Abstract
Non-invasive ventilation (NIV) has been widely supported in the past two decades as an effective application in avoiding the need for endotracheal intubation (ETI) and reducing associated mortality in acute hypoxemic respiratory failure (AHRF) patients. However, the efficacy of NIV in AHRF patients, non-related to chronic obstructive pulmonary disease (COPD) and trauma is still controversial in the field of medical research. This retrospective study aimed to evaluate the efficacy of NIV as an adjunctive therapy in non-COPD and non-traumatic AHRF patients. Data of 11 randomized control trials (RCTs), which were conducted between 1990 and 2010 to determine the efficacy of NIV in non-COPD and non-traumatic AHRF patients, were reviewed from the PUBMED, MEDLINE, Cochrane Library, and EMBASE databases. Parameters monitored in this study included the ETI rate, fatal complications, mortality rate of patients, and their ICU and hospital duration of stay. Overall results showed a statistically significant decrease in the rate of ETI, mortality, and fatal complications along with reduced ICU and hospital length of stay in non-COPD and non-trauma AHRF patients of various etiologies. This systematic review suggests that non-COPD and non-trauma AHRF patients can potentially benefit from NIV as compared with conventional treatment methods. Observations from various cohort studies, observational studies, and previously published literature advocate on the efficacy of NIV for treating non-COPD and non-traumatic AHRF patients. However, considering the diversity of studied populations, further studies and more specific trials on less heterogeneous AHRF patient groups are needed to focus on this aspect.
Keywords: Acute hypoxemic respiratory failure, endotracheal intubation, non-COPD, non-invasive ventilation, non-trauma
Acute respiratory failure (ARF) is a serious disorder that often requires endotracheal intubation (ETI) and mechanical ventilation for its management.[1] Although ETI is a routine practice in ICUs, worldwide, however, it may cause complications, both during intubation and after extubation.[2] To reduce ETI-associated morbidity, the application of non-invasive artificial ventilation over invasive methods is highly preferred and is usually practiced as treatment measures of AHRF since the past 2 decades.[3]
Non-invasive ventilation is the administration of mechanical ventilation to the lungs without using an invasive artificial airway, such as an endotracheal tube, laryngeal mask, or tracheostomy, for the management of ARF caused by various etiologies.[4,5] The provision of ventilator support to lungs is made through the patient's upper airway using a nasal mask or similar kind of device.[6,7] NIV not only reduces the need for ETI but also significantly reduces the mortality rate, ICU stay, and overall cost of hospitalization in patients.[8] The effectiveness of NIV in AHRF patients with exacerbations of chronic obstructive pulmonary disease (COPD) has been greatly supported in various literatures.[9,10,11] Although NIV is considered as an integral tool in the management of AHRF in patients with COPD; however, its efficacy in non-COPD and non-traumatic AHRF patients is still highly debated.[5]
The objective of this systematic review and meta-analysis was to determine whether the use of NIV can reduce the need for ETI, the length of ICU and hospital stay, and the associated complications and mortality rate among non-COPD and non-trauma patients.
Methods
Study design
This study performed a systematic review and meta-analysis of 11 RCTs to determine the effectiveness of NIV in the treatment of AHRF in non-COPD and non-trauma patients. The methodological approach followed in this study included the development of the selection criteria, definition of search strategies, quality assessment of all studies, data abstraction, and statistical data analysis.[12]
Statistical considerations
Results abstraction included presentation of proportions of those experiencing the outcome (ETI/MV) by study groups and means + standard deviation for continuous outcomes like ICU hospital stay. Meta-analysis was summarized by Forest plots (35) for the dichotomous outcomes of ETI/MV and/or mortality. The plots computed the odds (probability/1-probability) for these events and statistically modeled the log of these odds as a function of study group among the various studies. The odds ratio (OR) thus describes the risk of the outcome (ETI/MV or death) for one study group versus the other. OR values > 1 relay excess risk and those < 1 indicate reduced risk. Furthermore, OR 95% confidence limits disjointed from the value “1” indicate statistical significance using a 0.05 significance level.
Selection criteria
The criteria for selecting studies were defined before data collection in order to appropriately identify high-quality studies suitable for this analysis. For the selection purpose, the following inclusion criteria were defined.
Study Design: Systematic review and meta-analysis of selected RCTs.
Patient population: The study population consisted of a majority (>60%) of patients presented to hospitals with AHRF, not associated with an exacerbation of COPD or chest trauma and not requiring immediate ventilatory assistance.
