Abstract
Eosinophilic meningitis may be caused by non-infectious and infectious agents. Angiostrongylus cantonensis is the commonest causative agent of eosinophilic meningitis. Rats are the primary hosts of this parasite. Humans get infected by ingestion of raw or inadequately cooked hosts (snails or monitor lizard) or food contaminated with the infective third-stage larvae. A 16-year-old boy was admitted to our hospital with history of fever, headache, and altered sensorium. Magnetic resonance imaging of the brain showed unique findings. Cerebrospinal fluid (CSF) examination showed eosinophilia and the CSF wet mount identified a larva. Patient history revealed ingestion of monitor lizard 2 weeks prior to onset of symptoms. Hence, a diagnosis of eosinophilic meningitis caused by A. cantonensis was made. He was treated with oral albendazole and steroids, resulting in gradual improvement. A. cantonensis as a cause of eosinophilic meningitis is a possibility in patients who present with headache and vomiting after eating raw meat (monitor lizard). To the best of our knowledge, this is a very rare case being reported from India where the larva was identified during the microscopic examination of the CSF.
Keywords: Angiostrongylus cantonensis, eosinophilic meningitis, monitor lizard
INTRODUCTION

Angiostrongyliasis, gnathostomiasis, toxocariasis, lymphoma, and drugs can produce eosinophils in the cerebrospinal fluid (CSF).[1,2] Eosinophilic meningitis is defined as the presence of ≥10 eosinophils/ml in the cerebrospinal fluid (CSF) or at least 10% eosinophils in total CSF leukocyte count.[2] We describe a rare case of eosinophilic meningitis caused due to Angiostrongylus cantonensis (rat lungworm) following ingestion of raw monitor lizard meat. This case report presents unique magnetic resonance imaging (MRI) findings in a case of eosinophilic meningitis and identification of the larva in the CSF. To the best of our knowledge, this is a very rare case from India where the larva was identified on the CSF wet mount.
CASE REPORT
A 16-year-old boy presented with altered sensorium of 1 day duration. He had fever, headache, and vomiting for 7 days, prior to hospital admission. History of diplopia was also present. There was no history of seizures. On examination, he was febrile. Central nervous examination showed bilateral rectus palsy, left third cranial nerve palsy, and stiffness of the neck.
Laboratory investigations gave the following results: Hemoglobin 14.4 g/dl (normal range: 11–15 g/dl); total white blood cell count 10,000 cells/mm3 (normal range: 4000–10,000 cells/mm3); differential count – neutrophils: 91%, lymphocytes: 7%, monocytes: 2%; platelet count 335,000 cells/mm3 (normal range: 150,000–400,000 cells/mm3); and erythrocyte sedimentation rate 8 mm/1st hour (normal range: 0–20 mm). Serum electrolytes, renal and liver function tests were within normal limits. Chest X-ray did not reveal any abnormality. MRI of the brain showed subtle hyperintensities in the basal ganglia and periventricular regions as seen on the T2 and fluid-attenuated inversion recovery (FLAIR) images, which were isointense on T1-weighted images [Figure 1]. The foci were more distinct on the diffusion-weighted images (DWI) as foci of restricted diffusion appearing bright on the DWI series and hypointense on the apparent diffusion coefficient (ADC) series [Figure 2]. These lesions showed subtle enhancement and associated meningeal enhancement on contrast administration [Figure 3]. There was no meningeal or ependymal enhancement. CSF analysis showed the following results: Total cell count 620/μl (normal range 0–5 cells/μLl); differential count – eosinophils: 70%, lymphocytes: 30%; protein 62 mg/dl (normal range 15–60 mg/dl); and glucose 92 mg/dl (normal range 40–80 mg/dl).
Figure 1.
