Abstract
Peroxisome proliferator-activated receptor gamma (PPARγ) was identified as a cell-intrinsic regulator of Th17 cell differentiation. Th17 cells have been associated with several autoimmune diseases, including experimental autoimmune encephalomyelitis (EAE), inflammatory bowel disease (IBD), and collagen-induced arthritis. In this study, we confirmed PPARγ-mediated inhibition of Th17 cell differentiation and cytokine production at an early stage. Treatment with ciglitazone, a PPARγ ligand, reduced both IL-1β-mediated enhancement of Th17 differentiation and activation of Th17 cells after polarization. For Th17 cell differentiation, we found that ciglitazone-treated cells had a relatively low proliferative activity and produced a lower amount of cytokines, regardless of the presence of IL-1β. The inhibitory activity of ciglitazone might be due to decrease of CCNB1 expression, which regulates the cell cycle in T cells. Hence, we postulate that a pharmaceutical PPARγ activator might be a potent candidate for treatment of Th17-mediated autoimmune disease patients.
Keywords: Th17 cell, IL-17, PPARγ, CCNB1, Cell proliferation
INTRODUCTION
Peroxisome proliferator-activated receptor gamma (PPARγ) belongs to the nuclear receptor superfamily, which includes receptors for various lipid-soluble, small molecules that are most commonly generated as hormones or in the intermediary metabolic pathways (Evans et al., 2004). PPARγ is most highly expressed in adipose tissues (white- and brown-adipose tissue), is a master regulator of adipogenesis, and is a potent modulator of whole-body lipid metabolism, and insulin sensitivity (Evans et al., 2004; Tontonoz and Spiegelman, 2008). Recently, there have been a few reports of PPAR-γ mediated immune modulation. PPAR-γ activation in immune cells predominantly results in transrepression of proinflammatory gene expression (Schmidt et al., 2010) and plays a role as a negative regulator of dendritic cell maturation and function, contributing to CD4+ T cell anergy (Klotz et al., 2007; Szatmari et al., 2007).
Activation of PPARγ plays an anti-inflammatory role in various tissues, as demonstrated in liver inflammation (Moran-Salvador et al., 2011; Gazit et al., 2012), inflammatory bowel disease (IBD) (Hontecillas and Bassaganya-Riera, 2007; Guri et al., 2010; Mwinyi et al., 2012), and experimental autoimmune encephalomyelitis (EAE) (Niino et al., 2001; Diab et al., 2002). Recently, PPARγ was identified to be a cell-intrinsic regulator of Th17 cell differentiation. Th17 cells are known to be associated with EAE, IBD, allergic airway inflammation, experimental arthritis, and carrageenan-induced pleurisy (Becher and Segal, 2011; Newcomb and Peebles, 2013; Rossi and Bot, 2013; Ahmad et al., 2014). The agonists of PPARγ include endogenous ligands such as the linoleic acid derivative 13(S)-hydroxyoctadecadienoic acid (HODE) as well as several synthetic agonists such as pioglitazone, rosiglitazone, and troglitazone (Straus and Glass, 2007; Huang et al., 1999). Therefore, these ligands need to be investigated for their regulatory effects in Th17-mediated inflammatory diseases.
In this study, we confirmed a PPARγ-mediated inhibition against differentiation and cytokine production in both the absence and presence of IL1β. We propose that a PPARγ ligand negatively regulates CCNB1 expression, potentially regulating Th17 cell proliferation.
MATERIALS AND METHODS
Mice
Female C57BL/6 mice (age, 6–7 weeks) were purchased from Orient Bio Co. (Sungnam, Kyung Ki Do, Republic of Korea). All mice were kept at 23 ± 1°C with a 12 h light/dark cycle. They had free access to water and food and were acclimatized for at least 2 weeks before starting the experiments. All procedures using mice were reviewed and approved by the Animal Ethical Committee of Gyeongsang National University.