Interventions: Application of NIV as an adjunctive therapy to usual medical care (UMC) compared with UMC alone.
Outcomes: Primary outcome measure included the need for ETI as decided by clinical trialists and related complications, and the secondary outcomes included the mortality rate, length of stay in the ICU, and overall hospital stay.
Search strategy
A computerized literature search was performed in this study to identify potentially eligible studies. The PUBMED, MEDLINE, EMBASE databases, and Cochrane library were extensively searched for all possible literatures on NIV. In this review, the main focus of the search was limited only to RCTs [Figure 1]; however, other articles were also referred for gaining insight on the background of the current topic. The search was conducted using the following search keywords and Medical Subject Headings (MeSH) terms: NIV, NIPPV, BiPAP, and continuous positive airway pressure (CPAP).
Figure 1.
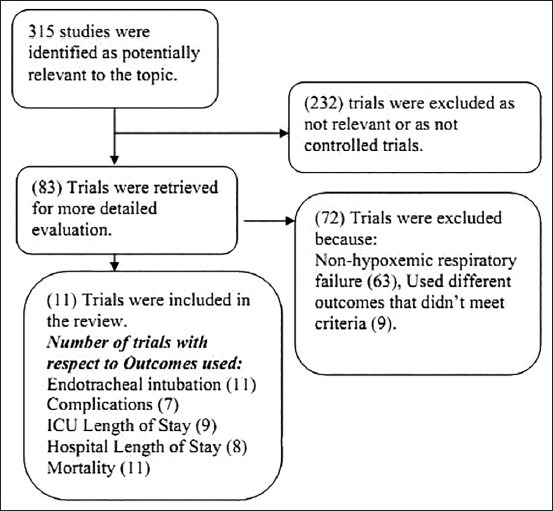
Data selection criteria for the current systematic review
In the current search strategy, formats with higher sensitivity were given the first priority, in order to increase the probability of identifying all relevant articles. All database searches were done for RCTs that were conducted between 1990 and 2010.
Study selection and data abstraction
In the first phase of selection, electronic searches identified 315 articles from the sources listed, using the specified search strategy. After screening titles and abstracts for relevance, 232 studies were excluded as they were either not relevant to the topic or were not RCTs. The full paper of the remaining 83 trials were retrieved and analyzed for more detailed evaluations. Of these 83 RCTs, 72 RCTs were excluded from the study as they did not meet the predefined selection criteria. In 63 RCTs, patients did not have AHRF, and in the rest 9 RCTs, the study population was mixed, i.e., AHRF patients were not reported separately or they used different outcomes that did not meet the set criteria. Thus, the remaining 11 RCTs that met the overall predefined selection criteria were retrieved from the Albertson Library, Boise State University website. These selected 11 RCTs considered five common outcome variables as monitoring parameters to access efficacy of NIV application in AHRF patients of non-COPD and non-trauma cases. The outcome variables are: ETI, related complications, ICU length of stay, hospital length of stay, and mortality rate [Figure 1].
Study description
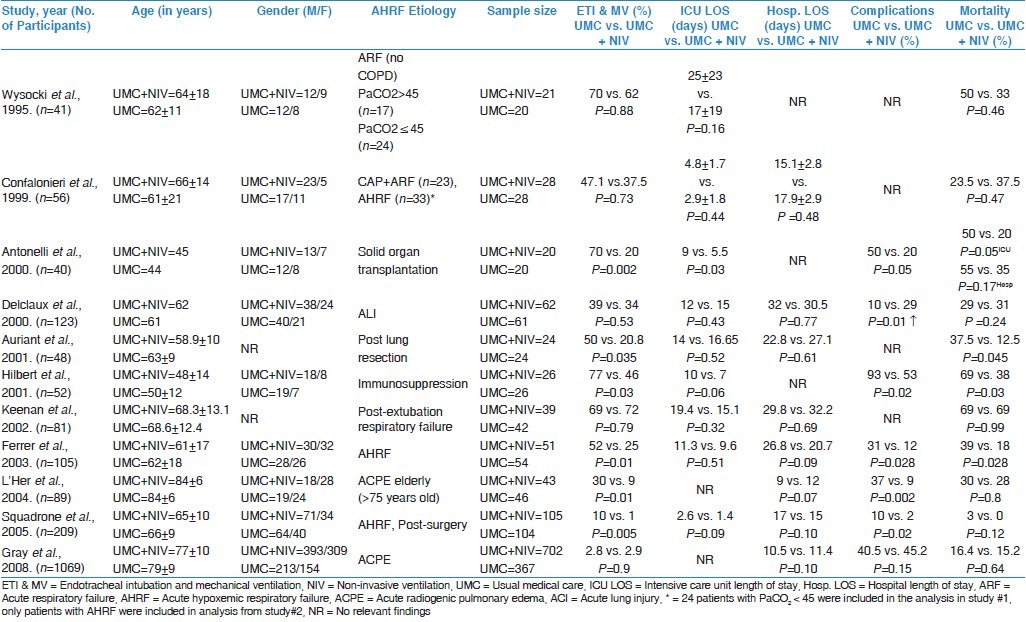
