SKIN DAMAGE
Although it is clear that VEGF can be expressed in keratinocytes, it is still controversial whether cutaneous VEGF is restorative or aggravative for skin damage. In normal epidermis, blood vessels are generally quiescent and angiogenesis is hardly occurring. Consistently, the level of VEGF in normal epidermis is found to be low (Weninger et al., 1996). However, in many skin conditions such as psoriasis, contact dermatitis, wound healing and cutaneous neoplasia that are closely associated with angiogenesis or chronic inflammation, there is prominent induction of VEGF in epidermal keratinocyte (Detmar, 1996; 2000) suggesting that increased VEGF plays key roles in these skin problems. The evidences and the suggested roles of up-regulated VEGF in abnormal skin condition are summarized in Table 1.
Table 1.
Keratinocyte derived VEGF under pathological conditions in skin
| Pathological condition | Evidences for VEGF involvement | Suggested roles of VEGF | Refs |
|---|---|---|---|
| Cutaneous inflammation | Increased expression of VEGF/VEGFR in skin lesion of inflammation VEGF induction by inflammatory cytokines such as ILs and TNF-α VEGF-induced immune cell accumulation in the skin |
Hyper-permeability Angiogenesis Lymphangiogenesis |
Detmar et al., 1994; Elias et al., 2008; Huggenberger and Detmar, 2011; Suzuki et al., 2014; Zhang et al., 2006 |
| Psoriasis | Increased expression of VEGF/VEGFR in skin lesion of psoriasis Increased level of systemic serum VEGF in psoriasis Aggravated psoriasis in VEGF transgenic animal Reduced psoriatic symptoms after systemic antagonism against VEGF-VEGFR Susceptibility change in psoriasis by VEGF genetic polymorphism |
Epidermal hyperplasia Hyper-permeability Inflammation Enragement of lymphatic vessels |
Bhushan et al., 1999; Canavese et al., 2010; Detmar et al., 1994, 1998; Elias et al., 2008; Nielsen et al., 2002; Rogers and D’Amato, 2006; Schonthaler et al., 2009; Weidemann et al., 2013; Xia et al., 2003; Young et al., 2006 |
| Phototoxicity | UV-induced VEGF induction in skins and keratinocytes Increased level of VEGFR by UV irradiation Increased susceptibility to photo-damage by VEGF |
Hyper-permeability Edema Erythema Epidermal hyperplasia Inflammation |
Blaudschun et al., 2000; Brauchle et al., 1996; Brenneisen et al., 2003; Gille et al., 2000; Hirakawa et al., 2005; Longuet-Perret et al., 1998; Yano et al., 2005 |
| Skin cancer | VEGF up-regulation in lesions of skin cancers Reduced invasion by blocking of VEGF-VEGFR Increased invasion by over-expressed VEGF |
Angiogenesis Lymphangiogenesis Inflammation Invasion |
Alitalo et al., 2013; Detmar et al., 1995; Gille et al., 2000; Hicklin and Ellis, 2005; Mantovani et al., 2008 |
Cutaneous inflammation
Inflammation is basically a self-defensive innate immune response against harmful stimuli including infectious agents, physical or chemical challenges. For infiltration of inflammatory cells to inflamed tissue, change in vascular permeability is inevitable. Besides the hyper-permeability, the close association between angiogenesis and inflammation is reported in various skin diseases that require vascular remodeling (Detmar et al., 1994; Karkkainen and Petrova, 2000). Angiogenesis and lymphangiogenesis occur in chronic cutaneous inflammations in atopic dermatitis and psoriasis (Detmar et al., 1994; Zhang et al., 2006; Elias et al., 2008; Huggenberger and Detmar, 2011). Hyper-permeability and angiogenesis/lymphangiogenesis are mainly mediated by VEGF, therefore it is convincing that VEGF is up-regulated in lesions with cutaneous inflammation (Detmar et al., 1994; Elias et al., 2008; Zhang et al., 2006). It is also interesting that pro-inflammatory cytokines up-regulate VEGF in keratinocytes. Temporal expression profile of cytokines in epidermal keratinocytes is important in the orchestration of inflammatory responses (Kataru et al., 2009). IL-1, 6, 8 and TNF-α are known to be potent inducers for keratinocyte-derived VEGF-A (Detmar et al., 1995). VEGF-C induction associated with up-regulated VEGFR-3 and dermal lymphangiogenesis were observed in the lesion of atopic dermatitis in IL-4 transgenic mouse (Shi et al., 2012), supporting the role of VEGF in IL-mediated lymphangiogenesis and inflammation. The role of VEGF as a chemotactic factor in skin inflammation was also reported (Suzuki et al., 2014)
