Abstract
Wnt/β-catenin signaling pathway was mutated in about 90% of the sporadic and hereditary colorectal cancers. The abnormally activated β-catenin increases the cancer cell proliferation, differentiation and metastasis through increasing the expression of its oncogenic target genes. In this study, we identified an inhibitor of β-catenin dependent Wnt pathway from rhizomes of Atractylodes macrocephala Koidzumi (Compositae). The active compound was purified by activity-guided purification and the structure was identified as 2,8-dimethyl-6-hydroxy-2-(4-methyl-3-pentenyl)-2H-chromene (atractylochromene, AC). AC suppressed β-catenin/T-cell factor transcriptional activity of HEK-293 reporter cells when they were stimulated by Wnt3a or inhibitor of glycogen synthase kinase-3β. AC down-regulated the nuclear level of β-catenin through the suppression of galectin-3 mediated nuclear translocation of β-catenin in SW-480 colon cancer cells. Furthermore, AC inhibits proliferation of colon cancer cell. Taken together, AC from A. macrocephala might be a potential chemotherapeutic agent for the prevention and treatment of human colon cancer.
Keywords: Atractylochromene, Wnt/β-catenin, Colon cancer, Proliferation
INTRODUCTION
Colorectal cancer is one of the most common cancers in the world and accounts for approximately 10% of all cancer related deaths (Jemal et al., 2011). It has been reported that Wnt/β-catenin signaling pathway is mutated about 90% in the sporadic and hereditary colorectal cancers (Miyaki et al., 1994; Morin, 1999; Fearnhead et al., 2001). The mutations cause to stabilize β-catenin protein and increase the nuclear translocation of β-catenin to induce target gene expression (Chen et al., 1995; Kobayashi et al., 2000). It has been reported that regulation of nuclear translocation of β-catenin is regulated by several genes, such as importinα/β, nucleoporins, FoxM1 and galectin-3 (Song et al., 2009; Morgan et al., 2014). The nuclear translocated β-catenin interacts with TCF/LEF and other cotranscriptional factors and promoters to express its oncogenic target genes, such as c-Myc, cyclin D1 and survivin, resulting in cancer cell proliferation, metabolism, and survival (He et al., 1998; Tetsu and McCormick, 1999; Zhang et al., 2001). Thus, there is a need for developing new chemotherapeutic agents against colon cancer by inhibition of Wnt/β-catenin signaling pathway.
Galectin-3 is a multifunctional protein and present in nucleus, cytoplasm, surface, and extracellular space of cells. Galectin-3 was reported to be involved in various tumors cells transformation, proliferation, survival, differentiation and metastasis (Inohara et al., 1998; Nangia-Makker et al., 2000; Song et al., 2014). Galectin-3 also has been involved in cancer cell resistance to chemotherapy (Fukumori et al., 2007; Nangia-Makker et al., 2007; Harazono et al., 2014). In colorectal cancer cells, galectin-3 phosphorylates serine 9 residue of glycogen synthase kinase-3β (GSK-3β) to activate Wnt pathway. Galectin-3 also plays important role as a β-catenin binding partner to assist its nuclear accumulation and activates TCF activity through Akt/PI3K pathway (Song et al., 2009).
