Abstract
SpyTag is a peptide which spontaneously forms an amide bond to its protein partner SpyCatcher. Here we fused SpyTag at the N-terminus of β-lactamase and SpyCatcher at the C-terminus, so the partners could cyclize to lock together the termini of the enzyme. Wild-type enzyme aggregates above 37 °C, with irreversible loss of activity. Cyclized β-lactamase was soluble even after heating at 100 °C; after cooling, the catalytic activity was restored. SpyTag/SpyCatcher-cyclization achieved > 60 °C increase in stability, much larger than from point mutation or alternative approaches to cyclization. Cyclized dihydrofolate reductase was similarly resilient. Analyzing unfolding calorimetrically and via mutants, cyclization did not increase the unfolding temperature of β-lactamase, but facilitated refolding after thermal stress. SpyTag and SpyCatcher sandwiching represents a simple and efficient route for enzyme cyclization, with potential to greatly enhance the robustness of biocatalysts.
Keywords: synthetic biology, protein engineering, enzymes, protein modifications, peptides
Biological catalysts frequently show superlative regioselectivity and stereoselectivity compared to chemical catalysts, but suffer from their instability.[1] Stabilization of enzymes without the use of chemical modifications can be achieved by looking for homologues in thermophiles,[2] inferring a consensus or ancestral sequence,[3–6] selection from libraries,[7,8] or structure-based design.[9,10] Proteins obtained from thermophiles usually achieve their optimum catalytic efficiency at a high temperature,[11] a costly trait for application of biotransformations. Therefore, it is desirable to obtain a protein with a high catalytic efficiency at ambient temperature, whilst maintaining the thermal tolerance of thermophile-derived proteins.
Directed evolution and rational design campaigns on enzymes have led to important successes, but require time-consuming individual optimization and gains are often marginal. For example, after >700 mutations on T4 lysozyme, the best thermostabilization from combining individual stabilizing point mutations was 8 °C, at a cost of losing most of the catalytic activity.[12] Therefore generic approaches, requiring neither extensive structural knowledge nor library selection, are needed to provide a faster and more broadly applicable route to enzyme stabilization.
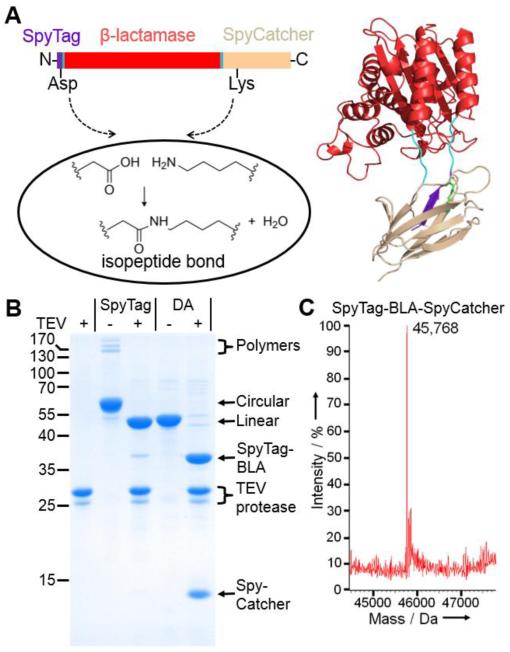
To overcome the limited stability of peptide interactions, we previously developed intermolecular spontaneous amide bond formation to a peptide tag, through dissection and modification of Gram-positive bacterial domains.[13–16] We engineered SpyTag, a 13 amino acid peptide, to rapidly form an irreversible amide bond to the 15 kDa protein SpyCatcher.[14] The system is genetically encodable and the tag and protein are functional on either terminus.[14] Since termini are one of the most flexible regions of proteins and fluctuations here may initiate unfolding,[17] we hypothesized that connecting the N- and C-termini of a protein through SpyTag/SpyCatcher cyclization could enhance stability (Figure 1a).
Figure 1.
SpyTag/SpyCatcher-mediated cyclization of β-lactamase. a) Cartoon of the cyclization of BLA. b) SDS-PAGE with Coomassie staining for TEV protease or SpyTag-BLA-TEV-SpyCatcher and SpyTag DA-BLA-TEV-SpyCatcher ± TEV protease. c) Mass spectrometry of SpyTag-BLA-SpyCatcher.
