Abstract
Aim
Oral mucositis is a common and debilitating side effect of haematopoietic stem cell transplantation. Our study investigated parents' and children's experiences of oral mucositis treatment and whether the parents' perceptions accurately reflected the children's views.
Methods
We analysed 71 questionnaires completed by the parents of children who had undergone haematopoietic stem cell transplantation, together with 38 questionnaires completed by children who were 7 years of age or over.
Results
The parent proxy and child self‐reports showed good to excellent agreement. For example, 86% of the parents and 83% of the children reported oral pain and 44% of the parents and 47% of the children reported difficulty swallowing often or very often. The majority of the parents (61%) were satisfied with the pain treatment that had been given to their child. However, the treatment provided for oral mucositis was not altogether consistent.
Conclusion
Oral mucositis affected the majority of the children undergoing haematopoietic stem cell transplantation, causing considerable pain and discomfort. The parent proxy reports proved to be reliable and are an important supplement to child self‐reports on symptoms related to oral mucositis. But there is a clear need to establish more evidence‐based care for children suffering from oral mucositis.
Keywords: Haematopoietic stem cell transplantation, Oral mucositis, Pain, Parent proxy, Questionnaire
Abbreviations
- HSCT
Haematopoietic stem cell transplantation
- ICC
Intraclass correlation
- OM
Oral mucositis
- VAS
Visual analogue scale
Key notes.
Oral mucositis is a common and debilitating side effect of haematopoietic stem cell transplantation in children.
Our study showed that parent proxy reports were a reliable and important supplement to child self‐reports on symptoms related to oral mucositis.
However, it also showed that the treatment of oral mucositis in children is not altogether consistent and there is a need for further randomised control trials to increase treatment evidence.
Introduction
Mucositis is a common adverse effect of cancer treatment and its symptoms include oral and/or gastrointestinal inflammation and ulceration. The incidence of oral mucositis (OM) in children ranges between 52% and 81% depending on the type of antineoplastic treatment 1. The majority of patients undergoing haematopoietic stem cell transplantation (HSCT) develop some degree of OM. There is a complex pathobiology behind OM. Chemotherapy and radiotherapy affect the mucosa and submucosa causing DNA‐strand breaks and generating reactive oxygen species. This initiates a cascade of events, which includes activation of transcription factors, up‐regulation of pro‐inflammatory cytokines and activation of macrophages and proteases, leading to tissue injury and causing symptoms such as erythema, oedema, ulceration, alterations to taste perception and mouth dryness 4. Oral mucositis often causes pain and difficulties in basal functions, such as talking and swallowing, which in turn affect drinking and eating. The condition often leads to local and systemic infections, fatigue and reduced psychological well‐being 4. Oral mucositis is reported to be one of the most painful and debilitating side effects of cancer treatment in paediatric patients 10. From a health care perspective, OM delays treatment, which reduces its intensity, and increases the incidence of infections, total parenteral nutrition use, drug consumption and hospitalisation 4. As well as causing increased morbidity and suffering, OM also increases health care costs and mortality 14. Preventive interventions and therapeutic treatments for OM have been evaluated by Cochrane reviews 15 and by the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer and the International Society of Oral Oncology 17. At present, standard oral care protocols for OM in children involve pharmacological and non‐pharmacological interventions that focus on good oral hygiene, with tooth brushing, flossing and non‐pharmacological rinses in combination with pain and supportive nutritional treatment. 21. The Mouth Care Group of the Children's Cancer and Leukaemia Group and the Paediatric Oncology Nurses' Forum have developed guidelines for the prevention and treatment of OM in children. They conclude that there is a need for further research to evaluate interventions that are infrequently used for children 22. However, it remains still unclear how many paediatric patients are affected by OM, to what extent and what, if any, consequences OM has on the child. This kind of knowledge is important to address physical and psychological symptoms in children with OM during HSCT treatment.
Assessing pain and discomfort is of the utmost importance for treating OM and is an important outcome measure in clinical trials for OM. Self‐reporting with visual analogue scales is recommended for children of more than 7 years old and facial pain scales can be used by children of more than 4 years old 23. Yet, there are circumstances when this is not possible and sometimes parents' perceptions of their children's symptoms and suffering (parent proxy) provide the only information to base treatment on 24. There are studies that validate how reliably parents' estimate their child's pain and health‐related quality of life 24. However, to our knowledge, there are no studies that explore the consistency between parents' and children's estimation of mucositis‐related symptoms.