Psoriasis
The link between pathological skin condition and VEGF is well-established in psoriasis by clinical and experimental data (Elias et al., 2008; Schonthaler et al., 2009), and it is even suggested that systemic VEGF antagonist can be a therapeutic option for psoriasis treatment (Canavese et al., 2010; Weidemann et al., 2013). The systemic serum VEGF level (Bhushan et al., 1999; Nielsen et al., 2002), as well as the local level of VEGF in hyperplastic epidermis is significantly increased in psoriasis, along with up-regulation of VEGFRs in ECs (Detmar et al., 1994; Bhushan et al., 1999). The observation that both systemic and local cutaneous levels of VEGF are increased under psoriasis further warrants the need to investigate the relationship between systemic and local VEGF in skin disorders. The clinical characteristics of psoriasis, which are epidermal hyperplasia, inflammation, hyper-permeable blood vessels, and enlargement of lymphatic vessels (Christensen et al., 2006), are closely associated with bioactivity of VEGF. The contribution of VEGF to psoriatic pathogenesis has been confirmed in experimental models, where typical features of human psoriasis were observed in transgenic mice with increased VEGF levels (Detmar et al., 1998; Xia et al., 2003). Keratinocytic VEGF was induced by vasoactive intestinal peptide (VIP), which is specifically found in psoriatic epidermis (Kakurai et al., 2009; Yu et al., 2010). Interestingly, several studies reported that the susceptibility to psoriasis might be affected by VEGF genetic polymorphism. Single nucleotide polymorphism of the VEGF gene was found to be more frequent in patients with psoriasis compared to healthy individuals, with increased level of VEGF (Young et al., 2006). Promoter variations in the VEGF gene was also reported to be associated with development of psoriatic symptoms (Rogers and D’Amato, 2006), further supporting the role of VEGF in the etiology of psoriasis.
Phototoxicity
UV irradiation is a major physical stimulus to skin, causing cutaneous phototoxicity such as photo-irritation, photo-sensitization, photo-aging, and photo-carcinogenesis (Syed et al., 2012). UV exposure induces generation of reactive oxygen species (ROS) affecting cellular macromolecules and DNA, and also activates multiple signaling pathways responsible for cell growth and proliferation. Different signaling pathways are known to be activated by UVA (320-400 nm) and UVB (280–320 nm) (Mildner et al., 1999; Syed et al., 2012). VEGF expression in cultured keratinocytes was induced by UVB irradiation (Brauchle et al., 1996; Yano et al., 2005), both indirectly by releasing soluble factors such as IL-1 and TNF-α or directly by activating transcription factors such as NFκB, AP-1 or AP-2 (Blaudschun et al., 2000; Gille et al., 2000; Brenneisen et al., 2003). While UVB induced VEGF up-regulation in primary human keratinocytes and in immortalized keratinocytes (HaCaT) (Longuet-Perret et al., 1998; Brenneisen et al., 2003), UVA increased VEGF level only in HaCaT cells (Longuet-Perret et al., 1998; Gille et al., 2000) suggesting specific regulation of cutaneous VEGF signaling in the immortalized keratinocytes which may favor tumorigenic transformation. UV irradiation induces skin alteration such as erythema, hyper-permeability, edema, and epidermal hyperplasia, which are closely associated with VEGF. Hirakawa et al. (2005) demonstrated that VEGF promotes sensitivity to UVB-induced cutaneous photo-damage in VEGF-transgenic mice, suggesting that VEGF may serve as a target for the prevention of photo-damage.
