Abstract
The seed of Vigna angularis has long been cultivated as a food or a folk medicine in East Asia. Genistein (4′,5,7-trihydroxyisoflavone), a dietary phytoestrogen present in this plant, has been known to possess various biological properties. In this study, we investigated the possible lifespan-extending effects of genistein using Caenorhabditis elegans model system. We found that the lifespan of nematode was significantly prolonged in the presence of genistein under normal culture condition. In addition, genistein elevated the survival rate of nematode against stressful environment including heat and oxidative conditions. Further studies demonstrated that genistein-mediated increased stress tolerance of nematode could be attributed to enhanced expressions of stress resistance proteins such as superoxide dismutase (SOD-3) and heat shock protein (HSP-16.2). Moreover, we failed to find genistein-induced significant change in aging-related factors including reproduction, food intake, and growth, indicating genistein exerts longevity activity independent of affecting these factors. Genistein treatment also led to an up-regulation of locomotory ability of aged nematode, suggesting genistein affects healthspan as well as lifespan of nematode. Our results represent that genistein has beneficial effects on the lifespan of C. elegans under both of normal and stress condition via elevating expressions of stress resistance proteins.
Keywords: Vigna angularis, Genistein, Caenorhabditis elegans, Lifespan extension, Stress tolerance
INTRODUCTION
Vigna angularis (Leguminosae) has long been cultivated throughout East Asia. The seed of this plant has been used as a food or a traditional medicine for the treatment of several diseases such as edema, beriberi, constipation, and diabetes (Yao et al., 2011). Previous pharmacological studies on the seed of V. angularis (aka: adzuki beans) revealed that it has various therapeutic properties on the aging-related diseases such as arthritis, osteoporosis, type 2 diabetes, and hypertension (Mukai and Sato, 2009; Itoh et al., 2014; Oh et al., 2014; Yao et al., 2011).
Polyphenols are plant secondary metabolites and have attracted interest with impressive health benefits (Crozier et al., 2009). Genistein, a polyphenol compound, belongs to the category of isoflavones, and has been found almost every leguminous plants including adzuki beans (Fig. 1). Early studies indicated that genistein protects age-associated degenerative disorders such as myocardial infarction, hepatic failure, diabetes, obesity and brain damage (Behloul and Wu, 2013; Lee et al., 2014; Lin et al., 2014; Wang et al., 2014). In addition, genistein proved to be effective in neuroprotection and bone loss in animal models via estrogen-mimicking activity (Moran et al., 2014; Song et al., 2014).
Fig. 1.
Structure of genistein.
However, no systematic study concerning the lifespan-extending activity of genistein has been published yet. Here we isolated genistein from the phenolic-rich ethyl acetate soluble fraction of V. angularis and evaluate its longevity effects using Caenorhabditis elegans model system. Furthermore, to validate the possible mechanism of genistein, expressions of stress resistance proteins and aging-related factors were analyzed.
MATERIALS AND METHODS
General
1H-and 13C-NMR spectra were determined on a JEOL JMNEX 400 spectrometer (Tokyo, Japan). Sephadex LH-20 was used for column chromatography (GE Healthcare, Uppsala, Sweden). The absorbance was determined using microplate reader (ELISA, Sunrise, Grödig, Austria). TLC was carried out on Merck (Darmstadt, Germany) precoated silica gel F254 plates and silica gel for column chromatography was Kiesel gel 60 (230-400 mesh, Merck, Darmstadt, Germany). Spots were detected under UV and by spraying with 10% H2SO4 in ethanol followed by heating for 3 min. All other chemicals and solvents were of analytical grade and used without further purification. Selected peptone and yeast extracts were obtained from BD bioscience (Sparks, USA). Agar, 2’,7’-dichlorodihydrofluoroscein diacetate, methyl viologen dichloride hydrate (paraquat), catalase, xanthine, xanthine oxidase, and nitroblue tetrazolium were purchased from Sigma (St. Louis, USA).
