Abstract
Background
The mTOR pathway, which consists of mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), is activated in polycystic kidney disease (PKD) kidneys. Sirolimus and everolimus indirectly bind and inhibit mTORC1. A novel group of drugs, the mTOR kinase inhibitors, directly bind to mTOR kinase, thus inhibiting both mTORC1 and 2. The aim of the study was to determine the therapeutic effect of an mTOR kinase inhibitor, PP242, in the Han:SPRD rat (Cy/+) model of PKD.
Methods
Male rats were treated with PP242 5 mg/kg/day IP or vehicle for 5 weeks.
Results
PP242 significantly reduced the kidney enlargement, the cyst density and the blood urea nitrogen in Cy/+ rats. On immunoblot of kidneys, PP242 resulted in a decrease in pS6, a marker of mTORC1 signaling and pAktSer473, a marker of mTORC2 signaling. mTORC plays an important role in regulating cytokine production. There was an increase in IL-1, IL-6, CXCL1 and TNF-α in Cy/+ rat kidneys that was unaffected by PP242. Apoptosis or proliferation is known to play a causal role in cyst growth. PP242 had no effect on caspase-3 activity, TUNEL positive or active caspase-3-positive tubular cells in Cy/+ kidneys. PP242 reduced the number of proliferating cells per cyst and per non-cystic tubule in Cy/+ rats.
Conclusions
In a rat model of autosomal dominant polycystic kidney disease, PP242 treatment (i) decreases proliferation in cystic and non-cystic tubules; (ii) inhibits renal enlargement and cystogenesis and (iii) significantly reduces the loss of kidney function.
Keywords: apoptosis, mTOR kinase, polycystic kidney, proliferation, sirolimus
INTRODUCTION
Automsomal dominant polycystic kidney disease (ADPKD) is the most common life threatening hereditary disease in the USA and is caused by a mutation in either the Pkd1 or 2 genes. ADPKD accounts for ∼5–10% of end-stage renal failure in the USA requiring dialysis and renal transplantation. While drugs like tolvaptan and sirolimus are in clinical studies in patients with ADPKD, there are no Federal Drug Administration (FDA)-approved therapies that slow cyst growth in ADPKD. Thus preclinical studies of potential therapeutic agents in ADPKD are important and may lead to therapies that slow cyst growth and improve kidney function in ADPKD.
A potential new therapy for ADPKD is the mTOR kinase inhibitors or TORK inhibitor. mTOR exists in association with two different complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 consists of mTOR and Raptor (regulatory associated protein of mTOR), while mTORC2 consists of mTOR and Rictor (rapamycin-independent companion of mTOR). The TORK inhibitors selectively bind to the ATP-binding site in the mTOR catalytic domain and thereby block both mTORC1 and 2 [1].
The rationale for testing TORK inhibitors in polycystic kidney disease (PKD) is as follows: firstly, activation of pro-proliferative mTORC1 has been demonstrated in PKD in rodents [2–4] and in humans [5]. Activation of pAktSer473, a marker of mTORC2, has also been demonstrated in ADPKD [2–4]. The effect of combined mTORC1 and mTORC2 inhibition in PKD is not known. Secondly, sirolimus-dependent inhibition of mTORC1 can result in increased activation of mTORC2 [6]. Sirolimus resulted in an increase in pAktSer473 in female Han:SPRD rats associated with no protection against PKD [4]. TORK inhibitors can result in inhibition of the activation of pAktSer473 induced by mTORC1 inhibition. Thirdly, mTORC1 inhibitors like sirolimus and everolimus reduce cyst growth and improve kidney function in animal studies, but the development of side effects was a dose limiting step in human studies [7, 8]. The mTOR kinase inhibitors or TORK inhibitors have a low side effect profile [1]. Fourthly, human and experimental data provide strong evidence that abnormal proliferation and apoptosis in tubular epithelial cells plays a crucial role in cyst development and/or growth in PKD [9]. Both mTORC1 and 2 are pro-proliferative and have a pro or anti-apoptotic effect depending on cell type and cell process [1, 10, 11]. Thus we hypothesized that an mTOR kinase inhibitor that inhibits both mTORC1 and 2 would result in inhibition of proliferation and apoptosis in cystic kidneys, less cyst growth and improvement of kidney function.
MATERIALS AND METHODS
Animals
The study was conducted in heterozygous (Cy/+) and normal littermate control (+/+) Han:SPRD rats. All normal rats and Cy/+ rats studied were males. The Cy/+ Han:SPRD rat develops clinically detectable PKD by 8 weeks of age as evidenced by a doubling of kidney size and kidney failure compared with +/+ control rats [12, 13]. A colony of Han:SPRD rats was established in our animal care facility from a litter that was obtained from the Polycystic Kidney Program at the University of Kansas Medical Center. Cy/+ rats were genotyped by detection of expression of Anks6(p.R823W) (Samcystin) by Transnetyz (Cordova, TN, USA) [14, 15]. The study protocol was approved by the University of Colorado Health Sciences Center Animal Care and Use Committee. Rats had free access to tap water and standard rat chow.
Experimental protocol
Male Cy/+ and +/+ rats were weaned at 3 weeks of age and then treated with PP242 at a dose of 5 mg/kg/day IP or vehicle (ethanol) for 5 weeks. 2-(4-Amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-3-yl)-1H-indol-5-ol, Dihydrate or PP242 is a cell-permeable pyrazolopyrimidine compound that acts as a potent, reversible, and ATP-competitive inhibitor of mTOR kinase thus inhibiting both mTORC1 and mTORC2. PP242 was obtained from ActiveBiochem (Maplewood, NJ, USA). At the end of the eighth week of age, rats were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg body weight) and kidneys were removed and weighed.
Separate experiments using sirolimus were performed at the time of the PP242 experiments in the same Han:SPRD rat model for the same time period. Male Cy/+ and +/+ rats were weaned at 3 weeks of age and then treated with sirolimus (0.05 or 0.1 mg/kg/day IP). At the end of the eighth week of age, rats were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg body weight) and kidneys were removed and weighed. Sirolimus was obtained from LC Laboratories (Woburn, MA, USA), and a 1 mg/mL stock solution in 100% ethanol was kept at 4°C.