Skin cancinogenesis
It is well-established that tumor cells show increased metabolic demands and initiate angiogenic response. Consistently, VEGF up-regulation was frequently observed in skin cancers and it has been considered to act as an endothelial-specific mitogen (Detmar et al., 1995, Hicklin and Ellis, 2005). It is well known that VEGF regulates endothelial cells in tumor angiogenesis and vascular permeability, but it has been recently found that VEGF also plays an integral role in tumor cell signaling in autocrine manner, such as promoting dedifferentiation and an epithelial-mesenchymal transition (Senger, 2010; Cao et al., 2012; Goel and Mercurio, 2013). Other cells in tumor microenvironment including immune cells and fibroblasts can also be regulated by VEGF, enhancing tumorigenesis (Quail and Joyce, 2013). In skin cancer including squamous cell carcinomas, VEGF-VEGFR signaling was found to be critical for invasion, and selective VEGF over-expression was sufficient for tumor invasiveness in vivo (Detmar, 2000). Angiogenesis and lymphangiogenesis were required for tumor growth, metastasis, and further infiltration of inflammatory cells (Mantovani et al., 2008; Alitalo et al., 2013). Blockade of VEGF-C and D signaling resulted in suppressed inflammatory tumor microenvironment, leading to significant inhibition of early skin cancer progression (Alitalo et al., 2013). Interestingly, UVA specifically induced VEGF in HaCaT cells (Gille et al., 2000), suggesting that VEGF signaling may differ in premalignant phenotype.
VEGF INDUCTION BY XENOBIOTICS
Besides pathological skin conditions, keratinocyte-derived VEGF can be induced by several xenobiotics supporting its potential as a novel biomarker in chemical-induced skin problem. VEGF over-expression in keratinocytes was observed by the treatment with phorbol esters, such as 12-O-tetradec-anoylphorbol-13-acetate (TPA) (Diaz et al., 2000). It is also known that oxidants such as H2O2 can enhance VEGF expression in keratinocyte (Brauchle et al., 1996; Sen et al., 2002), suggesting that cutaneous VEGF can be regulated by redox control. Peroxisome proliferator-activated receptor-gamma (PPARγ) agonist troglitazone significantly induced VEGF expression in keratinocytes mediated by p38 MAPK activation (Schiefelbein et al., 2008). Of note, there is an initial study to suggest VEGF as a potential soluble mediator for hyper-permeability and inflammation, where several contact allergens, metals and an irritant induced VEGF in keratinocytes (Palacio et al., 1997). Still, the mechanisms and the roles of chemically induced VEGF in keratinocytes are largely unknown, which warrant future researches.
INVOLVEMENT OF VEGFR
Although VEGFRs are predominantly expressed in ECs supporting the paracrine signaling of keratinocyte-derived VEGF, all types of identified VEGFRs are also expressed in keratinocytes in normal epidermis (Man et al., 2006). The VEGFR signaling in keratinocytes involves in the proliferation and migration of normal keratinocytes (Man et al., 2006). Using VEGFR-1 specific neutralizing antibody, it was demonstrated that VEGFR-1 in keratinocytes promoted re-epithelialization through a novel autocrine pathway (Wilgus et al., 2005). Besides the constitutive expression in normal keratinocytes, VEGFRs are found to be functionally over-expressed in pathological condition including psoriatic epidermis (Man et al., 2006; 2008; Zhu et al., 2013), and can be up-regulated by UV irradiation (Zhu et al., 2012).