The rhizomes of Atractylodes macrocephala Koidzumi (Compositae) has been used as a medicinal herb in Korea and China for relaxing pain, digestion and diuretic (Huh, J. (1613) Donguibogam). Diverse pharmacological activities were reported such as anti-inflammatory, anti-tumor, antiulcer, antioxidant, anti-obesity and neuroprotective effects (Mori et al., 1989; Matsuda et al., 1991; Dong et al., 2008; Kim et al., 2011; Li et al., 2012; Liu et al., 2014). However, there is no study about effects of A. macrocephala on anti-colon cancer. In this study, we isolated an inhibitor of Wnt/β-catenin pathway from rhizomes of A. macrocephala and observed anti-colon cancer activity
MATERIALS AND METHODS
Reagents
Rhizomes of A. macrocephala were purchased from Kimitongsang (Seoul, Korea) in April 2009 and authenticated by Prof. K. S. Yang at College of Pharmacy, Sookmyung Women’s University. A voucher specimen (No. SPH 09003) was deposited in the herbarium of Sookmyung Women’s University. β-catenin antibody were purchased from BD Transduction Laboratories (San Jose, CA, USA). Galectin-3 antibody was purchased from Santa Cruz Biotechnology (Dallas, Texas, USA). Lamin A/C and cyclin D1 antibodies were purchased from Cell Signaling (Danvers, MA, USA), and β-actin antibody was from Sigma-Aldrich (St Louis, MO, USA). HRP-conjugated goat anti-mouse IgG and goat anti-rabbit IgG was purchased from Enzo Life Science (East Farmingdale, NY, USA). Other chemical reagents including 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), were purchased from Sigma-Aldrich.
Extraction and isolation
The dried rhizomes of A. macrocephala (10 kg) were extracted with ethyl acetate to yield crude ethyl acetate extracts (280 g). The ethyl acetate extract (5 g) was subjected to silica gel column chromatography eluting with n-hexane-ethyl acetate (100:1→1:2) to give 13 fractions. The most potent active fraction 9 (120 mg) was further purified by silica gel column chromatography by using step gradient elution with n-hexaneacetone (40:1→10:1) to yield fraction 9-4 (37 mg). Fraction 9-4 was further purified by semi-preparative HPLC (INNO RPC18 column, 5 μm particle size, 10×250 mm, 75% MeOH as eluent at 2 ml/min, detection at 260 nm UV) to give pure atractylochromene (AC, 3.5 mg). The structure was confirmed by spectroscopic analysis.
Cell culture
Human colorectal adenocarcinoma cell line, SW-480 was purchased from Korea Cell Line Bank (Seoul, Korea). The HEK-293 β-catenin reporter cell line (TOPFlash) and media control L (L-control) cell line were kindly provided by Prof. Sangtaek Oh at Kookmin University (Seoul, Korea). Wnt3a-secreting L (L-Wnt3a) cell line was purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (10 μg/ml) at 37°C.
Cell viability assay
SW-480 cells were seeded in 96-well plates at a density of 2.9×103 cells per well and incubated at 37°C in 5% CO2 environment. Cells were treated with indicated concentrations of AC for 24 h, 48 h and 72 h. 50 μl of MTT reagent (5 mg/ml in phosphate buffered saline) was added to each well and incubated for 1.5 h at 37°C. The media were removed and DMSO was added to each well. Absorbance was measured using VERSAMax Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) at 570 nm.
Preparation of Wnt3a-conditioned medium
Wnt3a-conditioned medium (Wnt3a CM) was prepared according to the manufacturer's instruction. In brief, Wnt3a-secreting L cells were cultured in DMEM with 10% FBS for 4 days. The medium was harvested and sterilized using a 0.22-μm filter. The cells were added fresh medium and cultured for another 3 days, and the medium was collected and combined with the previous medium.
Luciferase reporter assay
Cells were seeded in 96-well plates at a density of 1.1×104 cells per well and incubated at 37°C in 5% CO2 environment. Then, cells were treated with 20 μg/ml of AC for 24 h and lysed with passive lysis buffer (Promega, Madison, WI, USA). Cell lysates were incubated with luciferase assay substrate (Promega) and the luciferase activity was measured by luminometer (Molecular Devices).
Western blot analysis
SW-480 cells were seeded in 6-well plates at density of 5.4× 104 cells per well and incubated at 37°C in 5% CO2 environment for 24 h. Cells were treated with 20 μg/ml of AC for 6, 9, 12, 15 h. The cells were harvested with ice-cold PBS and the cell pellets were incubated with cytosolic lysis buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, pH7.6) and centrifuged to get cytosolic fractions. The insoluble fractions were re-suspended with nuclear lysis buffer (20 mM HEPES, 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT) and centrifuged to isolate nuclear fractions. Whole cell lysates were prepared with RIPA buffer (25 mM Tris, 150 mM NaCl, 0.5% Triton X-100). The lysates were quantified with BCA protein assay kit (Pierce, Rockford, IL, USA) and loaded on SDS-PAGE after denaturation. The proteins were blotted to PVDF membrane and probed with the primary antibodies. The membranes were then incubated with horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG (Cell Signaling, Beverly, MA, USA) as the secondary antibody and visualized using the ECL chemiluminescence (GE healthcare, Piscataway, NJ, USA). All the primary antibodies were used in 1:1000 dilution and the secondary antibodies in 1:10000.