Plant materials, extraction and isolation
The seeds of V. angularis were provided from National Institute of Crop Science, Gyeongsangbuk-do of Korea in 2013. A voucher specimen was deposited in the herbarium of the College of Pharmacy, Woosuk University (WSU-13-008). The shade dried plant material (720 g) was extracted three times with methanol at 55°C and filtered. The extracts were combined and evaporated in vacuo at 50°C. The resultant methanolic extract (84 g) was successively partitioned as n-hexane (650 mg), methylene chloride (11.27 g), ethyl acetate (1.2 g), n-butanol (14.49 g) and H2O soluble fractions. Ethyl acetate soluble fraction showed the most potent lifespan extending activity in V. angularis (data are not shown). Sephadex LH-20 (MeOH) column of ethyl acetate soluble extract gave five fractions (EA1-EA5). Fraction EA2 (1.2 g) was chromatographed by silica gel column chromatography (CHCl3-MeOH-H2O, 40:10:1) to give five subfractions (EA21-EA25). Subfraction EA24 (120 mg) was chromatographed by silica gel column (CHCl3-MeOH-H2O, 30:10:1) and purified by RP Lobar-A column (MeOH-H2O, 40:60) to give compound 1 (21.2 mg).
Genistein (1)
1H-NMR (400 MHz, DMSO-d6) δ : 12.94 (1H, br s, 5-OH), 9.57 (1H, br s, 4’-OH), 8.30 (1H, s, H-2), 7.36 (2H, d, J=8.4 Hz, H-2’, 6’), 6.80 (2H, d, J=8.4 Hz, H-3’, 5’), 6.37 (1H, d, J=2.0 Hz, H-8), 6.21 (1H, d, J=2.0 Hz, H-6). 13C-NMR (100 MHz, DMSO-d6) δ : 157.3 (C-2), 122.2 (C-3), 180.1 (C-4), 161.9 (C-5), 98.9 (C-6), 164.2 (C-7), 93.6 (C-8), 157.5 (C-9), 104.4 (C-10), 121.1 (C-1′), 130.1 (C-2′), 115.0 (C-3′), 157.5 (C-4′), 115.0 (C-5′), 130.1 (C-6′). Structure characterization of compound 1 was carried out by interpretation of their spectral data comparison with the data reported in the literature.
C. elegans strains and maintenance
Bristol N2 (wild-type) was kindly provided by Dr. Myon-Hee Lee (East Carolina University, NC, USA). The worms were grown at 20°C on nematode growth medium (NGM) agar plate with Escherichia coli OP50 as described previously (Brenner, 1974). To prepare plates supplemented with genistein, the stock solution in DMSO was inserted into autoclaved NGM plates (at 50°C). A final DMSO concentration of 0.2% (v/v) was maintained under all conditions.
Lifespan assay
The lifespan assays were performed using wild-type at least 3 times independently at 20°C. To obtain age-synchronized nematodes, eggs were transferred to NGM plate in the absence or presence of sample after embryo isolation. Test worms were considered dead when they failed to respond to prodding with the tip of a platinum wire (Lithgow et al., 1995). The worms were transferred to fresh NGM plate every 2 days.
Determination of stress resistance
The age-synchronized N2 worms were bred on NGM agar plates with or without various concentrations of sample. For the heat tolerance assay the adult day 4 worms were transferred to fresh plates and then incubated at 36°C. The viability was scored over 16 h as previously described (Lee et al., 2005). Oxidative stress tolerance was assessed as described previously with minor modification (Mekheimer et al., 2012). Briefly, the adult day 7 worms were subjected to containing 85 mM paraquat liquid culture and then survivals were recorded over 46 h.
