Abstract
Vascular endothelial growth factor (VEGF) is an important regulator of neovascularization. Hypoxia inducible nitric oxide (NO) enhanced the expression of VEGF and thymosin beta-4 (Tβ4), actin sequestering protein. Here, we investigated whether NO-mediated VEGF expression could be regulated by Tβ4 expression in HeLa cervical cancer cells. Hypoxia inducible NO production and VEGF expression were reduced by small interference (si) RNA of Tβ4. Hypoxia response element (HRE)-luciferase activity and VEGF expression were increased by the treatment with N-(β-D-Glucopyranosyl)-N2-acetyl-S-nitroso-D, L-penicillaminamide (SNAP-1), to generate NO, which was inhibited by the inhibition of Tβ4 expression with Tβ4-siRNA. In hypoxic condition, HRE-luciferase activity and VEGF expression were inhibited by the treatment with NG-monomethyl-L-arginine (L-NMMA), an inhibitor to nitric oxide synthase (NOS), which is accompanied with a decrease in Tβ4 expression. VEGF expression inhibited by L-NMMA treatment was restored by the transfection with pCMV-Tβ4 plasmids for Tβ4 overexpression. Taken together, these results suggest that Tβ4 could be a regulator for the expression of VEGF via the maintenance of NOS activity.
Keywords: VEGF, Thymosin beta-4, Nitric oxide, Hypoxia, HIF-1α
INTRODUCTION
Thymosin beta-4 (Tβ4) is a small and naturally occurring 5 kDa peptide with 43 amino acids present in all cells except erythrocytes (Low et al., 1981; Huff et al., 2001). Tβ4 protein was cross-linked to monomer G-actin (Safer et al., 1991). Tβ4 has multiple diverse cellular functions including anti-apoptosis to an external stress (Sosne et al., 2004), paclitaxel-resistance through ROS production (Oh et al., 2006; Moon et al., 2007; Oh et al., 2010), and HIF-1α stabilization through Erk activation (Oh et al., 2008) via depolymerization of F-actin (Brakebusch and Fassler, 2005; Zvaifler, 2006). In addition, Tβ4 regulates cancer cell migration through various signaling pathways (Moon et al., 2010; Im et al., 2012; Ryu et al., 2012). Tβ4 triggers epithelial-mesenchymal transition (Huang et al., 2007), malignant progression and invasion in colon adenocarcinoma (Wang et al., 2003; Wang et al., 2004).
Vascular endothelial growth factor (VEGF) plays an important role in new blood vessel formation known as angiogenesis. Hypoxia-inducible factor (HIF)-1α stimulates VEGF gene transcription (Richard et al., 1999). Cytosolic HIF-1α protein is rapidly degraded by the ubiquitin-proteasome pathway in normoxia (Salceda and Caro, 1997) but its degradation is inhibited in hypoxia (Huang et al., 1998). VEGF up-regulation requires Tβ4 expression, which is enhanced in hypoxic condition (Gnecchi et al., 2006; Smart et al., 2007). Tβ4 also increased HIF-1α protein level and HRE activity in VEGF promoter (Oh et al., 2008). Tβ4-associated VEGF expression is indirectly mediated by HIF-1α stability (Jo et al., 2010). However, it is not enough information to connect Tβ4 and VEGF expression.
Nitric oxide (NO) is an uncharged free radical and synthesized from the amino acid L-arginine by NO synthase (Marsden et al., 1993; Chartrain et al., 1994; Hall et al., 1994). NO is a mediator of diverse physiological cellular functions including vasodilation, neurotransmission and anti-platelet aggregation (Moncada and Higgs, 2006). NO also plays a role in various cellular effects leading to DNA damage, cell death and antiapoptosis, which is dependent on cellular NO concentration (Wink et al., 1998). In addition, Previous report showed that paclitaxel induces VEGF expression through the production of reactive oxygen species (ROS), which lead to drug resistance (Kim et al., 2008). NO production is also increased in tumor cells under hypoxia condition (Maulik and Das, 2002), which upregulate VEGF (Xu et al., 2002; Fukumura et al., 2006; Hussain et al., 2008). However, little has been known about whether NO-mediated VEGF expression is controlled by Tβ4 expression under hypoxic condition.
Here, we studied whether VEGF expression in HeLa cervical cancer cells could be regulated by hypoxia-inducible NO via Tβ4 expression. Our data suggest that Tβ4 could be a regulator for the expression of VEGF through NO production.