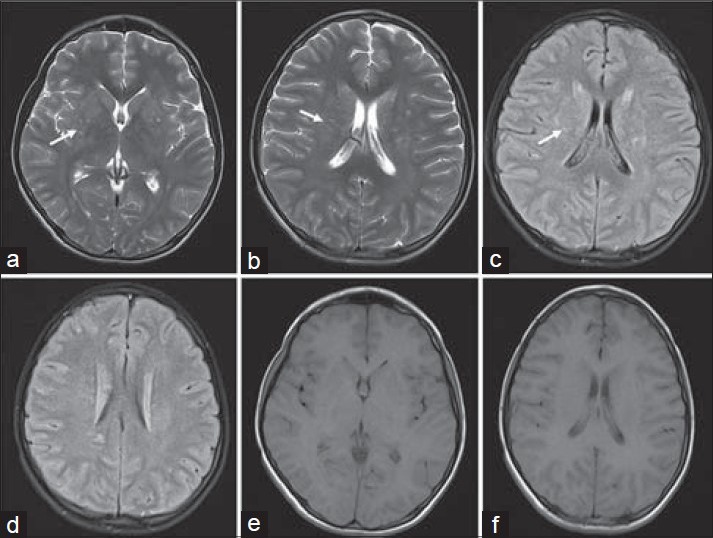
16-year-old boy presented with altered sensorium of 1 day duration diagnosed with eosinophilic meningitis caused by A. cantonensis. MR images, (a and b) axial T2 (c and d) axial FLAIR images, at the basal ganglia level demonstrate subtle scattered hyperintensities in the periventricular regions (white arrows). (e and f) Axial T1-weighted images do not show these features.
Figure 2.
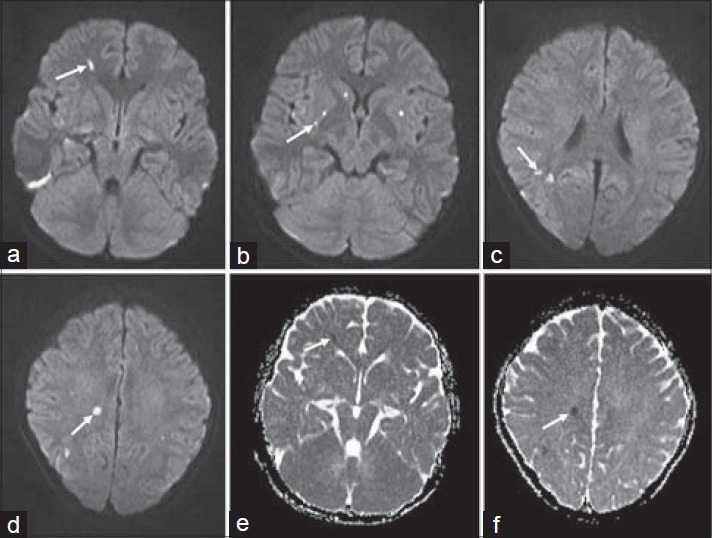
16-year-old boy presented with altered sensorium of 1 day duration diagnosed with eosinophilic meningitis caused by A. cantonensis. (a–d) MR diffusion-weighted images show foci of restricted diffusion appearing bright (white arrows). (e and f) Apparent diffusion coefficient (ADC) series shows hypointense areas (white arrows).
Figure 3.
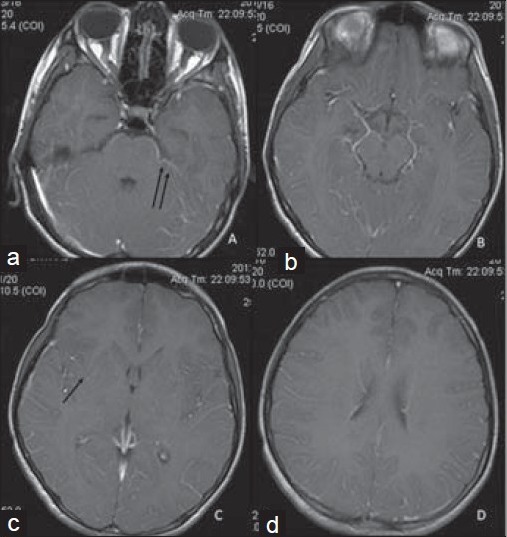
16-year-old boy presented with altered sensorium of 1 day duration diagnosed with eosinophilic meningitis caused by A. cantonensis. (a–d) MR post-gadolinium contrast-enhanced T1WI images show subtle meningeal enhancement (double black arrows) and faint enhancement of the lesions in the basal ganglia (single black arrow).
Gram stain and Ziehl–Neelsen stain did not reveal any organism, and CSF culture was negative. The CSF wet mount showed a larva which matched the larval form of A. cantonensis [Figure 4].