Measurement of antioxidant enzyme activities
To assess enzymatic activity, the worm homogenates were prepared. Briefly, the wild-type worms were harvested from plate with M9 buffer on the adult day 5 and washed 3 times. Then, the collected worms were resuspended in homogenization buffer (10 mM Tris-HCl, 150 mM NaCl, 0.1 mM EDTA, pH 7.5) and homogenized on ice. SOD activity was measured spectrophotometrically analysing the decolorization of formazan using enzymatic reaction between xanthine and xanthine oxidase. The reaction mixture contained 5 μL of worm homogenates and 120 μL of 1.6 mM xanthine, 0.48 mM nitrobluetetrazolium (NBT) in 10 mM phosphate buffer (pH 8.0). After preincubation at room temperature for 5 minutes, the reaction was initiated by adding 100 μL of xanthine oxidase (0.05 U/ml) and incubation at 37°C for 20 min. The reaction was stopped by adding 275 μL of 69 mM SDS, and the absorbance at 570 nm was measured. SOD activity was expressed as a percentage of the scavenged amount per control. Catalase activity was calculated by spectrophotometry as previously described (Aebi, 1984). Briefly, the prepared homogenates were mixed with the 25 mM H2O2 and after 5 min incubation, absorbance was determined at 240 nm. Catalase activity was expressed in U/mg protein (1 unit will decompose 1.0 μM of H2O2 per min at pH 7.0 at 25°C).
Fluorescence microscopy and visualization
The age-synchronized transgenic strains including CF1553 containing a SOD-3::GFP reporter and CL2070 containing HSP-16.2::GFP reporter were maintained in the presence or absence of genistein. Prior to microscopy observation, CL2070 mutants were received heat shock at 36°C for 2 h and allowed to recover at 20°C for 4 h. On the 3rd days of adulthood, both transgenic animals were anesthetized with sodium azide (2%) and mounted on 2% agarose pad. The GFP fluorescence of GFP-expressing populations was directly observed under a fluorescence microscope (Nikon Eclipse Niu, Japan). To determine the protein expression levels, photographs of the transgenic worms were taken and assayed using ImageJ software. All experiments were done in triplicate
Measurement of aging-related factors and locomotion
The age-synchronized N2 worms were bred on NGM agar plates with or without genistein. On the 4th day of adulthood, single worms were transferred to fresh plate followed by pharynx contractions and body movements of animals were counted under an inverted microscope for 1 min. For the growth alteration assay, photographs were taken of worms, and the body length of each animal was analyzed by the Nikon software (Nikon, Japan). Reproduction assay was conducted as follows. N2 worms were raised from embryo as in the lifespan assay. L4 larvae were individually transferred to the fresh plate every day to distinguish the parent from the progeny. The progeny was counted at the L2 or L3 stage. On the 7th days of adulthood, single worms were transferred to fresh plate followed by body movements were recorded under an inverted microscope for 20 seconds. The body movements of animals were analyzed by Nikon image software. All tests was completed in triplicate.
Data analysis
The data from the lifespan assay and stress resistance assays were plotted using Kaplan-Meier analysis and statistical significance was analyzed by log-rank test. Other data were presented as mean ± or standard error of the mean, as indicated. Statistical significance of differences between the control and treated groups were analyzed by one-way analysis of variance (ANOVA).
RESULTS
Effects of genistein on the lifespan of C. elegans
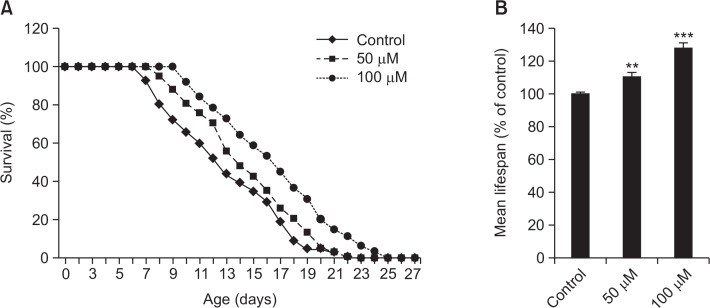
To determine the lifespan extension properties of genistein, lifespan assays were performed with wild-type N2 worms. As shown in Fig. 2A, concentration-dependent effect of genistein was observed. In addition, there was a significant increase (27.9% at 100 μM of genistein, p<0.001) in the estimated mean life of genistein treated worms compared to control worms (Fig. 2B). The mean life duration was 21.0 ± 0.3 days for control worms, and the mean life duration of genistein for the worms fed at 100 μM were 24.0 ± 0.7 days.
Fig. 2.