FUTURE DIRECTIONS
Identification of a biomarker for skin damage is an emerging issue for safety assessment of cosmetics or topically-administered medicinal compounds. Traditional skin toxicity test methods have been mostly performed in animal models. However, especially in cosmetic industries, the use of experimental animal is prohibited by the implementation of the 7th Amendment of the Cosmetic Directives in Europe (Directive 2003/15/EC), based on a growing attention on animal welfare. There have been intensive efforts to develop non-animal alternative tests for skin toxicity. The identification of reliable biomarkers for skin damage is prerequisite for development of new alternative methods. Also, a biomarker that can represent an integrated in vivo alteration would be useful to predict the potential biological effect and toxicity. Here we discussed the possibility of VEGF as a novel biomarker for keratinocytic damage in skin toxicity testing, based on the clear evidences on a significant role of VEGF in pathological alteration of keratinocytes under skin disorders.
Acknowledgments
This study was supported by a grant (13172MFDS987) from Ministry of Food and Drug Safety in 2014.
REFERENCES
- Alitalo AK, Proulx ST, Karaman S, Aebischer D, Martino S, Jost M, Schneider N, Bry M, Detmar M. VEGF-C and VEGF-D blockade inhibits inflammatory skin carcinogenesis. Cancer Res. 2013;73:4212–4221. doi: 10.1158/0008-5472.CAN-12-4539. [DOI] [PubMed] [Google Scholar]
- Bernard FX, Morel F, Camus M, Pedretti N, Barrault C, Garnier J, Lecron JC. Keratinocytes under fire of proinflammatory cytokines: Bona fide innate immune cells involved in the physiopathology of chronic atopic dermatitis and psoriasis. J. Allergy (Cairo) 2012;2012:718725. doi: 10.1155/2012/718725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaun C, Weninger W, Uthman A, Weich H, Tschachler E. Human keratinocytes express the three major splice forms of vascular endothelial growth factor. J Invest Dermatol. 1995;104:7–10. doi: 10.1111/1523-1747.ep12613450. [DOI] [PubMed] [Google Scholar]
- Bhushan M, McLaughlin B, Weiss JB, Griffiths CE. Levels of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Br J Dermatol. 1999;141:1054–1060. doi: 10.1046/j.1365-2133.1999.03205.x. [DOI] [PubMed] [Google Scholar]
- Blaudschun R, Brenneisen P, Wlaschek M, Meewes C, Scharffetter-Kochanek K. The first peak of the UVB irradiation-dependent biphasic induction of vascular endothelial growth factor (VEGF) is due to phosphorylation of the epidermal growth factor receptor and independent of autocrine transforming growth factor alpha. FEBS Lett. 2000;474:195–200. doi: 10.1016/S0014-5793(00)01605-7. [DOI] [PubMed] [Google Scholar]
- Brauchle M, Funk JO, Kind P, Werner S. Ultraviolet B and H2O2 are potent inducers of vascular endothelial growth factor expression in cultured keratinocytes. J Biol Chem. 1996;271:21793–21797. doi: 10.1074/jbc.271.36.21793. [DOI] [PubMed] [Google Scholar]
- Brenneisen P, Blaudschun R, Gille J, Schneider L, Hinrichs R, Wlaschek M, Eming S, Scharffetter-Kochanek K. Essential role of an activator protein-2 (AP-2)/specificity protein 1 (Sp1) cluster in the UVB-mediated induction of the human vascular endothelial growth factor in HaCaT keratinocytes. Biochem J. 2003;369:341–349. doi: 10.1042/BJ20021032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavese M, Altruda F, Ruzicka T, Schauber J. Vascular endothelial growth factor (VEGF) in the pathogenesis of psoriasis--a possible target for novel therapies? J Dermatol Sci. 2010;58:171–176. doi: 10.1016/j.jdermsci.