Figure 4.
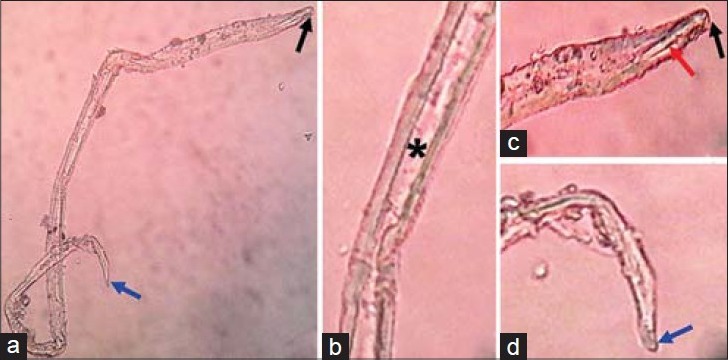
16-year-old boy presented with altered sensorium of 1 day duration diagnosed with eosinophilic meningitis caused by A. cantonensis. (a–d) Images of the microscopic examination of wet mount of CSF ×45 show third-stage larva of Angiostrongylus, knob-like tip (black arrow), rod-like structure (red arrow), intestine (asterisk), and terminal projection on the tip of the tail (blue arrow).
The patient gave history of consumption of raw meat of monitor lizard 14 days before the onset of symptoms. An immunologic study was not done, as it is not available in our state. The patient was treated with oral albendazole and prednisolone for 2 weeks, and there was gradual improvement of symptoms with complete resolution of cranial nerve palsies.
DISCUSSION
Cases of Angiostrongyliasis have been reported from Australia, the Caribbean, America, and Asia. Panackel et al., have reported five cases of eosinophilic meningitis from India. All the patients developed meningitis after ingestion of monitor lizard.[1]
The definitive hosts of A. cantonensis are rats.[3] Adult female worms lay their eggs in the pulmonary artery of rats. The first-stage larvae migrate to the pharynx, are swallowed and passed in the feces. Snails or slugs (intermediate hosts) ingest the first-stage larvae and these larvae develop into the infective, third-stage larvae. Humans are incidental hosts and become infected by ingesting raw or undercooked snails or paratenic hosts such as freshwater shrimp/crabs/frogs which have the third-stage larvae. Infective larval stages are also found in monitor lizard (Varanus bengalensis).[1,4] Prawns, crabs, and toads may also act as sources of infection to humans. Third-stage larvae penetrate the intestinal tissues of humans and are carried to the central nervous system (CNS). While in the CNS, the larvae continue to develop to the fourth and fifth stages.
Monitor lizards are eaten in parts of southern India as their meat according to local belief is supposed to have aphrodisiac properties.[5] In a case series of eosinophilic meningitis published from South India,[5] all male patients developed the illness after consumption of uncooked meat of monitor lizard. Our patient had also consumed raw meat of monitor lizard for its presumed aphrodisiac properties.
Angiostrongyliasis is an acute disease that spontaneously resolves within few weeks. The acute illness is characterized by headache, fever, meningitis, focal neurologic deficits, ocular lesions, radiculomyelitis, and palsies of the sixth and seventh cranial nerves.[6] The most common clinical manifestations mentioned in literature are headache, neck pain/stiffness, fever, and sensory symptoms. Our patient presented with fever, headache, and vomiting.
A presumptive diagnosis of angiostrongyliasis can be made in a patient presenting with eosinophilic meningitis with a history of exposure to an intermediate host. Peripheral eosinophilia can be present. Serological testing for diagnosis is available only in endemic areas. The detection of parasite in CSF is rare. Worms were only recovered from 28 (11%) of 259 human cases in Taiwan and only 10 (2%) of 484 human cases in Thailand.[3]
DWI is superior to conventional MRI for the detection of early signal abnormalities in viral encephalitis and meningitis.[7] MRI findings in angiostrongyliasis have been reported to be diverse and non-specific.[8] Multiple scattered hyperintensities with and without contrast enhancement are the most commonly described.[8] Sometimes computed tomogram (CT) and MRI may not reveal any abnormality. Our case showed scattered T2 hyperintensities and unique finding of DWI hyperintensities which represent the foci of microabscesses or developing infarcts. This finding of multiple foci of DWI hyperintensities representing microabscesses has been described in fungal infections and bacterial meningitis,[9] but has not yet been reported in angiostrongyliasis.