Effects of genistein on the lifespan of wild-type N2 nematodes. Worms were grown in the NGM agar plate at 20°C in the absence or presence of genistein. The number of worms used per each lifespan assay experiment was 27-41 and three independent experiments were repeated (N=3). (A) The mortality of each group was determined by daily counting of surviving and dead animals. (B) The mean lifespan of the N2 worms was calculated from the survival curves. Statistical difference between the curves was analyzed by log-rank test. Error bars represent the standard error of mean (S.E.M.). Differences compared to the control were considered signifiant at **p<0.01 and ***p<0.001 by one-way ANOVA.
Effects of genistein on the stress tolerance of C. elegans
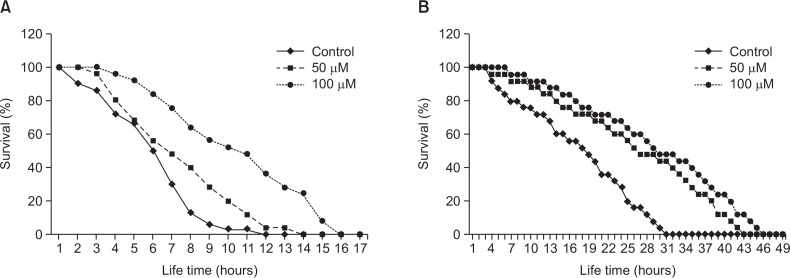
We determined the effect of genistein on two different kinds of stress conditions including thermal and oxidative stress using wild-type N2 worms. As can be seen in Fig. 3, we could observe that genistein exposure induced significant increases in thermotolerance, and consequently elevated survival rate of worms. Further, genistein exposure extended the maximum lifespan of worms by 68.4% (100 μM, p<0.001, Fig. 3A). Moreover, it was found that genistein treated N2 worms lived longer than control worms under oxidative stress conditions induced by 85 mM paraquat in a concentration-dependent manner (100 μM, p<0.01, Fig. 3B).
Fig. 3.
Effects of genistein on the stress tolerance of wild-type N2 nematodes. (A) To assess thermal tolerance, worms were incubated at 36°C and then their viability was scored. (B) For the oxidative stress assays, worms were transferred to 96-well plate containing 85 mM of paraquat liquid culture, and then their viability was scored. Statistical difference between the curves was analyzed by log-rank test. All experiments were done in triplicates.
Effects of genistein on the stress resistance proteins of C. elegans
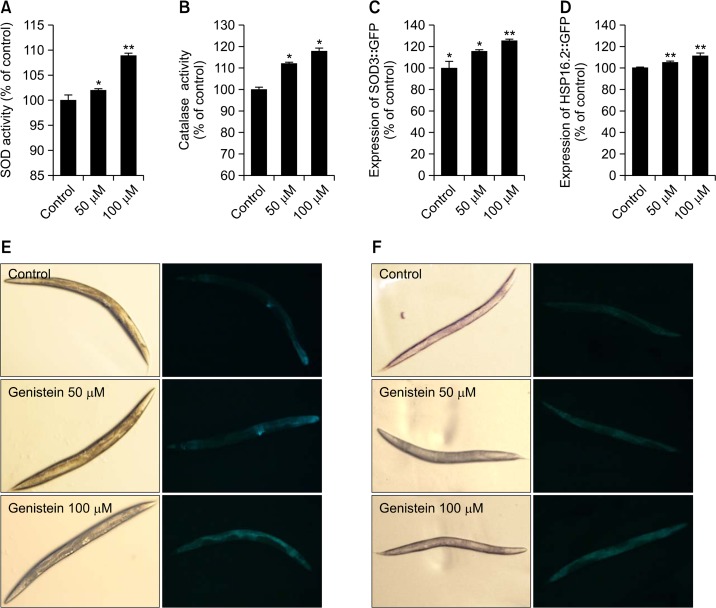
In order to verify the action of genistein on the lifespan and stress resistance of nematodes, effect of genistein on the antioxidant enzyme activities were investigated. The SOD and catalse enzymatic activities were measured spectrophotometrically using prepared worm homogenates. Results showed that genistein was able to elevate SOD and catalase activities of worms significantly by 7.07% and 17.8% at 100 μM, respectively (p<0.01, Fig. 4A and B). Then, to investigate whether genistein-mediated increased stress tolerance was due to regulation of stress-response genes, we quantified SOD-3 and HSP-16.2 expressions using transgenic strains including CF1553 and CL2070, respectively. Our data shows that genistein-treated CF1553 worms exhibited significantly higher SOD-3::GFP intensity (25.1% at 100 μM, p<0.01), compared with untreated control worms. (Fig. 4C, E) The CL2070 worms containing HSP-16.2::GFP reporter gene were received heat shock at 36°C for 2 h and allowed to recover at 20°C for 4 h, followed by quantifying fluorescence intensity. This heat shock-induced HSP-16.2::GFP expression was further up-regulated by 100 μM of genistein about 11.1% (p<0.01, Fig. 4D, F).