The 11 RCTs selected for this systematic review contain data from five different countries: –France (RCTs 1, 4, 5, 6, and 9); Italy (RCTs 2, 3, and 10); Spain (RCT 8); Canada (7); and United Kingdom (RCT 11). Diverse patient populations with AHRF were enrolled in these 11 RCTs. Five studies, namely RCT 2, 4, 8, 10, and 11 involved multiple center trials. RCTs 3 and 6 focused on immunosuppressed patients, RCT 9 and 11 on acute cardiogenic pulmonary edema (ACPE) patients, RCT 5 on patients who underwent post-lung resection surgery, RCT 2 on community-acquired pneumonia (CAP), RCT 7 on post-extubation respiratory failure, RCT 4 on acute lung injury (ALI), RCT 10 on post-abdominal surgery, and RCTs 1 and 8 on more heterogeneous groups of patients [Table 1].
Table 1.
Review of RCTs to assess the efficacy of UMC + NIV in AHRF patients in non-COPD and non-trauma cases
Types of NIV as implemented in the RCTs
NIV comes in two forms, non-invasive positive pressure ventilation (NIPPV) and CPAP. NIPPV is a combination of inspiratory pressure support (also known as inspiratory positive airway pressure [IPAP]) and positive end-expiratory pressure (PEEP) (also known as expiratory positive airway pressure [EPAP]) delivered to the patient through a mask interface. Biphasic positive airway pressure (BiPAP) and non-invasive pressure support ventilation (NIPSV) are also used to describe NIPPV. Continuous positive airway pressure provides a baseline constant positive airway pressure throughout inspiration and expiration, whereas BiPAP provides two levels of pressure: IPAP during inspiration and EPAP during expiration phase. The application of one or the other of these forms of NIV has been implemented in these 11 RCTs. The main focus of this study is to determine the potential of these forms of NIV in treating non-COPD and non-trauma AHRF patients.
Results and Discussion
Efficacy of NIV on immunosuppressed patients with AHRF
To assess the efficacy of NIV on immunosuppressed AHRF patients, RCTs by Antonelli et al. and Hilbert et al., involving 40 and 52 immunosuppressed AHRF patients, respectively, were reviewed.[13,14]
In the study by Antonelli et al., 40 patients, who had received a solid organ transplant (liver, kidney, or lung), were treated in the ICU for AHRF, post-transplantation.[13] Twenty patients each were randomly assigned to the UMC (n = 20) and UMC + NIV (n = 20) group. Fourteen patients (70%) of the UMC group required ETI as compared with four patients (20%) of the NIV group (P = 0.002). The ICU length of stay among the survivors was comparatively less in the UMC + NIV group than compared to the UMC group (UMC vs. UMC + NIV = 9 vs. 5.5 days; P = 0.03). The mortality rate of the UMC group patients in the ICU (UMC vs. UMC + NIV = 50% vs. 20%; P = 0.05) and hospital (UMC vs. UMC + NIV = 55% vs. 35%; P = 0.17) was greater compared to that of the UMC + NIV group. Serious fatal complications were significantly higher in the UMC group than in the UMC + NIV group (UMC vs. UMC + NIV = 50% vs. 20%; P = 0.05) [Table 1].