MATERIALS AND METHODS
Reagents
Anti-rabbit antibodies to HIF-1α or VEGF were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies that were reactive with actin and α-tubulin were obtained from Sigma-Aldrich (St. Louis, MO, USA). NG-monomethyl-L-arginine (L-NMMA) was obtained from Sigma-Aldrich (St. Lois, MO, USA). N-(β-D-Glucopyranosyl)-N2-acetyl-S-nitroso-D, L-penicillaminamide (SNAP-1) was obtained from Calbiochem (La Jolla, CA, USA). Except where indicated, all other materials including LiCl are obtained from Sigma-Aldrich chemical company (St. Louis, MO, USA).
Cell culture
HeLa cells were obtained from the Korea Research Institute of Bioscience and Biotechnology (KRIBB) cell bank (Daejeon, Korea). Cells were maintained and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (GIBCO, Grand Island, NY, USA), 2 mM L-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin. Cells were incubated under normoxia CO2 incubator with 5% CO2 and 95% air at 37°C. Cells were also incubated under hypoxia (0.5% O2) in an anaerobic incubator (Forma Scientific, Marietta, OH, USA) with 5% CO2, 10% H2, and 85% N2 at 37°C for an appropriate time. Then, cells were incubated at 37°C in an atmosphere of humidified normoxia incubator with 5% CO2 and 95% air.
Nitrite measurement
To assess NO production, accumulated nitrites were measured in the cell supernatant by the Griess reaction (Moon et al., 2011). In brief, 100 μl of supernatant from each well were mixed with 100 μl of Griess reagent (0.1% naphthylethylenediamine dihydrochloride and 1% sulfanilamide in 2% phosphoric acid) on 96-well microtiter plates. Absorbance was read at 540 nm, using an ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
Hypoxia response element (HRE) reporter assay
HRE reporter plasmid 5x VEGF-HRE-pSV40 min that was generated by cloning five tandem couples of HRE derived from the human VEGF promoter into the BglII site of pGL3 was kindly provided from Dr. Dong-Soo Im, KRIBB (Taejeon, Korea) (Cho et al., 2004). To measure the activity of VEGF transcription, confluent Hela cells were transfected with VEGF-HRE-pSV40 min plasmid using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
Reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was isolated from HeLa cells using TRIZOL reagent (Invitrogen, Calsbad, CA, USA). cDNA was synthesized from 1 μg of total RNA, using oligo-dT18 primers and reverse transcriptase in a final volume of 20 μl (Bioneer, Taejeon, Korea). For standard PCR, one μl of the first strand cDNA product was then used as a template for PCR amplification with Taq DNA polymerase (Bioneer, Taejeon, Korea). PCR amplification proceeded as follows using oligonucleotides specific for human Tβ4 (forward: 5′-atg tct gac aaa ccc gat atg gc-3′, reverse: 5′-tta cga ttc gcc tgc ttg ctt c-3′), VEGF (forward: 5′- tga cag gga aga gga gga ga-3′, reverse: 5′-tgg ttt caa tgg tgt gag ga-3′), and GAPDH (forward: 5′-gaa ggt gaa ggt cgg agt c-3′, reverse: 5′-gaa gat ggt gat ggg att tc-3′). PCR products were detected by running in 1.2% agarose gel electrophoresis.
Western blot analysis
Western blot analysis was carried out according to standard protocol. HeLa cells treated with various experimental condition were harvested and then lysed in ice-cold lysis buffer, containing 0.5% Nonidet P-40 (vol./vol.) in 20 mM Tris-HCl, at a pH of 8.3; 150 mM NaCl; protease inhibitors [2 μg/ml aprotinin, pepstatin; 1 μg/ml leupeptin; 1 mM phenylmethyl sulfonyl fluoride (PMSF)] and 1 mM Na3VO4, phospatase inhibitor. Lysates were incubated for 1 h in ice prior to centrifugation at 13,000 rpm for 20 min at 4°C. The protein concentration of the sample was measured using Bio-Rad protein assay dye reagent. Proteins in the supernatant were denatured by boiling for 5 min in Sodium Dodecyl Sulfate (SDS) sample buffer. Sample amount of proteins were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to nitrocellulose membranes by electro-blotting. Following this transfer, equal loading of protein was verified by Ponceau S staining. The membranes were blocked with 5% skim milk in Tris-buffered saline with Tween 20 (TBST) (10 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.5% Tween 20), then incubated with the indicated antibodies. Bound antibodies were visualized with HRPconjugated secondary antibodies with the use of enhanced chemiluminescence (ECL). Immune-reactive bands were detected using X-ray film.
Statistical analyses
Experimental differences were tested for statistical significance using ANOVA and Students’ t-test. p value of <0.05 was considered to be significant.