Cyst volume density
Hematoxylin-eosin stained sections were used to determine the cyst volume density (CVD). This was performed by a reviewer, blinded to the identity of the treatment modality, using point counting stereology [16]. Areas of the cortex at 90°, 180° and 270° from the hilum of each section were selected to guard against field selection variation.
Immunoblotting
Whole kidney was homogenized in radioimmunoprecipitation assay buffer plus protease inhibitors and immunoblotted as described previously [17]. For immunoblot analysis, actin was used as a loading control. Anti- pAktSer 473 (# 3787), anti-total Akt (# 9272), anti-pS6 (# 2211) and anti-total S6 (# 2217) antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA).
Caspase-3 assay
The activity of caspase-3 was determined by use of fluorescent substrates as we have previously described in detail [18]. Ac-Asp-Glu-Val--Asp-7-amido-4-methyl coumarin (Ac-DEVD-AMC) in 10% DMSO was used as a susceptible substrate for caspase-3. Peptide cleavage was measured over 1 h at 30°C using a Cytofluor 4000 series fluorescent plate reader (Perseptive Biosystems) at an excitation wavelength of 380 nm and an emission wavelength of 460 nm. Caspase-3 activity was expressed in nmol AMC released per minute of incubation time per milligram of lysate protein.
In situ detection of DNA fragmentation
The terminal deoxynucleotidyltransferase (TdT) mediated dUTP nick-end labeling (TUNEL) method was used to detect in situ DNA strand breaks as we have previously described [19]. The Deadend™ Colorimetric TUNEL assay kit (Promega, Madison, WI, USA) was used. Positive and negative controls for TUNEL stain were performed. All cells with apoptotic morphology (cellular rounding and shrinkage, pyknotic nuclei or formation of apoptotic bodies) that stained positive with the TUNEL assay were counted. The number of TUNEL positive cells per cyst was counted using a Nikon Eclipse E400 microscope equipped with a digital camera connected to Spot Advanced imaging software (Version 3.5) by an observer blinded to the treatment modality, as we have previously described [13, 19]. Twelve areas in the cortex per sample were randomly selected at 90°, 180° and 270° from the hilum of each section to guard against field selection variation. To avoid confusion between non-cystic tubules and small cysts, as well as potential changes in tubular cells lining massive cysts, apoptotic cells were counted in ‘medium sized cysts’ of ∼50–250 µm diameter. Twenty to thirty cysts were counted per sample.
Immunofluorescence studies
Kidney tissues were embedded in OCT, snap-frozen in liquid nitrogen and stored at −80°C until sectioning. Five micrometer cryostat sections were fixed in 70% acetone/30% methanol and prepared for immunofluorescence studies as previously described by us [17]. The primary antibody used was an anti-cleaved caspase-3 antibody (#9579) that was purchased from Cell Signaling Technology (Beverly, MA, USA). The number of caspase-3 positive cells per cyst was counted as described above for the quantitation of TUNEL staining.
Immunohistochemistry
Immunohistochemical detection of proliferating cell nuclear antigen (PCNA) staining was performed using an anti-PCNA antibody (M0879, Dako, 1:200). The sections were incubated with alkaline-phosphatase labeled polymer (DAKO EnVision System, Cat# K4016, DAKO, Carpinteria, CA, USA) and visualized with the substrate chromogen, fast red [19]. Negative control sections showed no staining.
Tubular cell proliferation
The number of PCNA-positive cells was counted using an Aperioscanner (Aperio Technologies, Vista, CA, USA) at ×20 magnification by an observer blinded to the treatment modality [19]. Non-cystic tubules were defined as tubules <50 µm diameter. At least 10 high power fields devoid of cysts in the cortex per sample were randomly selected and PCNA-positive cells were counted. To avoid confusion between non-cystic tubules and small cysts as well as potential changes in tubular cells lining massive cysts, PCNA-positive tubular cells were counted in ‘medium sized cysts’ of ∼250 µm diameter. At least 20 cysts per sample in the cortex were randomly selected and counted.
Measurement of cytokines
A multiplex sandwich immunoassay was used to measure 10 inflammatory cytokines: IL-1, IL-2, IL-4, IL-5, IL-6, CXCL1 (also known as IL-8 in humans and KC in mice), IL-10, IL-13, IFN-γ, TNF-α using a multiarray electrochemiluminescence panel (Meso Scale Discovery, MULTI-SPOT Assay System, V-plex Proinflammatory Panel-1 for rats, Catalog no: K15044D-1, Rockville, MD, USA).
Chemistry
Serum urea nitrogen levels were measured using a Beckman autoanalyzer (Beckman Instruments, Fullerton, CA, USA).
Statistical analysis
Non-normally distributed data were analyzed by the nonparametric unpaired Mann–Whitney test. Multiple group comparisons were performed using a one-way analysis of variance (ANOVA) with post-test according to Newman–Keuls (Table 1) or a two-way analysis of variance (ANOVA) (GraphPad Prism Version 4) (Table 2). A P-value of <0.05 was considered statistically significant. Values are expressed as means ± SE.
Table 1.
Pro-inflammatory cytokines in Cy/+ rat kidneys
| +/+ Veh (n = 4) | Cy/+ Veh (n = 5) | Cy/+ PP (n = 7) | |
|---|---|---|---|
| IL-1 (pg/mg) | 5.4 ± 0.9 | 9.7 ± 1.1* | 10 ± 1.2* |
| IL-2 (pg/mg) | 14.1 ± 3.9 | 20.9 ± 4.3 | 23.6 ± 4.2 |
| IL-4 (pg/mg) | 0.14 ± 0.02 | 0.2 ± 0.07 | 0.3 ± 0.06 |
| IL-5 (pg/mg) | 2.0 ± 0.3 | 3.2 ± 0.6 | 3.5 ± 0.6 |
| IL-6 (pg/mg) | 2.6 ± 0.4 | 5.7 ± 1.3* | 4.1 ± 0.4 |
| IL-10 (pg/mg) | 0.5 ± 0.1 | 0.8 ± 0.2 | 1.2 ± 0.3 |
| IL-13 (pg/mg) | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.2 |
| CXCL1 (pg/mg) | 1.1 ± 0.2 | 3.7 ± 0.9* | 3.3 ± 0.8 |
| TNF-α (pg/mg) | 0.3 ± 0.1 | 1.3 ± 0.4* | 0.8 ± 0.1 |
| IFN-γ (pg/mg) | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.7 ± 0.1 |
*P < 0.05 versus +/+ Veh. Veh = vehicle.