The study by Hilbert et al. involved 52 immunosuppressed patients who were admitted to the ICU with AHRF associated with pulmonary infiltrates and fever.[14] Twenty-six patients were treated in the UMC group and 26 were treated in the UMC + NIV group. Twenty patients (77%) of the UMC group required ETI, whereas only 12 patients (46%) in the UMC + NIV group showed the need for ETI (P = 0.03). The ICU mortality rate of patients in the UMC group was 69% (18 patients) as compared with 10 (38%) in the UMC + NIV group (P = 0.03). The ICU length of stay among survivors was 10 ± 4 days in the UMC group as compared to 7 ± 3 days in the UMC + NIV group (P = 0.06). The overall hospital mortality rate was 21 (81%) in the UMC group as compared to 13 (50%) in the UMC + NIV group (P = 0.02) [Table 1].
In the above studied cases, patients of the UMC + NIV group showed statistically significant results in terms of the reduced need of ETI, rate of complications, and most importantly, decreased mortality rates [Figure 2]. Study results of these two RCTs suggest that NIV can be used as an effective treatment in addition to UMC for immunosuppressed patients. The success of the NIV in these vulnerable populations was probably due to the avoidance of complications associated with the invasive mechanical ventilation.[15]
Figure 2.
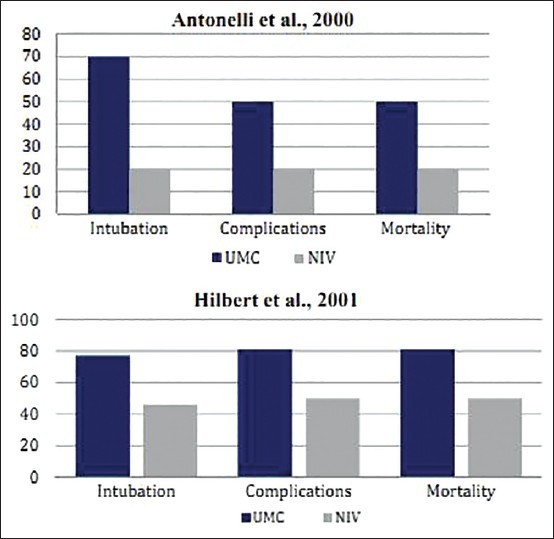
Comparative results of RCTs by Antonelli et al. and Hilbert et al. on immunosuppressed AHRF patients showing differences in the rate of ETI, fatal complications, and mortality in patients when treated with UMC and UMC + NIV
Efficacy of NIV on patients with ALI or acute respiratory distress syndrome (ARDS)
This multicenter RCT was conducted by Delclaux et al. on 123 adult patients who were admitted to hospitals for treatment of acute respiratory insufficiency, secondary to pulmonary edema.[16] Around 83%, i.e., 102 patients were presented with ALI (PaO2/FiO2≤ 300 mm Hg), whereas 21 patients (17%) were identified having pure cardiac decompensation. These cardiac patients were equally distributed among the UMC (n = 60) and UMC + NIV (n = 62) groups. No significant differences were found between the two treatment groups for any of the clinical outcome measures studied, including the rate of ETI (UMC vs. UMC + NIV = 39% vs. 34%; P = 0.53), length of stay in ICU (UMC vs. UMC + NIV = 12 vs. 15 days; P = 0.43), hospital length of stay (UMC vs. UMC + NIV = 32 vs. 30.5 days; P = 0.77), and hospital mortality rate (UMC vs. UMC + NIV = 29% vs. 31%; P = 0.24). Moreover, ETI-related complications were more prevalent in the UMC + NIV group as compared to the UMC group (UMC vs. UMC + NIV = 10% vs. 29%; P = 0.01) [Table 1].
This RCT revealed no benefits of NIV application on AHRF patients with ALI. Furthermore, majority of patients from the NIV + UMC group developed higher rates of adverse effects [Figure 3]. A probable reason could be due to the delay of conventional mechanical ventilation to these patients that was needed to improve the ventilatory support, rather than oxygenation alone. The result of this study, demonstrating the failure of NIV, is in absolute agreement with findings of some previously published multicenter cohort studies that were conducted on AHRF patients having ALI, which adopted NIV as the treatment line.[17,18,19]
Figure 3.