RESULTS
Tβ4 regulates NO production and VEGF expression under hypoxic condition
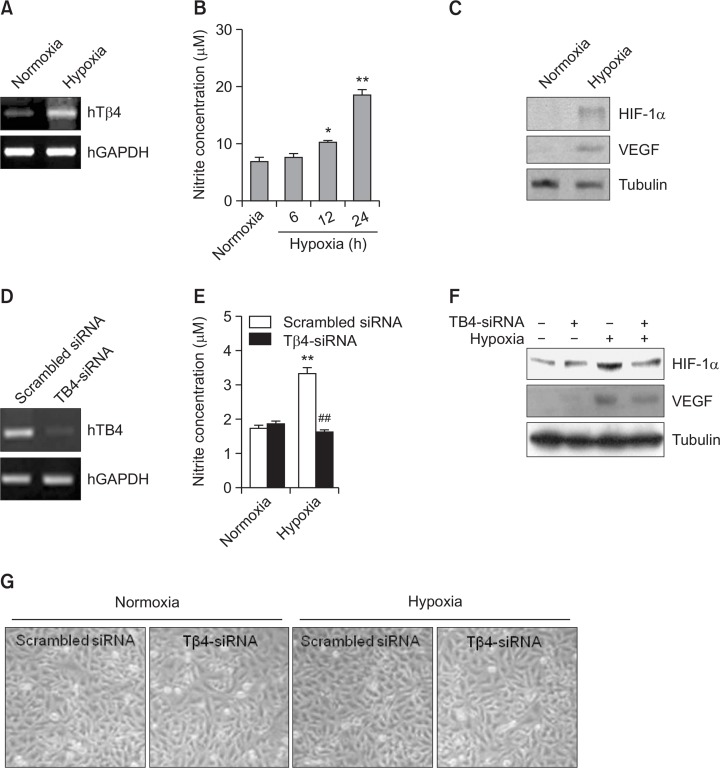
Given that Tβ4 expression and nitric oxide (NO) production were increased under hypoxia condition (Maulik and Das, 2002; Moon et al., 2010), we also examined an increase in Tβ4 expression (Fig. 1A) and NO production (Fig. 1B) in HeLa cervical cancer cells. Hypoxia condition was confirmed by the increase in HIF-1α and VEGF protein level in HeLa cells under hypoxia condition (Fig. 1C). Then, to examine the effect of Tβ4 on VEGF expression, Tβ4-siRNA was used to inhibit Tβ4 expression (Fig. 1D). NO production in HeLa cells under hypoxia condition was inhibited by the transfection with Tβ4-siRNA cells (Fig. 1E). We also observed that HIF-1α and VEGF protein level increased under hypoxic condition was attenuated by the inhibition of Tβ4 expression with Tβ4-siRNA cells (Fig. 1F). No changes in cellular morphology were detected in cells transfected with Tβ4-siRNA. However, a little reduction of cell density was observed by the incubation of Tβ4-siRNA-transfected cells under hypoxic condition (Fig. 1G). It suggests that hypoxia-inducible Tβ4 could be involved in VEGF expression.
Fig. 1.
VEGF is increased by Tβ4 expression under hypoxic condition. (A-C) HeLa cells were incubated under normoxia or hypoxia condition. RNA was purified with TRIZOL reagent. Tβ4 transcript level was measured by RT-PCR (A). NO production was detected as nitrite accumulated in culture supernatant by using Griess reagents. Data in bar graph represent mean ± SED. *p<0.05; **p<0.01, statistical significance vs. normoxia control group (B). HIF-1α and VEGF in cell lysate were detected by western blot analysis (C). (D-G) Tβ4 expression in HeLa cells was inhibited by the transfection with Tβ4-siRNA. RNA was purified with TRIZOL reagent. Tβ4 transcript level was measured by RT-PCR (D). Cells were incubated under normoxic or hypoxic condition (E-G). NO production was detected as nitrite accumulated in culture supernatant by using Griess reagents. Data in bar graph represent mean ± SED. **p<0.01, statistical significance vs. normoxia control group. ##p<0.01, statistical significance vs. scrambled siRNA-treated group under hypoxic condition (E). HIF-1α and VEGF protein levels in cell lysates were detected by western blot analysis (F). Cellular morphology was photographed with a phase-contrast microscope. Pictures were taken at the same magnification, 200x. Data are representative of four experiments (G).