RESULTS
Isolation of AC from A. macrocephala
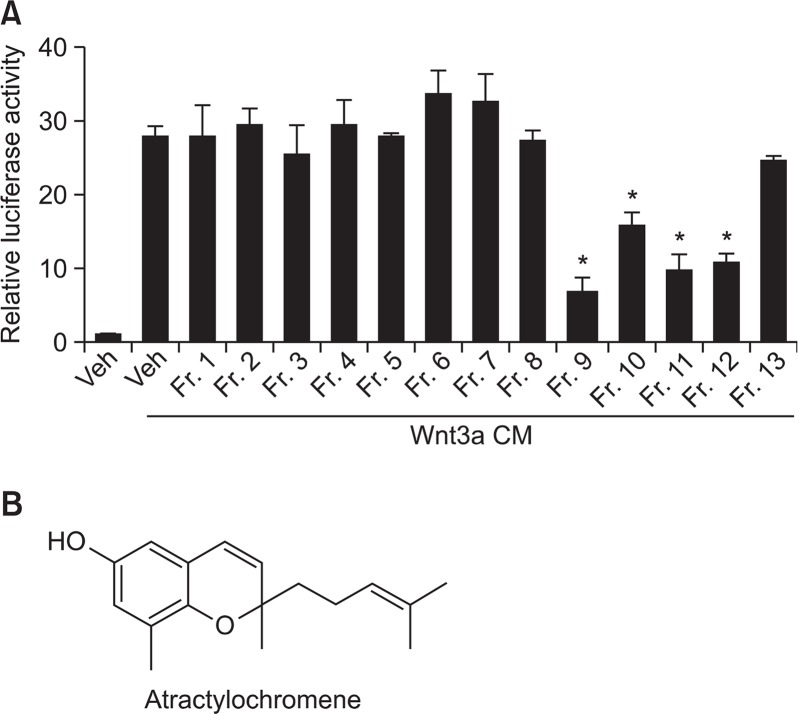
To isolate inhibitors of Wnt/β-catenin signaling from the extracts of A. macrocephala, we performed activity-guided isolation. HEK-293 reporter cells that are stably transfected with TOPFlash, a synthetic β-catenin/TCF-dependent luciferase reporter, and human Frizzled-1 expression plasmids were used as a cell-based screening system (Li et al., 2014). After treatment of Wnt3a CM and test sample to the cells for 15 h, TOPFlash reporter activity was determined by measuring firefly luciferase activity. We found that fraction 9 (20 μg/ml) obtained from column chromatography of ethyl acetate extracts showed significant inhibitory effects on reporter gene activity (Fig. 1A). From the further chromatographic purification of fraction 9, we isolated an inhibitor of Wnt/β-catenin signaling pathway. The chemical structure was identified as 2,8-dimethyl-6-hydroxy-2-(4-methyl-3-pentenyl)-2H-chromene (atractylochromene, AC) (Fig. 1B) by spectroscopic analysis and confirmed by comparison with the reported data (Resch et al., 1998).
Fig. 1.
Purification of atractylochromene as an inhibitor of TOP-Flash activity. The rhizomes of A. macrocephala were extracted with ethyl acetate as described in materials and methods. After treatment of Wnt3a CM and test samples (column fractions of ethyl acetate extracts) to TOPFlash reporter stable HEK-293 cells for 15 h, TOPFlash reporter activity was determined by measuring firefly luciferase activity (A). Structure of atractylochromene (B). Data shows the relative luciferase activity by mean ± S.D. of three experiments. The asterisks indicate a significant difference from the Wnt3a CM treated vehicle group (*p<0.01).