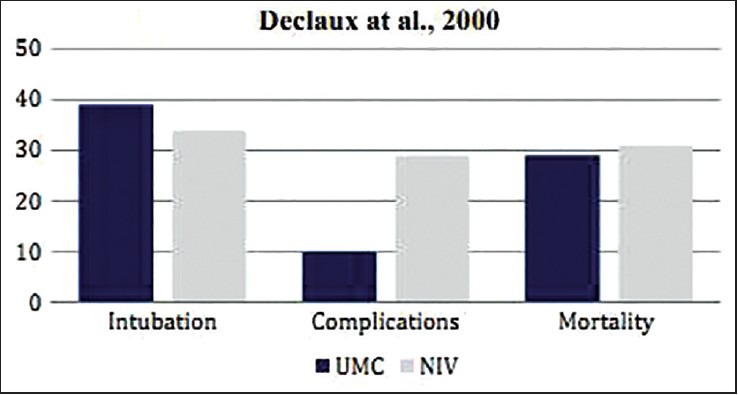
Comparative result of the RCT by Declaux et al. on AHRF patients with ALI showing differences in the rate of ETI, fatal complications, and mortality in patients when treated with UMC and UMC + NIV
Efficacy of NIV on post-extubation AHRF patients
Keenan et al. conducted an RCT on 81 patients who required ventilatory support for more than 48 hours, post-extubation.[20] These patients, who either had a history of congestive heart failure or chronic lung disease, had eventually developed respiratory distress. Forty two patients were treated in the UMC group and 39 patients in the UMC + NIV group. There was no statistically significant difference in the ETI rates between both groups (UMC vs. UMC + NIV = 69% vs. 72% patients; P = 0.79). The ICU length of stay was shorter for patients in the UMC + NIV group as compared to the UMC group (UMC vs. UMC + NIV = 19.4 vs. 15.1 days; P = 0.32). Contrarily, the duration of hospital stay for the UMC group was shorter than that of the UMC + NIV group (UMC vs. UMC + NIV = 29.8 vs. 32.2 days; P = 0.69). However, both groups demonstrated the same mortality rate (UMC vs. UMC + NIV = 69% vs. 69%; P = 0.99) [Table 1, Figure 4].
Figure 4.
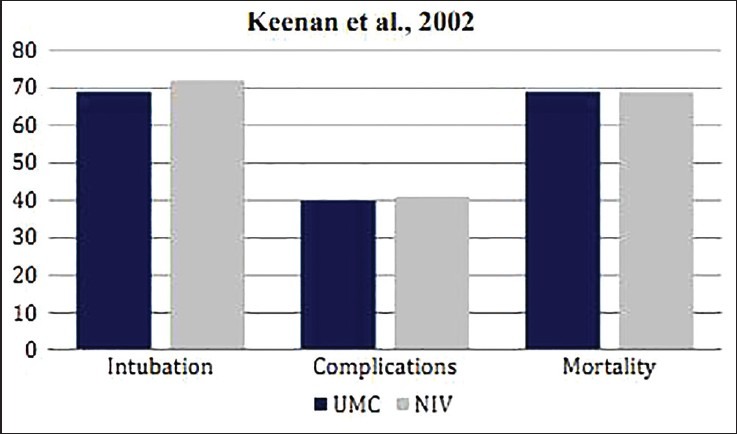
Comparative result of the RCT by Keenan et al. on post-extubation AHRF patients showing differences in the rate of ETI, fatal complications, and mortality in patients when treated with UMC and UMC + NIV
The study by Keenan et al. study showed no specific advantages of NIV on post-extubation AHRF patients. However, another multicenter RCT (not included in this review) that was done on 224 patients went further into this issue. Its results demonstrated higher mortality rate in the UMC + NIV group than in the UMC group (UMC vs. UMC + NIV = 14% vs. 25%; P = 0.048).[21] Analyzing results of both cases and looking at the mortality rates, the efficiency of NIV as a treatment therapy poses controversial for post-extubation AHRF patients.
Efficacy of NIV on AHRF patients with CAP
This multicenter cohort study by Confalonieri et al. included 56 AHRF patients with CAP.[22] The population was equally divided into two groups: 28 patients to be treated with UMC alone and 28 patients with NIV intervention. Twenty-three patients presented a history COPD and therefore, were excluded from the study. The remaining 33 non-COPD patients, who were segregated in groups of 17 and 16 in the UMC and UMC + NIV groups, respectively, were further analyzed. In the UMC group, eight patients (47.1%) required ETI, while only six patients (37.5%) in the UMC + NIV group needed intubation (P = 0.73). The ICU length of stay for patients was shorter in the UMC + NIV group as compared to the UMC group alone (UMC vs. UMC + NIV = 4.8 ± 1.7 vs. 2.9 ± 1.8 days; P = 0.44). Conversely, the overall duration of hospital stay was shorter for patients in the UMC group as compared to the UMC + NIV group (UMC vs. UMC + NIV = 15.1 ± 2.8 vs. 17.9 ± 2.9 days; P = 0.48). The mortality rate was 23.5% (n = 4) for the UMC + NIV group, whereas it was 37.5% (n = 6) for the UMC group (P = 0.47) [Table 1].
