Abstract
Purpose
Lymph-node metastasis is considered as critical prognostic factor in colorectal cancer. A preoperative evaluation of lymph-node metastasis can also help to determine the range of distant lymph node dissection. However, the reliability of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) in the detection of lymph-node metastasis is not fully known.
Methods
The medical records of 433 patients diagnosed with colorectal cancer were reviewed retrospectively. FDG-PET/CT and CT were performed on all patients. Lymph nodes were classified into regional and distant lymph nodes according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edition.
Results
The patients included 231 males (53.3%) and 202 females (46.7%), with a mean age of 64.7 ± 19.0 years. For regional lymph nodes, the sensitivity of FDG-PET/CT was lower than that of CT (57.1% vs. 73.5%, P < 0.001). For distant lymph nodes, the sensitivity of FDG-PET/CT was higher than that of CT (64.7% vs. 52.9%, P = 0.012). The sensitivity of FDG-PET/CT for regional lymph nodes was higher in patients with larger primary tumors. The positivity of lymph-node metastasis for FDG-PET/CT was affected by carcinoembryonic antigen levels, tumor location, and cancer stage for regional lymph nodes and by age and cancer stage for distant lymph nodes (P < 0.05).
Conclusion
The sensitivity of FDG-PET/CT for regional lymph-node metastasis was not superior to that of CT. However, FDG-PET/CT provides helpful information for determining surgical plan especially in high risk patients group.
Keywords: Colorectal neoplasms, Positron emission tomography, Lymph node, Metastasis
INTRODUCTION
Colorectal cancer is one of the most common cancers, constituting a major part of global cancer deaths [1]. Because the lymphatic metastasis of cancer cells is one of the major factors that impact the prognosis of colorectal cancer [2], the accuracy of nodal staging is important to predict the prognosis of colorectal cancer. In particular, the location of metastatic lymph nodes, such as the metastatic lymph nodes along the aorta or the major vessels that branch from the aorta, can be a poorer prognostic factor than the number of metastatic lymph nodes [3]. However, although the dissection of the lymph nodes along the aorta or the major supplying vessels is not the standard procedure of colorectal cancer surgery and is accompanied by operative risks or complications, a decision to perform a lymph-node dissection is usually a prudent one. Therefore, the accuracy of preoperative lymph-node staging plays an important role in determining the extent of dissection required.
The preoperative lymphatic stage can be estimated with imaging studies, such as computed tomography (CT), magnetic resonance imaging, and ultrasonography, but many researchers have reported the low sensitivity of these imaging studies as a decisive tool for determining lymphatic status [4, 5]. In addition, although 18F-fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) is well-known for its effectiveness in diagnosing primary tumors or distant organ metastasis, there are no well-defined findings for its reliability and effectiveness regarding the lymph-node status in patients with colorectal cancer. Therefore, in this study, we investigated the reliability of FDG-PET/CT in determining the lymph-node staging for patients with colorectal cancer, and we addressed the additional issue of improving the utility of FDG-PET/CT in preoperative lymph-node staging.
METHODS
The medical records of 433 patients who were diagnosed with colorectal cancer and underwent radical surgery from January 2009 to December 2013 were reviewed. Surgeries that did not involve dissection of the lymph nodes, such as transanal/transrectal local excisions or palliative surgery, like ileostomy, colostomy, and bypass surgery, were excluded.
FDG-PET/CT was performed using a Siemens Biograph mCT/128 PET/CT scanner (Siemens Medical Solutions, Hoffman Estates, Knoxville, TN, USA). All patients fasted for at least 6 hours; their serum glucose levels were then checked just before the examination to ensure the levels were below 150 mg/dL. 18F-FDG, 4.81 MBq/kg, was injected, and CT scans were performed after 1 hour. Then, 1.5-minute emission scans were taken from the base of the skull to the proximal thigh. These images were also reconstructed. The interpretation of these FDG-PET/CT images was performed by one nuclear radiologist, and consistent criteria were used for interpretation. Also, the interpretation of the CT images was performed by one radiologist using consistent criteria. Patients with suspicious lymph nodes and metastatic lymph nodes in the FDG-PET/CT and CT results, as interpreted by our professional radiologist, were enrolled in our study.
Lymph nodes were classified as regional lymph nodes and distant lymph nodes in accordance with the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edition [2]. Regional lymph nodes are lymph nodes located near the peripheral vessels of the colon or rectum where the primary tumors occur, and distant lymph nodes are lymph nodes along the aorta or the supplying vessels that branch from the aorta. Distant lymph nodes are defined differently according to the locations of the primary tumors, as stated in the AJCC Cancer Staging Manual [2].