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Cao Y, E G, Wang E, Pal K, Dutta SK, Bar-Sagi D, Mukhopadhyay D. VEGF exerts an angiogenesis-independent function in cancer cells to promote their malignant progression. Cancer Res. 2012;72:3912–3918. doi: 10.1158/0008-5472.CAN-11-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Christensen TE, Callis KP, Papenfuss J, Hoffman MS, Hansen CB, Wong B, Panko JM, Krueger GG. Observations of psoriasis in the absence of therapeutic intervention identifies two unappreciated morphologic variants, thin-plaque and thick-plaque psoriasis, and their associated phenotypes. J Invest Dermatol. 2006;126:2397–2403. doi: 10.1038/sj.jid.5700489. [DOI] [PubMed] [Google Scholar]
- Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- Detmar M. Molecular regulation of angiogenesis in the skin. J Invest Dermatol. 1996;106:207–208. doi: 10.1111/1523-1747.ep12340457. [DOI] [PubMed] [Google Scholar]
- Detmar M. The role of VEGF and thrombospondins in skin angiogenesis. J. Dermatol. Sci. 2000;24(Suppl 1):S78–84. doi: 10.1016/S0923-1811(00)00145-6. [DOI] [PubMed] [Google Scholar]
- Detmar M, Brown LF, Berse B, Jackman RW, Elicker BM, Dvorak HF, Claffey KP. Hypoxia regulates the expression of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) and its receptors in human skin. J Invest Dermatol. 1997;108:263–268. doi: 10.1111/1523-1747.ep12286453. [DOI] [PubMed] [Google Scholar]
- Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, Berse B, Dvorak HF. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111:1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- Detmar M, Yeo KT, Nagy JA, Van de Water L, Brown LF, Berse B, Elicker BM, Ledbetter S, Dvorak HF. Keratinocyte-derived vascular permeability factor (vascular endothelial growth factor) is a potent mitogen for dermal microvascular endothelial cells. J Invest Dermatol. 1995;105:44–50. doi: 10.1111/1523-1747.ep12312542. [DOI] [PubMed] [Google Scholar]
- Diaz BV, Lenoir MC, Ladoux A, Frelin C, Demarchez M, Michel S. Regulation of vascular endothelial growth factor expression in human keratinocytes by retinoids. J Biol Chem. 2000;275:642–650. doi: 10.1074/jbc.275.1.642. [DOI] [PubMed] [Google Scholar]
- Elias PM, Arbiser J, Brown BE, Rossiter H, Man MQ, Cerimele F, Crumrine D, Gunathilake R, Choi EH, Uchida Y, Tschachler E, Feingold KR. Epidermal vascular endothelial growth factor production is required for permeability barrier homeostasis, dermal angiogenesis, and the development of epidermal hyperplasia: implications for the pathogenesis of psoriasis. Am J Pathol. 2008;173:689–699. doi: 10.2353/ajpath.2008.080088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck C. Soluble biomarkers in psoriasis. Eur J Dermatol. 2011;21:844–850. doi: 10.1684/ejd.2011.1482. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Finkenzeller G, Sparacio A, Technau A, Marme D, Siemeister G. Sp1 recognition sites in the proximal promoter of the human vascular endothelial growth factor gene are essential for platelet-derived growth factor-induced gene expression. Oncogene. 1997;15:669–676. doi: 10.1038/sj.onc.1201219. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- Geretti E, Shimizu A, Klagsbrun M. Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis. 2008;11:31–39. doi: 10.1007/s10456-008-9097-1. [DOI] [PubMed] [Google Scholar]
- Gille J, Reisinger K, Asbe-Vollkopf A, Hardt-Weinelt K, Kaufmann R. Ultraviolet-A-induced transactivation of the vascular endothelial growth factor gene in HaCaT keratinocytes is conveyed by activator protein-2 transcription factor. J Invest Dermatol. 2000;115:30–36. doi: 10.1046/j.1523-1747.2000.00020.x. [DOI] [PubMed] [Google Scholar]
