Abstract
In this study, we investigated the inhibitory activities on gastritis and gastric ulcer using liriodendrin which is a constituent isolated from Kalopanax pictus. To elucidate its abilities to prevent gastric injury, we measured the quantity of prostaglandin E2 (PGE2) as the protective factor, and we assessed inhibition of activities related to excessive gastric acid be notorious for aggressive factor and inhibition of Helicobacter pylori (H. pylori) colonization known as a cause of chronic gastritis, gastric ulcer, and gastric cancer. Liriodendrin exhibited higher PGE2 level than rebamipide used as a positive control group at the dose of 500 μM. It was also exhibited acid-neutralizing capacity (10.3%) and H+/K+-ATPase inhibition of 42.6% (500 μM). In pylorus-ligated rats, liriodendrin showed lower volume of gastric juice (4.38 ± 2.14 ml), slightly higher pH (1.53 ± 0.41), and smaller total acid output (0.47 ± 0.3 mEq/4 hrs) than the control group. Furthermore liriodendrin inhibited colonization of H. pylori effectively. In vivo test, liriodendrin significantly inhibited both of HCl/EtOH-induced gastritis (46.9 %) and indomethacin-induced gastric ulcer (46.1%). From these results, we suggest that liriodendrin could be utilized for the treatment and/or protection of gastritis and gastric ulcer.
Keywords: Liriodendrin, Prostaglandin E2, H+/K+-ATPase, Gastritis, Gastric ulcer
INTRODUCTION
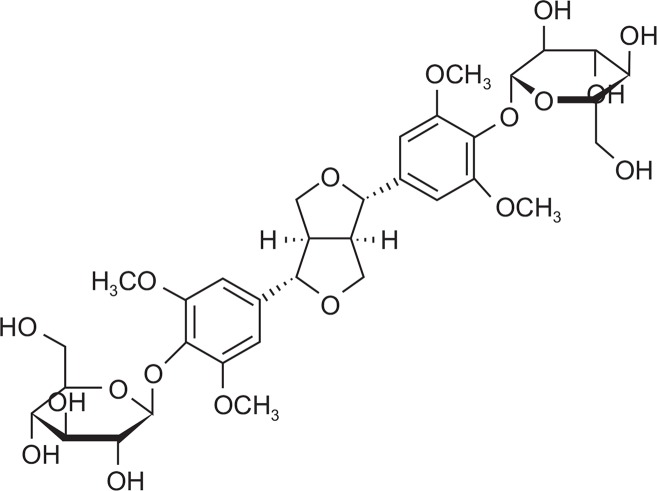
Gastritis means the gastric mucous membrane inflammation having symptoms of heartburn, nausea, vomit, indigestion, displeasure of the upper part of the belly after meals and etc. (Bebb et al., 2003) and is caused by the imbalance between defensive and offensive factors which keep the gastric intramucosal wall (Shay et al., 1945). The National Health Insurance Corporation reported in 2010 that approximately 5.4 million patients visit hospitals due to gastritis and duodenitis (Korean Standard Classification of Diseases: k29) which was ranked 6th in the statistics of disease (Health insurance statistics bulletin, 2010). There are four defense mechanisms for gastric lesions; prostaglandin (PG) analogue (Rovert et al., 1979; Hollander et al., 1984), sucralfate (Tarmawski et al., 1985), sulfhydryl compound and bismuth compound. PGE2 biosynthesis is the representative defensive factor and is known as the important one to maintain the resistance of the mucous membrane. It increases the mucus and bicarbonate secretion, guarantees the healthy circulation of mucous membrane by keeping the resistance of its microvessel endothelial cell and maintaining the resistance of the epithelial cell (Rovert, 1979). H+/K+ATPase is called the proton pump which secretes gastric acid in the stomach wall cell. Gastric acid is induced by acethylcholine, gastrin and histamine. There are receptors on the surface of membrane in the wall cell and the combinatorial material of each receptor is carried and combined through the blood flow. Then, H+/K+ATPase activated on the surface of membrane in the nanocyte is released to the stomach and increases the acidity with the medium of transmitter substance in the cell (Seol, 2006; Walker et al., 2012). In order effectively to control the gastric acid output it is adequate to make use of proton pump inhibitors (PPI) directly inhibiting H+/K+ATPase which is at the last stage of gastric acid output rather than histamine receptor inhibitor (Wallmark et al., 1985). Helicobacter pylori (H. pylori) causes the inflammation by cytokine-induced production through the direct contact with gastric epithelial cell. Gastric mucosal injury by H. pylori is divided into two: direct and indirect injury. Direct injury comes from various types of toxic factors generated by H. pylori itself and indirect one comes from the changes of physiologic action of gastric mucous membrane or from the chronic generation of inflammatory response to gastric mucous membrane (Itoh et al., 1999). H. pylori infection influences several pathologic alterations, including atrophic gastritis, peptic ulcer, gastric adenocarcinoma, and gastrointestinal motility which results in indigestion (Muhammad et al., 2013). Digestion is the driving force enabling the human body to maintain the daily life. If digestion troubles, this results in interfering with disintegration, absorption, distribution and metabolism of nutrients and accumulating the poison into the human body put under stress, which leads to disease. In general, H. pylori gastritis causes acute gastritis having intense inflammation and develops gastric ulcer or chronic gastritis in process of time. However, acute gastritis mostly does not have detectable symptoms. Longtime propagation of H. pylori leads the increase of lymphocyte in plasma and of polymorphonuclear and granular leukocytes in mucous layer. This chronic state of inflammatory reaction can result in atrophic mucosa and metaplasia and 15% of the patients can get stomach ulcer and duodenal ulcer and even can be the cause of gastric cancer (Van Doorn et al., 1998). Liriodendrin [(+)-Syringaresinol di-O-β-D-glucopyranoside] is the active constituent of Kalopanax pictus having the structure of lignan of disaccharide (Fig. 1), and known as the treatment for liver protection, edema control and analgesia effect (Lee et al., 1995; Jung et al., 2003). Recent study is reported that liriodendrin is effective on heart protection such as arrhythmia reduction, ventricular fibrillation control and etc (Feng et al, 2010).