SNAP-1, NO donor-mediated VEGF expression is dependent on Tβ4 expression
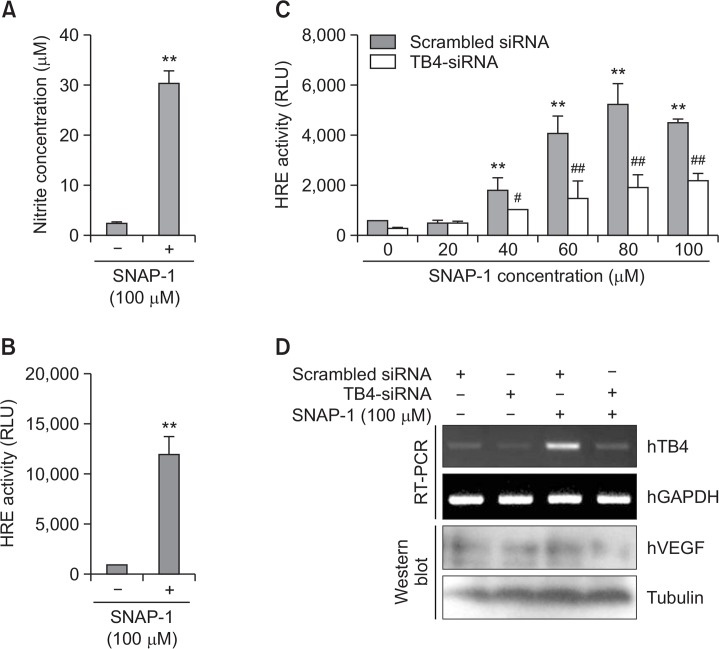
To confirm the effect of NO on VEGF through Tβ4 expression in HeLa cells, we used N-(β-D-Glucopyranosyl)-N2-acetyl- S-nitroso-D,L-penicillaminamide (SNAP-1) as NO donor (Fig. 2A). SNAP-1 treatment enhanced VEGF transcription as judged by hypoxia response element (HRE) reporter activity in VEGF promoter (Fig. 2B). NO-mediated VEGF expression increased by SNAP-1 was attenuated by the transfection with Tβ4-siRNA (Fig. 2C, D). Inhibition of Tβ4 expression was proved by examination (Fig. 2D). Data demonstrate that NO-mediated VEGF expression is dependent of Tβ4 expression
Fig. 2.
VEGF expression is upregulated by SNAP-1, NO donor. (A) HeLa cells were treated with 100 μM SNAP-1. NO production was detected as nitrite accumulated in culture supernatant by using Griess reagents. (B) HeLa cells were transfected with pGL2 plasmid of hypoxia response element (HRE)-luciferase (Luc) and treated with 100 μM SNAP-1. Luc activity was measured with luminometer using Luc substrate. Data in bar graph represent mean ± SED. **p<0.01, statistical significance vs. SNAP-1-untreated group (A and B). (C-D) HeLa cells were co-transfected with Tβ4-siRNA and pGL2-HRE-Luc plasmid. Then, cells were treated with various concentrations of SNAP-1. Luc activity was measured with luminometer using Luc substrate. Data in bar graph represent mean ± SED. Luc activity was measured with luminometer using Luc substrate. Data in bar graph represent mean ± SED. **p<0.01, statistical significance vs. SNAP-1-untreated group. #p<0.05; ##p<0.01, statistical significance vs. scrambled siRNA-treated group at each concentration of SNAP-1 (C). RNA was purified with TRIZOL reagent as described in materials and methods. Tβ4 transcript level was measured by RT-PCR and VEGF levels were detected by western blot analysis (D).
Hypoxia-induced VEGF expression is inhibited by L-NMMA, NOS inhibitor
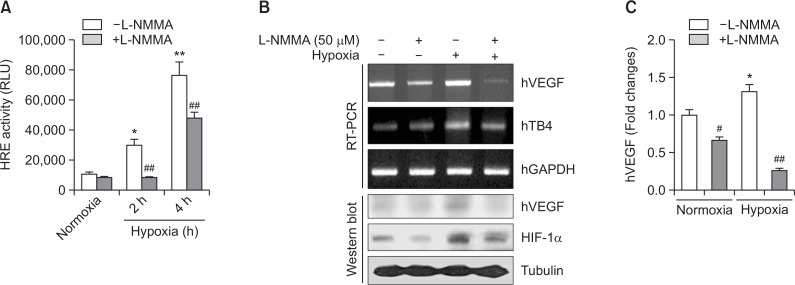
To test the effect of hypoxia-inducible NO on VEGF expression through Tβ4 expression in HeLa cells, we treated cells with NG-monomethyl-L-arginine (L-NMMA). Hypoxia response element (HRE) reporter activity in VEGF promoter was reduced by the treatment with L-NMMA under hypoxia condition (Fig. 3A). Hypoxia-induced increase in Tβ4 and HIF-1α was attenuated by the treatment with L-NMMA (Fig 3B). In addition, transcriptional and protein level of VEGF were also inhibited by the treatment with L-NMMA under hypoxia condition (Fig. 3B, C). Data demonstrate that hypoxia-inducible VEGF expression might be resulted from the regulation of NO production under hypoxia condition. It also suggests that VEGF expression could be controlled by NO-mediated Tβ4 expression and HIF-1α stabilization.