Table 2.
PP242 decreases 2KW, 2K/TBW (%), CVD and BUN in Cy/+ rat kidneys
| +/+ Veh (n = 8) | +/+ PP (n = 6) | Cy/+ Veh (n = 7) | Cy/+ PP (n = 13) | |
|---|---|---|---|---|
| TBW (g) | 277 ± 15 | 247 ± 24 | 267 ± 23 | 281 ± 6 |
| 2KW | 2.3 ± 0.1 | 2.1 ± 0.2 | 6.8 ± 0.2* | 4.7 ± 0.2** |
| 2K/TBW (%) | 0.8 ± 0.02 | 0.9 ± 0.02 | 2.2 ± 0.04* | 1.6 ± 08** |
| CVD (%) | 0.8 ± 0.1 | 0.8 ± 0.1 | 32 ± 1.9* | 25 ± 2† |
| BUN (mg/dL) | 23 ± 1 | 18 ± 1 | 56 ± 6* | 30 ± 3‡ |
Veh, vehicle; 2KW, two kidney weight; 2K/TBW (%), two kidney weight to total body weight ratio; CVD, cyst volume density and BUN, blood urea nitrogen.
*P < 0.0001 versus +/+.
**P < 0.001 versus Cy/+ Veh.
†P = 0.03 versus Cy/+ Veh.
‡P < 0.01 versus Cy/+ Veh, NS versus +/+.
RESULTS
Effect of PP242 on body weight, two kidney weight, two kidney/total body weight ratio, CVD and blood urea nitrogen
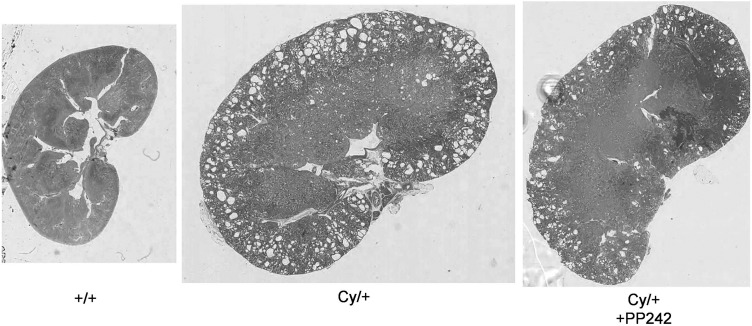
Unlike the weight loss we have previously reported with sirolimus in Cy/+ rats [13], PP242 did not cause significant weight loss. Cy/+ rats had a more than doubling of 2 kidney weight (2KW) and 2 kidney/total body weight ratio (2K/TBW) compared with +/+ controls. PP242 significantly reduced the 2KW and 2K/TBW (Table 2). PP242 significantly reduced the CVD and blood urea nitrogen (BUN) in Cy/+ rats (Table 2). Representative kidney sections of +/+, Cy/+ and Cy/+ rats treated with PP242 and stained with hematoxylin, at the same magnification are demonstrated in Figure 1.
FIGURE 1:
PP242 decreases cyst volume. Representative kidney sections stained with hematoxylin-eosin, at the same magnification demonstrate less cysts in PP242-treated Cy/+ rat kidneys.
Effect of sirolimus on two kidney weight and two kidney/total body weight ratio
Separate experiments using sirolimus were performed at the time of the PP242 experiments. Two kidney weight (g) was 2.2 ± 0.6 in +/+ (n = 5), 2.4 ± 0.2 in +/+ treated with sirolimus 0.1 mg/kg/day (n = 4), 5.1 ± 0.6 in Cy/+ (P < 0.001 versus +/+, n = 5), 1.8 ± 0.1 in Cy/+ treated with sirolimus 0.05 mg/kg/day (P < 0.01 versus Cy/+, n = 3) and 1.9 ± 0.2 in Cy/+ treated with sirolimus 0.1 mg/kg/day (P < 0.001 versus Cy/+, n = 7).
2K/TBW (%) was 0.8 ± 0.02 in +/+ (n = 5), 0.8 ± 0.02 in +/+ treated with sirolimus 0.1 mg/kg/day (n = 4), 1.75 ± 0.2 in Cy/+ (P < 0.001 versus +/+, n = 5), 1.2 ± 0.03 in Cy/+ treated with sirolimus 0.05 mg/kg/day (P < 0.001 versus Cy/+, n = 3) (58% reduction in 2K/TBW compared to 43% reduction in 2K/TBW with PP242) and 1.1 ± 0.05 in Cy/+ treated with sirolimus 0.1 mg/kg/day (P < 0.001 versus Cy/+, n = 7) (68% reduction in 2K/TBW compared to 43% reduction in 2K/TBW with PP242).