Fig. 1.
Chemical structure of liriodendrin isolated from Kalopanax pictus.
In this study, we investigated the level of PGE2, cytotoxicity to the gastric cancer cells, acid-neutralizing capacity, H+/K+ATPase activity, gastric secretion, H. pylori colonization inhibitory activity, gastric emptying activity. In vivo test, HCl/ EtOH-induced gastritis and indomethacin-induced gastric ulcer experiment were performed.
MATERIALS AND METHODS
Liriodendrin
Liriodendrin is offered by Dr. Kim Young Ho, College of Pharmacy, Choongnam University, Daejeon, Korea (2009).
Reagents and laboratory equipments
Ampicillin, 3-(4,5-dimethylthiazol-2,5-diphenyltetrazolium bromide (MTT), trypan blue, cimetidine, and hydrotalcite were purchased from Sigma (Sigma-Aldrich Inc., MO, USA). Cell culture medium and reagents, such as RPMI 1640, fetal bovine serum (FBS), penicillin/streptomycin, and trypsin-EDTA were purchased from GIBCO (Invitrogen Inc., NY, USA). AnaeroPack Campylo, PGE2 assay kit was obtained from Mitsubishi Gas Cjemical Co., Inc. and R&D Systems, Inc. respectively. HCl, EtOH, and other solvents were purchased from Duksan pure Chemical Co. Ltd. (Kyunggi-do, Korea). All other reagents were the pharmaceutical or analytical grade. UV-spectrophotometric plate reader (ASYS UVM340), centrifuge 5810R (eppendorf), pH meter (IQ scientific instruments Inc), CO2 incubator (Forma scientific), inverted microscope (Olympus), liquid nitrogen dewars (CHART MVE) were used as laboratory equipments.
Animals
Male Sprague-Dawley rats, weighing 170–190 g, were purchased from Samtako, Kyunggi-do, Korea, and were acclimatized to standard laboratory conditions (22 ± 2°C, 55 ± 5% humidity and 12 h light/dark cycle) for 14 days in the animal facility in Duksung Women’s University. The animals were allowed free access to food (standard pellet diet) and water ad libitum. All of this study was carried out in compliance with the Testing Guidelines for Safety Evaluation of Drugs (Notification No. 1999–61) issued by the Korea Food and Drug Administration, the Good Laboratory Practice Regulations for Non-clinical Laboratory Studies (Notification No. 2000–63) issued by the Korea Food and Drug Administration, and the Principles of Good Laboratory Practice issued by the Organization for Economic Cooperation and Development.
Gastric cancer cell lines and H. pylori strain
AGS gastric cancer cells were obtained from Korean Cell Line Bank (KCLB, Seoul, Korea). These cells were cultured with RPMI-1640 containing 10% FBS, penicillin (100 units/ mL), and streptomycin (100 lg/mL) in a 5% CO2 humidified incubator at 37°C. For subculture, AGS cells were rinsed twice with phosphate buffered saline (PBS, pH 7.4) to remove all traces of serum (which can inhibit trypsin) and were subdivided using 0.05% trypsin with 0.53 mM EDTA. H. pylori strain (ATCC 43504) was obtained from American Tissue Culture Collection (ATCC, Rockville, MD, USA).
Measurement of prostaglandin E2
The production of PGE2, a protective factor against the gastric lesion, was measured using commercially available assay (PGE2: R&D Systems, KGE004, Wiesbaden, Germany). As a blank we measured cell culture medium from wells without cells that had been treated the same way as the samples according to the procedure described by the manufacturer. Briefly, AGS cells were cultured in 96-well culture plates (1×104/ well). The supernatant (100 μl) of cell culture that treated with samples were added to the plate, and mixed with 50 μl of a primary mouse monoclonal PGE2-antibody and PGE2-conjugated with horseradish peroxidase (HRP), and were incubated for 2 hrs on the shaker at room temperature. And then the supernatant was discarded and washed 4 times. The mixture with hydrogen peroxide and chromagen (tetramethylbenzidine), a substrate, was added to all wells for the termination of the reaction, and absorbance was read at 450 nm within 30 min.
MTT assay
Cytotoxicity to AGS cells (gastric cancer cell lines) was examined using the modified MTT assay. Cells were seeded at a density of 1×104 cells/well in 96-well culture plates (Corning Inc., USA), and were cultured for 24 hrs at 37°C in a 5% CO2 humidified incubator. The samples were added to the plate in the manner of serial dilution and incubated for 24 hrs. MTT agent was added at a final concentration of 0.5 mg/ml and the plates were incubated for 4 hours at 37°C. After discarding all media from the plates, 0.1 ml of DMSO was added to all wells. The plates were held for 5 min at room temperature with shaking in order to completely dissolve formazan. The absorbance of the MTT formazan was determined at 540 nm using a UV-spectrophotometric plate reader (Choi et al., 2004).