Fig. 4.
Effects of genistein on the stress resistance proteins of wild type N2 nematodes. (A) The enzymatic reaction of xanthine with xanthine oxidase was used to generate •O2- and the SOD activity was estimated spectrophotometrically through formazan formation by NBT reduction. SOD activity was expressed as a percentage of the scavenged amount per control. (B) Catalase activity was calculated from the concentration of residual H2O2, as determined by a spectrophotometric method. Catalase activity was expressed in U/mg protein. Effects of genistein on the expression of SOD-3 and HSP-16.2 was determined using transgenic nematodes. Mean GFP intensity of CF1553 (C) and CL2070 (D) mutants were represented as mean ± S.E.M. of values from 18 to 26 animals per each experiment. The GFP intensity was quantified using Image software by determining average pixel intensity. Images of SOD-3::GFP (E) and HSP-16.2::GFP (F) expressions of CF1553 worms in the presence or absence of genistein. Data are expressed as the mean ± standard deviation of three independent experiments (N=3). Differences compared to the control were considered significant at *p<0.05, **p<0.01 by one-way ANOVA.
Effects of genistein on the aging-related factors of C. elegans
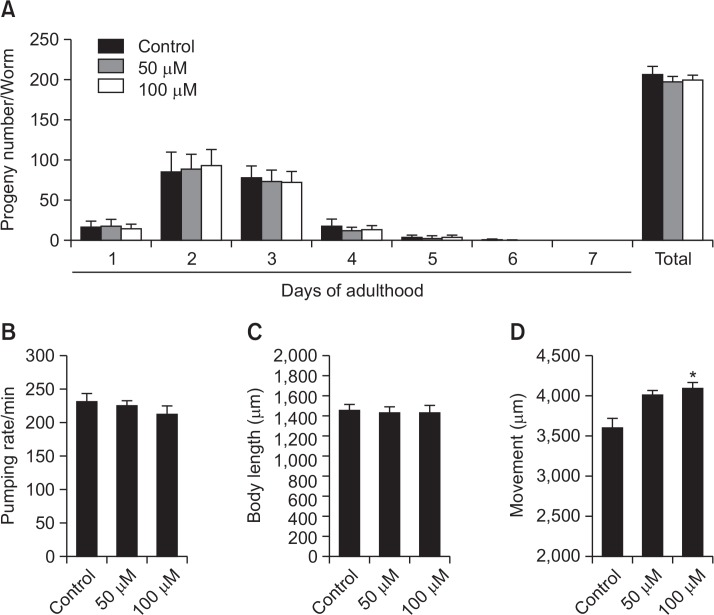
In order to verify the mechanism of genistein on the lifespan of nematodes, we observed genistein-induced change in parameters of aging-related factors such as progeny, pharyngeal pumping, and body length. We found that genistein slightly decreases the reproduction rate and food intake, compared to control worms without statistical significance (Fig. 5A, B). In addition, we could not detect any differences in growth rate between genistein-fed worms and control worms (Fig. 5C). These results demonstrate that the alteration of aging-related factors is not responsible for genistein’s longevity action in C. elegans.
Fig. 5.
Effects of genistein on the various aging-related factors of wild-type N2 nematodes. (A) Daily and total reproductive outputs were counted. The progeny was counted at the L2 or L3 stage. (B) On the 4th days of adulthood, the pharyngeal pumping rates were measured. (C) For the growth alteration assay, photographs were taken of worms and the body length of each animal was analyzed. (D) The body movement were counted under a dissecting microscope for 1 min. Data are expressed as the mean ± S.E.M. of three independent experiments (N=3). Differences compared to the control were considered significant at *p<0.05 by one-way ANOVA.