The primary aim of this study was to describe, from a parent proxy and child perspective, how OM was treated in children and adolescents (hereafter referred to as children) undergoing HSCT and their perceptions of that treatment. The secondary aim was to investigate the agreement between parent proxy and child reports of OM‐related symptoms.
Patients and Methods
Design
The study had a cross‐sectional, descriptive and comparative design.
Settings and participants
The study was carried out to inform future randomised clinical trials of OM interventions in children. Between 50 and 60 children undergo HSCT every year in Sweden, with four hospitals conducting the transplants. Parental questionnaires were sent out to all living patients who had undergone HSCT in Sweden between 2008 and 2010 and who were aged between birth and 18 years of age at the time of the transplant. A separate child self‐report questionnaire was also sent out to those children who were aged 7 or above at the time of the study. We asked them to fill out the questionnaires separately, with just one parent completing the parent proxy questionnaire. A total of 127 parent proxy and 73 child questionnaires were sent out, with two reminders sent out, after 3 and 6 weeks. The study was approved by the Regional Ethics Review Committee in Uppsala, Sweden.
Materials
The questionnaires include questions about: background data, treatment‐related data, distress caused by HSCT‐related difficulties, OM‐related symptoms and pain, the impact of OM on physical performance and psychological well‐being and current OM‐related problems (see Appendix S1 for questionnaire content). Visual analogue scales (VAS) ranging from 0 to 100 were used to retrospectively assess the perceived distress caused by HSCT‐related difficulties and OM‐related pain. To assess the perceived occurrence and degree of OM‐related symptoms and the impact of OM on psychological status and treatment‐related data, a zero to three scale was used, where zero represented ‘not at all’, one ‘a little’, two ‘a lot” and three ‘very much’. The perceived impact of OM on general condition and physical performance was assessed on a zero to four scale, where zero represented ‘never’, one ‘seldom’, two ‘sometimes’, three ‘often’ and four ‘very often”. Questions concerning prophylactic interventions and therapeutic treatment for OM were not included in the child version of the questionnaire to minimise attrition. As a result, some questions were only answered by the parents. Clinicians who were experts in OM and HSCT were involved in the development of the questionnaire to achieve face validity. The readability was tested on children and adults before the questionnaires were sent.
Statistical methods
Descriptive analyses were carried out using SPSS version 20.0 (© Copyright IBM Corporation 2012, Armonk, NY, USA). A two‐way mixed model intraclass correlation (ICC) test was performed to test for potential agreement between the parental and child assessments of the occurrence and degree of OM‐related symptoms. According to the guidelines of interpretation, we considered that an ICC of <0.40 indicated poor agreement, values of between 0.40 and 0.75 indicated fair to good agreement and an ICC of >0.75 indicated excellent agreement 28. Wilcoxon signed rank test was used to test for potential differences between the parents' and children's reports and the significance level was set at p < 0.05.
Results
Of the 127 parent proxy questionnaires sent out, 71 (56%) were returned and included in the analysis. Of the 56 not included, 51 did not respond, three declined and two only sent back the child version. Of the 73 child questionnaires sent out, 38 (52%) were returned and included in the analysis. Of the 35 not included, 33 did not respond, one declined and one only sent back the parent proxy version.
Table 1 presents the characteristics of the children included in the analysis, as reported in the parent proxy and child questionnaires. Table 2 presents the distribution of diagnoses.
Table 1. Characteristics and background data for children in the parent proxy and child survey respectively.
| Demographic data | Parent proxy survey (N = 71) | Child survey (N = 38) | |
|---|---|---|---|
| Age (years) | mean (SD) | 9.0 (5.0) | 12.7 (3.5) |
| Age range (years) | 1–18 | 7–18 | |
| Gender, boys/girls | n (%) | 49 (69)/22 (31) | 27 (71)/11 (29) |
| Age at diagnosis (years) | mean (SD) | 6.7 (5.0) | 8.7 (4.2) |
| Age at HSCT (years) | mean (SD) | 7.1 (5.0) | 10.7 (3.7) |
| Reason for HSCT, cancer/other | n (%) | 46 (65)/25 (35) | 31 (82)/7 (18) |
| Type of HSCT (Auto/allo)a | n (%) | 16 (23)/54 (77) |
HSCT = Haematopoietic stem cell transplantation.