Acid-neutralizing capacity
The sample (0.5 mg) was added to 50 μl of 0.1 N HCl and incubated for 1 hr at 37°C with shaking. The acid-neutralizing capacity was determined by titration with 0.1 N NaOH using methyl orange as the indicator. Hydrotalcite was used as the positive control.
H+/K+-ATPase activities
H+/K+-ATPase activity was measured as previously described by Saccomani (1981). In other words, the frozen mouse gastric membrane protein determined by the bradford law and were tested with 300 μg protein. 20 mM Tris-HCl (pH 7.4) and sample was added to the assay medium at 37°C for 30 min makes incubation. 2 mM MgCl2, 2 mM ATP, 2 mM KCl, and 20 mM Tris-HCl (pH 7.4) containing the substrate mixture was then added 0.5 mL each incubated at 37°C for 30 min. Here of 22% trichloroacetic acid 0.2 mL reaction termination at 4°C for 15 min and centrifuged at 3,000 rpm and take the supernatant generated by ATP hydrolysis are subjected to the determination of inorganic phosphate. Determination of inorganic phosphate obtained through over the course of the molybdic acid solution 0.4 mL in the supernatant was added, absorbance was measured at 400 nm.
Gastric secretion
After 24 hrs fasting with free access to water prior to the experiment, the rats were administered with the samples in-traduodenally (Shay et al., 1945). Four hours after pyloric ligation, the animals were sacrificed, and the stomach contents were collected and centrifuged at 3,000 rpm for 10 min. The total volume of gastric juice and pH were measured, and the acid output (mEq/4hrs) was determined by titration with 0.1 N NaOH using phenol red as an indicator.
Anti-H. pylori activity
The inhibitory effects on the growth of H. pylori were investigated. 600 μl of samples were mixed in a petri dish with 5.4 ml brucella agar medium containing 7% horse serum. H. pylori (5×105 CFU) was seeded in this medium and placed for 3 days in an incubator at 37°C (AnaeroPak Campylo : 85% N2, 10% CO2, and 5% O2). H. pylori viability was determined by colony counts after 3 days incubation. Ampicillin was used as a positive control (Bae et al., 2001; Kim et al, 2003).
Evaluation of gastric emptying
Using the method reported by Scarpignato et al. (1980), the mice were fasted for 24 hrs with free access to water prior to the experiment. Gastric emptying was performed by administering a 0.05% (w/v) phenol red solution (0.5 ml/mouse), 30 min after treatment with samples. 20 min later, the mice were sacrificed, and the stomach was immediately removed and cut into several pieces in 5 ml of 0.01 N NaOH, followed by addition of 0.2 ml 20% trichloroacetic acid in 1 ml of homogenate. The mixture was centrifuged for 10 min at 10,000 rpm, and the supernatant (0.05 ml) added to 0.5 N NaOH (0.2 ml). The absorbance of this mixed solution was measured with a spectrometer at a wavelength of 560 nm. The gastric emptying rate (%) was calculated=100-(A/B)×100, where A is the amount of phenol red remaining in the stomach 20 min after administration of the phenol red solution, and B is the amount of phenol red in the stomach immediately after administration of the phenol red solution.
HCl/EtOH-induced gastritis in rats
After 24 hrs fasting with free access to water prior to the experiment, the samples were administered orally to the rats. Thirty minutes later, 1 ml of a HCl/EtOH solution (150 mM HCl in 60% EtOH) was administered orally. After 1 hr, each animal was sacrificed by ether inhalation and its stomach was excised, inflated by injecting 2 ml of normal saline and then fixed for 30 min in a 2% formalin solution. The stomach was incised along the greater curvature and the glandular portion was examined for hemorrhage. The length (mm) of each lesion was measured under a dissecting microscope (10X), and the total value is expressed as the lesion index (Mizui and Dodeuchi, 1983). The inhibition rate (%) was calculated=[lesion length (control)-lesion length (drug)]/[lesion length (control)]×100.
Indomethacin-induced gastric ulcer in rats
Using the method reported by Kasuya et al. (1979), the rats were fasted for 24 hrs with free access to water prior to the experiment. The sample was dosed orally and 30 min later, indomethacin (35 mg/kg in 50 mM sodium biocarbonate solution) was injected subcutaneously. The animals were sacrificed 7 hrs after the indomethacin injection. The excised stomach was placed in a 2% formalin solution for 30 min. The stomach was incised along the greater curvature and the glandular portion was examined for hemorrhage. Each lesion was measured and the total value is expressed as the lesion index. Cimetidine was used as the positive control drug. The area (mm2) of mucosal erosive lesions was measured under a dissecting microscope (10X) and cimetidine was used as the positive control drug. The inhibition rate (%) was calculated=[lesion area (control)-lesion area (drug)]/[lesion area (control)]×100.
Statistical analysis
All experiments were carrid out more than three times. The data was analyzed using a Student’s t-test. p-values 0.05 were considered significant. When gastric lesions were induced by the various methods, the inhibitory effects of 4GBA on gastritis and gastric ulcers were determined as the inhibition ratio (%) as follows:
RESULTS AND DISCUSSION
Prostaglandin E2 measurement
PGE2 function gastric hyperacidity inhibition and preservation of cellular integrity of gastric mucous membrane, protect gastric mucous membrane cell from damage of ischemic gastric cell by increase of microvascular blood flow. The results are as follows; liriodendrin increased to 217.6 pg/ml and 297.9 pg/ml of PGE2, at doses of 250 μM, 500 μM, respectively, which increased to about 22.7%, 68.0% compared control group (177.3 pg/ml). Rebamipide, gastric mucosal membrane protect drug, increases PGE2 via cyclooxygenase-2 (COX-2) inhibition activity. Rebamipide at dose of 500 μM increased 248.4 pg/m PGE2. Given that result, we found that liriodendrin had excellent PGE2 increase effect (Fig. 2).