Fig. 3.
L-NMMA, NOS inhibitor attenuated hypoxia-inducible VEGF expression. (A) HeLa cells were transfected with pGL2 plasmid of hypoxia response element (HRE)-luciferase (Luc) and incubated under normoxic or hypoxic condition in the presence or absence of L-NMMA. Luc activity was measured with luminometer using Luc substrate. Data in bar graph represent mean ± SED. *p<0.05; **p<0.01, statistical significance vs. normoxia control group. ##p<0.01, statistical significance vs. L-NMMA-untreated group at each time point under hypoxic condition. (B-C) HeLa cells were treated with L-NMMA and incubated under normoxic or hypoxic condition. RNA was purified with TRIZOL reagent. hVEGF and Tβ4 transcript level was measured by RT-PCR (B, top). hVEGF and HIF-1α in cell lysates were detected by western blot analysis (B, bottom). hVEGF transcripts were normalized and data in bar graph represent mean ± SED. *p<0.05, statistical significance vs. normoxia control group. #p<0.05; ##p<0.01, statistical significance vs. L-NMMA-untreated control at each normoxic or hypoxic condition (C).
VEGF expression is restored by the transfection of pCMV- Tβ4 plasmids in L-NMMA-treated cells
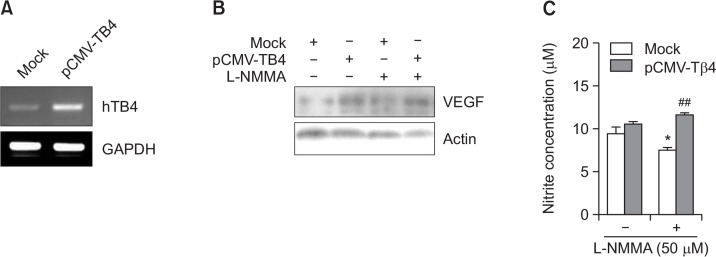
To examine the role of Tβ4 on NO-dependent VEGF expression, Tβ4 was over-expressed by the transfection of pCMV-Tβ4 plasmids (Fig. 4A). VEGF protein level was increased by pCMV-Tβ4 transfection and then it was reduced by the treatment with L-NMMA. In contrast, L-NMMA-treated decrease in VEGF level was restored by the transfection of pCMV-Tβ4 plasmids (Fig. 4B). In addition, NO production was reduced by the treatment with L-NMMA and it was reversed by the transfection of pCMV-Tβ4 plasmids (Fig. 4C). These results evidenced that VEGF expression could be dependent on NO level. It suggests that NO-dependent VEGF expression might be regulated by Tβ4 expression.
Fig. 4.
VEGF expression reduced in L-NMMA-treated cells was restored by the transfection with pCMV-Tβ4 plasmid. (A-C) HeLa cells were transfected with control pCMV (Mock) or pCMV-Tβ4 plasmids. Then, HeLa cells were treated with L-NMMA and incubated under normoxic or hypoxic condition. RNA was purified with TRIZOL reagent. Tβ4 transcript level was measured by RT-PCR (A). Cells were incubated in the presence or absence of L-NMMA and cell lysates were prepared from HeLa cells transfected with Mock control or pCMV-Tβ4 plasmids. Then, hVEGF protein levels were detected by western blot analysis (B). NO production was detected as nitrite accumulated in culture supernatant by using Griess reagents. Data in bar graph represent mean ± SED. *p<0.05, statistical significance vs. L-NMMA-untreated control group. ##p<0.01, statistical significance vs. Mock control in L-NMMA-treated group (C).
DISCUSSION
The increased HIF-1 activity provides a molecular basis for other adaptations of cancer cells to hypoxia that are critical for establishment of a primary tumor and its progression to the lethal phenotype (Semenza, 2000). VEGF plays a key role in angiogenesis and its gene transcription is activated by translocation of HIF-1 (Richard et al., 1999; Zhong et al., 1999). Hypoxic condition increases VEGF, Tβ4 expression (Gnecchi et al., 2006; Smart et al., 2007; Oh et al., 2008) and NO production (Maulik and Das, 2002). NO can promote tumor invasion and metastasis by activating various enzymes. VEGF expression is also upregulated by NO production, which lead to tumor angiogenesis (Xu et al., 2002; Fukumura et al., 2006; Hussain et al., 2008). Although it has been known that NO plays a key role in VEGF expression, it is required to define the mechanism of action or intermediate protein on NO-mediated VEGF expression. Here, we investigated whether NO-mediated VEGF expression under hypoxic condition is controlled by Tβ4 expression in HeLa cervical cancer cells. Our data showed that NO production and VEGF expression was reduced by Tβ4-siRNA under hypoxic condition (Fig. 1). SANP-1, NO donor, increases HRE activity and the expression of VEGF (Fig. 2). VEGF expression reduced by L-NMMA, NOS inhibitor, is reversed by overexpression of Tβ4 (Fig. 3, 4). It suggests that NO-dependent VEGF expression is regulated via Tβ4 expression under hypoxic condition.