Protein cyclization has been previously achieved via carbodiimide cross-linking,[18] sortase,[19] or most often using inteins.[20–23] TEM-1 β-lactamase (BLA) is an important model system for enzyme evolution, as well as having clinical relevance from the emergence of bacteria with broad spectrum antibiotic resistance.[6,24] Previous cyclization of BLA via a split intein achieved a 5 °C increase in thermal tolerance.[21] We genetically fused SpyTag to the N-terminus of BLA and SpyCatcher to the C-terminus (Figure 1a). This SpyTag-BLA-SpyCatcher construct expressed efficiently in Escherichia coli. To test whether we had successfully cyclized BLA, we generated negative controls unable to react in both SpyTag and SpyCatcher: we mutated the reactive Asp in SpyTag to Ala (SpyTag DA) or the catalytic Glu residue in SpyCatcher to Gln (SpyCatcher EQ).[14] When we ran the linear mutants with the cyclized construct on SDS-PAGE, we found that the cyclized form had a lower mobility, consistent with efficient cyclization (75%) (Figure 1b and Figure S1a).
To further confirm that we had successfully cyclized BLA, we introduced a TEV protease cleavage site between the BLA and SpyCatcher. Upon TEV protease cleavage, the cyclized SpyTag-BLA-TEV-SpyCatcher now migrated to the same apparent molecular weight as the linear construct (Figure 1b). We also witnessed the disappearance of polymeric forms (generated from a low level of intermolecular reaction of SpyTag-BLA-SpyCatcher) and the appearance of new bands from polymer cleavage (Figure 1b).
In addition, we verified the molecular weight of SpyTag-BLA-SpyCatcher, as well as the DA and EQ controls, using electrospray mass spectrometry (calculated Mw of SpyTag-BLA-SpyCatcher before reaction 45,786.4 Da; calculated Mw of SpyTag-BLA-SpyCatcher after loss of H2O from spontaneous amide bond formation 45,768.4 Da; observed Mw 45,768 Da) (Figure 1c and Figure S1).
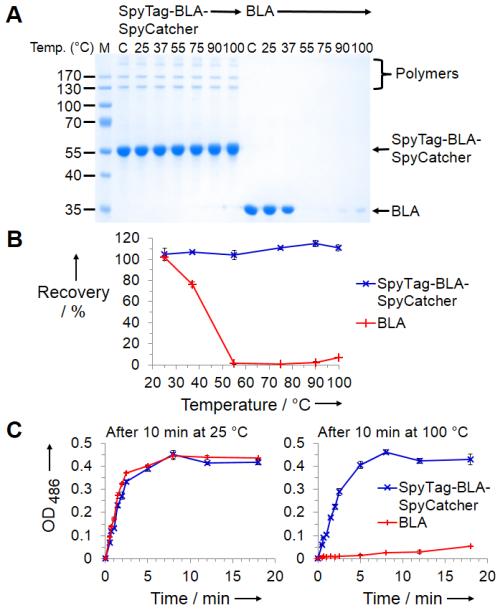
To test the effect of cyclization on protein thermal stability we incubated SpyTag-BLA-SpyCatcher in phosphate buffered saline over a wide range of temperatures, in comparison to unmodified BLA. Surprisingly we found that SpyTag-BLA-SpyCatcher was not lost from solution at any of the temperatures tested, even up to 100 °C. On the other hand, BLA started aggregating at 37 °C and had completely aggregated at 55 °C (Figure 2a & b). Therefore SpyTag/SpyCatcher cyclization conferred an increase in temperature for aggregation of > 60 °C. It was conceivable that the different stability resulted from different protease contamination of preparations. However, after mixing BLA and SpyTag-BLA-SpyCatcher, there was still much more loss of BLA than SpyTag-BLA-SpyCatcher upon heating (Figure S2).
Figure 2.
Increased thermal tolerance of cyclized enzyme. a) Cyclized or original enzyme was heated at the indicated temperature for 10 min, centrifuged and the supernatant analyzed by SDS-PAGE with Coomassie staining. M: molecular weight markers. C: control without any incubation. b) Quantification of soluble fractions from a) (mean of triplicate ± 1 s.d.). c) Nitrocefin assay of enzyme activity after 10 min incubation at 25 or 100 °C (mean of triplicate ± 1 s.d.). Some error bars are too small to be seen.