Fig. 2.
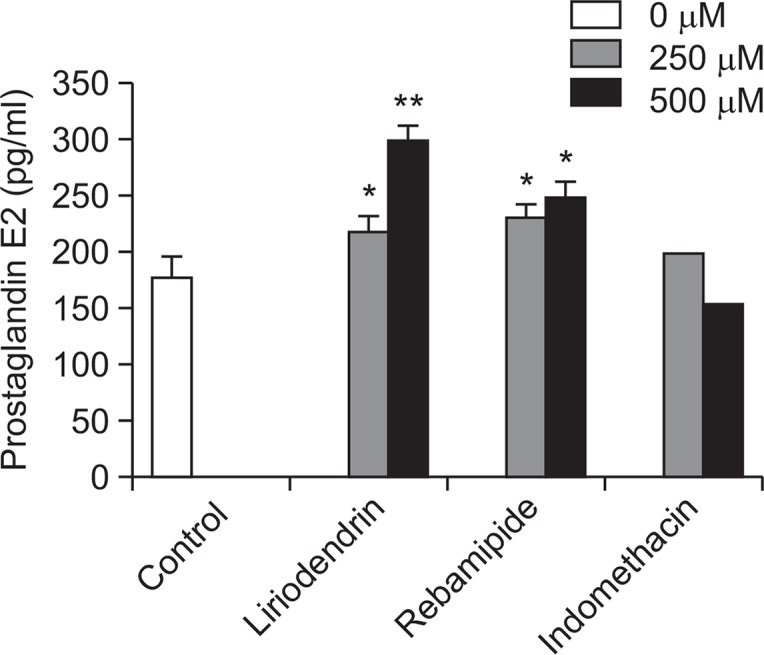
The effect of liriodendrin on prostaglandin E2 synthesis. The values are mean ± S.E.M. of 3 experiments. Significant difference *p<0.05; **p<0.01 compared to the control group.
MTT assay
MTT assay is widely used for determinig the cytotoxicity, This is the way to evaluate the activity of intracellular organelles which can get cytotoxicity quantitatively using a colorimetric method. This is the method of measuring absorbance of formazan which is produced when the succinate dehydrogenase in mitochondria reduction of MTT agent. Cells being treated liriodendrin incubated during 24 hrs. Liriodendrin revealed approximately 92.3%, 89.4%, 88.7% and 83.2% at doses of 25, 50, 100, and 200 μM, respectively (Fig. 3). From these results, liriodendrin wasn’t show significant cytotoxicity.
Fig. 3.
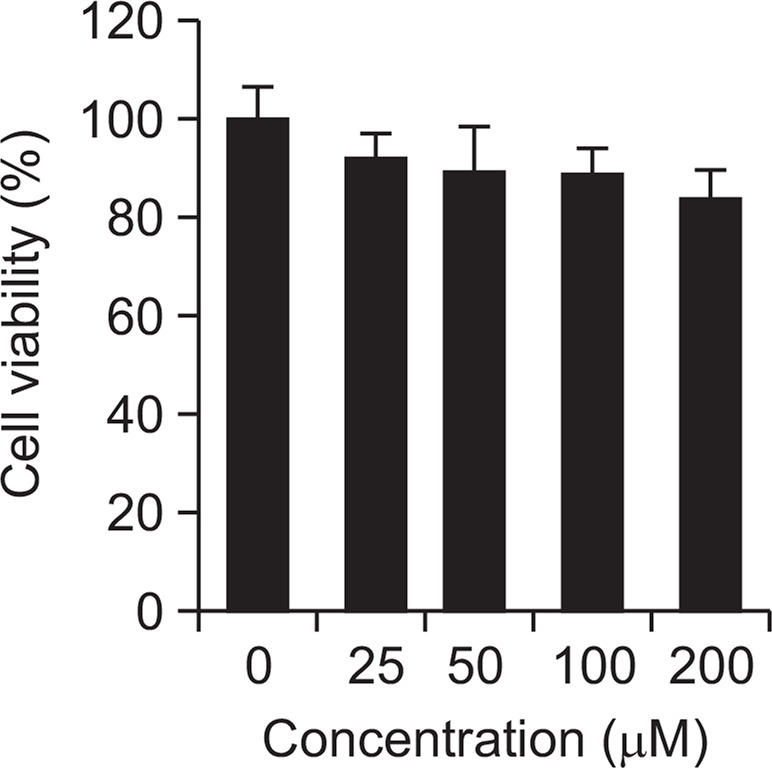
Cell viability of liriodendrin at AGS cells. The values are mean ± S.E.M. of 3 experiments.
Acid-neutralizing capacity
There is no evidence that chronic gastritis or non-ulcerative indigestive patients have over-secretion of gastric acid compare to normal subjects, but antacids is effective gastritis and gastric ulcer treatment through control of gastric acidity. Antacids neutralize gastric acid and relieve the pain promptly. Making artificial gastric juice having the same condition with gastritis, we got some antacid-neutralizing capacity in liriodendrin which is reacts with gastric acid. Liriodendrin showed 10.3% of acid-neutralizing capacity which had less capacity than hydrotalcite 95.62% as the positive control, but we assume it could be effective in a overdose or long-term use (Table 1).