AC inhibits TOPFlash reporter activity
As shown in Fig. 2A, treatment of AC inhibits TOPflash activity in Wnt3a CM-treated stable reporter HEK-293 cells in a dose-dependent manner. The activity of FOPFlash, a negative control reporter with mutated β-catenin/TCF binding elements, was not altered by the treatment of AC and Wnt3a CM (data not shown). To test whether the GSK-3β was involved in the inhibition of β-catenin response transcription, we treated HEK cells with LiCl as an inhibitor of GSK-3β. AC treatment also inhibits TOPflash activity in LiCl-treated HEK-293 cells (Fig. 2B).
Fig. 2.
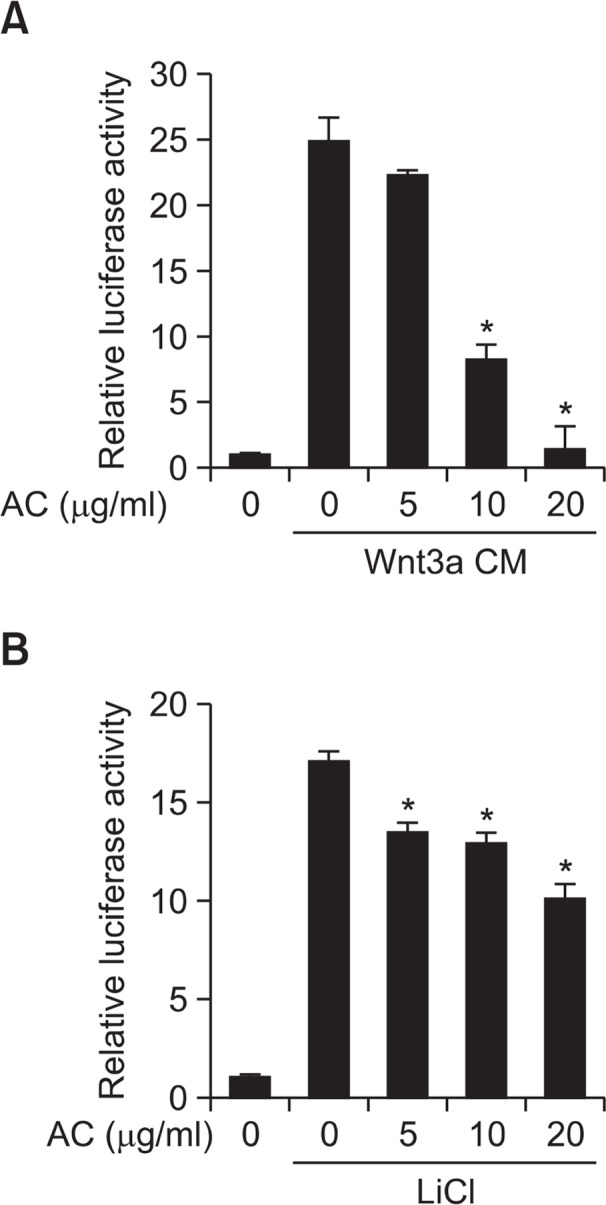
Atractylochromene inhibits TOPFlash activity. After treatment of Wnt3a CM (A) or LiCl (20 mM) (B), and atractylochromene (20 μg/ml) to the TOPFlash reporter stable HEK-293 cells for 15 h, TOPFlash reporter activity was determined by measuring firefly luciferase activity. Data shows the relative luciferase activity by mean ± S.D. of three experiments. The asterisks indicate a significant difference from the Wnt3a CM treated vehicle group (*p<0.01).