To test whether SpyTag-BLA-SpyCatcher retained activity as well as solubility, we measured enzymatic activity with the colorimetric substrate nitrocefin, after incubation at 25 or 100 °C. Nearly all activity of BLA was lost after high temperature incubation, whereas nearly all activity of SpyTag-BLA-SpyCatcher was retained after 100 °C exposure (Figure 2c).
To understand to what extent the fused components conferred resistance to aggregation, we tested the non-cyclized SpyTag DA-BLA-SpyCatcher in equivalent assays. Interestingly, SpyTag DA-BLA-SpyCatcher did show enhanced resistance to aggregation compared to BLA. However, covalent cyclization was important, because the recovery of activity following heating of SpyTag DA-BLA-SpyCatcher was inferior to SpyTag-BLA-SpyCatcher (Figure S3). SpyTag-BLA-SpyCatcher EQ (with the E77Q mutation blocking SpyCatcher reaction)[14] had low resistance to aggregation and weak recovery of catalytic activity (Figure S4). Fusing the whole CnaB2 domain (BLA-CnaB2, having isopeptide formation within the CnaB2 domain but not connecting the termini of BLA)[14] gave excellent resistance to aggregation, but only moderate recovery of catalytic activity (Figure S4).
To see if the effect of SpyTag/SpyCatcher could be extended to another class of enzyme, we tested our approach on dihydrofolate reductase (DHFR). SpyTag-DHFR-SpyCatcher cyclized in high yield, as shown by SDS-PAGE and mass spectrometry (Figure S5). SpyTag-DHFR-SpyCatcher, like the SpyTag DA-DHFR-SpyCatcher control, resisted aggregation at 100 °C (Figure S5).[25] However, SpyTag-DHFR-SpyCatcher retained its catalytic activity following the 100 °C heating, unlike SpyTag DA-DHFR-SpyCatcher (Figure S5).
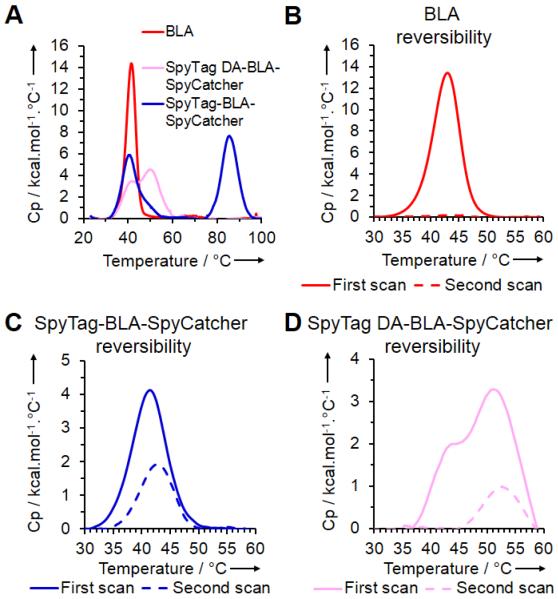
To dissect the mechanism of SpyTag/SpyCatcher stabilization, we used Differential Scanning Calorimetry (DSC) to test the thermal unfolding profile of SpyTag-BLA-SpyCatcher, SpyTag DA-BLA-SpyCatcher and BLA. SpyTag-BLA-SpyCatcher and SpyTag DA-BLA-SpyCatcher generated two peaks (Figure 3a). The common peak for all constructs, corresponding to BLA domain unfolding, had a melting temperature (Tm) of 39.6 °C for SpyTag-BLA-SpyCatcher, 39.6 °C for SpyTag DA-BLA-SpyCatcher, and 41.3 °C for BLA (Figure 3a). The second peak for SpyTag-BLA-SpyCatcher with a Tm of 85.4 °C is likely to correspond to unfolding of the reconstituted SpyTag/SpyCatcher domain. The second peak for SpyTag DA-BLA-SpyCatcher, related to the non-covalent SpyTag DA/SpyCatcher complex, had a Tm of 54.0 °C (Figure 3a).
Figure 3.
Thermal unfolding transitions of β-lactamase, SpyTag-BLA-SpyCatcher and SpyTag DA-BLA-SpyCatcher. a) DSC giving the specific heat capacity (Cp) of each enzyme construct scanned from 20-110 °C at 1 °C/min. b) DSC of BLA scanned from 20-60 °C at 2 °C/min, before cooling and then repeating the scan. c) DSC as in b) for SpyTag-BLA-SpyCatcher. d) DSC as in b) for SpyTag DA-BLA-SpyCatcher.