Table 1.
Acid-neutralizing capacity of liriodendrin
| Material | NaOH consumption volume (μl) | Inhibition (%) |
|---|---|---|
| Control | 103.0 ± 2.0 | - |
| Liriodendrin | 92.4 ± 2.1* | 10.3 |
| Hydrotalcite | 56.2 ± 1.6** | 45.4 |
The values are mean ± S.E.M. of 3 experiments. Significant difference
p<0.01;
p<0.001 compared to the control group.
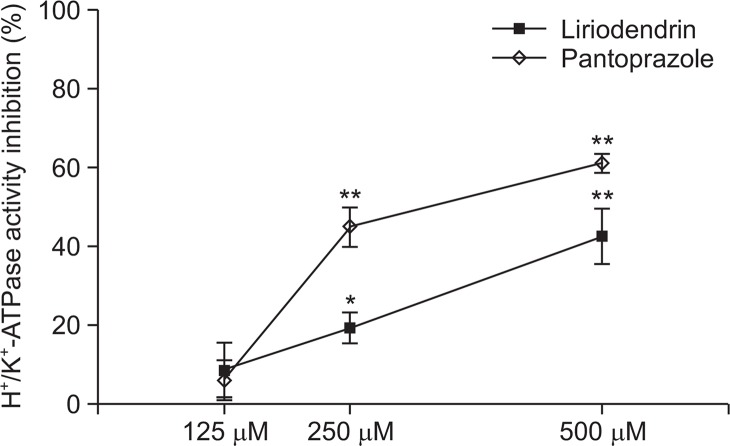
H+/K+-ATPase activity
H+/K+-ATPase pump is gastric acid secretion enzyme in parietal cell, also known as proton pump. Proton pump inhibitors, omeprazole was introduced in the market first in 1988, used widely for over 20 years which is proved effective in treatment of mucous membrane and improvement of symptoms in peptic ulcer and reflux esophagitis (Raghunath et al, 2005). PPI per orally is absorbed in intestine and reach in parietal cell of fundic glands membrane by blood flow, which combine cystein residue of H+/K+-ATPase which is activated by gastric acid in the secretary tubule, therefore inhibit acid secretion effectively by inhibition of this enzyme. By measurement of H+/K+-ATPase activity, pantoprazole (control) showed. 61.3% (500 μM), 45.1% (250 μM) inhibition effect, in case of liriodendrin 42.6% (500 μM), 19.3% (250 μM). We found liriodendrin has gastric acid secretion inhibit effect by the H+/K+-ATPase activity (Fig. 4).
Fig. 4.
Inhibitory effect of liriodendrin on H+/K+-ATPase activity. The values are mean ± S.E.M. of 3 experiments. Significant difference *p<0.05; **p<0.01 compared to the control group.
Gastric secretion in pylorus-ligated rats
Ulcers may arise when there is an imbalance between the aggressive and defensive factors that renders the mucosa susceptible to damage (Mcquaid and Isenberg, 1992). In the first stage of gastric ulcer, pylorus ligation induces strong stimulation of gastric secretion. To investigate the gastric lesions inhibition mechanism, gastric juice volume, pH and total acid output were measured. Compared to control group (8.45 ± 1.0), liriodendrin (100 mg/kg) decreased volume of gastric juice (4.38 ± 2.14 ml, inhibition of 48.16%), increased to pH to 1.53 ± 0.41 compared to control group (1.13 ± 0.05). Total acid output of liriodendrin significantly decreased to 0.47 ± 0.30 mEq/4hrs (Table 2).
Table 2.
Effect of liriodendrin on gastric secretion in pylorus-ligated rats
| Treatment | Dose (mg/kg) | Volume (ml) | pH | Total acid output (mEq/4 hrs) |
|---|---|---|---|---|
| Control | - | 8.5 ± 1.0 | 1.1 ± 0.1 | 1.3 ± 0.4 |
| Liriodendrin | 100 | 4.5 ± 2.1** | 1.5 ± 0.4* | 0.5 ± 0.3** |
| Cimetidine | 150 | 3.2 ± 1.7*** | 2.5 ± 1.3* | 0.3 ± 0.1*** |
The values are mean ± S.E.M. of 6 animals. Significant difference.
p<0.05;
p<0.01;
p<0.001 compared to the control group.
Colonization inhibiting effect at H. pylori
One of the aggressive factors is infection by H. pylori, which is also a cause of gastric cancer. Gastric cancer is a known cause of death from malignant disease. Gastric mucosa infected with H. pylori exhibit increases in reactive oxygen species (ROS), which induce DNA damage. Eradication of H. pylori prevents gastritis, as well as gastric cancer. As Antibiotic activities of liriodendrin (100 μM) against H. pylori was detected, the number of colony decreased (+) comparing control group (+++) (Table 3). Result demonstrating its potential to decrease the risk of H.pylori pathogen-derived gastritis and inhibit the development of gastric cancer.
Table 3.
Colonization inhibiting effect by liriodendrin for H. pylori
| Material | Dose (μM) | Colonization |
|---|---|---|
| Control | - | +++ |
| Liriodendrin | 10 | ++ |
| 50 | + | |
| 100 | + | |
| Ampicilin* | 100 | - |
μg/ml.
colonies (4–5×105CFU);
colonies (2–4×105CFU);
colonies (0–2×105CFU);
-: none.