CVD (%) was 38.2 ± 1 in Cy/+ and 15.4 ± 0.8 in Cy/+ treated with sirolimus 0.01 mg/kg/day (59% reduction in CVD compared to 23% reduction in CVD with PP242).
mTORC1 and 2 signaling
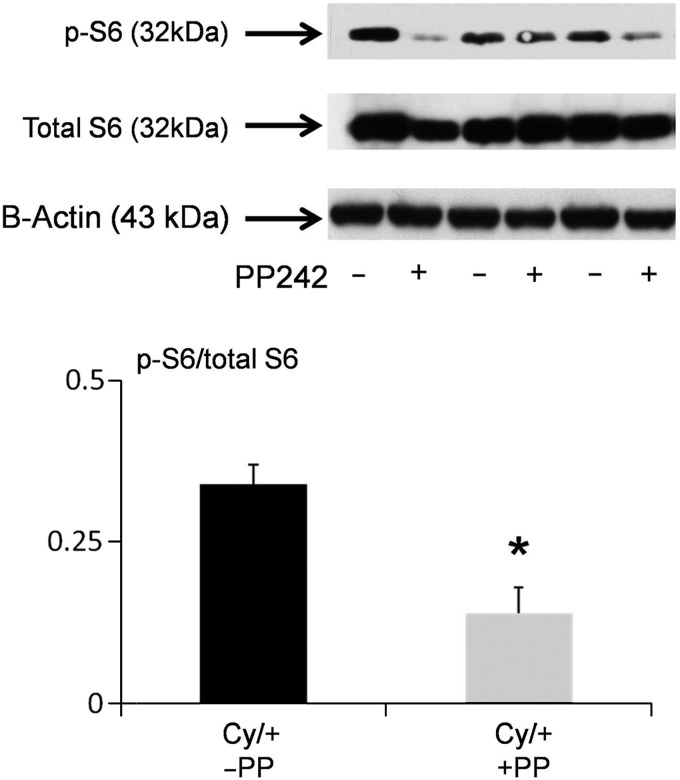
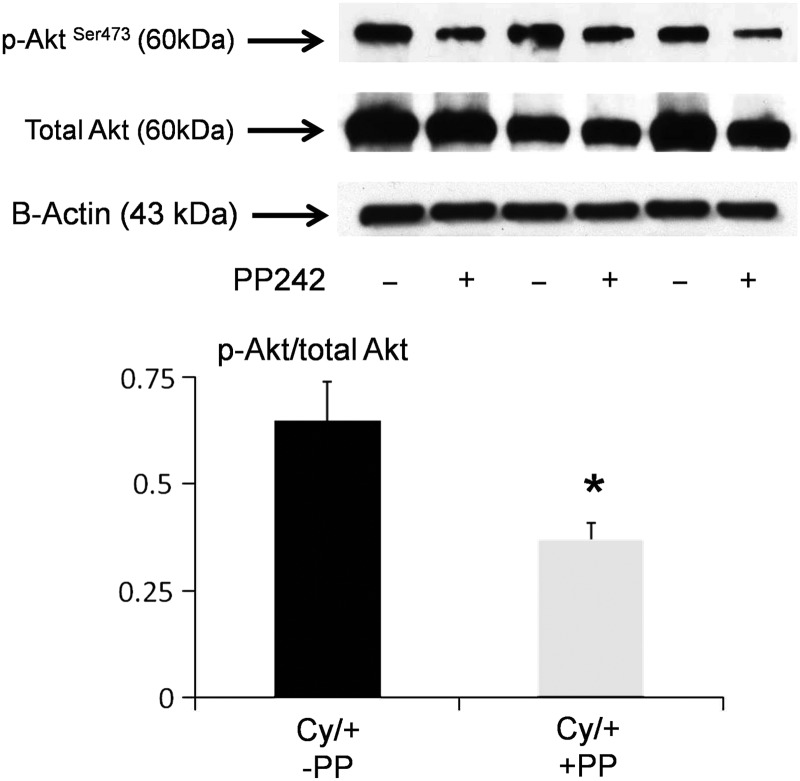
pS6 is a marker of mTORC1 signaling and pAktSer473 is a marker of mTORC2 signaling [1]. PP242 resulted in a significant decrease in pS6 (Figure 2) and pAktSer473 (Figure 3) in Cy/+ kidneys.
FIGURE 2:
PP 242 decreases pS6. pS6 is a marker of mTORC1 signaling. We have previously demonstrated that pS6 is increased in male Cy/+ versus +/+ rats [4]. On immunoblot, PP242 resulted in a significant decrease in pS6 in Cy/+ kidneys. In densitometric analysis, data are presented as pS6/total S6 plotted on the y-axis. B-actin, used as a loading control, was not different between the groups. *P < 0.05 versus no PP242 (n = 3).
FIGURE 3:
PP242 decreases pAktSer473. pAktSer473 is a marker of mTORC2 signaling. We have previously demonstrated that pAktSer473 is increased in male Cy/+ versus +/+ rats [4]. On immunoblot, PP242 resulted in a significant decrease in pAktSer473 in Cy/+ kidneys. In densitometric analysis, data are presented as pAktSer473/ total Akt plotted on the y-axis. B-actin, used as a loading control, was not different between the groups. *P < 0.05 versus no PP242 (n = 3).
Cytokines
The cytokines IL-1, IL-2, IL-4, IL-5, IL-6, CXCL1 (also known as IL-8 in humans and KC in mice), IL-10, IL-13, IFN-γ, TNF-α were measured in wild-type kidneys and Cy/+ kidneys treated with vehicle or PP242. Of the 10 cytokines measured, there was an increase in IL-1, IL-6, CXCL1 and TNF-α in Cy/+ rat kidneys that was unaffected by PP242 (Table 1).
Tubular cell apoptosis
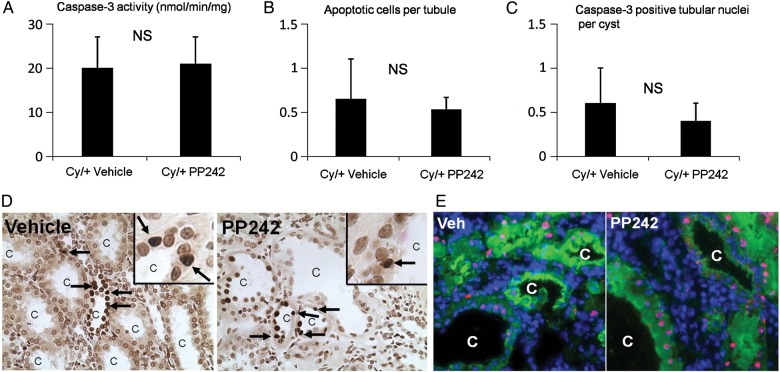
Caspase-3 is the major mediator of apoptosis [20]. PP242 had no significant effect on caspase-3 activity, tubular apoptosis on TUNEL staining or activated caspase-3 in tubular cells lining the cysts. Caspase-3-like activity in cytosolic extracts of whole Cy/+ kidneys was not affected by PP242 (Figure 4A). The number of apoptotic tubular cells per cyst on TUNEL staining in Cy/+ kidneys was not affected by PP242 (Figure 4B). The number of caspase-3 positive tubular nuclei per cyst in Cy/+ kidneys was not affected by PP242 (Figure 4C). Representative pictures of TUNEL staining and active caspase-3 staining are shown in Figure 4D and E.