The study result of Confalonieri et al. did not show any significant benefits of NIV in AHRF patients who were presented with CAP, with no underlying COPD disease [Figure 5]. However, another similar study, by the same author, showed positive results in terms of the efficacy of NIV treatment.[23] The application of NIV in a non-RCT of AIDS patients, presented with severe pneumocystis pneumonia, showed improvement in their outcomes as compared to patients who received invasive mechanical ventilation.
Figure 5.
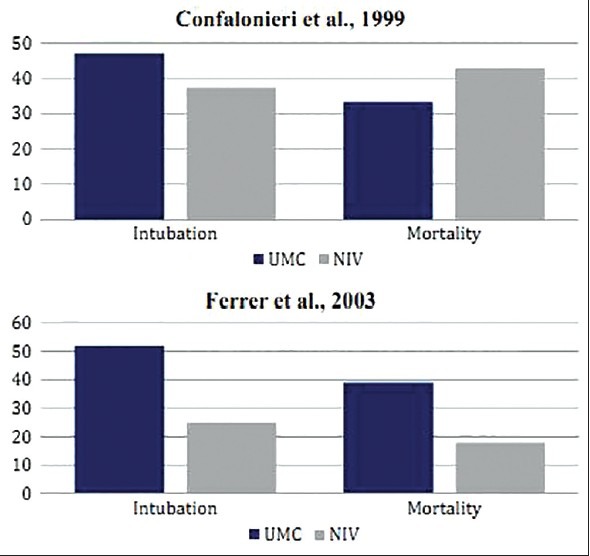
Comparative results of RCTs by Confalonieri et al. and Ferrer et al. on heterogeneous AHRF patients showing differences in the rate of ETI, fatal complications, and mortality in patients when treated with UMC and UMC + NIV
Interestingly enough, in another RCT by Ferrer et al. that involved 105 patients (UMC vs. UMC + NIV = 54 vs. 51 patients), presented with AHRF due to heterogeneous causes, significant results were noticed.[24] Authors indicated a significantly lower rate of intubation (UMC vs. UMC + NIV = 52% vs. 25%; P = 0.01), fatal complications (UMC vs. UMC + NIV = 31% vs. 12%; P = 0.028), hospital length of stay (UMC vs. UMC + NIV = 26.8 vs. 20.7 days; P = 0.09), and mortality (UMC vs. UMC + NIV = 39% vs. 18%; P = 0.028) in patients who were treated with NIV in addition to UMC as compared to the UMC group alone [Table 1, Figure 5].
Although some contradictory results like these debates regarding the risk-free use of NIV, however, the overall statistics of the use of NIV justifies its potential for its application on AHRF patients with CAP.
Efficacy of NIV in AHRF patients with ACPE
To assess the efficacy of NIV on AHRF patients with ACPE, two RCTs by L’Her et al. and Gray et al., which were conducted on patients admitted to emergency departments for immediate treatment, were reviewed.[25,26]
L’Her et al. conducted their study on 89 patients (age ≥75 years) who were admitted to emergency departments presented with AHRF related to ACPE.[25] The population was randomly assigned to receive UMC (n = 46) and UMC + NIV, particularly CPAP therapy (n = 43). Fourteen patients (30%) of the UMC group required ETI, while only four patients (9%) in the NIV group underwent intubation (P = 0.01). There were 17 patients (37%) who experienced serious complications in the UMC group, while the number was limited to only four (9%) in the NIV group (P = 0.002). The early 48-hour mortality was significantly lower in the NIV group as compared to the UMC + NIV group (UMC vs. UMC + NIV = 24% vs. 7%; P = 0.017). However, no significant difference was observed in the overall mortality rate between the two groups (UMC vs. UMC + NIV = 30% vs. 28%; P = 0.8). In-hospital length of stay was 9 ± 7 days in the UMC group versus 12 ± 11 days in the NIV group (P = 0.07) [Table 1].