It is possible to suggest a few mechanism of action on Tβ4 expression. Since NO activate guanylate cyclase producing cGMP and protein kinase G (PKG) (Deguchi et al., 2004; Murad, 2006). So, cGMP or PKG could be involved in Tβ4 expression through the activation of transcription factor. Further study is required to define transcription factors to activate Tβ4 promoter. Another mechanism is direct activation of transcription factor by NO (Chamorro-Jorganes et al., 2011; Kanao et al., 2012), which might increase Tβ4 expression. HIF-1α also might be a candidate to bind Tβ4 promoter under hypoxic condition. Since Tβ4 increased HIF-1α protein level (Oh et al., 2008) and the increase in HIF-1 activity is associated with a molecular adaptation of cancer cells to hypoxia (Semenza, 2000), positive feedback regulation might be involved in between Tβ4 expression and HIF-1α stabilization. Then, Tβ4 lead to HIF-1α stabilization and sequentially lead to an increase in Tβ4 expression by binding HIF-1α on Tβ4 promoter. It is also possible that nitric oxide synthase (NOS) expression could be regulated by HIF-1α acting on NOS promoter, which lead to NO production in hypoxia condition. These suggest that VEGF expression could be controlled by Tβ4 expression via NO production under hypoxic condition. However, it remains to be clarified 1) whether a Tβ4-binding element is present in the promoter of VEGF, 2) which sequence in the Tβ4 promoter binds NO-associated transcription factor or HIF-1α, and 3) the mechanism of action underlying Tβ4-regulated VEGF expression.
Previous reports showed that various kinases except PKG could be activated by NO. NO and/or cGMP activate c-Src/PI3K- and PKG-dependent ERK 1/2 (Tejedo et al., 2004), and p21Ras-Raf-1 kinase-MEK-ERK1/2 (Oliveira et al., 2003). NO also activate CaMKII by an increase in Ca2+ leak from sarcoplasmic reticulum (Curran et al., 2014) So, it is possible to explain that hypoxia-inducible VEGF expression might be due to the participation of various signaling molecules including ERK in Tβ4-regulated NO production. Further study is required to define the main signaling molecules on Tβ4-mediated NO production.
Collectively, although it has not been defined whether VEGF promoter bind Tβ4 and what mechanism of action is involved in NO-mediated VEGF expression, hypoxia-inducible NO could influence the increase in VEGF expression through Tβ4. Data suggest that Tβ4 may participate in VEGF expression by a hypoxia-inducible NO.
Acknowledgments
This work was supported by Grants from Mid-career Researcher Program (#2012-R1A2A2A01005449), and Nuclear R&D Program (#2013M2B2A9A03051296 and 2010-0018545) through National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST).
Footnotes
CONFLICT OF INTEREST
The manuscript has been reviewed and approved by all authors. No potential conflicts of interest were disclosed.