AC inhibits nuclear translocation of β-catenin in SW480 cells
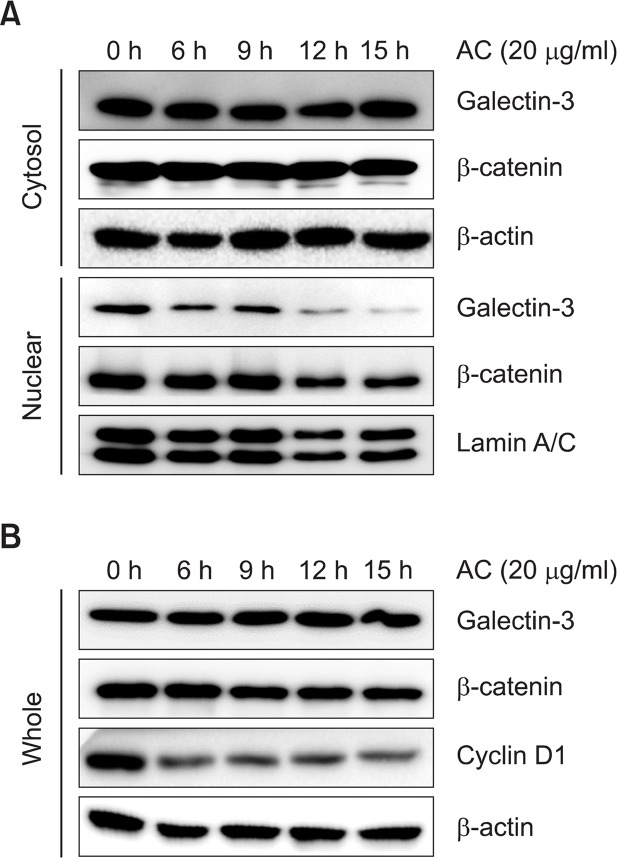
To further confirm the inhibitory effect of AC on Wnt/β-catenin signaling pathway, β-catenin constitutively activated SW-480 colon cancer cell line were used. Treatment of AC (20 μg/ml) decreased the nuclear level of β-catenin in SW-480 cells in a time-dependent manner. But the levels of β-catenin in cytosol and whole cell lysate were not affected by AC (Fig. 3A and 3B). And a target gene of β-catenin, cyclin D1 was decreased by treatment of AC in SW-480 cells. The level of galectin-3, one of the β-catenin nuclear translocation modulators, was also decreased in nuclear of AC-treated SW-480 cells, but not in cytosolic fraction and whole cell lysates (Fig. 3A, B). These data indicate that AC inhibits Wnt/β-catenin signaling pathway through modulating the nuclear translocation of β-catenin and galectin-3 in colon cancer cells.
Fig. 3.
Atractylochromene inhibits β-catenin signaling pathway in SW-480 cells. SW-480 cells were treated with 20 μg/ml of atractylochromene for the indicated time, and cells were harvested to prepare nuclear and cytosolic fractions (A), and whole cell lysates (B). Relative expression levels of target proteins were detected by western blot analysis.
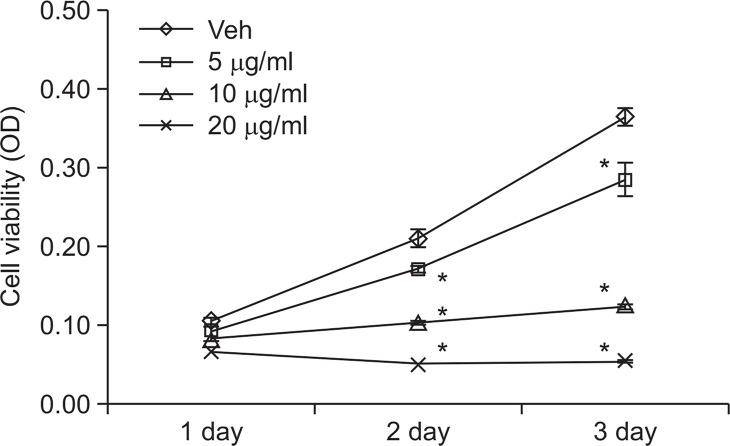
AC inhibits SW-480 cell proliferation
As AC inhibits Wnt/β-catenin signaling pathway in SW-480 cells, we have tested the effects of AC on proliferation of colon cancer cells. As shown in Fig. 4, the cell viabilities of SW-480 cells were decreased dramatically by treatment of AC in a dose dependent manner ranging from 5 to 20 μg/ml.