The patients were classified into the elderly group and the control group according to an age of 65 years and into the obese group and the normal group according to a body mass index (BMI) of 25 kg/m2. The patients were also divided into two groups by a carcinoembryonic antigen (CEA) level of 5 ng/dL, which was accepted as being clinically meaningful. On the histopathologic results, T stage 3 or 4 was interpreted as being a pericolic/perirectal infiltration of the primary tumor, which was checked by comparison with the results of the CT scan. The size of the primary tumor was expressed by the product of the length and the width of the tumor on the pathologic results. The mean standardized uptake value (SUV) of the primary tumor was calculated as about 15. The patients were then divided into two groups according to the SUV of 15, and the results were compared.
Noncontinuous variables, like the sex of the patient and the stage and the location of the primary tumor, were analyzed by using the chi-square test. Continuous variables, like the age, the duration of the follow-up and the number of lymph nodes, were expressed as "mean ± standard deviation", and these variables were analyzed using the independent t-test. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the FDG-PET/CT and the CT were identified and compared by using the McNemar test. Statistical analyses were performed using the IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA), and P-values < 0.05 were considered statistically significant.
RESULTS
Of 433 patients, there were 231 males (53.3%) and 202 females (46.7%). The mean age was 64.7 ± 19.0 years. The mean BMI was 26.1 ± 30.6 kg/m2, and 163 of patients (37.6%) were obese. The mean level of CEA was 46.7 ± 288.6 ng/dL at the time of diagnosis. Two hundred forty-seven patients (63.3%) were diagnosed with colon cancer, and 159 patients (36.7%) were diagnosed with rectal cancer. On the distribution of stages, there were 8 patients at stage 0 (1.8%), 77 at stage I (17.8%), and 136 at stage II (31.4%), for a total of 221 patients (51.0%). There were 143 patients at stage III (33.0%) and 69 patients at stage IV (15.9%), for a total of 212 patients (49.0%). The mean duration of follow-up was 31.0 ± 17.1 months; the recurrent cases were 109 (25.2%), and the mortality occurred in 91 cases (21.0%) during the follow-up (Table 1).
Table 1.
Demographic characteristics of the patients
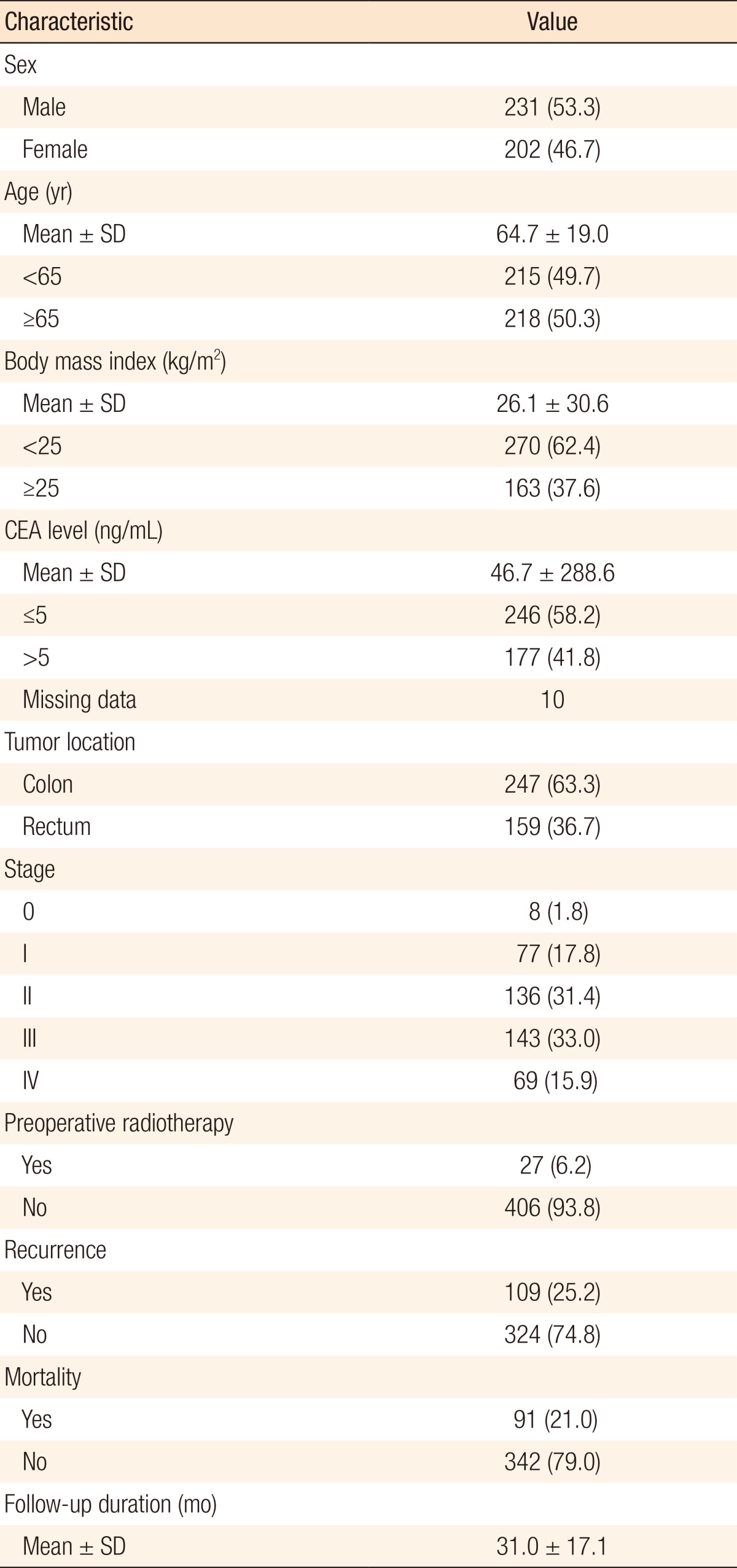
Values are presented as number (%) unless otherwise indicated.