REFERENCES
- Brakebusch C, Fassler R. beta 1 integrin function in vivo: adhesion, migration and more. Cancer Metastasis Rev. 2005;24:403–411. doi: 10.1007/s10555-005-5132-5. [DOI] [PubMed] [Google Scholar]
- Chamorro-Jorganes A, Calleros L, Griera M, Saura M, Luengo A, Rodriguez-Puyol D, Rodriguez-Puyol M. Fibronectin upregulates cGMP-dependent protein kinase type Ibeta through C/EBP transcription factor activation in contractile cells. Am J Physiol Cell Physiol. 2011;300:C683–691. doi: 10.1152/ajpcell.00251.2010. [DOI] [PubMed] [Google Scholar]
- Chartrain NA, Geller DA, Koty PP, Sitrin NF, Nussler AK, Hoffman EP, Billiar TR, Hutchinson NI, Mudgett JS. Molecular-cloning, structure, and chromosomal localization of the human inducible nitric-oxide synthase gene. J Biol Chem. 1994;269:6765–6772. [PubMed] [Google Scholar]
- Cho WK, Seong YR, Lee YH, Kim MJ, Hwang KS, Yoo J, Choi S, Jung CR, Im DS. Oncolytic effects of adenovirus mutant capable of replicating in hypoxic and normoxic regions of solid tumor. Mol Ther. 2004;10:938–949. doi: 10.1016/j.ymthe.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Curran J, Tang L, Roof SR, Velmurugan S, Millard A, Shonts S, Wang H, Santiago D, Ahmad U, Perryman M, Bers DM, Mohler PJ, Ziolo MT, Shannon TR. Nitric oxidedependent activation of CaMKII increases diastolic sarcoplasmic reticulum calcium release in cardiac myocytes in response to adrenergic stimulation. PLoS ONE. 2014;9:e87495. doi: 10.1371/journal.pone.0087495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi A, Thompson WJ, Weinstein IB. Activation of protein kinase G is sufficient to induce apoptosis and inhibit cell migration in colon cancer cells. Cancer Res. 2004;64:3966–3973. doi: 10.1158/0008-5472.CAN-03-3740. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat. Rev. Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- Hall AV, Antoniou H, Wang Y, Cheung AH, Arbus AM, Olson SL, Lu WC, Kau CL, Marsden PA. Structural organization of the human neuronal nitric-oxide synthase gene (Nos1) J Biol Chem. 1994;269:33082–33090. [PubMed] [Google Scholar]
- Huang HC, Hu CH, Tang MC, Wang WS, Chen PM, Su Y. Thymosin beta4 triggers an epithelial-mesenchymal transition in colorectal carcinoma by upregulating integrin-linked kinase. Oncogene. 2007;26:2781–2790. doi: 10.1038/sj.onc.1210078. [DOI] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff T, Muller CS, Otto AM, Netzker R, Hannappel E. beta-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33:205–220. doi: 10.1016/S1357-2725(00)00087-X. [DOI] [PubMed] [Google Scholar]
- Hussain SP, He P, Subleski J, Hofseth LJ, Trivers GE, Mechanic L, Hofseth AB, Bernard M, Schwank J, Nguyen G, Mathe E, Djurickovic D, Haines D, Weiss J, Back T, Gruys E, Laubach VE, Wiltrout RH, Harris CC. Nitric oxide is a key component in inflammation-accelerated tumorigenesis. Cancer Res. 2008;68:7130–7136. doi: 10.1158/0008-5472.CAN-08-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im YS, Ryu YK, Moon EY. Mouse melanoma cell migration is dependent on production of reactive oxygen species under normoxia condition. Biomol Ther. 2012;20:165–170. doi: 10.4062/biomolther.2012.20.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo JO, Kim SR, Bae MK, Kang YJ, Ock MS, Kleinman HK, Cha HJ. Thymosin beta4 induces the expression of vascular endothelial growth factor (VEGF) in a hypoxia-inducible factor (HIF)-1alpha-dependent manner. Biochim. Biophys. Acta. 2010;1803:1244–1251. doi: 10.1016/j.bbamcr.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Kanao T, Sawada T, Davies SA, Ichinose H, Hasegawa K, Takahashi R, Hattori N, Imai Y. The nitric oxide-cyclic GMP pathway regulates FoxO and alters dopaminergic neuron survival in Drosophila. PLoS One. 2012;7:e30958. doi: 10.1371/journal.pone.0030958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Oh JM, Jin DH, Yang KH, Moon EY. Paclitaxel induces vascular endothelial growth factor expression through reactive oxygen species production. Pharmacology. 2008;81:317–324. doi: 10.1159/000119756. [DOI] [PubMed] [Google Scholar]
- Low TL, Hu SK, Goldstein AL. Complete amino acid sequence of bovine thymosin beta 4: a thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populations. Proc Natl Acad Sci USA. 1981;78:1162–1166. doi: 10.1073/pnas.78.2.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden PA, Heng HHQ, Scherer SW, Stewart RJ, Hall AV, Shi XM, Tsui LC, Schappert KT. Structure and chromosomal localization of the human constitutive endothelial nitric-oxide synthase gene. J Biol Chem. 1993;268:17478–17488. [PubMed] [Google Scholar]