The second case is a multicenter study by Gray et al. in which total 1069 patients were randomly assigned to the UMC (n = 367) and UMC + NIV (n = 702) groups.[26] Observations noted at the end of first 1 week (UMC vs. UMC + NIV = 9.8% vs. 9.5%; P = 0.87) and 1 month (UMC vs. UMC + NIV = 16.4% vs. 15.2%; P = 0.64) showed no significant differences in the mortality rate of patients receiving the UMC and those undergoing the UMC + NIV treatment. Other outcomes, like the ETI rate (UMC vs. UMC + NIV = 2.8% vs. 2.9%; P =0.9), length of hospital stay (UMC vs. UMC + NIV = 10.5 vs. 11.4 days; P = 0.10), and rate of fatal complications (UMC vs. UMC + NIV = 40.5% vs. 45.2%; P = 0.15) also showed no significant differences for both groups [Table 1].
The RCT by L’Her et al. showed statistically significant improvements in terms of rates of mortality, need for intubation, and serious complications in elderly patients in the first 48 hours after getting admitted to the emergency department with ACPE and treated with CPAP as compared to another group treated with the UMC [Figure 6]. However, no sustained benefits were observed in CPAP-treated patients during their overall hospital stay. In contrast, in the study by Gray et al. on AHRF patients with ACPE, NIV induced a more rapid improvement in respiratory distress and metabolic disturbance, but had no effect on short-term mortality [Figure 6]. This is in agreement with another large randomized patient study, which included 130 patients from multiple emergency departments of Italy showed that there were improvements only in PaO2/FiO2 ratios, but showed a reduction in hypercapnic patients and not the other patients with PaCO2≤45 mm Hg.[27] On the other hand, another meta-analysis provides strong evidence on the efficacy of NIV in treating AHRF patients with ACPE.[28]
Figure 6.
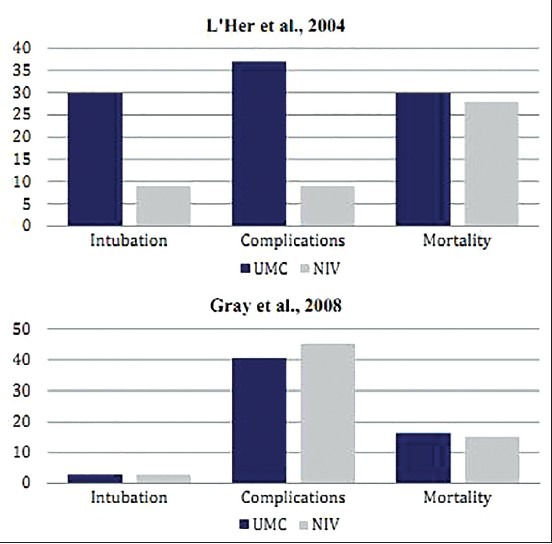
Comparative results of RCTs by L’Her et al. and Gray et al. on AHRF patients with ACPE showing differences in the rate of ETI, fatal complications, and mortality in patients when treated with UMC and UMC + NIV
Efficacy of NIV in post-surgical AHRF patients
To test the efficacy of NIV on postoperative AHRF patients, RCTs by Auriant et al. and Squadrone et al. that were conducted on patients who underwent lung resection and major abdominal surgery, respectively, were reviewed.[29,30]
The RCT by Auriant et al. involved 48 AHRF patients, who underwent surgical resection for treatment of lung cancer.[29] All patients were extubated in the operating room after the surgery. Patients were randomly assigned to the UMC (n = 24) and UMC + NIV (n = 24) groups. The UMC + NIV group showed significantly reduced intubation (UMC vs. UMC + NIV = 50% vs. 20.8%; P = 0.035) and mortality rates (UMC vs. UMC + NIV = 37.5% vs. 12.5%; P =0.045) as compared to the UMC group alone. However, the ICU (UMC vs. UMC + NIV = 14 vs. 16.65 days; P =0.52) and hospital (UMC vs. UMC + NIV = 22.8 vs. 27.1 days; P = 0.61) length of stay were similar in both groups [Table 1].
Squadrone et al. conducted their study on 209 patients who underwent major abdominal surgery.[30] Patients were extubated at the end of the surgical procedure and underwent a 1-hour screening test of breathing oxygen via a Venturi mask at an inspiratory fraction of 0.3. Patients were included in the study if they developed PaO2/FiO2≤300 mm Hg while breathing.[30] There were 104 and 105 patients in the UMC and UMC + NIV group (mode of NIV was CPAP in this study), respectively. The rate of ETI (UMC vs. UMC + NIV = 10% vs. 1%; P = 0.005) and associated fatal complications (UMC vs. UMC + NIV = 10% vs. 2%; P = 0.002) were significantly much lower in the UMC + NIV group as compared to the UMC group. The ICU length of stay was 2.6 days in the UMC group versus 1.4 days in the UMC + NIV group (P = 0.09). However, the hospital length of stay was almost similar in the two groups. No mortality (0%) occurred in the UMC + NIV group while three patients (3%) of the UMC group died (P = 0.12) [Table 1].