Samples (600 μl) of each concentration were injected to 5.4 ml of brucella agar medium contained 7% horse serum in the petri dish. H. pylori of 5×105 CFU was seeded in this media and then incubated for 3 days at 37°C incubator (AnaeroPak Campylo: 85% N2, 10% CO2, 5% O2).
Gastric emptying
The typical symptoms of peptic ulcer are epigastrium distress, epigastrium pain, heart burn, heavy stomach and anorexia. Most of chronic gastritis patients have functional disease also therefore they are treated by combination of antacids and prokinetic drugs because they feel early satiety and postprandial inflation. Gastric emptying experiment has been done for indigestion mainly caused by poor gastrointestinal motility (Malagelada and Stanghellini,1985). Mosapride as a positive control is prokinetic drug which affect 5HT4 receptor, so it boosts gastric emptying and esophageal movement, increases lower esophageal sphincter pressure, stimulates the whole intestine movement (Kato et al, 1995; Odaka et al., 2006). The result of test showed gastric emptying of control was 50.2%, mosapride 79.4%. Liriodendrin showed gastric emptying of 66.3%, it proved pro-kinetic effect of liriodendrin (Fig. 5).
Fig. 5.
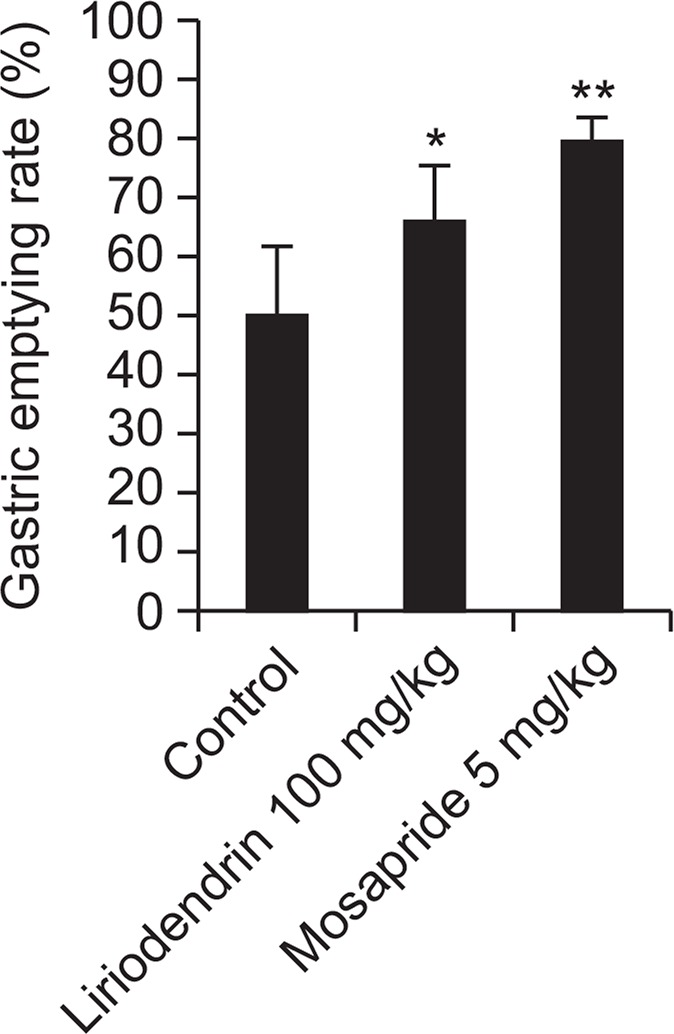
The effect of liriodendrin on gastric emptying in mice. The values are mean ± S.E.M. of 6 animals. Significant difference *p< 0.05; **p<0.01 compared to the control group.
HCl/EtOH-induced gastritis
Oral administration of EtOH accelerates gastric mucosal necrosis and apoptosis by damage in gastric mucosal defense system. Thus, EtOH-induced gastritis in vivo model is considered as a reliable tool of the pathogenesis of gastritis. In this study, we investigated the effects of liriodendrin against HCl/ EtOH-induced gastritis in rats. Cimetidine at a dose of 150 mg/kg as a positive control showed superior inhibition (35.7%) of gastric lesion than control group. Gastric lesion index of cimetidine and control were 62.2 ± 10.25 mm, 110.2 ± 14.65 mm, respectively. Liriodendrin significantly inhibited by 46.9 % against gastric lesion index 58.5 ± 15.55 mm (Table 4, Fig. 6). Therefore liriodenrin showed anti-ulcer activity against HCl/ EtOH-induced gastric lesions.
Table 4.
Effects of liriodendrin on HCl/EtOH-induced gastritis in rats
| Treatment | Dose (mg/kg) | Lesion index (mm) | Inhibition (%) |
|---|---|---|---|
| Control | - | 110.2 ± 14.6 | - |
| Liriodendrin | 100 | 58.5 ± 15.6*** | 46.9 |
| Cimetidine | 150 | 62.2 ± 10.3** | 35.7 |
| Hydrotalcite | 100 | 75.6 ± 22.2* | 31.4 |
The values are mean ± S.E.M. of 6 animals. Significant difference.
p<0.05;
p<0.01;
p<0.001 compared to the control group.
Fig. 6.