- Gille J, Swerlick RA, Caughman SW. Transforming growth factor-alpha-induced transcriptional activation of the vascular permeability factor (VPF/VEGF) gene requires AP-2-dependent DNA binding and transactivation. EMBO J. 1997;16:750–759. doi: 10.1093/emboj/16.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat. Rev. Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänel KH, Cornelissen C, Lüscher B, Baron JM. Cytokines and the skin barrier. Int J Mol Sci. 2013;14:6720–6745. doi: 10.3390/ijms14046720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Fujii S, Kajiya K, Yano K, Detmar M. Vascular endothelial growth factor promotes sensitivity to ultraviolet B-induced cutaneous photodamage. Blood. 2005;105:2392–2399. doi: 10.1182/blood-2004-06-2435. [DOI] [PubMed] [Google Scholar]
- Huggenberger R, Detmar M. The cutaneous vascular system in chronic skin inflammation. J Investig Dermatol Symp Proc. 2011;15:24–32. doi: 10.1038/jidsymp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- Kakurai M, Demitsu T, Umemoto N, Kobayashi Y, Inoue-Narita T, Fujita N, Ohtsuki M, Furukawa Y. Vasoactive intestinal peptide and inflammatory cytokines enhance vascular endothelial growth factor production from epidermal keratinocytes. Br J Dermatol. 2009;161:1232–1238. doi: 10.1111/j.1365-2133.2009.09439.x. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Petrova TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19:5598–5605. doi: 10.1038/sj.onc.1203855. [DOI] [PubMed] [Google Scholar]
- Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim TY. Regulation of vascular endothelial growth factor expression by insulin-like growth factor-II in human keratinocytes, differential involvement of mitogen-activated protein kinases and feedback inhibition of protein kinase C. Br J Dermatol. 2005;152:418–425. doi: 10.1111/j.1365-2133.2004.06397.x. [DOI] [PubMed] [Google Scholar]
- Kozlowska U, Blume-Peytavi U, Kodelja V, Sommer C, Goerdt S, Jablonska S, Orfanos CE. Vascular endothelial growth factor expression induced by proinflammatory cytokines (interleukin 1 alpha, beta) in cells of the human pilosebaceous unit. Dermatology. 1998;196:89–92. doi: 10.1159/000017878. [DOI] [PubMed] [Google Scholar]
- Kubo A, Nagao K, Amagai M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest. 2012;122:440–447. doi: 10.1172/JCI57416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Longuet-Perret I, Schmitt D, Viac J. Tumour necrosis factor-alpha is involved in the contrasting effects of ultraviolet B and ultraviolet A1 radiation on the release by normal human keratinocytes of vascular permeability factor. Br J Dermatol. 1998;138:221–224. doi: 10.1046/j.1365-2133.1998.02064.x. [DOI] [PubMed] [Google Scholar]
- Ma L, Xue HB, Guan XH, Shu CM, Zhang JH, Yu J. Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol. 2014;175:25–31. doi: 10.1111/cei.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man XY, Yang XH, Cai SQ, Bu ZY, Zheng M. Overexpression of vascular endothelial growth factor (VEGF) receptors on keratinocytes in psoriasis: regulated by calcium independent of VEGF. J Cell Mol Med. 2008;12:649–660. doi: 10.1111/j.1582-4934.2007.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man XY, Yang XH, Cai SQ, Yao YG, Zheng M. Immunolocalization and expression of vascular endothelial growth factor receptors (VEGFRs) and neuropilins (NRPs) on keratinocytes in human epidermis. Mol Med. 2006;12:127–136. doi: 10.2119/2006-00024.Man. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mildner M, Weninger W, Trautinger F, Ban J, Tschachler E. UVA and UVB radiation differentially regulate vascular endothelial growth factor expression in keratinocyte-derived cell lines and in human keratinocytes. Photochem Photobiol. 1999;70:674–679. doi: 10.1111/j.1751-1097.1999.tb08269.x. [DOI] [PubMed] [Google Scholar]