Fig. 4.
Atractylochromene inhibits SW-480 cell proliferation. SW-480 cells were treated with indicated dose of atractylochromene for the indicated time. Cell viability was measured by MTT assay. The asterisks indicate a significant difference from the vehicle treated group (*p<0.01).
Taken together, AC inhibits Wnt/β-catenin signaling pathway to suppress proliferation of colon cancer cells, and galectin-3 is maybe involved in its action mechanism.
DISCUSSION
It has been reported that about 90% of Wnt/β-catenin signaling pathway is mutated in sporadic and hereditary colorectal cancers (Miyaki et al., 1994; Jemal et al., 2011). The mutated Wnt/β-catenin pathway causes to stabilize and abnormally activates β-catenin signal (Chen et al., 1995). The aberrant activation of β-catenin increases its translocation to nucleus (Kobayashi et al., 2000), and increases the expression of oncogenic target gene, such as cyclin D1 (Tetsu and McCormick, 1999) in colorectal cancer. Thus, targeting of Wnt/β-catenin signaling pathway is a good strategy for developing new chemotherapeutic agents against colon cancer. We have tried to find new modulators of Wnt/β-catenin signaling pathway from medicinal plants by using cell-based reporter assay as screening system. We purified an active compound from the ethyl acetate extracts of A. macrocephala and identified the structure as a branched chromene derivative, AC. AC inhibits TOPFlash activity in a dose-dependent manner (Fig. 2) and suppresses the expression of β-catenin target gene, cyclin D1 (Fig. 3B) in SW-480 colon cancer cells.
When Wnt pathway is activated, β-catenin can be released from the APC/Axin/GSK-3β destructive complex and translocated to the nucleus to activate expression of target genes. The nuclear translocation is regulated by several genes, such as FoxM1, importinα/β, nucleoporins and galectin-3. Galectin-3 is associated with the development of several cancers including colorectal cancer (Song et al., 2014). Treatment of AC inhibits nuclear translocations of β-catenin and galectin-3 in SW-480 cells (Fig. 3A) to suppress β-catenin oncogenic target gene, cyclin D1. This parallel nuclear level of β-catenin and galectin-3 means that AC modulates nuclear translocation of β-catenin by using galectin-3 as a binding partner for translocation (Fig. 3). Galectin-3 was reported to accumulate β-catenin in human colon cancer by regulating GSK-3β activity (Song et al., 2009). An inhibitor of GSK-3β, LiCl can increase the nuclear accumulation of β-catenin through suppressing degradation of β-catenin in cytoplasm. AC significantly inhibits LiCl-induced TOPFlash activities, but not stronger than that observed in Wnt3a-induced system (Fig. 2B). Synthetic thiodigalactosides (Delaine et al., 2008) and modified citrus pectin (Nangia-Makker et al., 2002) have been developed as specific inhibitors of galectin-3 to suppress cancers. Recent review summarized galectin-3 inhibitors and their application to cancer therapy (Blanchard et al., 2014; Nangia-Makker et al., 2007). Most of the reported galectin-3 inhibitors have carbohydrate-based structures and genistein was reported to induce cell cycle arrest in galectin-3 mediated manner (Lin et al., 2000). This is the first report on the galectin-3 mediated regulation of Wnt/β-catenin signaling by plant-derived chromene compound. However, the exact regulation mechanism should be further investigated.
AC has been reported as anti-inflammatory and anti-oxidant agent (Resch et al., 1998; Li et al., 2012). AC also have inhibitory effects on 5-lipoxygenase and cyclooxygenase-1 activities (Resch et al., 1998). However, there is no report about effects of AC on colon cancer cell proliferation. In this study, we report that AC has inhibitory effects on colon cancer cell proliferation through inhibiting Wnt/β-catenin signaling pathway and its target gene cyclin D1 expression (Fig. 4). In colon cancer, β-catenin directly up-regulates cyclin D1 expression to promote proliferation of colon cancer cell (Tetsu and Mc-Cormick, 1999).