SD, standard deviation; CEA, carcinoembryonic antigen.
In the histopathologic results, the mean number of harvested lymph nodes was 16.5 ± 10.4, and lymph-node metastasis was confirmed in 200 patients. Of these cases, regional lymph-node metastasis was identified in 196 patients (45.3%), and distant lymph-node metastasis was identified in 17 patients (3.9%). When confirming regional lymph-node metastasis with FDG-PET/CT, the sensitivity was 57.1%, the specificity was 67.5%, the PPV was 59.3%, and the NPV was 65.6%. When confirming regional lymph-node metastasis with CT, the sensitivity was 73.5%, the specificity was 49.8%, the PPV was 54.8%, the NPV was 69.4%. When identifying regional lymph-node metastasis, the sensitivity was lower and the specificity was higher in FDG-PET/CT than in CT (P < 0.001). When confirming distant lymph-node metastasis with FDG-PET/CT, the sensitivity was 64.7%, the specificity was 65.6%, the PPV was 7.1%, and the NPV was 97.8%. When confirming distant lymph-node metastasis with CT, the sensitivity was 52.9%, the specificity was 72.4%, the PPV was 7.3%, and the NPV was 97.4%. When confirming distant lymph-node metastasis, the sensitivity was higher and the specificity was lower in FDG-PET/CT than in CT (P = 0.012) (Table 2).
Table 2.
Comparison of reliability between FDG-PET/CT and CT in detecting lymph-node metastasis
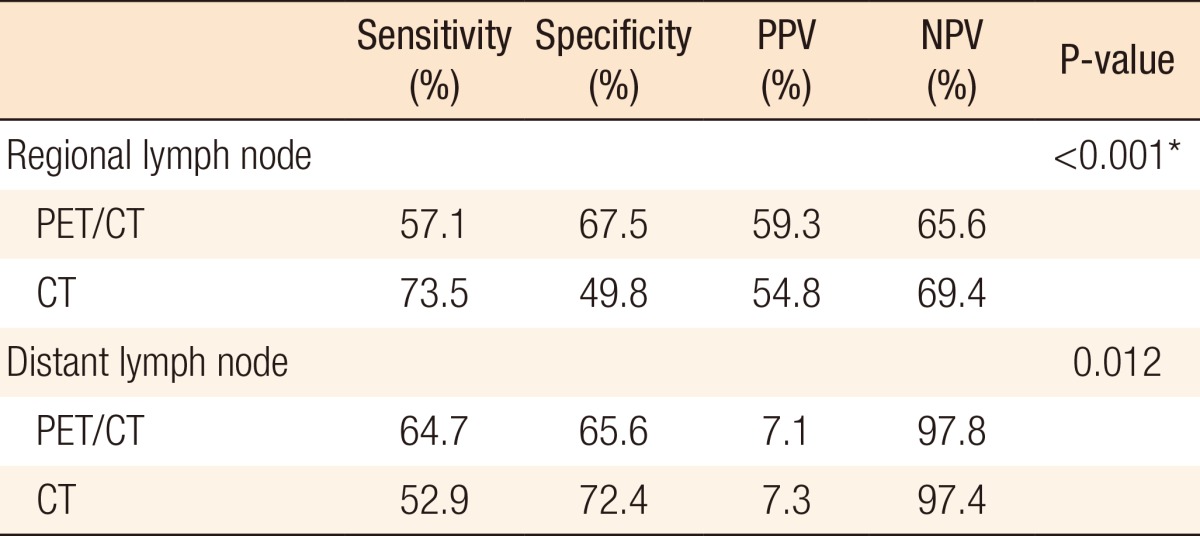
FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; PPV, positive predictive value; NPV, negative predictive value.