Effects of genistein on the locomotory activities in C. elegans
C. elegans decline in movement with age is similar to human decrepitude (Herndon et al., 2002). To determine the effects of genistein on the age-associated functional changes in C. elegans, we measured the body movement of worms after genistein treatment. Interestingly, genistein-fed worms effectively delayed age-related deterioration of body movement, compared with untreated aged worms (Fig. 5D). Our data shows that genistein enhances the travel range of worms per 20 seconds about 11.5% at 50 μM and 13.7% at 100 μM, respectively. (p<0.05, Fig. 5D).
DISCUSSION
Here in this work, we isolated genistein from the seed of V. angularis and elucidated its longevity activity in C. elegans. Our lifespan assay results represent that lifespan of wild-type worms was significantly lengthened in the presence of genistein in a concentration-dependent manner under normal culture conditions. In addition, genistein considerably enhanced the survival rate of worms under both of paraquat-induced oxidative stress and heat stress conditions. Since there is a clear correlation between lifespan-extension and the stress resistance (Kenyon, 2010), protective ability against stress condition may positively affect genistein-mediated prolonged lifespan.
To explain how genistein eliminates oxidative stress, we analyzed the antioxidant enzyme activities using worm homogenates. Our results shows that both of SOD and catalase enzyme activities were significantly up-regulated by genistein, indicating attenuation of hydroxyl radical levels results in diminished oxidative stress. Genistein is a phenolic compounds, and thus, has very strong antioxidant capacity. Therefore, genistein may also mimic antioxidant enzyme activities via direct scavenging of reactive oxygen species. To exclude this possibility we tested whether genestein affects the gene expressions of SOD-3 using GFP-expressing transgenic worms. It appears that genistein-fed worms had higher GFP intensity compared with the control, indicating increased SOD-3 expression. Consistent with this finding, previous report demonstrates that activities of SOD, catalase, and glutathione peroxidase were enhanced by genistein in rats (Ding and Liu, 2011). Moreover, Fan’s group also noted that genistein could activate hepatic glutathione peroxidase in acetaminophentreated mice (Fan et al., 2013). Since oxidative stress is a major factor limiting lifespan in invertebrates as well as mammals (Kampkotter et al., 2007), in vivo antioxidant properties of genistein might be attributed to extended lifespan and increased survival rate under oxidative stress condition.
We then tried to reveal possible involvement of HSP-16.2 in the genistein-mediated stress resistance. Heat shock proteins (HSPs) are expressed under stress condition, and thus, are believed to be a stress-sensitive reporters which can predict the longevity (Swindell, 2009). The HSP-16.2 family are homologous to chaperone protein, αβ-crystallin in C. elegans and higher HSP-16.2 levels predict longer remaining lifespan (Rea et al., 2005). We found that HSP-16.2 expression induced by heat shock was significantly elevated in the genistein-fed worms, suggesting longevity and enhanced stress tolerance of genistein may also be explained by this property.
We further investigated whether genistein affects agingrelated factors such as reproduction, food intake, and growth. Previous studies suggest that reductions in reproduction, food intake, and body size are closely interconnected with longevity (Bordone and Guarente, 2005; Partridge et al., 2005; Morck and Pilon, 2006). In this study, there was no significant variation in the body length, number of progeny and pharyngeal pumping between genistein-fed worms and control worms. Together these results provide evidence that genistein extends lifespan independent of altering aging-related factors. Although we could not detect any significant decrease in reproductive ability of worms, previous studies suggest that phytoestrogen exposure negatively affects reproductive health (Jefferson et al., 2012). One possible explanation for this opposite results is that C. elegans has different type of reproductive system (hermaphrodite and male) compared to mammals.
To explore whether genistein affects functional aging, locomotion assay was conducted. Interestingly, genistein significantly up-regulated the body movement of worms, indicating genistein-mediated lifespan-extension was not possibly associated with decreased metabolic rate. This result also suggests that genistein provide a beneficial effects on healthspan as well as lifespan.