Auto = Autologous, Allo = allogeneic.
Table 2. Distribution of diagnosis for children in the parent proxy and child survey respectively.
| Diagnosis | Parent proxy survey (N = 71) | Child survey (N = 38) | ||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Cancer | ||||
| Leukaemia | 22 | (31) | 19 | (50) |
| CNS tumour | 14 | (21) | 2 | (5) |
| MDS | 6 | (9) | 4 | (10) |
| Hodgkin lymphoma | 2 | (3) | 2 | (5) |
| Rhabdomyosarcoma | 1 | (1) | 1 | (3) |
| Neuroblastoma | – | 2 | (5) | |
| Missing | 1 | (1) | 1 | (3) |
| Other | ||||
| Aplastic anaemia | 5 | (7) | 2 | (5) |
| SCID | 4 | (6) | – | |
| WAS | 3 | (4) | – | |
| HLH | 3 | (4) | – | |
| Fanconi anaemia | 2 | (3) | 1 | (3) |
| Thalassaemia | 2 | (3) | 1 | (3) |
| CAMT | 1 | (1) | – | |
| SLE | 1 | (1) | – | |
| Sickle cell anaemia | 1 | (1) | 1 | (3) |
| Hurler's syndrome | 1 | (1) | – | |
| Other | 2 | (3) | 2 | (5) |
MDS = Myelodysplastic syndrome, SCID = Severe combined immunodeficiency, WAS = Wiscott–Aldrich syndrome, HLH = Haemophagocytic lymphohistiocytosis, CAMT = Congenital amegakaryocytic thrombocytopenia, SLE = Systemic lupus erythematosus.
Haematopoietic stem cell transplantation‐related difficulties
Parents (Fig. 1) and children were asked about the child's distress due to HSCT‐related physical and psychological difficulties. Children aged 7 or older reported a mean distress VAS score of 52 (SD 37) for oral pain, 45 (SD 36) for pain in their muscles and joints, 38 (SD 35) for pain during medical procedures, 72 (SD 27) for nausea, 40 (SD 38) for worried being sick and 66 (SD 35) for ‘sad not being home’.
Figure 1.
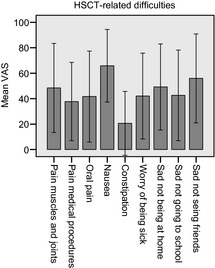
Parent proxy mean visual analogue scale score (0–100, where 0 = ‘no trouble at all’ and 100 = ‘worst trouble possible’) with regard to haematopoietic stem cell transplantation ‐related physical and psychological difficulties. Error bars showing ±1 SD.
Oral mucositis‐related symptoms
Parents (Table 3) and children were asked about the occurrence and degree of OM‐related symptoms during HSCT treatment. The children said they suffered a lot/very much from various symptoms and these were: taste perception (66%), oral pain (43%), mouth blisters (41%) and mouth sores (24%).
Table 3. Parent proxy (n = 71) reports on perceived occurrence and degree of oral mucositis‐related symptoms during haematopoietic stem cell transplantation.
| Dry lips | Taste perception alteration | Mouth blisters | Mouth ulcerations | Oral pain | Throat pain | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Total | 67 | (100) | 62 | (100) | 65 | (100) | 61 | (100) | 66 | (100) | 58 | (100) |
| Not at all/A little | 43 | (64) | 13 | (21) | 41 | (63) | 44 | (72) | 33 | (50) | 35 | (60) |
| A lot/Very much | 24 | (36) | 49 | (79) | 24 | (37) | 17 | (28) | 33 | (50) | 23 | (40) |
Parents (Fig. 2) and children were asked to rate the degree of oral pain on a VAS scale and the children reported a mean oral pain of 56 (SD 36.9). The scores for the individual elements were as follows: 49 (SD 37) for tooth brushing, 38 (SD 36) for oral examinations, 34 (SD 33) for talking, 57 (SD 39) for eating solid food, 34 (SD 37) for drinking cold drinks and 42 (SD 39) for drinking hot drinks. A third of the parents (33%) who reported that their child had had oral pain said that it was constant and 44% said that it had been recurrent.