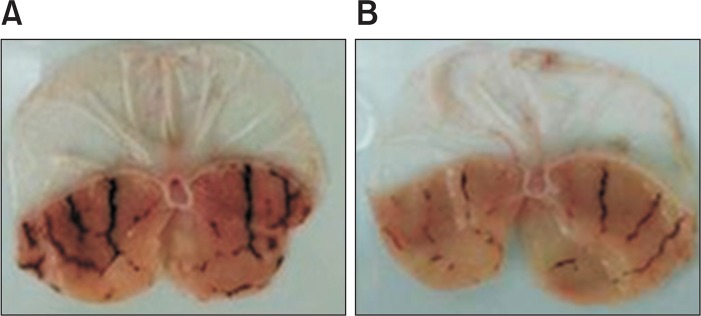
The effect of liriodendrin on HCl/EtOH-induced gastritis in rats. A: Control group; B: Liriodendrine treatment group (100 mg/kg) Typical macro view of stomachs: HCl/EtOH-induced gastric damage was observed in the gastric mucosa as elongated black-red lines parallel to the long axis of the stomach of the rats and pretreatment with liriodendrin markedly reduced the number and severity of these lesions.
Indomethacin-induced gastric ulcer
Subcutaneous injection of indomethacin induces mucosal blood flow decrease characterized by oxidative damage and gastric lesion by decrease by inhibiting PG secretion. According to study of Kunikata et al. (2001), subcutaneous injection of indomethacin significantly induced ulcerative lesions therefore we experimented that method. After administration of indomethacin, ulcerative lesions of the control group were observed at 29.7 ± 3.06 mm2. Liriodendrin (100 mg/kg) treated group were observed ulcerative lesions of 16.0 ± 2.55 mm2 and showed the inhibitory effect of 46.1%. Compared with the administration of cimetidine (14.04 ± 5.7 mm2), significant effect could be confirmed (Table 5, Fig. 7). Indomethacin, NSAIDs can induce gastric ulcer and severe gastric mucosal lesions. Liriodendrin exhibited indomethacin-induced gastric ulcer inhibitory activity therefore it showed liriodenrin has antigastric ulcer effects.
Table 5.
Effects of liriodendrin on indomethacin-induced gastric ulcer in rats
| Treatment | Dose (mg/kg) | Lesion index (mm2) | Inhibition (%) |
|---|---|---|---|
| Control | - | 29.7 ± 3.0 | - |
| Liriodendrin | 100 | 16.0 ± 2.6** | 46.1 |
| Cimetidine | 200 | 14.0 ± 5.7* | 52.7 |
The values are mean ± S.E.M. of 6 animals. Significant difference.
p<0.05;
p<0.001 compared to the control group.
Fig. 7.
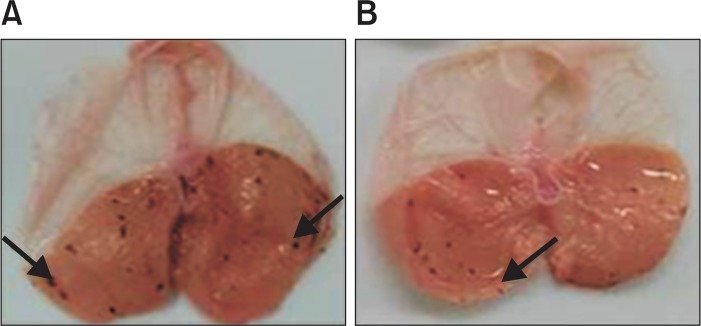
The effect of liriodendrin on indomethacin-induced gastric ulcer in rats. A: Control group; B: Liriodendrine treatment group (100 mg/kg), Typical macro view of stomachs: Indomethacin-induced black-red hemorrhagic lesions were pointed by the arrows in the gastric mucosa and pretreatment with liriodendrin markedly reduced the number and severity of these lesions.
As a result, we investigated anti-gastritis and anti-ulcer, antacid, defense factor increasing effect by increase PGE2 level and gastric acid inhibition activity by H+/K+-ATPase inhibition. Further study will be needed about development of liriodendrin as treatment/adjunctive of gastritis and gastric ulcer having better effective and less side effects.
REFERENCES
- Bae EA, Han MJ, Baek NI, Kim DH. In vitro anti Helicobacter pylori activity of panaxtriol isolated from ginseng. Arch Pharm Res. 2001;24:297–299. doi: 10.1007/BF02975095. [DOI] [PubMed] [Google Scholar]
- Bebb J, James MW, Atherton J. Gastritis and Peptic Ulcer. Medicine. 2003;31:15–18. doi: 10.1383/medc.31.1.15.28592. [DOI] [Google Scholar]
- Choi CH, Cha YJ, An CS, Kim KJ, Kim KC, Moon SP, Lee ZH, Min YD. Molecular mechanisms of heptaplatin effective against cisplatin-resistant cancer cell lines: less involvement of metallothionein. Cancer Cell Int. 2004;4:6. doi: 10.1186/1475-2867-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Li BG, Gao XP, Qi HY, Zhang GL. A new triterpene and an antiarrhythmic liriodendrin from Pittosporum brevicalyx. Arch Pharm Res. 2010;33:1927–1932. doi: 10.1007/s12272-010-1206-1. [DOI] [PubMed] [Google Scholar]
- Hollander D, Tarnawski A, Cummings D. Cytoprotectiveaction of antacids against alcohol induced gastric mucosal injury:Morphological ultrasturctural and functional time sequence analysis. Gastroenterology. 1984;86:1114. doi: 10.1016/s0016-5085(85)80191-8. [DOI] [PubMed] [Google Scholar]
- Itoh T, Wakatsuki Y, Yoshida M, Usui T, Matsunaga Y, Kaneko S, Chiba T, Kita T. The vast majority of gastric T cells are polarized to produce T helper 1 type cytokines upon antigenic stimulation despite the absence of Helicobacter pylori infection. Gastroenterology. 1999;34:560–570. doi: 10.1007/s005350050373. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Park HJ, Kim RG, Shin KM, Ha J, Choi JW, Kim HJ, Lee YS, Lee KT. In vivo anti-inflammatory and antinociceptive effects of liriodendrin isolated from the stem bark of Acanthopanax senticosus. Planta Med. 2003;69:610–616. doi: 10.1055/s-2003-41127. [DOI] [PubMed] [Google Scholar]