Cytokines and antibodies
Recombinant murine IL-1β and IL-6 were purchased from PeproTech (Rocky Hill, NJ, USA). Recombinant human TGF-β1 was purchased from R&D systems (Minneapolis, MN, USA). PPARγ ligands (ciglitazone, rosiglitazone, and troglitazone) and trypan blue solution were obtained from Sigma- Aldrich (St. Louis, MO, USA). FITC-conjugated anti-mouse CD4 antibody, PE-conjugated anti-mouse IL-17 antibody, anti-mouse CD3 (145-2C11) antibody, and anti-mouse CD28 (37.51) antibody were purchased from BD Bioscience (Mississauga, ON, CA).
In vitro differentiation of Th17 T cells
Naïve CD4+ T cells (CD4+CD62L+) were isolated from the lymph nodes and spleen of C57BL/6 mouse using MACS (Miltenyi Biotec, Auburn, CA, USA). Naïve CD4+ T cells were cultured in IMDM (Sigma) supplemented with 10% FBS, 4 mM l-glutamine, 1 mM HEPES, 50 μM 2-mercaptoethanol, 100 units/mL Penicillin, 100 μg/ml Streptomycin, and 0.025 μg/mL amphotericin B at 37°C (CO2 concentration at 5%). The cells were cultured with plate-bound anti-CD3 (145-2C11, 1 μg/mL) and anti-CD28 (37.51, 0.5 μg/mL) antibodies in flat-bottomed 96-well plates. Th17 cells were induced to undergo differentiation with rhTGF-β1 (2 ng/mL), rmIL-6 (40 ng/mL), 10% 11B11 culture supernatant (anti-IL-4 antibody), and 10% XMG1.2 culture supernatant (anti-IFN-γ antibody). In some instances, IL-1β (10 ng/ml) was added during and after Th17 differentiation.
Measurement of cytokine production
IL-17 and IL-22 production in the culture medium was determined using ELISA kit (eBiosciences, San Diego, CA, USA).
Flow cytometry analysis
For intracellular staining, cells were stimulated with 50 nM phorbol 12-myristate 13-acetate (Sigma-Aldrich), 1 μg/mL ionomycin (Calbiochem, Nottingham, UK), and Golgi-Stop (BD Biosciences) for 5 h. After stimulation, the cells were washed and treated with 200 μl of 2.4G2 culture supernatant (anti Fc receptor antibody) for 20 min at 4°C. Without washing, FITCconjugated anti-mouse CD4 antibody was added to the cells and incubated for 30 min, followed by washing. For fixation and permeabilization, Cytofix/Cytoperm (BD bioscience) was used. After 20 min, cells were washed with permeabilization/ wash buffer and stained with PE-conjugated anti mouse IL-17 antibody for intracellular cytokine staining.
Proliferation assay
Proliferation studies were performed using CellTiter 96® Aqueous One Solution cell proliferation assay (Promega, USA). Naïve T cells were seeded in 96-wells cell culture plates (1 × 105 cells/well) within the respective Th17 differentiation media (Group A: TGF-β + IL-6 + DMSO; Group B: TGF-β + IL-6 + ciglitazone). On day 4 and day 7, cells were incubated with 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)- 2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent at 37°C for 4 hours, and absorbance was measured using a spectrophotometer at wavelength 490 nm. Sometimes polarized Th17 cells were restimulated on anti-CD3Ab coated plates for 2 days, and then the proliferation of cells was analyzed. To confirm the proliferation of Naïve T cells under Th17 differentiation condition, the cells were stained with carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, USA) for the cell proliferation assay. The stained cells were analyzed using a FACS Calibur (BD Biosciences) and analyzed with Win MDI 2.9 software (TSRI Flow Cytometry Core Facility, La Jolla, CA, USA).