To test the BLA domain’s capability to refold, we used DSC to scan from 20 to 60 °C (where the signature from BLA unfolding is observed), allowed the sample to cool down, and then scanned over this temperature range again. BLA gave no peak on the second scan, consistent with an inability to refold (Figure 3b). In contrast, SpyTag-BLA-SpyCatcher produced a signal of similar shape but reduced intensity on the second scan (Figure 3c), consistent with the ability of the BLA domain to refold successfully in this cyclized context. SpyTag DA-BLA-SpyCatcher gave no peak in the range 40-45 °C, corresponding to the absence of BLA refolding; the peak from 45-60 °C corresponds to the SpyTag DA/SpyCatcher moiety (Figure 3d). Therefore, cyclization through SpyTag-SpyCatcher chemistry did not appear to increase resistance to unfolding, but instead increased the capability to refold.
In conclusion, we have developed a method to dramatically increase a protein’s ability to recover from denaturation, using cyclization via spontaneous isopeptide bond formation. SpyTag/SpyCatcher cyclization conferred resistance to aggregation and enabled the recovery of catalytic activity following heating. DSC showed that the BLA domain’s Tm was largely unaffected by cyclization, so that the enhanced resilience is likely to be from an increase in the cyclized protein’s ability to refold. The greatly enhanced effect on stability to aggregation of SpyTag/SpyCatcher-cyclization compared to other cyclization strategies may relate to the shielding of inter-protein associations by the presence of a stable domain,[26] but intriguingly DSC indicates that the effect of stabilization extends beyond temperatures where the SpyCatcher/SpyTag complex can unfold.
Disulfide bonds may also be engineered to lock protein termini, but can interfere with existing disulfides, will not form in reducing environments, can break via β-elimination at elevated temperatures,[27] and often only give modest stability enhancements.[12] While this work was in progress, elastin-like peptides containing terminal SpyTag and SpyCatcher were shown to cyclize, but no functional effect of cyclization was established.[28] For some protein architectures it will be hard to achieve SpyTag/SpyCatcher cyclization, but ~50% of single domain proteins in the PDB have N- and C-terminal structural elements within 5 Å,[29] and future work may be able to harness the reactivity of SpyTag and SpyCatcher in internal regions of proteins.[14,28] Since protein stability can enable tolerance of mutations advantageous for function but deleterious for folding to the native structure,[30] it is also interesting to explore in future work whether SpyTag/SpyCatcher-stabilization facilitates library-based evolution of novel protein function.
Supplementary Material
Footnotes
Funding was provided by BBSRC (C.S.), Sekisui Diagnostics (C.S., S.P.B.), the Clarendon Fund (J.O.F.), New College Oxford (J.O.F.), Worcester College Oxford (M.H.) and Oxford University Department of Biochemistry (M.H.). We thank Nick Major of Sekisui Diagnostics for helpful advice and the Molecular Biophysics Facility at Oxford University Department of Biochemistry.
Experimental Section
Materials and methods are found in the Supporting information.
References
- [1].Polizzi KM, Bommarius AS, Broering JM, Chaparro-Riggers JF. Curr. Opin. Chem. Biol. 2007;11:220–225. doi: 10.1016/j.cbpa.2007.01.685. [DOI] [PubMed] [Google Scholar]
- [2].Chien A, Edgar DB, Trela JM. J. Bacteriol. 1976;127:1550–1557. doi: 10.1128/jb.127.3.1550-1557.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lehmann M, Kostrewa D, Wyss M, Brugger R, D’Arcy A, Pasamontes L, van Loon AP. Protein Eng. 2000;13:49–57. doi: 10.1093/protein/13.1.49. [DOI] [PubMed] [Google Scholar]