- Kasuya Y, Urushidani T, Okabe S. Effects of various drugs and vagotomy on indomethacin-induced gastric ulcers in the rat. Jpn J Pharmacol. 1979;29:670–673. doi: 10.1254/jjp.29.670. [DOI] [PubMed] [Google Scholar]
- Kato S, Morie T, Yoshida N. Synthesis and biological activities of metabolites of mosapride, a new gastroprokinetic agent. Chem Pharm Bull. 1995;43:699–702. doi: 10.1248/cpb.43.699. [DOI] [PubMed] [Google Scholar]
- Kim JM, Shin JE, Han MJ, Baek NI, Kim DH. Inhibitory effect of ginseng polyacetylenes on infection and vacuolation of Helicobacter pylori. Nat prod sci. 2003;9:158–160. [Google Scholar]
- Kunikata T, Araki H, Takeeda M, Kato S, Takeuchi K. Prostaglandin E prevents indomethacin-induced gastric and intestinal damage through different EP receptor subtypes. J. Physiol. Paris. 2001;95:157–163. doi: 10.1016/S0928-4257(01)00021-3. [DOI] [PubMed] [Google Scholar]
- Lee E, Choi MY, Park HJ, Cha BC, Cho SH. Chemical constituents and biological activity of Kalopanacis Cortex. Kor J Pharmacogn. 1995;26:122–129. [Google Scholar]
- Malagelada JR, Stanghellini V. Manometric evaluation of functional upper gut symptoms. Gastoenterology. 1985;88:1223–1231. doi: 10.1016/s0016-5085(85)80083-4. [DOI] [PubMed] [Google Scholar]
- Mcquaid KR, Isenberg JI. Medical therapy of peptic ulcer disease. Surg Clin North Am. 1992;72:285–316. doi: 10.1016/s0039-6109(16)45680-x. [DOI] [PubMed] [Google Scholar]
- Mizui T, Dodeuchi M. Effect of polyamines on acidified EtOH-induced gastric lesion in rats. Jpn J Pharmacol. 1983;33:939–945. doi: 10.1254/jjp.33.939. [DOI] [PubMed] [Google Scholar]
- Muhammad JS, Sugiyama T, Zaidi SF. Gastric pathophysiological ins and outs of Helicobacter pylori. J Pak Med Assoc. 2013;63:1528–1533. [PubMed] [Google Scholar]
- Odaka T, Suzuki T, Seza A, Yamaguchi T, Saisho H. Serotonin 5- HT4 receptor agonist (mosapride citrate) Nihon Rinsho. 2006;64:1491–1494. [PubMed] [Google Scholar]
- Raghunath AS, O'Morain C, Mcloughlin RC. The longterm use of proton-pump inhibitors. Aliment Pharmacol Ther. 2005;22:55–63. doi: 10.1111/j.1365-2036.2005.02611.x. [DOI] [PubMed] [Google Scholar]
- Rovert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761–767. [PubMed] [Google Scholar]
- Rovert A, Nezaims JE, Lancaste C, Hanchar AJ. Cytoprotection by prostaglandins in rats; Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl and thermal injury. Gastroenterology. 1979;77:433–443. [PubMed] [Google Scholar]
- Saccomani G, Barcellona ML, Sachs G. Reactivity of gastric (H+/K+)-ATPase to N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline. J Biol Chem. 1981;256:12405–12410. [PubMed] [Google Scholar]
- Scarpignato C, Capovilla T, Bertaccini G. Action of caerulein on gastric emptying of the conscious rat. Arch Int Pharmacodyn Ther. 1980;246:286–294. [PubMed] [Google Scholar]
- Seol SY. Mechanism of actions and clinical applications of proton pump inhibitors. Korean J Gastroenterol. 2006;48:4–8. [PubMed] [Google Scholar]
- Shay H, Komarov SA, Fels SS, Meranze D. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterol. 1945;4:43–61. [Google Scholar]
- Tarnawski A, Hollander D, Gergely H, Stachura J. Comparison of antacid, sucralfate, cimetidine and ranitidine in protection of the gastric mucosa against EtOH injury. Am J Med. 1985;79:19–23. doi: 10.1016/0002-9343(85)90567-4. [DOI] [PubMed] [Google Scholar]
- van Doorn NE, van Rees EP, Namavar F, de Graaff J. Local cellular immune response in the acute phase of gastritis in mice induced chemically and by Helicobacter pylori. J Med Microbiol. 1998;47:863–870. doi: 10.1099/00222615-47-10-863. [DOI] [PubMed] [Google Scholar]
- Walker J, Hell J, Liszt KI, Dresel M, Pignitter M, Hofmann T, Somoza V. Identification of beer bitter acids regulating mechanisms of gastric acid secretion. J Agric Food Chem. 2012;60:1405–1412. doi: 10.1021/jf204306z. [DOI] [PubMed] [Google Scholar]
- Wallmark B, Larsson H, Humble L. The relationship between gastric acid secretion and gastric H+,K+-ATPase activity. J Biol Chem. 1985;260:13681–13684. [PubMed] [Google Scholar]