The final diagnosis of angiostrongyliasis is not based on the MRI findings, but on serology, CSF cell counts and microscopic examination, and blood counts.[10] CSF mount can help in isolation of the larva and confirms the diagnosis. The standard treatment for eosinophilic meningitis caused by A. cantonensis has been controversial. Treatment options consist of serial lumbar punctures, anti-helminthic therapy, steroid therapy, or combination of these. We treated our patient for 2 weeks with oral albendazole and steroids.
In the present case, the patient developed signs and symptoms of meningitis after eating monitor lizard, had CSF eosinophilia, and demonstrable larva in the CSF. Hence, angiostrongyliasis was identified as the cause of eosinophilic meningitis in this case.
CONCLUSION
Food-borne zoonotic disease can be prevented or reduced in endemic areas by increasing awareness and public knowledge about the dangers of eating raw or undercooked meat. Physicians must consider a diagnosis of eosinophilic meningitis in patients who present with headache and neurological deficits after eating monitor lizard.
ACKNOWLEDGMENTS
The authors thank Dr Shrijeet Chakraborti (Pathology) and Dr Suchitra Shenoy (Microbiology) for their assistance in preparing the manuscript.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2014/4/1/76/148303
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Panackel C, Vishad, Cherian G, Vijayakumar K, Sharma RN. Eosinophilic meningitis due to Angiostrongylus cantonensis. Indian J Med Microbiol. 2006;24:220–1. [PubMed] [Google Scholar]
- 2.Senthong V, Chindaprasirt J, Sawanyawisuth K. Differential diagnosis of CNS angiostrongyliasis: A short review. Hawaii J Med Public Health. 2013;72(Suppl 2):52–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Cowie RH. Biology, systematics, life cycle, and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawaii J Med Public Health. 2013;72(Suppl 2):6–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Radomyos P, Tungstrongchitr A, Praewanich R, Khewwatchan P, Kantangkul T, Junlananto P, et al. Occurrence of the infective stage of Angiostrongylus cantonensis in the yellow tree monitor (Varanus bengalensis) in five provinces of Thailand. Southeast Asian J Trop Med Public Health. 1994;25:498–500. [PubMed] [Google Scholar]
- 5.Parameswaran K. Case series of eosinophilic meningoencephalitis from South India. Ann Indian Acad Neurol. 2006;9:217–22. [Google Scholar]
- 6.Tsai HC, Liu YC, Kunin CM, Lee SS, Chen YS, Lin HH, et al. Eosinophilic meningitis caused by Angiostrongylus cantonensis: Report of 17 cases. Am J Med. 2001;111:109–14. doi: 10.1016/s0002-9343(01)00766-5. [DOI] [PubMed] [Google Scholar]
- 7.Maschke M, Kastrup O, Forsting M, Diener HC. Update of neuroimaging in infectious central nervous system disease. Curr Opin Neurol. 2004;17:475–80. doi: 10.1097/01.wco.0000137540.29857.bf. [DOI] [PubMed] [Google Scholar]
- 8.Nalini A, Ramakrishna A, Dekumoy P, Kumar RR, Pakdee W, Saini J, et al. VS. Severe form of radiculo-myelo-neuropathy with meningo-encephalitis secondary to Angiostrongylus cantonensis infection: Unusual corpus callosal lesions and serial magnetic resonance imaging findings. Neurol India. 2013;61:414–8. doi: 10.4103/0028-3886.117613. [DOI] [PubMed] [Google Scholar]
- 9.Starkey J, Moritani T, Kirby P. MRI of CNS fungal infections: Review of aspergillosis to histoplasmosis and everything in between. Clin Neuroradiol. 2014;24:217–30. doi: 10.1007/s00062-014-0305-7. [DOI] [PubMed] [Google Scholar]
- 10.Jin E, Ma D, Liang Y, Ji A, Gan S. MRI findings of eosinophilic myelomeningoencephalitis due to Angiostrongylus cantonensis. Clin Radiol. 2005;60:242–50. doi: 10.1016/j.crad.2004.05.012. [DOI] [PubMed] [Google Scholar]