Quantitative real-time RT-PCR analysis (real-time RT-PCR)
Total RNA was isolated using the RNeasy kit (Qiagen). RNA was quantitated using a NanoDrop spectrophotometer (NanoDrop Technologies). RNA concentrations were equalized before making cDNA. cDNA was synthesized with an oligo dT primer and reverse transcriptase (Fermentas, Newington, NH, USA). Quantitative PCR was performed with CFX 384 real-time RT-PCR detection system (Bio-rad) using an Evagreen qRT-PCR master mixture (Bio-rad Laboratories, CA, USA). The results were normalized to β-actin levels. The primer sequences were as follows: β-actin, 5′-GGTCATCACTATTGGCAACG- 3′ and 5′-ACGGATGTCAACGTCACACT-3′; CCNB1, 5′-ATGGTGCATTTTGCTCCTTC-3′ and 5′-CTTTGTGAGGCCACAGTTCA- 3′; CCNE2, 5′-CAGACTCTCCGCAAGAAACC- 3′ and 5′-GCCAAACCTCCTGTGAACAT-3′. Data were acquired on a CFX 384 and analyzed with the CFX manager software (Bio-rad).
Statistical analysis
Data were analyzed using unpaired Student’s t-test or ANOVA. Results were considered statistically significant if p <0.05.
RESULTS
PPARγ ligands inhibit Th17 cell differentiation in a concentration-dependent manner
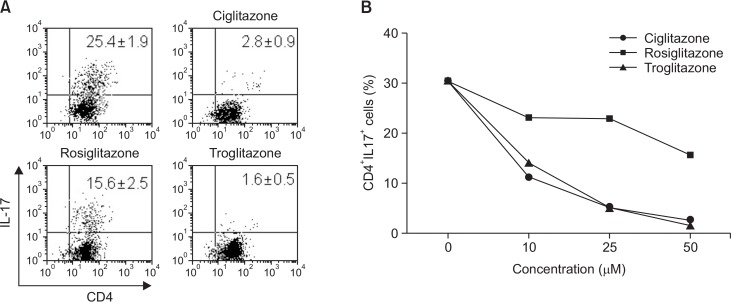
To confirm whether various PPARγ ligands could inhibit Th17 cell differentiation, we treated cells with PPARγ ligands (25 μM; Ciglitazone, Rosiglitazone, and Troglitazone) under conditions of Th17 cell differentiation for 7 days. The cells were subsequently stained with α-CD4 and α-IL-17 antibodies, and were analyzed for determination of Th17 cell differentiation. As shown in Fig. 1A., the PPARγ ligands inhibited Th17 cell differentiation. The inhibitory effect was dependent on the concentration of ligands, and ciglitazone and troglitazone were found to be more effective than rosiglitazone (Fig. 1B).
Fig. 1.
PPARγ ligands inhibit Th17 cells differentiation. (A) Naïve CD4+ T cells were purified and differentiated into Th17 cells with or without 25 μM of ciglitazone, rosiglitazone, and troglitazone. After 7 days, IL-17 producing CD4+ cells were analyzed using FACS. (B) Data from IL-17-producing cells with various concentrations of PPARγ ligand are indicated. One-way ANOVA was used for statistical analysis (p<0.01).
PPARγ ligand inhibits IL-1β-mediated enhancement of Th17 cell differentiation
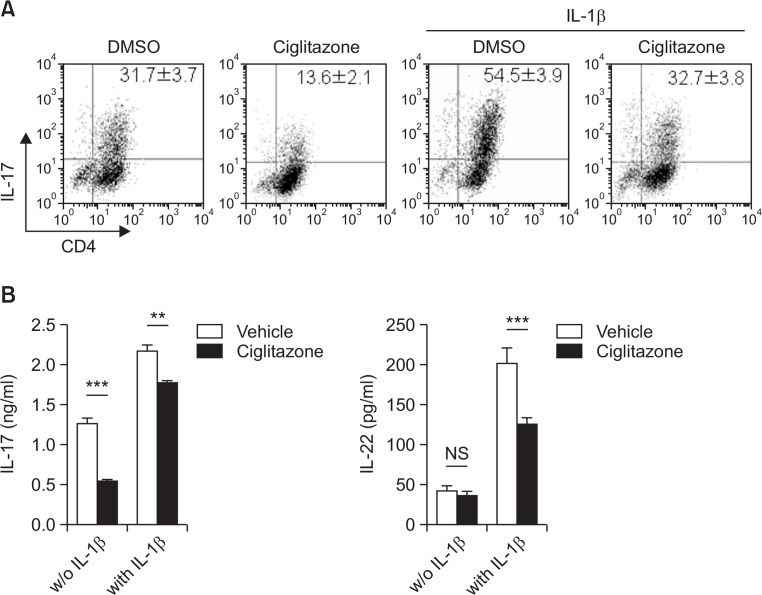
IL-1β plays an important role in regulation of early Th17 cell differentiation, and in inducing autoimmune disease and EAE (mediated by Th17 cells) (Chung et al., 2009). In this study, we tested whether a PPARγ ligand could inhibits IL-1β-mediated enhancement of Th17 cell differentiation. At the beginning of Th17 cell differentiation, naïve T cells were incubated in the presence or absence of IL-1β supplemented with a PPARγ ligand (ciglitazone). Ciglitazone effectively attenuated Th17 cell differentiation and offset the enhancement of Th17 differentiation induced by IL-1β (Fig. 2A). IL-22 and IL-17 levels, which are enhanced by IL-1β, were attenuated by ciglitazone (Fig. 2B).