- [4].Gaucher EA, Govindarajan S, Ganesh OK. Nature. 2008;451:704–707. doi: 10.1038/nature06510. [DOI] [PubMed] [Google Scholar]
- [5].Watanabe K, Ohkuri T, Yokobori S, Yamagishi A. J. Mol. Biol. 2006;355:664–674. doi: 10.1016/j.jmb.2005.10.011. [DOI] [PubMed] [Google Scholar]
- [6].Risso VA, Gavira JA, Mejia-Carmona DF, Gaucher EA, Sanchez-Ruiz JM. J. Am. Chem. Soc. 2013;135:2899–2902. doi: 10.1021/ja311630a. [DOI] [PubMed] [Google Scholar]
- [7].Reetz MT. J. Am. Chem. Soc. 2013;135:12480–12496. doi: 10.1021/ja405051f. [DOI] [PubMed] [Google Scholar]
- [8].Goldsmith M, Tawfik DS. Curr. Opin. Struct. Biol. 2012;22:406–412. doi: 10.1016/j.sbi.2012.03.010. [DOI] [PubMed] [Google Scholar]
- [9].Iyer PV, Ananthanarayan L. Process Biochem. 2008;43:1019–1032. [Google Scholar]
- [10].Korkegian A, Black ME, Baker D, Stoddard BL. Science. 2005;308:857–860. doi: 10.1126/science.1107387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vieille C, Zeikus GJ. Microbiol. Mol. Biol. Rev. 2001;65:1–43. doi: 10.1128/MMBR.65.1.1-43.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baase WA, Liu L, Tronrud DE, Matthews BW. Protein Sci. Publ. Protein Soc. 2010;19:631–641. doi: 10.1002/pro.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zakeri B, Howarth M. J. Am. Chem. Soc. 2010;132:4526–4527. doi: 10.1021/ja910795a. [DOI] [PubMed] [Google Scholar]
- [14].Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, Howarth M. Proc. Natl. Acad. Sci. 2012;109:E690–E697. doi: 10.1073/pnas.1115485109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kang HJ, Baker EN. Trends Biochem. Sci. 2011;36:229–237. doi: 10.1016/j.tibs.2010.09.007. [DOI] [PubMed] [Google Scholar]
- [16].Hendrix RW, Johnson JE. Adv. Exp. Med. Biol. 2012;726:351–363. doi: 10.1007/978-1-4614-0980-9_15. [DOI] [PubMed] [Google Scholar]
- [17].Jacob E, Unger R. Bioinforma. Oxf. Engl. 2007;23:e225–230. doi: 10.1093/bioinformatics/btl318. [DOI] [PubMed] [Google Scholar]
- [18].Goldenberg DP, Creighton TE. J. Mol. Biol. 1983;165:407–413. doi: 10.1016/s0022-2836(83)80265-4. [DOI] [PubMed] [Google Scholar]
- [19].Parthasarathy R, Subramanian S, Boder ET. Bioconjug. Chem. 2007;18:469–476. doi: 10.1021/bc060339w. [DOI] [PubMed] [Google Scholar]
- [20].Evans TC, Jr, Benner J, Xu MQ. J. Biol. Chem. 1999;274:3923–3926. doi: 10.1074/jbc.274.7.3923. [DOI] [PubMed] [Google Scholar]
- [21].Iwai H, Plückthun A. FEBS Lett. 1999;459:166–172. doi: 10.1016/s0014-5793(99)01220-x. [DOI] [PubMed] [Google Scholar]
- [22].Camarero JA, Muir TW. J. Am. Chem. Soc. 1999;121:5597–5598. [Google Scholar]
- [23].Scott CP, Abel-Santos E, Wall M, Wahnon DC, Benkovic SJ. Proc. Natl. Acad. Sci. 1999;96:13638–13643. doi: 10.1073/pnas.96.24.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bershtein S, Goldin K, Tawfik DS. J. Mol. Biol. 2008;379:1029–1044. doi: 10.1016/j.jmb.2008.04.024. [DOI] [PubMed] [Google Scholar]
- [25].Bershtein S, Wu W, Shakhnovich EI. Proc. Natl. Acad. Sci. 2012;109:4857–4862. doi: 10.1073/pnas.1118157109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li L, Fierer JO, Rapoport TA, Howarth M. J. Mol. Biol. 2014;426:309–317. doi: 10.1016/j.jmb.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Volkin DB, Klibanov AM. J. Biol. Chem. 1987;262:2945–2950. [PubMed] [Google Scholar]
- [28].Zhang W-B, Sun F, Tirrell DA, Arnold FH. J. Am. Chem. Soc. 2013;135:13988–13997. doi: 10.1021/ja4076452. [DOI] [PubMed] [Google Scholar]
- [29].Krishna MMG, Englander SW. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1053–1058. doi: 10.1073/pnas.0409114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bloom JD, Labthavikul ST, Otey CR, Arnold FH. Proc. Natl. Acad. Sci. 2006;103:5869–5874. doi: 10.1073/pnas.0510098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.