Figure 2.
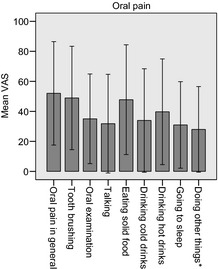
Parent proxy mean visual analogue scale score (0–100 score where 0 = ‘no pain at all’ and 100 = ‘worst pain possible’) on oral mucositis‐related pain during haematopoietic stem cell transplantation. Error bars showing ±1 SD. *‘doing other things’ includes examples of playing and watching TV.
Impact of OM on general condition and physical performance
Parents and children were asked about the impact of OM on the child's general condition and physical performance. Difficulties swallowing often/very often due to OM were reported by 44% of the parents and 47% of the children and never/seldom by 34% of the parents and 31% of the children. Difficulties talking often/very often were reported by 27% of the parents and 21% of the children and never/seldom by 60% of the parents and 50% of the children. Difficulties sleeping often/very often were reported by 18% of the parents and 6% of the children and never/seldom by 65% of the parents and 63% of the children. A third of the parents (33%) reported that their child had needed nutrition via tube, due to OM, and 53% said that their child had needed total parenteral nutrition because of OM.
Effect of OM on psychological status
Half (50%) of the parents and 37% of the children reported that OM had a high impact on psychological well‐being and 42% of the parents and 26% of the children reported a high impact on interest in, and commitment to, activities.
Information, prophylactic intervention and oral assessment
The majority of parents (94%) said that they had been informed about OM prior to HSCT (6% had not) and 83% had received information about oral care (17% had not). Most parents (84%) said that their child had been orally assessed by dental professionals such as a dentist, dental hygienist and/or dental nurse, before conditioning (16% had not). Daily assessment of oral status during hospitalisation was reported by 70% of the parents and daily assessment of oral pain by 31%. One‐third of parents said that prophylactic intervention for OM had been used, including nystatine, ointment, ice chips/ice lollipops (cryo‐therapy) and benzydamide hydrochloride (mouthwash) or a combination cryo‐therapy and mouthwash. The use of prophylactic interventions did not vary with age or treatment site and the treatments seemed to be randomly administered.
Treatment data
When it came to treating OM, 47% of parents reported that ointment had been used, 49% reported mouthwash, 38% reported ice cubes/ice lollipops and 47% reported pain medication. The therapeutic treatments for OM did not vary with age or treatment site and were not altogether consistently administered. According to the parents, 56% of the children who had been prescribed mouthwash had managed to follow the instructions and rinse their mouth completely or quite a lot without a problem. However, 44% had not been able to rinse their mouth out at all or only to a small extent. Just over a third (35%) of the parents reported that their child had not been able to follow the instructions for brushing their teeth at all or only to a small extent. The most common reasons for bad compliance to treatment were oral pain and poor general condition. Just over half of the parents (52%) said that their child needed medication for oral pain and morphine was the only treatment that was reported to be highly beneficial. Nearly two thirds of the parents (61%) were satisfied with the pain treatment their child was given, but 39% were dissatisfied.
Agreement between parents' and children's reports of mucositis‐related symptoms and difficulties
To test the agreement between the parents' and children's reports of OM‐related symptoms, a two‐way mixed model ICC was calculated and Wilcoxon signed rank test was performed (Table 4). The matched parent and child reports were included in the analysis.