*P < 0.05.
Among the patients who had pericolic/perirectal infiltrations on their histopathologic results or CT scans, the sensitivity was 58.6%, the sensitivity was 61.7%, the PPV was 65%, and the NPV was 55.1% to detect regional lymph-node metastasis with FDG-PET/CT. Among the cases where the size of the primary tumor was more than 20 cm2, the sensitivity was 44.6%, the specificity was 64.4%, the PPV was 47.5%, and the NPV was 61.7% to detect regional lymph-node metastasis with FDG-PET/CT. In the cases where the SUV of the primary tumor was more than 15, the sensitivity was 79.6%, the specificity was 49.4%, the PPV was 50%, and the NPV was 79.2% to detect regional lymph-node metastasis with FDG-PET/CT (Table 3).
Table 3.
Comparison of the reliability of PDG-PET/CT according to the state of the primary tumors in regional lymph nodes
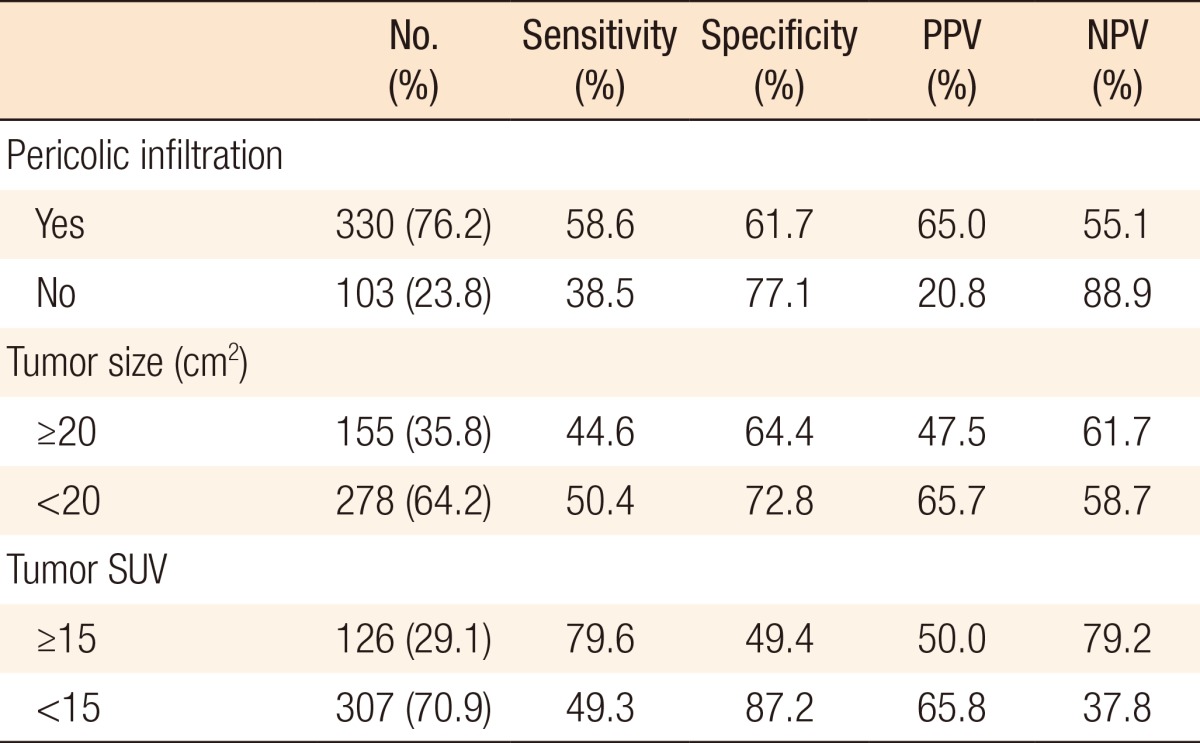
FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; PPV, positive predictive value; NPV, negative predictive value; SUV, standardized uptake value.
For investigating the factors that affect the interpretation of FDG-PET/CT for lymph-node metastasis, we performed univariate and multivariate analyses with some variables. For regional lymph nodes, factors such as the BMI, the CEA level, the location of the primary tumor, and the stage showed some significant results with the univariate analysis. On the multivariate analysis, the CEA level (P = 0.006), the location of the primary tumor (P = 0.023), and the stage (P < 0.001) were statistically significant for interpreting the regional lymph-node metastasis (Table 4). For distant lymph nodes, factors such as age, level of CEA, and stage showed significant results. The age (P < 0.001) and the stage (P = 0.040) were also statistically significant for interpreting distant lymph-node metastasis (Table 5).