Fig. 2.
Ciglitazone inhibited the enhancement of Th17 differentiation induced by IL-1β. Naïve CD4+ T cells were differentiated to Th17 in the presence or absence of IL-1β for 4 days. To test whether a PPARγ ligand inhibits IL-1β-mediated Th17 differentiation, 25 μM ciglitazone was added to the differentiation medium. On day 4, cells were harvested for CD4 and IL-17 staining (A), and cell supernatant was used for determination of IL-17 and IL-22 using ELISA (B). Statistically significant differences are indicated by asterisks (**p<0.01; ***p<0.001).
Ciglitazone attenuated the maintenance of Th17 phenotype by IL-1β after polarization
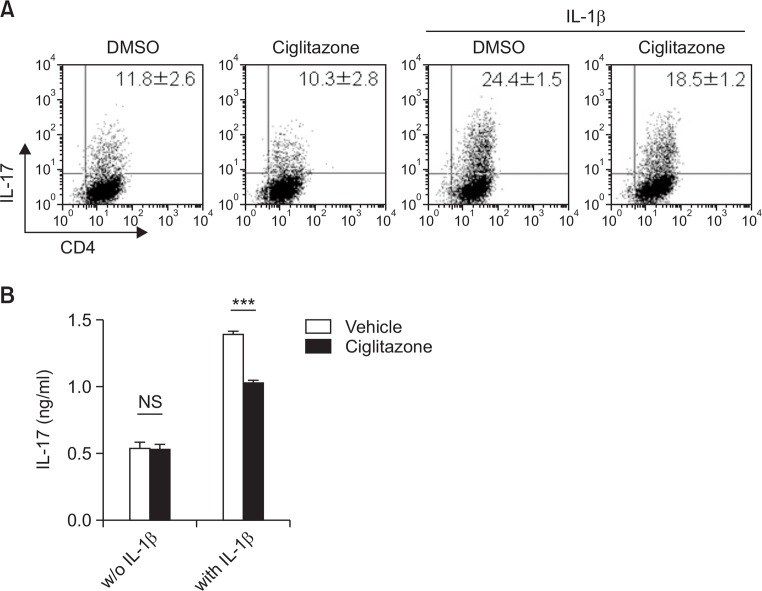
To investigate whether ciglitazone could inhibit the maintenance of Th17 phenotype after polarization, we treated Th17 cells differentiated and restimulated with anti-CD3 antibody with ciglitazone, in the presence or absence of IL-1β. Ciglitazone inhibited the amount of IL-17-producing Th17 cells enhanced by IL-1β, while it had no effect against polarized Th17 cells in the absence of IL-1β (Fig. 3). These data suggested that ciglitazone might have no effect against already polarized Th17 cells, and might inhibit only IL-1β-mediated stimulatory signaling after polarization.
Fig. 3.