FIGURE 4:
PP242 has no effect on caspase-3 activity, apoptosis and active caspase-3 staining. Caspase-3 activity in Cy/+ rat kidneys was unaffected by PP242, n = 5 (A). The number of apoptotic cells per cyst of Cy/+ kidneys as detected by TUNEL staining was unaffected by PP242, n = 5 (B). The number of caspase-3 positive nuclei per cyst was unaffected by PP242, n = 4 (C). NS = not significant. Representative pictures of TUNEL staining are shown in (D). Arrows = TUNEL positive apoptotic cells. C = cyst. Inset shows high power of apoptotic nuclei. Representative pictures of active caspase-3 staining is shown in (E). Cy/+ kidneys were stained with Lectin (green), DAPI (blue), active caspase-3 (red). Tubular nuclei staining with both DAPI and active caspase-3 are pink. C = cyst.
Tubular cell proliferation
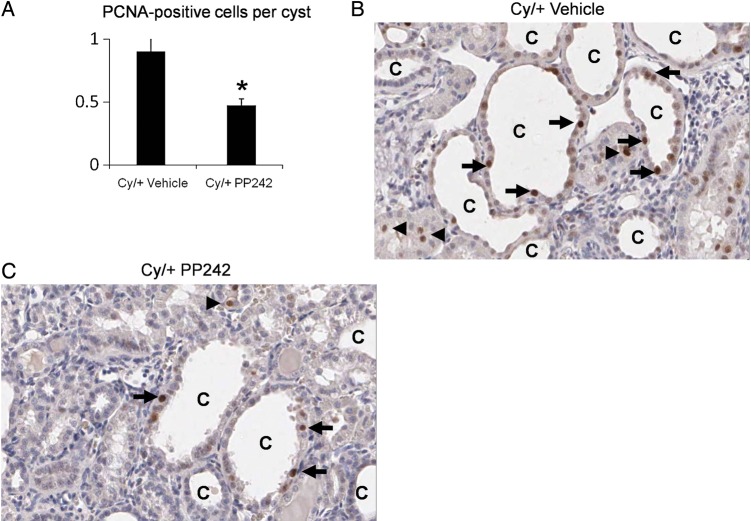
The number of PCNA-positive cells per cyst in the cortex in Cy/+ rats was significantly reduced by PP242 (Figure 5A). Representative pictures are shown in Figure 5B and C.
FIGURE 5:
PP242 decreases PCNA-positive cells per cyst. The number of PCNA-positive cells in tubular epithelial cells lining the cysts was decreased in Cy/+ treated with PP242 (Cy/+ PP242) versus Cy/+ treated with vehicle (Cy/+ vehicle) (A). *P < 0.01 versus Cy/+ vehicle, n = 5. Representative pictures of PCNA staining in Cy/+ Vehicle (B) and Cy/+ PP242 (C) are demonstrated. Arrow = PCNA-positive cell. C = cyst. Arrowhead = PCNA-positive cell in non-cystic tubule.
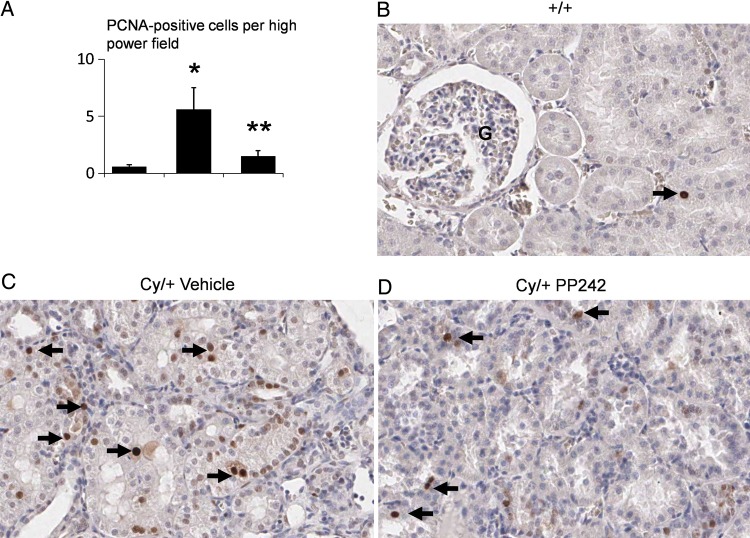
The number of PCNA-positive cells per high power field in non-cystic tubules in the cortex was significantly reduced by PP242 (Figure 6A). Representative pictures are shown in Figure 6B, C and D.
FIGURE 6:
PP242 decreases PCNA-positive cells in non-cystic tubules. The number of PCNA-positive cells per high power field was increased in Cy/+ treated with vehicle compared with wild type (+/+) (A). The number of PCNA-positive cells per tubule was decreased in Cy/+ treated with PP242 (Cy/+ PP242) versus Cy/+ treated with vehicle (Cy/+ vehicle) (A). *P < 0.01 versus +/+. **P < 0.05 versus Cy/+ vehicle, n = 5. Representative pictures of PCNA staining in +/+ (B), Cy/+ vehicle (C) and Cy/+ PP242 (D) are demonstrated. Arrow = PCNA-positive cell.