Table 4.
Factors that affect the positive detection rate of regional lymph nodes for FDG-PET/CT
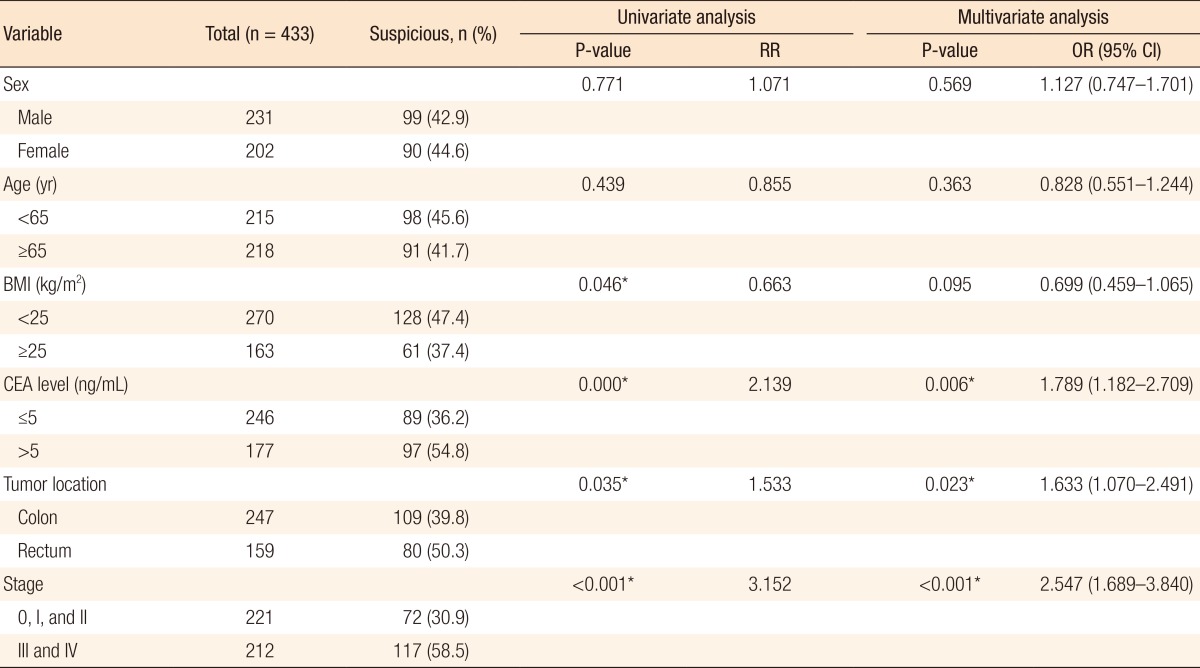
FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; RR, relative risk; OR, odds ratio; CI, confidence interval; BMI, body mass index; CEA, carcinoembryonic antigen.
*P < 0.05.
Table 5.
Factors that affect the positive detection rate of distant lymph nodes for FDG-PET/CT
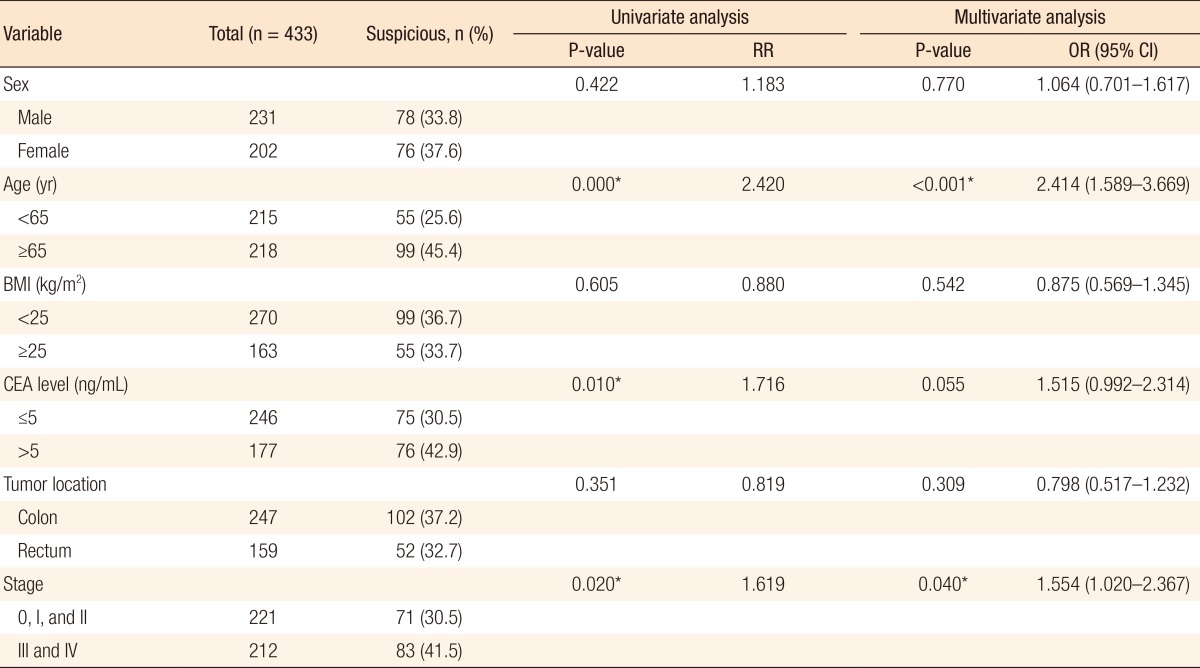
FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; RR, relative risk; OR, odds ratio; CI, confidence interval; BMI, body mass index; CEA, carcinoembryonic antigen.