IL-1β-mediated activation of Th17 cells after polarization is attenuated by ciglitazone. Naïve CD4+ T cells were differentiated using the Th17 cell differentiation medium. (A) On day 4, the cells were harvested and then further restimulated with plate bound anti-CD3 overnight in the presence or absence of IL-1β or/and ciglitazone (25 μM). Following 2 days restimulation, IL-17 producing cells were analyzed using FACS. (B) Restimulated cell supernatant was collected and IL-17 production was measured. Statistically significant differences are indicated by asterisks (***p<0.001).
Ciglitazone attenuated Th17 cell function through regulation of cell cycle
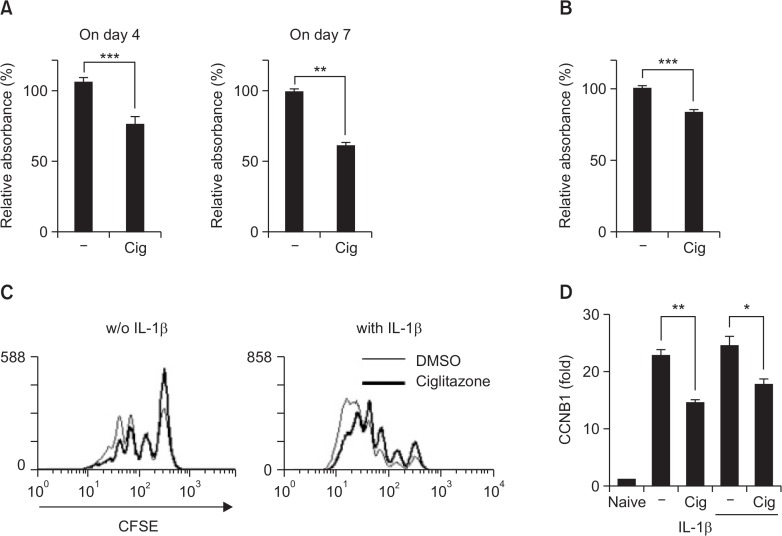
Under Th17 cell differentiation conditions, ciglitazone showed an inhibitory activity in terms of IL-17-producing cell numbers and the amounts of cytokines in the presence or absence of IL-1β. At first we did MTS assay to determine proliferative activity of the cells under Th17 differentiation (Fig. 4A) and Th17 activation condition (Fig. 4B). Ciglitazone-mediated inhibitory effect was shown at both of them and these results proposed that ciglitazone might inhibit the proliferation of differentiating cells into Th17 and of polarized Th17. To confirm whether ciglitazone can decrease cell proliferation during Th17 differentiation, we labeled cells with CFSE and then induced differentiation under indicated conditions. As shown in Figure 4C, ciglitazone-treated cells (bold line) had a relatively low proliferative activity in the presence or absence of IL-1β. The inhibitory activity of ciglitazone might be due to a decrease in CCNB1, which regulated the G2/M phase of the cell cycle (Fig. 4D). These observations indicate that ciglitazone might attenuate IL-17-producing Th17 cell number, and the enhancement of Th17 cells by IL-1β through regulation of the cell cycle in T cells under these conditions.
Fig. 4.
Ciglitazone decreased cell proliferation during Th17 cell differentiation. Naïve T cells were seeded in 96-wells cell culture plates (1×105 cells/well) within the indicated Th17 differentiation condition. On day 4 and day 7, cells were incubated with MTS reagent for 4 hr (A). Sometimes the differentiated Th17 cells were restimulated with anti-CD3 Ab for 2 days and the proliferation was analyzed (B). To confirm whether ciglitazone can decrease cell proliferation during Th17 differentiation, CFSE-labeled Naïve CD4+ T cells were cultured under Th17 differentiation in the presence or absence of IL-1β or/and ciglitazone (25 μM). (C) On day 4, the intensity of CFSE in IL-17 producing cells were analyzed using FACS. (D) After 2 days of Th17 cell differentiation, mRNA expression was assessed by real-time RT-PCR. Statistically significant differences are indicated by asterisks (*p<0.05; **p<0.01; ***p<0.001).