Table 4. Comparison between parent proxy‐ and self‐reports (>7 years old) on perceived occurrence and degree of oral mucositis‐related symptoms during haematopoietic stem cell transplantation. (0 = not at all, 1 = a little, 2 = a lot, 3 = very much).
| N | Mean parents (SD) | Mean children (SD) | Mean difference | Siga | ICCb (95% CI) | |
|---|---|---|---|---|---|---|
| Dry lips | 31 | 1.91 (0.91) | 0.97 (0.75) | 0.06 | 0.15 | 0.82 (.65 – .91) |
| Taste perception alteration | 32 | 2.38 (1.50) | 2.31 (1.49) | 0.07 | 0.63 | 0.57 (.28 – .76) |
| Mouth blisters | 32 | 1.53 (1.08) | 1.56 (0.98) | −0.03 | 0.71 | 0.90 (.80 – .95) |
| Mouth ulcerations | 31 | 0.87 (1.06) | 0.84 (1.16) | 0.03 | 0.71 | 0.91 (.81 – .95) |
| Oral pain | 33 | 1.58 (1.06) | 1.48 (1.09) | 0.10 | 0.46 | 0.73 (.51 – .85) |
| Throat pain | 32 | 1.09 (1.25) | 0.97 (1.09) | 0.12 | 0.21 | 0.89 (.78 – .94) |
| Difficulties swallowing | 33 | 1.39 (1.30) | 1.30 (1.31) | 0.9 | 0.41 | 0.90 (.81 – .95) |
Wilcoxon's signed rank test.
Mixed model intraclass correlation.
Discussion
The parents and children reported that oral pain was one of the most troublesome side effects of HSCT, which is in line with previous research 10. The majority reported some degree of oral pain, taste perception alterations and mouth blisters. Most of the oral pain was as a result of eating solid food and tooth brushing, resulting in moderate mean pain scores. However, the range of responses showed a large variation, with some respondents reporting very high levels of pain. It is worth noting that the general level of oral pain reported by parents and children was fairly high and it was reported even when the child was engaged in activities such as playing or watching TV. Many of the parents and children reported mouth dryness, ulcerations and throat pain. Two thirds of the parents reported that their child had difficulties swallowing and just over 40% that the child had problems talking, sometimes to very often, due to OM. Half of the parents reported that OM had a high impact on their child's psychological well‐being. Thus, according to both the parents and children the occurrence of OM‐related symptoms and oral pain was high and it caused considerable pain and disability for those who were affected. Only 61% of the parents were satisfied with the pain treatment that had been given to their child for OM, with the remaining 39% reporting dissatisfaction with the treatment. This supports suggestions from previous studies about the difficulties of treating mucositis pain.
Although the majority of parents and children reported that they had been given information about OM, oral care and oral assessment prior to HSCT, almost a fifth reported that they had not been informed. A third of the parents said that prophylactic interventions had been carried out and a half reported therapeutic treatment. However, neither the interventions nor treatment had been administered to the children in a consistent way. It is a challenge to find treatments and interventions that children can tolerate. Good oral hygiene with tooth brushing and oral rinsing is part of the standard oral care protocol recommended for preventing and treating OM 18. However, more than one third of the children were unable to follow these recommendations, mostly due to pain and their poor general condition. Daily assessment of OM during hospitalisation was reported by two thirds of parents, while daily oral pain assessment was only reported by one third. These results indicate that there might be difficulties in following the standard oral care protocols that are highly recommended in literature 17. Taken together, these findings indicate a distinct need for a thorough evaluation of the interventions and treatments being used for children with OM.
There was an excellent level of agreement between the parent proxy and child reports for all the OM‐related symptoms, with the exception of taste perception alterations and oral pain, where the level of agreement was good. This is not surprising when you consider how subjective taste and pain symptoms are. The high level of agreement associated with the other OM‐related symptoms could also be because the parents and children did not follow the instructions to answer the two questionnaires separately. We must also bear in mind that parent proxy and self‐reports partly reflect different perspectives and that one cannot completely replace the other. Despite this, the study supports the recommendation that parent proxy reports should be used in clinical and research settings as a supplement to child self‐reports of pain 26.
The sample represents children, aged from birth to the age of 18, undergoing HSCT due to cancer or other diseases and the results may therefore be generalised to this group. The response rate was 56% for the parents and 52% for the children. This may seem like a low response rate, but with the increasing number of surveys being carried out in society and health care these days, we believe it is acceptable 29. The response rate might imply a risk of bias for the results. However, the fact that the patient sample was representative of the whole cohort, with regard to age, gender, time since diagnosis, reasons for HSCT and type of HSCT, and that there was a variation in the level of distress and pain, suggests that this may not be the case. The fact that no clinical data were collected from the patient charts could be considered a weakness. However, the focus of this study was perceptions of OM during HSCT by parents and children. It was not the aim of this study to investigate the risk factors for OM in children undergoing HSCT. However, we do need to develop a better understanding of the pathobiology and risk factors for OM in children so that preventive interventions can be given to the most vulnerable children.