*P < 0.05.
DISCUSSION
As previously mentioned, preoperative staging, especially of the lymph node status, is helpful to predict the prognosis of patients and plays an important role in determining more appropriate treatment plans. Particularly, determining the extent of dissection required can influence the operative risk and postoperative morbidities [6]; therefore, preoperative lymph-node staging should be performed with more sensitive and reliable diagnostic tools.
FDG-PET is useful as a preoperative study to diagnose the primary tumor and distant organ metastasis [7, 8] and is helpful to detect recurrence after the operation [9]. However, reports have suggested that such imaging studies have low sensitivities as a diagnostic tool for lymphatic status [10, 11]. This is because FDG-PET images cannot reveal the anatomical structures precisely, making it hard to distinguish a regional lymph node from the primary tumor [12]. Moreover, because the FDP-PET image is a manifestation of the GLUT1 (protein glucose transport 1) in the tissue, in some organs that biologically overexpress the GLUT1- or in the case of an inflammatory reaction, which reads as an overexpression of GLUT 1-interpretation of the FDP-PET image can be difficult [13] (Figs. 1, 2).
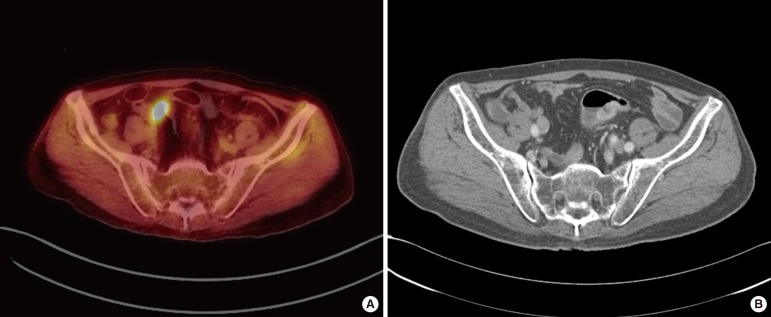
Fig. 1.
False positive results in 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT): (A) FDG uptake of lymph node was identified in FDG-PET/CT and (B) no enlarged lymph node in CT.
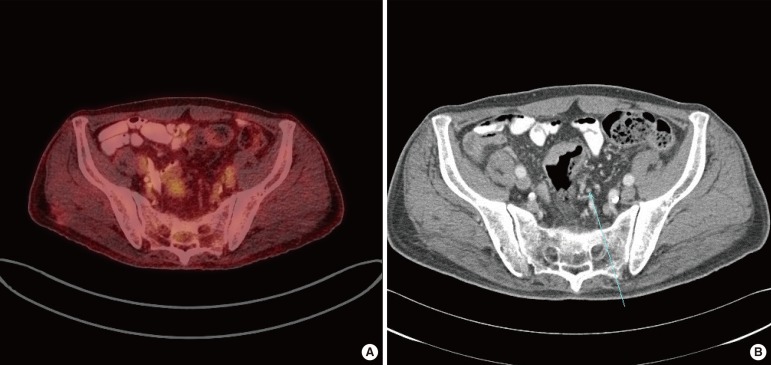
Fig. 2.
False negative results in 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT): (A) no visual FDG uptake was seen in FDG-PET/CT and (B) enlarged lymph nodes (arrow) were identified in CT and metastasis of that lymph node was confirmed in the histopathologic results.
The combination of the PET image, which results in a metabolic reaction, and the CT image yields a more accurate image of the data for anatomical structures. Therefore, the expected accuracy of FDP-PET/CT in evaluating lymph-node status should be higher than that of either technique alone. However, previous studies yielded varying results. While Tateishi et al. [14] reported a sensitivity of 85.3% and a specificity of 42.1% for lymph-node metastasis, Kim et al. [15] reported a sensitivity of 61.1% and a specificity of 87.9%, and Shin et al. [16] showed a sensitivity of 43% and a specificity of 80%.