DISCUSSION
Th17 cells have been known as a key mediator in the pathology of several autoimmune diseases, including EAE, IBD, and collagen-induced arthritis in humans (Lock et al., 2002; Tzartos et al., 2008) as well as in mouse models (Cua et al., 2003; Murphy et al., 2003; Yen et al., 2006). Therefore, regulation or inhibition of Th17-differentiation and activation might be an important strategy to treat autoimmune diseases. In this study, we confirmed that a PPARγ ligand could inhibit Th17 cell differentiation in both the presence and absence of IL1β, and could attenuate Th17 activation induced by IL-1β after polarization.
There are some reports where PPARγ activation could impair differentiation of Th17 cells. The inhibitory function of PPARγ on Th17 cells has focused on RORγt expression, which was necessary for Th17 cell differentiation (Yang et al., 2008). PPARγ activation is thought to prevent removal of SMRT (silencing mediator of retinoid and thyroid hormone receptor). SMRT is bound to the RORγt promoter and inhibits transcription of the gene. Thus, PPARγ activation can suppress RORγt expression, thereby inducing Th17 cell differentiation (Hwang, 2010; Klotz and Knolle, 2011), whereas the expression of the transcription factors determining Th1 (T-bet), Th2 (GATA-3), and Treg (Foxp3), was not influenced by the PPAR activation (Klotz et al., 2009). We also confirmed that PPARγ ligand could induce/inhibit Th17 cell differentiation at an early stage (Fig. 1). Although modulation of Th17 cell differentiation is important to regulate several autoimmune diseases, IL-1 signaling was known to be important in pathology mediated by Th17 cells as well as in early Th17 cell differentiation (Chung et al., 2009). Ciglitazone, a PPARβ ligand, showed an inhibitory activity against IL-1β-mediated enhancement of Th17 cell differentiation (Fig. 2) and activation of Th17 after polarization (Fig. 3). Under conditions of Th17 cell differentiation, ciglitazone treated cells had less cell density than that of the control group. Upon examining cell proliferation of IL-17 producing cells, we found ciglitazone inhibited both the proliferation rate of IL-17 producing cells and the expression of CCNB1, which regulates the cell cycle. Although we could not identify the mechanisms by which the PPARγ ligand could attenuate CCNB1 expression in Th17 cells, there are earlier reports of PPARγ ligand-mediated inhibition of cyclin B expression resulting in attenuated cell proliferation in several cell lines (Strakova et al., 2004; Yeh et al., 2011).
Unfortunately, it is known that pharmaceutical PPARγ ligands induce severe side effects such as fluid retention, weight gain, bone loss, and congestive heart failure in clinical applications. Nevertheless, recent studies also provide opportunities to develop newer classes of molecules that reduce or eliminate the side effects (Ahmadian et al., 2013). Thus, we propose that pharmaceutical PPARγ activators might be potent candidates for treatment Th17-mediated autoimmune disease, meriting further investigation.
Acknowledgments
This study was supported by Next-Generation BioGreen21 (SSAC, PJ00957102), Rural Development Administration, Republic of Korea.