No validated questionnaire concerning these issues exists. Therefore, new questions were developed. It was not within the scope of this project to validate a new instrument, but experts were consulted to achieve face validity. The time frame of the previous 3 years was chosen to include as many children as possible, while keeping memory bias at a reasonable level. Despite this, the time that had passed since treatment may have influenced the reports. The use of a VAS is recommended for assessing acute, chronic and recurrent pain in children over the age of 7 23 and the validity of performing these assessments retrospectively could be challenged. However, studies have shown high accuracy when children have been asked to remember pain 30 and this supports the use of retrospective pain assessments in this study.
In conclusion, OM affected many of the children in our study who underwent HSCT and caused them great pain and disability. The study supports the notion that further research is needed to establish more evidence‐based care for OM that addresses both the physical and psychological symptoms that children with OM experience. The use of children's own reports of pain and discomfort is advocated whenever possible. However, parent proxy reports play an important role in both clinical and research settings, especially for the youngest children.
Supplementary Material
Appendix S1 Content of parent proxy‐ and child questionnaire respectively.
Acknowledgements
The study was funded by The Swedish Childhood Cancer Foundation. We would also like to thank Jacek Winiarski from the Department of Clinical Science, Intervention and technology, Karolinska Institutet, for his support and for providing patients.
References
- 1.Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber‐Durlacher JE, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007; 109: 820–31. [DOI] [PubMed] [Google Scholar]
- 2.Fadda G, Campus G, Lugliè P. Risk factors for oral mucositis in paediatric oncology patients receiving alkylant chemotherapy. BMC Oral Health 2006; 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung L, Tomlinson GA, Greenberg ML, Koren G, Judd P, Ota S, et al. Serial controlled N‐of‐1 trials of topical vitamin E as prophylaxis for chemotherapy‐induced oral mucositis in paediatric patients. Eur J Cancer 2007; 43: 1269–75. [DOI] [PubMed] [Google Scholar]
- 4.Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer‐Jensen M, et al. Perspectives on cancer therapy‐induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004; 100: 1995–2025. [DOI] [PubMed] [Google Scholar]
- 5.Vagliano L, Feraut C, Gobetto G, Trunfio A, Errico A, Campani V, et al. Incidence and severity of oral mucositis in patients undergoing haematopoietic SCT – results of a multicenter study. Bone Marrow Transplant 2001; 46: 727–32. [DOI] [PubMed] [Google Scholar]
- 6.Stone R, Fliedner MC, Smiet ACM. Management of oral mucositis in patients with cancer. Eur J Oncol Nurs 2005; 9: S24–32. [DOI] [PubMed] [Google Scholar]
- 7.Bellm LA, Epstein JB, Rose‐Ped AM, Martin P, Fuchs HJ. Patients' reports of complications of bone marrow transplantation. Support Care Cancer 2000; 8: 33–9. [DOI] [PubMed] [Google Scholar]
- 8.Rose‐Ped AM, Bellm LA, Epstein JB, Trotti A, Gwede C, Fuchs HJ. Complications of radiation therapy for head and neck cancers. The patient's perspective. Cancer Nurs 2002; 25: 461–7. [DOI] [PubMed] [Google Scholar]
- 9.Borbasi S, Cameron K, Quested B, Olver I, To B, Evans D. More than a sore mouth: patients' experience of oral mucositis. Oncol Nurs Forum 2002; 29: 1051–7. [DOI] [PubMed] [Google Scholar]
- 10.Ljungman G, Kreuger A, Gordh T, Berg T, Sörensen S, Rawal N. Treatment of pain in pediatric oncology: a Swedish nationwide survey. Pain 1996; 68: 385–94. [DOI] [PubMed] [Google Scholar]
- 11.Ljungman G, Gordh T, Sörensen S, Kreuger A. Pain in pediatric oncology: interviews with children, adolescents and their parents. Acta Paediatr 1999; 88: 623–30. [DOI] [PubMed] [Google Scholar]