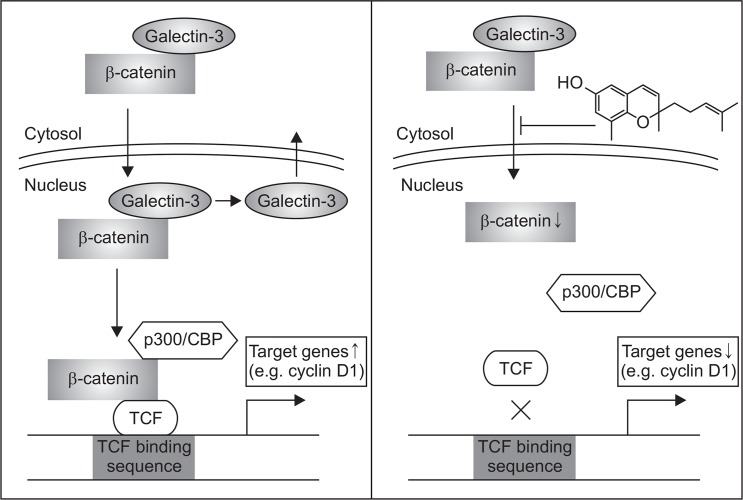
In conclusion, AC purified from A. macrocephala inhibits cyclin D1 expression through inhibiting nuclear translocation of β-catenin and galectin-3 to inhibit colon cancer cell proliferation (Fig. 5). AC may be a new lead compound for the development of therapeutic agents against colon cancer through down-modulating Wnt/β-catenin signaling pathway.
Fig. 5.
Schematic diagram illustrating the proposed reaction point at which AC blocks the Wnt/β-catenin pathway in colon cancer cells.
Acknowledgments
This work was supported by Sookmyung Women’s University Research Grant in 2013.
REFERENCES
- Blanchard H, Yu X, Collins PM, Bum-Erdene K. Galectin-3 inhibitors: a patent review (2008-present) Expert Opin Ther Pat. 2014;24:1–13. doi: 10.1517/13543776.2014.947961. [DOI] [PubMed] [Google Scholar]
- Chen HC, Chou CK, Lee SD, Wang JC, Yeh SF. Active compounds from Saussurea lappa Clarks that suppress hepatitis B virus surface antigen gene expression in human hepatoma cells. Antiviral Res. 1995;27:99–109. doi: 10.1016/0166-3542(94)00083-K. [DOI] [PubMed] [Google Scholar]
- Delaine T, Cumpstey I, Ingrassia L, Le Mercier M, Okechukwu P, Leffler H, Kiss R, Nilsson UJ. Galectin-inhibitory thiodigalactoside ester derivatives have antimigratory effects in cultured lung and prostate cancer cells. J Med Chem. 2008;51:8109–8114. doi: 10.1021/jm801077j. [DOI] [PubMed] [Google Scholar]
- Dong H, He L, Huang M, Dong Y. Anti-inflammatory components isolated from Atractylodes macrocephala Koidz. Nat Prod Res. 2008;22:1418–1427. doi: 10.1080/14786410801931629. [DOI] [PubMed] [Google Scholar]
- Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- Fukumori T, Kanayama HO, Raz A. The role of galectin-3 in cancer drug resistance. Cancer Metastasis Rev. 2007;10:101–108. doi: 10.1016/j.drup.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harazono Y, Nakajima K, Raz A. Why anti-Bcl-2 clinical trials fail: a solution. Cancer Metastasis Rev. 2014;33:285–294. doi: 10.1007/s10555-013-9450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Inohara H, Akahani S, Raz A. Galectin-3 stimulates cell proliferation. Exp Cell Res. 1998;245:294–302. doi: 10.1006/excr.1998.4253. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kim CK, Kim M, Oh SD, Lee SM, Sun B, Choi GS, Kim SK, Bae H, Kang C, Min BI. Effects of Atractylodes macrocephala Koidzumi rhizome on 3T3-L1 adipogenesis and an animal model of obesity. J Ethnopharmacol. 2011;137:396–402. doi: 10.1016/j.jep.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Honma T, Matsuda Y, Suzuki Y, Narisawa R, Ajioka Y, Asakura H. Nuclear translocation of beta-catenin in colorectal cancer. Br. J. Cancer. 2000;82:1689–1693. doi: 10.1054/bjoc.1999.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lee HJ, Ahn YH, Kwon HJ, Jang CY, Kim WY, Ryu JH. Tussilagone suppresses colon cancer cell proliferation by promoting the degradation of beta-catenin. Biochem Biophys Res Commun. 2014;443:132–137. doi: 10.1016/j.bbrc.2013.11.062. [DOI] [PubMed] [Google Scholar]