Footnotes
Current address
D.H.K; Republic of Korea and Division of bacterial respiratory diseases, National Institute of Health, Korea CDC, Osong 363-951, Republic of Korea; H-J. Ihn; Division of Vaccine research, National Institute of Health, Korea CDC, Osong 363-951, Republic of Korea
REFERENCES
- Ahmad SF, Zoheir KM, Abdel-Hamied HE, Attia SM, Bakheet SA, Ashour AE, Abd-Allah AR. Grape seed proanthocyanidin extract protects against carrageenan-induced lung inflammation in mice through reduction of pro-inflammatory markers and chemokine expressions. Inflammation. 2014;37:500–511. doi: 10.1007/s10753-013-9764-2. [DOI] [PubMed] [Google Scholar]
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B, Segal BM. T(H)17 cytokines in autoimmune neuro-inflammation. Curr Opin Immunol. 2011;23:707–712. doi: 10.1016/j.coi.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Gazit V, Huang J, Weymann A, Rudnick DA. Analysis of the role of hepatic PPARγ expression during mouse liver regeneration. Hepatology. 2012;56:1489–1498. doi: 10.1002/hep.25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guri AJ, Mohapatra SK, Horne WT, 2nd, Hontecillas R, Bassaganya-Riera J. The role of T cell PPAR gamma in mice with experimental inflammatory bowel disease. BMC Gastroenterol. 2010;10:60. doi: 10.1186/1471-230X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator- activated receptor gamma is required for regulatory CD4+ T cell-mediated protection against colitis. J Immunol. 2007;178:2940–2949. doi: 10.4049/jimmunol.178.5.2940. [DOI] [PubMed] [Google Scholar]
- Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- Hwang ES. Transcriptional regulation of T helper 17 cell differentiation. Yonsei Med J. 2010;51:484–491. doi: 10.3349/ymj.2010.51.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L, Dani I, Edenhofer F, Nolden L, Evert B, Paul B, Kolanus W, Klockgether T, Knolle P, Diehl L. Peroxisome proliferator-activated receptor gamma control of dendritic cell function contributes to development of CD4+ T cell anergy. J Immunol. 2007;178:2122–2131. doi: 10.4049/jimmunol.178.4.2122. [DOI] [PubMed] [Google Scholar]
- Klotz L, Knolle P. Nuclear receptors: Th17 cell control from within. FEBS Lett. 2011;585:3764–3769. doi: 10.1016/j.febslet.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Klotz L, Burgdorf S, Dani I, Saijo K, Flossdorf J, Hucke S, Alferink J, Nowak N, Beyer M, Mayer G, Langhans B, Klockgether T, Waisman A, Eberl G, Schultze J, Famulok M, Kolanus W, Glass C, Kurts Knolle PA. The nuclear receptor PPARγ selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009;20:2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Moran-Salvador E, Lopez-Parra M, Garcia-Alonso V, Titos E, Martinez-Clemente M, Gonzalez-Periz A, Lopez-Vicario C, Barak Y, Arroyo V, Claria J. Role for PPARγ in obesity- induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 2011;25:2538–2550. doi: 10.1096/fj.10-173716. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwinyi J, Grete-Wenger C, Eloranta JJ, Kullak-Ublick GA. The impact of PPARγ genetic variants on IBD susceptibility and IBD disease course. PPAR Res. 2012;2012:349469. doi: 10.1155/2012/349469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb DC, Peebles RS., Jr Th17-mediated inflammation in asthma. Curr Opin Immunol. 2013;25:755–760. doi: 10.1016/j.coi.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niino M, Iwabuchi K, Kikuchi S, Ato M, Morohashi T, Ogata A, Tashiro K, Onoe K. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by an agonist of peroxisome proliferator-activated receptor-gamma. J Neuroimmunol. 2001;116:40–48. doi: 10.1016/S0165-5728(01)00285-5. [DOI] [PubMed] [Google Scholar]
- Rossi M, Bot A. The Th17 cell population and the immune homeostasis of the gastrointestinal tract. Int Rev Immunol. 2013;32:471–474. doi: 10.3109/08830185.2013.843983. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Brune B, von Knethen A. The nuclear hormone receptor PPARgamma as a therapeutic target in major diseases. ScientificWorldJournal. 2010;10:2181–2197. doi: 10.1100/tsw.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakova N, Ehrmann J, Dzubak P, Bouchal J, Kolar Z. The synthetic ligand of peroxisome proliferator-activated receptor-gamma ciglitazone affects human glioblastoma cell lines. J Pharmacol Exp Ther. 2004;309:1239–1247. doi: 10.1124/jpet.103.063438. [DOI] [PubMed] [Google Scholar]
- Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Szatmari I, Torocsik D, Agostini M, Nagy T, Gurnell M, Barta E, Chatterjee K, Nagy L. PPARgamma regulates the function of human dendritic cells primarily by altering lipid metabolism. Blood. 2007;110:3271–3280. doi: 10.1182/blood-2007-06-096222. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh SL, Yeh CL, Chan ST, Chuang CH. Plasma rich in quercetin metabolites induces G2/M arrest by upregulating PPAR-gamma expression in human A549 lung cancer cells. Planta Med. 2011;77:992–998. doi: 10.1055/s-0030-1250735. [DOI] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]