In contrast to our study, Altun’s group reported that genistein treatment decreased the lifespan of Drosophila (Altun et al., 2011). They estimated that genistein-induced oxidative damage was followed by shortened lifespan of fly. However, it is somewhat surprising, because genistein has been exhibited strong antioxidant potential in various in vitro and in vivo experimental models (Lee, 2006; Ma et al., 2010; Gong et al., 2014; Javanbakht et al., 2014). Furthermore, it is well known that estrogens up-regulate longevity-associated genes (Borras et al., 2005). Previous in vitro study revealed that genistein, a phytoestrogen, mimics estrogen activity and upregulates the expression of longevity-related genes similar to 17β-estradiol via interactions with estrogen receptor and activation of ERK1/2 and NF-κB (Borras et al., 2006).
In conclusion, herein our work, genistein displayed lifespanextension of C. elegans via its antioxidant potential and regulating stress resistance protein. Yet, since the present data are preliminary, the question as to whether genistein provide positive or negative action against aging in mammals is still open and further studies are required.
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009218)” Rural Development Administration, Republic of Korea.
REFERENCES
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Altun D, Uysal H, Askin H, Ayar A. Determination of the effects of genistein on the longevity of Drosophila melanogaster meigen (Diptera; Drosophilidae) Bull Environ Contam Toxicol. 2011;86:120–123. doi: 10.1007/s00128-010-0159-x. [DOI] [PubMed] [Google Scholar]
- Behloul N, Wu G. Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol. 2013;698:31–38. doi: 10.1016/j.ejphar.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Borras C, Gambini J, Gomez-Cabrera MC, Sastre J, Pallardo FV, Mann GE, Vina J. 17beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging Cell. 2005;4:113–118. doi: 10.1111/j.1474-9726.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- Borras C, Gambini J, Gomez-Cabrera MC, Sastre J, Pallardo FV, Mann GE, Vina J. Genistein, a soy isoflavone, upregulates expression of antioxidant genes: involvement of estrogen receptors, ERK1/2, and NFkappaB. FASEB J. 2006;20:2136–2138. doi: 10.1096/fj.05-5522fje. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- Ding W, Liu Y. Genistein attenuates genioglossus muscle fatigue under chronic intermittent hypoxia by down-regulation of oxidative stress level and up-regulation of antioxidant enzyme activity through ERK1/2 signaling pathway. Oral Dis. 2011;17:677–684. doi: 10.1111/j.1601-0825.2011.01822.x. [DOI] [PubMed] [Google Scholar]
- Fan YJ, Rong Y, Li PF, Dong WL, Zhang DY, Zhang L, Cui MJ. Genistein protection against acetaminopheninduced liver injury via its potential impact on the activation of UDPglucuronosyltransferase and antioxidant enzymes. Food Chem Toxicol. 2013;55:172–181. doi: 10.1016/j.fct.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Gong DK, Liu BH, Tan XH. Genistein prevents cadmium-induced neurotoxic effects through its antioxidant mechanisms. Drug Res. (Stuttg) 2014 doi: 10.1055/s-0034-1372595. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Itoh T, Nakamura M, Nakamichi H, Ando M, Tsukamasa Y, Furuichi Y. Regulation of the differentiation of osteoblasts and osteoclasts by a hot-water extract of adzuki beans (Vigna angularis) Biosci Biotechnol Biochem. 2014;78:92–99. doi: 10.1080/09168451.2014.877182. [DOI] [PubMed] [Google Scholar]