- Li X, Lin J, Han W, Mai W, Wang L, Li Q, Lin M, Bai M, Zhang L, Chen D. Antioxidant ability and mechanism of rhizoma Atractylodes macrocephala. Molecules. 2012;17:13457–13472. doi: 10.3390/molecules171113457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HM, Moon BK, Yu F, Kim HR. Galectin-3 mediates genistein-induced G(2)/M arrest and inhibits apoptosis. Carcinogenesis. 2000;21:1941–1945. doi: 10.1093/carcin/21.11.1941. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhao H, Ji ZH, Yu XY. Neuroprotection of atractylenolide III from atractylodis macrocephalae against glutamate-induced neuronal apoptosis via inhibiting caspase signaling pathway. Neurochem Res. 2014;39:1753–1758. doi: 10.1007/s11064-014-1370-7. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Li YH, Taniguchi K, Yamahara J, Tamai Y. [Imaging analysis of antiulcer action and the active constituent of Atractylodis rhizoma] Yakugaku Zasshi. 1991;111:36–39. doi: 10.1248/yakushi1947.111.1_36. [DOI] [PubMed] [Google Scholar]
- Miyaki M, Konishi M, Kikuchi-Yanoshita R, Enomoto M, Igari T, Tanaka K, Muraoka M, Takahashi H, Amada Y, Fukayama M, et al. Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res. 1994;54:3011–3020. [PubMed] [Google Scholar]
- Morgan RG, Ridsdale J, Tonks A, Darley RL. Factors affecting the nuclear localization of beta-catenin in normal and malignant tissue. J Cell Biochem. 2014;115:1351–1361. doi: 10.1002/jcb.24803. [DOI] [PubMed] [Google Scholar]
- Mori H, Xu Q, Sakamoto O, Uesugi Y, Koda A, Nishioka I. Mechanisms of antitumor activity of aqueous extracts from Chinese herbs: their immunopharmacological properties. Jpn J Pharmacol. 1989;49:423–431. doi: 10.1254/jjp.49.423. [DOI] [PubMed] [Google Scholar]
- Morin PJ. beta-catenin signaling and cancer. Bioessays. 1999;21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangia-Makker P, Nakahara S, Hogan V, Raz A. Galectin-3 in apoptosis, a novel therapeutic target. J Bioenerg Biomembr. 2007;39:79–84. doi: 10.1007/s10863-006-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch M, Steigel A, Chen ZL, Bauer R. 5-Lipoxygenase and cyclooxygenase-1 inhibitory active compounds from Atractylodes lancea. J Nat Pro. 1998;61:347–350. doi: 10.1021/np970430b. [DOI] [PubMed] [Google Scholar]
- Song L, Tang JW, Owusu L, Sun MZ, Wu J, Zhang J. Galectin-3 in cancer. Clin. Chim. Acta. 2014;431:185–191. doi: 10.1016/j.cca.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Song S, Mazurek N, Liu C, Sun Y, Ding QQ, Liu K, Hung MC, Bresalier RS. Galectin-3 mediates nuclear betacatenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009;69:1343–1349. doi: 10.1158/0008-5472.CAN-08-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich SM, Fields JZ, Boman BM. Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;61:8664–8667. [PubMed] [Google Scholar]