- 12.Hedström M, Ljungman G, von Essen L. Perceptions of distress among adolescents recently diagnosed with cancer. J Pediatr Hematol Oncol 2005; 27: 15–22. [DOI] [PubMed] [Google Scholar]
- 13.Cheng KK. Oral mucositis: a phenomenological study of pediatric patients' and their parents' perspectives and experiences. Support Care Cancer 2009; 17: 829–37. [DOI] [PubMed] [Google Scholar]
- 14.Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem‐cell transplantation. J Clin Oncol 2001; 19: 2201–5. [DOI] [PubMed] [Google Scholar]
- 15.Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny A, Littlewood A, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2011, . Art. No.:CD000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarkson JE, Worthington HV, Eden OB. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2010, . Art. No.: CD001973. [DOI] [PubMed] [Google Scholar]
- 17.McGuire D, Fulton J, Park J, Brown C, Correa M, Eilers J, et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients. Support Care Cancer 2013; 21: 3191–207. [DOI] [PubMed] [Google Scholar]
- 18.Nicolatou‐Galitis O, Sarri T, Bowen J, Di Palma M, Kouloulias VE, Niscola P, et al. Systematic review of anti‐inflammatory agents for the management of oral mucositis in cancer patients. Support Care Cancer 2013; 21: 3179–89. [DOI] [PubMed] [Google Scholar]
- 19.Yarom N, Ariyawardana A, Hovan A, Barash A, Jarvis V, Jensen SB, et al. Systematic review of natural agents for the management of oral mucositis in cancer patients. Support Care Cancer 2013; 21: 3209–21. [DOI] [PubMed] [Google Scholar]
- 20.Saunders DP, Epstein JB, Allemano J, Bossi P, van de Watering MD, Rao NG, et al. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients. Support Care Cancer 2013; 21: 3191–207. [DOI] [PubMed] [Google Scholar]
- 21.Cheng KK, Molassiotis A, Chang AM, Wai WC, Cheung SS. Evaluation of an oral care protocol intervention in the prevention of chemotherapy‐induced oral mucositis in paediatric cancer patients. Eur J Cancer 2001; 37: 2056–63. [DOI] [PubMed] [Google Scholar]
- 22.Glenny AM, Gibson F, Auld E, Coulson S, Clarkson JE, Craig JV, et al. The development of evidence‐based guidelines on mouth care for children, teenagers and young adults treated for cancer. Eur J Cancer 2010; 46: 1399–412. [DOI] [PubMed] [Google Scholar]
- 23.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMPACT recommendations. J Pain 2008; 9: 771–83. [DOI] [PubMed] [Google Scholar]
- 24.Vetter TR, Bridgewater CL, McGwin G. An observational study of patient versus parental perceptions of health‐related quality of life in children and adolescents with a chronic pain condition: who should the clinician believe? Health Qual Life Outcomes 2012; 10: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen LL, Vowles KE, Eccleston C. Adolescent chronic pain‐related functioning: concordance and discordance of mother‐proxy and self‐report ratings. Eur J Pain 2010; 14: 882–6. [DOI] [PubMed] [Google Scholar]
- 26.Russell K, Hudson M, Long A, Phipps S. Assessment of health‐related quality of life in children with cancer: consistency and agreement between parent and child reports. Cancer 2006; 106: 2267–74. [DOI] [PubMed] [Google Scholar]
- 27.Varni JW, Limbers CA, Burwinkle TM. Parent proxy‐report of their children's health‐related quality of life: an analysis of 13,878 parents' reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 2007; 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosner B. Fundamentals of Biostatistics, 6th ed Belmont: Duxbury Press, 2005. [Google Scholar]
- 29.Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol 1997; 50: 1129–36. [DOI] [PubMed] [Google Scholar]
- 30.Zonneveld L, McGrath PJ, Reid GJ, Sorbi MJ. Accuracy of children's pain memories. Pain 1997; 71: 297–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Content of parent proxy‐ and child questionnaire respectively.