- Nakai K, Yoneda K, Moriue T, Igarashi J, Kosaka H, Kubota Y. HB-EGF-induced VEGF production and eNOS activation depend on both PI3 kinase and MAP kinase in HaCaT cells. J Dermatol Sci. 2009;55:170–178. doi: 10.1016/j.jdermsci.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Nielsen HJ, Christensen IJ, Svendsen MN, Hansen U, Werther K, Brunner N, Petersen LJ, Kristensen JK. Elevated plasma levels of vascular endothelial growth factor and plasminogen activator inhibitor-1 decrease during improvement of psoriasis. Inflamm Res. 2002;51:563–567. doi: 10.1007/PL00012428. [DOI] [PubMed] [Google Scholar]
- Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Palacio S, Schmitt D, Viac J. Contact allergens and sodium lauryl sulphate upregulate vascular endothelial growth factor in normal keratinocytes. Br J Dermatol. 1997;137:540–544. doi: 10.1111/j.1365-2133.1997.tb03783.x. [DOI] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MS, D’Amato RJ. The effect of genetic diversity on angiogenesis. Exp Cell Res. 2006;312:561–574. doi: 10.1016/j.yexcr.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- Schiefelbein D, Seitz O, Goren I, Dissmann JP, Schmidt H, Bachmann M, Sader R, Geisslinger G, Pfeilschifter J, Frank S. Keratinocyte-derived vascular endothelial growth factor biosynthesis represents a pleiotropic side effect of peroxisome proliferator-activated receptor-gamma agonist troglitazone but not rosiglitazone and involves activation of p38 mitogen-activated protein kinase: implications for diabetes-impaired skin repair. Mol Pharmacol. 2008;74:952–963. doi: 10.1124/mol.108.049395. [DOI] [PubMed] [Google Scholar]
- Schonthaler HB, Huggenberger R, Wculek SK, Detmar M, Wagner EF. Systemic anti-VEGF treatment strongly reduces skin inflammation in a mouse model of psoriasis. Proc Natl Acad Sci USA. 2009;106:21264–21269. doi: 10.1073/pnas.0907550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scortegagna M, Cataisson C, Martin RJ, Hicklin DJ, Schreiber RD, Yuspa SH, Arbeit JM. HIF-1alpha regulates epithelial inflammation by cell autonomous NFkappaB activation and paracrine stromal remodeling. Blood. 2008;111:3343–3354. doi: 10.1182/blood-2007-10-115758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, Roy S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J Biol Chem. 2002;277:33284–33290. doi: 10.1074/jbc.M203391200. [DOI] [PubMed] [Google Scholar]
- Senger DR. Vascular endothelial growth factor: much more than an angiogenesis factor. Mol. Biol. Cell. 2010;21:377–379. doi: 10.1091/mbc.E09-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Shi VY, Bao L, Chan LS. Inflammation-driven dermal lymphangiogenesis in atopic dermatitis is associated with CD11b+macrophage recruitment and VEGF-C up-regulation in the IL-4-transgenic mouse model. Microcirculation. 2012;19:567–579. doi: 10.1111/j.1549-8719.2012.00189.x. [DOI] [PubMed] [Google Scholar]
- Skobe M, Brown LF, Tognazzi K, Ganju RK, Dezube BJ, Alitalo K, Detmar M. Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AID-Sassociated Kaposi’s sarcoma. J Invest Dermatol. 1999;113:1047–1053. doi: 10.1046/j.1523-1747.1999.00798.x. [DOI] [PubMed] [Google Scholar]