- Javanbakht MH, Sadria R, Djalali M, Derakhshanian H, Hosseinzadeh P, Zarei M, Azizi G, Sedaghat R, Mirshafiey A. Soy protein and genistein improves renal antioxidant status in experimental nephrotic syndrome. Nefrologia. 2014;34:483–490. doi: 10.3265/Nefrologia.pre2014.Jun.12051. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Patisaul HB, Williams CJ. Reproductive consequences of developmental phytoestrogen exposure. Reproduction. 2012;143:247–260. doi: 10.1530/REP-11-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampkotter A, Gombitang Nkwonkam C, Zurawski RF, Timpel C, Chovolou Y, Watjen W, Kahl R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch Toxicol. 2007;81:849–858. doi: 10.1007/s00204-007-0215-4. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Lee EY, Shim YH, Chitwood DJ, Hwang SB, Lee J, Paik YK. Cholesterol-producing transgenic Caenorhabditis elegans lives longer due to newly acquired enhanced stress resistance. Biochem Biophys Res Commun. 2005;328:929–936. doi: 10.1016/j.bbrc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Lee JS. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life Sci. 2006;79:1578–1584. doi: 10.1016/j.lfs.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lee JH, Asahara T, Kim YS, Jeong HC, Ahn Y, Jung JS, Kwon SM. Genistein promotes endothelial colony-forming cell (ECFC) bioactivities and cardiac regeneration in myocardial infarction. PLoS One. 2014;9:e96155. doi: 10.1371/journal.pone.0096155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Zhang S, Huang R, Wei L, Liang C, Chen Y, Lv S, Liang S, Wu X, Huang Q. Protective effect of genistein on lipopolysaccharide/D-galactosamine-induced hepatic failure in mice. Biol Pharm Bull. 2014;37:625–632. doi: 10.1248/bpb.b13-00908. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Yuan L, Yu H, Ding B, Xi Y, Feng J, Xiao R. Genistein as a neuroprotective antioxidant attenuates redox imbalance induced by beta-amyloid peptides 25-35 in PC12 cells. Int J Dev Neurosci. 2010;28:289–295. doi: 10.1016/j.ijdevneu.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Mekheimer RA, Sayed AA, Ahmed EA. Novel 1,2,4-triazolo[1,5-a]pyridines and their fused ring systems attenuate oxidative stress and prolong lifespan of Caenorhabiditis elegans. J Med Chem. 2012;55:4169–4177. doi: 10.1021/jm2014315. [DOI] [PubMed] [Google Scholar]
- Moran J, Garrido P, Cabello E, Alonso A, Gonzalez C. Effects of estradiol and genistein on the insulin signaling pathway in the cerebral cortex of aged female rats. Exp. Gerontol. 2014;58C:104–112. doi: 10.1016/j.exger.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Morck C, Pilon M. C. elegans feeding defective mutants have shorter body lengths and increased autophagy. BMC Dev Biol. 2006;6:39. doi: 10.1186/1471-213X-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai Y, Sato S. Polyphenol-containing azuki bean (Vigna angularis) extract attenuates blood pressure elevation and modulates nitric oxide synthase and caveolin-1 expressions in rats with hypertension. Nutr Metab Cardiovasc Dis. 2009;19:491–497. doi: 10.1016/j.numecd.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Oh HM, Lee SW, Yun BR, Hwang BS, Kim SN, Park CS, Jeoung SH, Kim HK, Lee WS, Rho MC. Vigna angularis inhibits IL-6-induced cellular signalling and ameliorates collagen-induced arthritis. Rheumatology (Oxford) 2014;53:56–64. doi: 10.1093/rheumatology/ket302. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Liang X, Zhou Y. Estrogen-mimicking isoflavone genistein prevents bone loss in a rat model of obstructive sleep apnea-hypopnea syndrome. Int J Clin Exp Pathol. 2014;7:1687–1694. [PMC free article] [PubMed] [Google Scholar]
- Swindell WR. Heat shock proteins in long-lived worms and mice with insulin/insulin-like signaling mutations. Aging (Albany NY) 2009;1:573–577. doi: 10.18632/aging.100058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wei H, Cai M, Lu Y, Hou W, Yang Q, Dong H, Xiong L. Genistein attenuates brain damage induced by transient cerebral ischemia through up-regulation of ERK activity in ovariectomized mice. Int J Biol Sci. 2014;10:457–465. doi: 10.7150/ijbs.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Cheng X, Wang L, Wang S, Ren G. A determination of potential alpha-glucosidase inhibitors from Azuki Beans (Vigna angularis) Int J Mol Sci. 2011;12:6445–6451. doi: 10.3390/ijms12106445. [DOI] [PMC free article] [PubMed] [Google Scholar]