In this study, for regional lymph nodes, the sensitivity of FDG-PET/CT was 57.1%, the specificity was 67.5%, the PPV was 59.3%, and the NPV was 65.6%. For the regional lymph-node evaluation with a CT scan, the sensitivity was 73.5%, the specificity was 49.8%, the PPV was 54.8%, and the NPV was 69.4%. We expected to detect more precisely the presence of regional lymph nodes around the primary tumor by utilizing the advantages of PET and CT, but the sensitivity of FDG-PET/CT turned out to be lower and the specificity to be higher than that the corresponding value for a CT scan. When identifying distant lymph nodes, the sensitivity of FDG-PET/CT was 64.7%, the specificity was 65.7%, the PPV was 7.1%, and the NPV was 97.8%, while the sensitivity of CT was 52.9%, the specificity was 72.4%, the PPV was 7.3%, and the NPV was 97.4%.
There was a significant difference between the predictive values of lymph-node metastasis in FDG-PET/CT and CT scan (regional lymph node, P < 0.000; distant lymph node, P = 0.012) (Table 2). Kwak et al. [17] reported that the sensitivity of FDG-PET/CT was inferior to that of CT for proximal lymph nodes, or regional lymph nodes and that the sensitivity of FDG-PET/CT was superior to that of CT for distal lymph nodes or distant lymph nodes. Tsunoda et al. [12] also suggested that the sensitivity of FDG-PET/CT was lower for regional lymph nodes than for distant lymph nodes because it was difficult to detect the FDG uptake around the primary tumor when the volume of the primary tumor itself was larger.
Therefore, we compared the differences in the sensitivity, specificity, PPV, and NPV of FDG-PET/CT according to the pericolic/perirectal infiltration, the size, and the SUV of the primary tumors. However, contrary to expectations, the sensitivity was higher (58.6% vs. 38.5%) and the specificity was lower (61.7% vs. 77.1%) in the pericolic/perirectal infiltration-positive cases than in the pericolic/perirectal infiltration-negative cases. We also expected that the higher the SUV of the primary tumor was, the more difficult the detection of lymph-node metastasis would be because of the difficulty to distinguish the FDG uptake of regional lymph nodes. However, the higher the SUV was, the higher the sensitivity was (79.6% vs. 49.3%), and the lower the specificity was (49.4% vs. 87.2%). In addition, the sensitivity and the specificity were compared based on the size of the primary tumor. The sensitivity was 44.6% and the specificity was 64.4% in patients with a larger primary tumor while the sensitivity was 50.4% and the specificity was 72.8% in patients with a smaller primary tumor (Table 3). In this study, the low sensitivity of regional lymph-node metastasis for FDG-PET/CT was affected by the size of the primary tumor. The larger the size of the primary tumor, the more difficult it became to distinguish regional lymph nodes from FDG uptake. Therefore, the reliability of FDG-PET/CT for lymph-node staging was inferior in the patients with larger primary tumors.
To establish the factors that affect the interpretation of lymph-node metastasis positivity in FDG-PET/CT, we performed univariate and multivariate analyses using variables such as sex, age, BMI, CEA level, and location ab stage of the primary tumor. In the results, suspicious regional lymph-node metastasis on FDG-PET/CT was more detectable in colon cancers than in rectal cancers (P = 0.023). We concluded that the physiologic uptake could not be differentiated from the pathological uptake because of the anatomical complexity of vascularity or the mesentery on FDG-PET/CT. In addition, the higher the stage, the more suspicious the lymph-node metastasis was on FDG-PET/CT (regional lymph nodes, P < 0.001; distant lymph nodes, P = 0.040), which meant that FDG-PET/CT could represent lymph-node metastasis accurately. The higher the CEA level, the more suspicious the regional lymph-node metastasis was on FDG-PET/CT in both the univariate and the multivariate analyses (P < 0.05). Between the CEA level and the suspicion of distant lymph-node metastasis on FDG-PET/CT, there was statistical significance in the univariate analysis (P = 0.010), but not in the multivariate analysis (P = 0.055) (Tables 4, 5).
In summary, FDG-PET/CT can reflect lymph-node metastasis; however, the sensitivity and the specificity were not found to be superior to those of conventional CT. FDG-PET/CT is recommended in cases of increased CEA, colon cancer, and advanced stage to detect regional lymph-node metastasis and in cases of older age and advanced stage to detect the distant lymph-node metastasis. The factors that can affect the false positive detection or the false negative detection of FDG-PET/CT need to be clarified with a well-designed, randomized, controlled study.