DISCUSSION
PP242 is a widely studied TORK inhibitor in animal studies and is readily commercially available [21]. PP242 is a pyrazolopyrimidine analog that inhibits mTOR with an IC50 of 8 nM and is selective relative to other PI3Ks [1]. Compared to sirolimus, PP242 has stronger anti-proliferative effects, is a more effective mTORC1 inhibitor, decreases phosphorylation of 4E-BP1, is not immunosuppressive on bone marrow, T and B cells [1, 10, 11] and does not result in positive feedback activation of Akt [1]. TORK inhibitors selectively bind to the ATP-binding site in the mTOR catalytic domain and thereby block both mTORC1 and 2. It has been stated that ‘the discovery of specific, active-site mTOR inhibitors has opened a new chapter in the 40-plus year old odyssey that began with the discovery of sirolimus from a soil sample on Easter Island’ [22]. Thus we considered the TORK inhibitors to be ideal new anti-proliferative drugs to be tested in PKD.
We tested whether the TORK inhibitor PP242 slows cyst growth and improves kidney function in PKD. The rationale for using TORK inhibitors in PKD is as follows. There is increased mTORC1 signaling in PKD. Drugs like sirolimus and everolimus bind to FKBP12 which binds Raptor and indirectly inhibits mTORC1, not mTORC2. Sirolimus does not directly target mTORC2-dependent Akt function [22]. In fact, sirolimus-dependent inhibition of mTORC1 can result in increased activation of mTORC2 [6]. In this regard, we have demonstrated that there is increased pAktSer473 (a marker of mTORC2 activation) in female Han:SPRD rats due to sirolimus associated with no protection against PKD [4]. We and others have demonstrated increased mTORC2 signaling in PKD [2, 3, 4]. There is increased pAktSer473, a marker of mTORC2 signaling in Pkd1 and 2 knockout models [2, 3, 4]. Phosphorylation at Ser473 primes Akt for further phosphorylation at Thr308, in the catalytic domain. Knockout of mTORC2 does not affect mTORC1 suggesting that mTORC2 activates a pool of Akt that is not upstream of mTORC1 [23]. Akt regulates cell survival, proliferation and growth. In a landmark paper in Science in 2005, it was shown that the Rictor-mTOR complex directly phosphorylates Akt on Ser473 and that a reduction in Rictor expression inhibited pAktSer473 [24]. The effect of pAktSer473 inhibition in PKD is not known. Thus the first aim of the study was to determine whether the TORK inhibitor PP242 slows cyst growth and improves kidney function in the Cy/+ rat model of ADPKD. We demonstrate for the first time that a novel drug, a ‘second-generation’ mTOR inhibitor, slows cyst growth and improves kidney function in PKD.
The decrease in kidney weight and cyst volume with PP242 was no better than we have previously reported with sirolimus in the same Cy/+ rat model [13] and in separate studies repeated with sirolimus at the time of the PP242 studies. There are possible reasons for this. Firstly, the dose of PP242 used in the present study was 5 mg/kg/day. Doses as high as 20 mg/kg IP [1, 21] have been used in vivo in mice. Secondly sirolimus completely inhibited pS6 in Cy/+ rats with PKD [4]. In the present study there was less inhibition of pS6 by PP242 than we previously reported with sirolimus [4]. Thirdly, PP242 reduced proliferation in cystic and non-cystic tubules, but did not have an effect on tubular apoptosis. In this regard, inhibition of apoptosis with pharmacological [19] or genetic techniques [20] has been associated with less cyst growth. The present study was designed to determine whether a novel method on mTOR inhibition by inhibiting mTOR kinase was effective in reducing PKD. In the future, preclinical studies of the effect of the newly developed TORKs in head to head to comparison with sirolimus will test the therapeutic potential of TORKs for human PKD. Next, we measured pro-inflammatory cytokines in Cy/+ rat kidneys. Evidence suggests that interstitial inflammation in human and animal model PKD is driven by cytokines like TNF-α [25]. We measured an array of 10 pro-inflammatory cytokines in Cy/+ kidneys. Of the 10 cytokines measured, there was an increase in IL-1, IL-6, CXCL1 and TNF-α in Cy/+ kidneys. Pro-inflammatory cytokines can activate mTORC1 [26]. The mTOR pathway can regulate the expression of inflammatory mediators like cytokines and chemokines [27]. The second-generation mTOR kinase inhibitor INK128 exhibits anti-inflammatory activity in LPS-activated macrophages [28]. However, the mTOR pathway can also limit pro-inflammatory mediators thus exerting an anti-inflammatory effect [29, 30]. On LPS stimulation, mTORC2-deficient fibroblasts and dendritic cells exhibit an increased inflammatory response, suggesting that mTORC2 is anti-inflammatory [31]. Based on these studies, it is possible that mTOR kinase inhibition could increase pro-inflammatory cytokines. On the contrary, if mTOR is pro-inflammatory in PKD then mTOR inhibition may decrease pro-inflammatory cytokines. Besides a non-significant decrease in TNF-α, PP242 had no significant effect on pro-inflammatory cytokines in Cy/+ kidneys suggesting that the protective effect of PP242 in PKD is not mediated by a decrease in the 10 pro-inflammatory cytokines that were studied.
Next we determined the effect of PP242 on caspase-3 and apoptosis in PKD kidneys. There are PKD studies where increased apoptosis is associated with less cyst growth [2, 32]. However, there is extensive in vitro and in vivo evidence that apoptosis inhibition results in less cyst formation in PKD [19, 20, 33]. We have demonstrated that a caspase inhibitor results in less apoptosis, less proliferation and less PKD in male Cy/+ rats [19] and that knockout of the caspase-3 gene markedly prolongs survival in PKD mice [20]. Thus we originally thought that TORK inhibitors may inhibit apoptosis in PKD. However, our data demonstrate that PP242 has no effect on caspase-3 and apoptosis in PKD. In support of a lack of effect of mTORC2 on apoptosis in PKD, it has been shown that mTORC2 inhibition by Genz-12334, which retards accumulation of glucosylceramide, results in very effective blockade of PKD without an effect on apoptosis [34]. In Pkd2WS25/- mice, sirolimus (0.5 mg/kg) improved PKD but had no effect on tubular cell apoptosis and caspase-3 activity [3].