- Maulik N, Das DK. Redox signaling in vascular angiogenesis. Br J Pharmacol. 2002;33:1047–1060. doi: 10.1016/s0891-5849(02)01005-5. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147:S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EY, Im YS, Ryu YK, Kang JH. Actin-sequestering protein, thymosin beta-4, is a novel hypoxia responsive regulator. Clin. Exp. Metastasis. 2010;27:601–609. doi: 10.1007/s10585-010-9350-z. [DOI] [PubMed] [Google Scholar]
- Moon EY, Song JH, Yang KH. Actin-sequestering protein, thymosin-beta-4 (TB4), inhibits caspase-3 activation in paclitaxel-induced tumor cell death. Oncol Res. 2007;16:507–516. doi: 10.3727/096504007783438349. [DOI] [PubMed] [Google Scholar]
- Moon EY, Yi GH, Kang JS, Lim JS, Kim HM, Pyo S. An increase in mouse tumor growth by an in vivo immunomodulating effect of titanium dioxide nanoparticles. J Immunotoxicol. 2011;8:56–67. doi: 10.3109/1547691X.2010.543995. [DOI] [PubMed] [Google Scholar]
- Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355:2003–2011. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- Oh JM, Ryoo IJ, Yang Y, Kim HS, Yang KH, Moon EY. Hypoxia-inducible transcription factor (HIF)-1 alpha stabilization by actin-sequestering protein, thymosin beta-4 (TB4) in Hela cervical tumor cells. Cancer Lett. 2008;264:29–35. doi: 10.1016/j.canlet.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Oh JM, Ryu YK, Lim JS, Moon EY. Hypoxia induces paclitaxel-resistance through ROS production. Biomol Ther. 2010;18:145–151. doi: 10.4062/biomolther.2010.18.2.145. [DOI] [Google Scholar]
- Oh SY, Song JH, Gil JE, Kim JH, Yeom YI, Moon EY. ERK activation by thymosin-beta-4 (TB4) overexpression induces paclitaxel-resistance. Exp Cell Res. 2006;312:1651–1657. doi: 10.1016/j.yexcr.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Oliveira CJ, Schindler F, Ventura AM, Morais MS, Arai RJ, Debbas V, Stern A, Monteiro HP. Nitric oxide and cGMP activate the Ras-MAP kinase pathway-stimulating protein tyrosine phosphorylation in rabbit aortic endothelial cells. Free Radic Biol Med. 2003;35:381–396. doi: 10.1016/S0891-5849(03)00311-3. [DOI] [PubMed] [Google Scholar]
- Richard DE, Berra E, Pouyssegur J. Angiogenesis: how a tumor adapts to hypoxia. Biochem Biophys Res Commun. 1999;266:718–722. doi: 10.1006/bbrc.1999.1889. [DOI] [PubMed] [Google Scholar]
- Ryu YK, Lee YS, Lee GH, Song KS, Kim YS, Moon EY. Regulation of glycogen synthase kinase-3 by thymosin beta-4 is associated with gastric cancer cell migration. Int. J. Cancer. 2012;131:2067–2077. doi: 10.1002/ijc.27490. [DOI] [PubMed] [Google Scholar]
- Safer D, Elzinga M, Nachmias VT. Thymosin beta 4 and Fx, an actin-sequestering peptide, are indistinguishable. J. Biol. Chem. 1991;266:4029–4032. [PubMed] [Google Scholar]
- Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF- 1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: using two hands to flip the angiogenic switch. Cancer Metastasis Rev. 2000;19:59–65. doi: 10.1023/A:1026544214667. [DOI] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- Sosne G, Siddiqi A, Kurpakus-Wheater M. Thymosin-beta4 inhibits corneal epithelial cell apoptosis after ethanol exposure in vitro. Invest Ophthalmol Vis Sci. 2004;45:1095–1100. doi: 10.1167/iovs.03-1002. [DOI] [PubMed] [Google Scholar]
- Tejedo JR, Cahuana GM, Ramirez R, Esbert M, Jimenez J, Sobrino F, Bedoya FJ. nitric oxide triggers the phosphatidylinositol 3-kinase/Akt survival pathway in insulin-producing RINm5F cells by arousing Src to activate insulin receptor substrate-1. Endocrinology. 2004;145:2319–2327. doi: 10.1210/en.2003-1489. [DOI] [PubMed] [Google Scholar]
- Wang WS, Chen PM, Hsiao HL, Ju SY, Su Y. Overexpression of the thymosin beta-4 gene is associated with malignant progression of SW480 colon cancer cells. Oncogene. 2003;22:3297–3306. doi: 10.1038/sj.onc.1206404. [DOI] [PubMed] [Google Scholar]
- Wang WS, Chen PM, Hsiao HL, Wang HS, Liang WY, Su Y. Overexpression of the thymosin beta-4 gene is associated with increased invasion of SW480 colon carcinoma cells and the distant metastasis of human colorectal carcinoma. Oncogene. 2004;23:6666–6671. doi: 10.1038/sj.onc.1207888. [DOI] [PubMed] [Google Scholar]
- Wink DA, Vodovotz Y, Laval J, Laval F, Dewhirst MW, Mitchell JB. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res. 2002;12:311–320. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- Zvaifler NJ. Relevance of the stroma and epithelial-mesenchymal transition (EMT) for the rheumatic diseases. Arthritis Res Ther. 2006;8:210. doi: 10.1186/ar1963. [DOI] [PMC free article] [PubMed] [Google Scholar]