The purpose of this study was to establish the reliability of FDG-PET/CT for detecting lymp-node metastasis, but FDG-PET/CT did not give results superior to those of conventional CT. However, FDG-PET/CT showed higher sensitivity for distant lymph-node metastasis than CT. A surgical plan based on preoperative FDG-PET/CT can provide support by determining the range of lymph-node dissection required and ultimately can help to decrease surgical risks and improve oncologic safety.
ACKNOWLEDGMENTS
This research was supported by Dr. Bo-Young Oh with data analysis.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 3.Hermanek P, Altendorf A. Classification of colorectal carcinomas with regional lymphatic metastases. Pathol Res Pract. 1981;173:1–11. doi: 10.1016/S0344-0338(81)80002-7. [DOI] [PubMed] [Google Scholar]
- 4.Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging: a meta-analysis. Radiology. 2004;232:773–783. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 5.Heriot AG, Grundy A, Kumar D. Preoperative staging of rectal carcinoma. Br J Surg. 1999;86:17–28. doi: 10.1046/j.1365-2168.1999.00996.x. [DOI] [PubMed] [Google Scholar]
- 6.Leggeri A, Roseano M, Balani A, Turoldo A. Lumboaortic and iliac lymphadenectomy: what is the role today. Dis Colon Rectum. 1994;37(2 Suppl):S54–S61. doi: 10.1007/BF02048433. [DOI] [PubMed] [Google Scholar]
- 7.Kato H, Miyazaki T, Nakajima M, Takita J, Kimura H, Faried A, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer. 2005;103:148–156. doi: 10.1002/cncr.20724. [DOI] [PubMed] [Google Scholar]
- 8.Erturk SM, Ichikawa T, Fujii H, Yasuda S, Ros PR. PET imaging for evaluation of metastatic colorectal cancer of the liver. Eur J Radiol. 2006;58:229–235. doi: 10.1016/j.ejrad.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 9.Valk PE, Abella-Columna E, Haseman MK, Pounds TR, Tesar RD, Myers RW, et al. Whole-body PET imaging with [18F]fluorodeoxyglucose in management of recurrent colorectal cancer. Arch Surg. 1999;134:503–511. doi: 10.1001/archsurg.134.5.503. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Nabi H, Doerr RJ, Lamonica DM, Cronin VR, Galantowicz PJ, Carbone GM, et al. Staging of primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose whole-body PET: correlation with histopathologic and CT findings. Radiology. 1998;206:755–760. doi: 10.1148/radiology.206.3.9494497. [DOI] [PubMed] [Google Scholar]
- 11.Kantorova I, Lipska L, Belohlavek O, Visokai V, Trubac M, Schneiderova M. Routine (18)F-FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. J Nucl Med. 2003;44:1784–1788. [PubMed] [Google Scholar]
- 12.Tsunoda Y, Ito M, Fujii H, Kuwano H, Saito N. Preoperative diagnosis of lymph node metastases of colorectal cancer by FDG-PET/CT. Jpn J Clin Oncol. 2008;38:347–353. doi: 10.1093/jjco/hyn032. [DOI] [PubMed] [Google Scholar]
- 13.Avril N. GLUT1 expression in tissue and (18)F-FDG uptake. J Nucl Med. 2004;45:930–932. [PubMed] [Google Scholar]
- 14.Tateishi U, Maeda T, Morimoto T, Miyake M, Arai Y, Kim EE. Non-enhanced CT versus contrast-enhanced CT in integrated PET/CT studies for nodal staging of rectal cancer. Eur J Nucl Med Mol Imaging. 2007;34:1627–1634. doi: 10.1007/s00259-007-0455-9. [DOI] [PubMed] [Google Scholar]
- 15.Kim DJ, Kim JH, Ryu YH, Jeon TJ, Yu JS, Chung JJ. Nodal staging of rectal cancer: high-resolution pelvic MRI versus 18F-FDGPET/CT. J Comput Assist Tomogr. 2011;35:531–534. doi: 10.1097/RCT.0b013e318225720f. [DOI] [PubMed] [Google Scholar]
- 16.Shin SS, Jeong YY, Min JJ, Kim HR, Chung TW, Kang HK. Preoperative staging of colorectal cancer: CT vs. integrated FDG PET/CT. Abdom Imaging. 2008;33:270–277. doi: 10.1007/s00261-007-9262-9. [DOI] [PubMed] [Google Scholar]
- 17.Kwak JY, Kim JS, Kim HJ, Ha HK, Yu CS, Kim JC. Diagnostic value of FDG-PET/CT for lymph node metastasis of colorectal cancer. World J Surg. 2012;36:1898–1905. doi: 10.1007/s00268-012-1575-3. [DOI] [PubMed] [Google Scholar]