Increased cell proliferation of cystic and non-cystic tubular epithelial cells plays a crucial role in cyst growth PKD [9]. Both mTORC1 and 2 are pro-proliferative signaling pathways. Genetic manipulations like induction of C-myc or targeting of the rasT24 oncogene that induce the proliferation of tubular epithelial cells in mice cause cysts to form in the kidney [35, 36]. The therapeutic effect of PP242 in PKD is likely related to its anti-proliferative effect. While the proliferation index is consistently highest in cystic tubular epithelium, non-cystic tubules from mice with PKD [37] and Han:SPRD rats [13] have higher proliferation rates than tubules from age-matched controls. In Pkd2WS25/- mice, increased cell proliferation is an early event preceding cyst formation [37]. These studies suggest that tubular cell proliferation precedes cyst formation. PP242 resulted in a decrease in proliferation in non-cystic tubules in Cy/+ rats.
The present study highlights important aspects of using TORK inhibitors in ADPKD. Firstly, the studies of sirolimus and everolimus in human ADPKD were plagued with side effects [7, 8]. Eighty percent of ADPKD patients treated with everolimus had a serious adverse event. The side effect profile of TORK inhibitors is potentially better than sirolimus due to a lesser immunosuppressive effect (no bone marrow suppression, no effect on T and B cell proliferation) [11]. Sirolimus therapy in rats, but not mice, resulted in a 10–20% weight loss despite no apparent difference in food intake [13, 38]. Weight loss has not been reported with the TORK inhibitors and did not occur in the present study. The development of TORK inhibitors that may have fewer side effects than sirolimus and everolimus in PKD may result in very effective mTOR inhibition without the side effects that plagued the human studies. The potential use of TORK inhibitors in ADPKD may change current clinical practice regarding the use of mTOR inhibitors in PKD. Secondly, PP242 results in inhibition of pAktSer473, a marker of mTORC2 signaling, in addition to mTORC1 signaling. As there are no specific inhibitors of mTORC2, the effect of specific mTORC2 inhibition on PKD is not known. In this regard, in MDCK type II cells transfected with PC-1, there was increased pAktSer473 suggesting that loss of PC-1, as occurs in PKD, may be associated with decreased mTOR signaling as measured by pAktSer473 [39, 40]. However, in the present study, additional inhibition of pAktSer473 with PP242 was associated with a decrease in PKD. Thirdly, new TORK inhibitors are being developed that may be used in PKD in the future. AZD8055 (from AstraZeneca) is more anti-proliferative than sirolimus in several in vivo cancer models [10, 41, 42]. The potential clinical use of TORK inhibitors is demonstrated by five phase I/II clinical studies of the TORK inhibitor AZD8055 in patients with various advanced solid tumors (see clinicaltrials.gov). There are other TORK inhibitors that are in development. Torin2 is a second-generation mTOR inhibitor that is very suitable for in vivo work [43]. INK-128 (ActiveBiochem) is a potent and selective mTOR inhibitor that is in clinical studies (see clinicaltrials.gov) [6].
In conclusion, the mTOR kinase inhibitors or TORK inhibitors represent a novel therapy in PKD. In view of the absence of effective therapies in ADPKD and the potential safety of TORK inhibitors, the effect of newer TORK inhibitors in other models of PKD should determine the therapeutic potential and possible side effects in human PKD.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
C.L.E. was supported by 1RO1 DK074835 from the National Institutes of Health (NIH) and a Grant-in-aid from the American Heart Association (AHA). A.O. was supported by fellowships from the Turkish Society of Nephrology and the International Society of Nephrology.
REFERENCES
- 1.Feldman ME, Apsel B, Uotila A, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shillingford JM, Piontek KB, Germino GG, et al. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol. 2010;21:489–497. doi: 10.1681/ASN.2009040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zafar I, Ravichandran K, Belibi F, et al. Sirolimus attenuates disease progression in an orthologous mouse model of human autosomal dominant polycystic kidney disease. Kidney Int. 2010;78:754–761. doi: 10.1038/ki.2010.250. [DOI] [PubMed] [Google Scholar]
- 4.Belibi F, Ravichandran K, Zafar I, et al. mTORC1/2 and rapamycin in female Han:SPRD rats with polycystic kidney disease. Am J Physiol Renal Physiol. 2011;300:F236–F244. doi: 10.1152/ajprenal.00129.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer DC, Jacoby U, Pape L. Activation of the AKT/mTOR pathway in autosomal recessive polycystic kidney disease (ARPKD) Nephrol Dial Transplant. 2009;24:1819–1827. doi: 10.1093/ndt/gfn744. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Thoreen C, Wang J, et al. mTOR mediated anti-cancer drug discovery. Drug Discov Today Ther Strateg. 2011;6:47–55. doi: 10.1016/j.ddstr.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walz G, Budde K, Mannaa M. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 8.Serra AL, Poster D, Kistler AD. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 9.Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 10.Albert S, Serova M, Dreyer C, et al. New inhibitors of the mammalian target of rapamycin signaling pathway for cancer. Expert Opin Investig Drugs. 2010;19:919–930. doi: 10.1517/13543784.2010.499121. [DOI] [PubMed] [Google Scholar]
- 11.Janes MR, Limon JJ, So L. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–213. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowley BD, Jr, Gudapaty S, Kraybill AL, et al. Autosomal-dominant polycystic kidney disease in the rat. Kidney Int. 1993;43:522–534. doi: 10.1038/ki.1993.79. [DOI] [PubMed] [Google Scholar]
- 13.Tao Y, Kim J, Schrier RW, et al. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease (PKD) J Am Soc Nephrol. 2005;16:46–51. doi: 10.1681/ASN.2004080660. [DOI] [PubMed] [Google Scholar]