- Suto K, Yamazaki Y, Morita T, Mizuno H. Crystal structures of novel vascular endothelial growth factors (VEGF) from snake venoms: insight into selective VEGF binding to kinase insert domain-containing receptor but not to fms-like tyrosine kinase-1. J Biol Chem. 2005;280:2126–2131. doi: 10.1074/jbc.M411395200. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hirakawa S, Shimauchi T, Ito T, Sakabe J, Detmar M, Tokura Y. VEGF-A promotes IL-17A-producing gammadelta T cell accumulation in mouse skin and serves as a chemotactic factor for plasmacytoid dendritic cells. J Dermatol Sci. 2014;74:116–124. doi: 10.1016/j.jdermsci.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Syed DN, Afaq F, Mukhtar H. Differential activation of signaling pathways by UVA and UVB radiation in normal human epidermal keratinocytes. Photochem Photobiol. 2012;88:1184–1190. doi: 10.1111/j.1751-1097.2012.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. (Lond) 2005;109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- Trompezinski S, Berthier-Vergnes O, Denis A, Schmitt D, Viac J. Comparative expression of vascular endothelial growth factor family members, VEGF-B, -C and -D, by normal human keratinocytes and fibroblasts. Exp Dermatol. 2004;13:98–105. doi: 10.1111/j.0906-6705.2004.00137.x. [DOI] [PubMed] [Google Scholar]
- Weidemann AK, Crawshaw AA, Byrne E, Young HS. Vascular endothelial growth factor inhibitors: investigational therapies for the treatment of psoriasis. Clin Cosmet Investig Dermatol. 2013;6:233–244. doi: 10.2147/CCID.S35312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir L, Robertson D, Leigh IM, Vass JK, Panteleyev AA. Hypoxia-mediated control of HIF/ARNT machinery in epidermal keratinocytes. Biochim. Biophys. Acta. 2011;1813:60–72. doi: 10.1016/j.bbamcr.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Weninger W, Uthman A, Pammer J, Pichler A, Ballaun C, Lang IM, Plettenberg A, Bankl HC, Sturzl M, Tschachler E. Vascular endothelial growth factor production in normal epidermis and in benign and malignant epithelial skin tumors. Lab Invest. 1996;75:647–657. [PubMed] [Google Scholar]
- Wilgus TA, Matthies AM, Radek KA, Dovi JV, Burns AL, Shankar R, DiPietro LA. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am J Pathol. 2005;167:1257–1266. doi: 10.1016/S0002-9440(10)61213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–417. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol. 2005;152:115–121. doi: 10.1111/j.1365-2133.2005.06368.x. [DOI] [PubMed] [Google Scholar]
- Young HS, Summers AM, Read IR, Fairhurst DA, Plant DJ, Campalani E, Smith CH, Barker JN, Detmar MJ, Brenchley PE, Griffiths CE. Interaction between genetic control of vascular endothelial growth factor production and retinoid responsiveness in psoriasis. J Invest Dermatol. 2006;126:453–459. doi: 10.1038/sj.jid.5700096. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Ren XH, Xu YH, Chen LM, Zhou CL, Li CY. Vasoactive intestinal peptide induces vascular endothelial growth factor production in human HaCaT keratinocytes via MAPK pathway. Neuropeptides. 2010;44:407–411. doi: 10.1016/j.npep.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Zgraggen S, Ochsenbein AM, Detmar M. An important role of blood and lymphatic vessels in inflammation and allergy. J. Allergy (Cairo) 2013;2013:672381. doi: 10.1155/2013/672381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Matsuo H, Morita E. Increased production of vascular endothelial growth factor in the lesions of atopic dermatitis. Arch Dermatol Res. 2006;297:425–429. doi: 10.1007/s00403-006-0641-9. [DOI] [PubMed] [Google Scholar]
- Zhu JW, Wu XJ, Lu ZF, Luo D, Cai SQ, Zheng M. Role of VEGF receptors in normal and psoriatic human keratinocytes: evidence from irradiation with different UV sources. PLoS One. 2013;8:e55463. doi: 10.1371/journal.pone.0055463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JW, Wu XJ, Luo D, Lu ZF, Cai SQ, Zheng M. Activation of VEGFR-2 signaling in response to moderate dose of ultraviolet B promotes survival of normal human keratinocytes. Int J Biochem Cell Biol. 2012;44:246–256. doi: 10.1016/j.biocel.2011.10.022. [DOI] [PubMed] [Google Scholar]