- 14.Brown JH, Bihoreau MT, Hoffmann S. Missense mutation in sterile alpha motif of novel protein SamCystin is associated with polycystic kidney disease in (cy/+) rat. J Am Soc Nephrol. 2005;9:937–945. doi: 10.1681/ASN.2005060601. [DOI] [PubMed] [Google Scholar]
- 15.Nagao S, Morita M, Kugita M, et al. Polycystic kidney disease in Han:SPRD Cy rats is associated with elevated expression and mislocalization of Sam Cystin. Am J Physiol Renal Physiol. 2010;299:F1078–F1086. doi: 10.1152/ajprenal.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowley BD, Jr, Rupp JC, Muessel MJ, et al. Gender and the effect of gonadal hormones on the progression of inherited polycystic kidney disease in rats. Am J Kidney Dis. 1997;29:265–272. doi: 10.1016/s0272-6386(97)90039-1. [DOI] [PubMed] [Google Scholar]
- 17.Dursun B, He Z, Somerset H, et al. Caspases and calpain are independent mediators of cisplatin-induced endothelial cell necrosis. Am J Physiol Renal Physiol. 2006;291:F578–F587. doi: 10.1152/ajprenal.00455.2005. [DOI] [PubMed] [Google Scholar]
- 18.Melnikov VY, Faubel SG, Siegmund B, et al. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest. 2002;110:1083–1091. doi: 10.1172/JCI15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao Y, Kim J, Faubel S, et al. Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease (PKD) Proc Natl Acad Sci USA. 2005;102:6954–6959. doi: 10.1073/pnas.0408518102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao Y, Zafar I, Kim J, et al. Deletion of the caspase-3 gene markedly prolongs survival in the cpk mouse model of polycystic kidney disease (PKD) J Am Soc Nephrol. 2007;19:749–755. doi: 10.1681/ASN.2006121378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoang B, Frost P, Shi Y, et al. Targeting TORC2 in multiple myeloma with a new mTOR kinase inhibitor. Blood. 2010;116:4560–4568. doi: 10.1182/blood-2010-05-285726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shor B, Gibbons JJ, Abraham RT, et al. Targeting mTOR globally in cancer; thinking beyond rapamycin. Cell Cycle. 2009;8:3831–3837. doi: 10.4161/cc.8.23.10070. [DOI] [PubMed] [Google Scholar]
- 23.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 25.Ta MH, Harris DC, Rangan GK. Role of interstitial inflammation in the pathogenesis of polycystic kidney disease. Nephrology (Carlton) 2013;18:317–330. doi: 10.1111/nep.12045. [DOI] [PubMed] [Google Scholar]
- 26.Lee DF, Kuo HP, Chen CT. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 27.Katholnig K, Linke M, Pham H, et al. Immune responses of macrophages and dendritic cells regulated by mTOR signalling. Biochem Soc Trans. 2013;41:927–933. doi: 10.1042/BST20130032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan H, Xu LH, Ouyang DY, et al. The second-generation mTOR kinase inhibitor INK128 exhibits anti-inflammatory activity in lipopolysaccharide-activated RAW 264.7 cells. Inflammation. 2014;37:756–765. doi: 10.1007/s10753-013-9794-9. [DOI] [PubMed] [Google Scholar]
- 29.Weichhart T, Haidinger M, Katholnig K. Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood. 2011;117:4273–4283. doi: 10.1182/blood-2010-09-310888. [DOI] [PubMed] [Google Scholar]
- 30.Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23:744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Brown J, Wang H, Suttles J, et al. Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1. J Biol Chem. 2011;286:44295–44305. doi: 10.1074/jbc.M111.258053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shillingford JM, Murcia NS, Larson CH. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edelstein CL. What is the role of tubular epithelial cell apoptosis in polycystic kidney disease (PKD)? Cell Cycle. 2005;4:e141–e145. doi: 10.4161/cc.4.11.2185. [DOI] [PubMed] [Google Scholar]
- 34.Natoli TA, Smith LA, Rogers KA. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat Med. 2010;16:788–792. doi: 10.1038/nm.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trudel M, Barisoni L, Lanoix J, et al. Polycystic kidney disease in SBM transgenic mice: role of c-myc in disease induction and progression. Am J Pathol. 1998;152:219–229. [PMC free article] [PubMed] [Google Scholar]
- 36.Schaffner DL, Barrios R, Massey C, et al. Targeting of the rasT24 oncogene to the proximal convoluted tubules in transgenic mice results in hyperplasia and polycystic kidneys. Am J Pathol. 1993;142:1051–1060. [PMC free article] [PubMed] [Google Scholar]
- 37.Chang MY, Parker E, Ibrahim S, et al. Haploinsufficiency of Pkd2 is associated with increased tubular cell proliferation and interstitial fibrosis in two murine Pkd2 models. Nephrol Dial Transplant. 2006;21:2078–2084. doi: 10.1093/ndt/gfl150. [DOI] [PubMed] [Google Scholar]
- 38.Zafar I, Belibi FA, He Z, et al. Long-term rapamycin therapy in the Han:SPRD rat model of polycystic kidney disease (PKD) Nephrol Dial Transplant. 2009;24:2349–2353. doi: 10.1093/ndt/gfp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boletta A. Emerging evidence of a link between the polycystins and the mTOR pathways. Pathogenetics. 2009;2:6. doi: 10.1186/1755-8417-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boletta A, Qian F, Onuchic LF, et al. Polycystin-1, the gene product of PKD1, induces resistance to apoptosis and spontaneous tubulogenesis in MDCK cells. Mol Cell. 2000;6:1267–1273. doi: 10.1016/s1097-2765(00)00123-4. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Q, Weiss JM, Back T, et al. mTOR kinase inhibitor AZD8055 enhances immunotherapeutic activity of an agonist CD40 antibody in cancer treatment. Cancer Res. 2011;71:4074–4084. doi: 10.1158/0008-5472.CAN-10-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chresta CM, Davies BR, Hickson I. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 43.Liu Q, Chang JW, Wang J, et al. Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1, 6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem. 2010;53:7146–7155. doi: 10.1021/jm101144f. [DOI] [PMC free article] [PubMed] [Google Scholar]