Both studies showed statistically significant differences with regard to intubation rates [Figure 7]. The beneficial effects of NIV, demonstrated in the study by Auriant et al., were probably because of the presence of ACPE, which usually responds well to NIV therapy. Their study result suggests that NIV is safe and effective in reducing the need for ETI and improving survival of AHRF patients after lung resection. Their findings suggest NIV as a secondary therapy to the standard conservative therapy of AHRF due to lung resection. NIV has also been supported by similar studies to successfully treat atelectasis (collapsed lung units), which is very common in post-abdominal surgery.[31] Auriant et al. probably did not assess the rate of fatal complications in their study, which however, showed a significant decrease in the study by Squadrone et al. A significant reduction in the mortality rate was observed in the post-lung resection surgery population when treated with NIV, which however, did not show any statistically significant improvement in the post-abdominal surgery case [see Table 1, Figure 7]. Results of the second study suggest that CPAP may decrease the incidence of ETI and other severe complications in patients who develop hypoxemia after elective major abdominal surgery.
Figure 7.
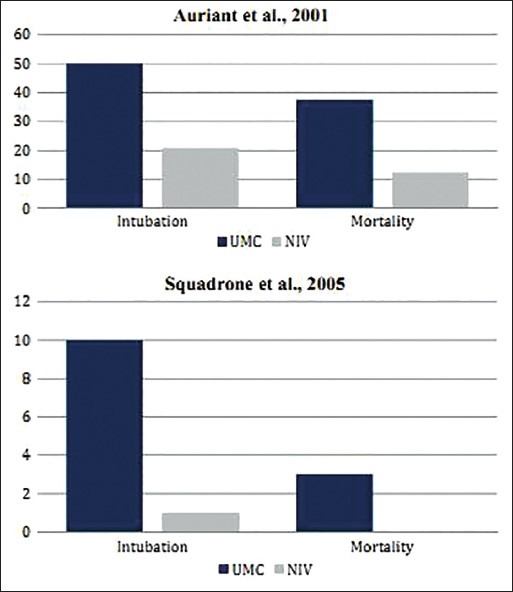
Comparative results of RCTs by Auriant et al. and Squadrone et al. on postoperative AHRF patients showing differences in the rate of ETI, fatal complications, and mortality in patients when treated with UMC and UMC + NIV
Efficacy of NIV on heterogeneous group of patients
This RCT that was conducted by Wysocki et al. on a more heterogeneous group of 41 non-COPD patients with ARF gave contradictory results for its two patient groups.[32] Seventeen patients having PaCO2 >45 mm gave positive results, whereas 24 patients having PaCO2≤45 mm gave negative results in terms of application of NIV. To statistically evaluate the results for efficacy of NIV, 20 and 21 patients were randomly assigned under the UMC and UMC + NIV groups, respectively. When the overall statistics were determined, no significant differences were observed between the rate of ETI (UMC vs. UMC + NIV = 62% vs. 70%, P = 0.88), the length of ICU stay (UMC vs. UMC + NIV = 17 ± 19 vs. 25 ± 23 days, P = 0.16), and the mortality rate (UMC vs. UMC + NIV = 33% vs. 50%, P = 0.46) between patients treated with NIV and those treated conventionally.
From the result of this RCT by Wysocki et al., it can be concluded that NIV is of no benefit when systematically used in all forms of ARF not associated with COPD. However, NIV is particularly useful when applied on non-COPD ARF patients with PaCO2 >45 mm Hg.
Meta-analysis forest plots findings
As indicated in Figure 8 and for the outcome of Endotracheal Intubation, studies by by Auriant et al., Squadrone et al. Confalonieri et al., Hilbert et al., Antonelli et al. and Ferrer et al., all significantly (p-values < 0.05) favored NIV/NISPV as an adjunctive therapy to UMC versus UMC/conventional therapy alone. Figure 9 on the other hand for the outcome of mortality shows that only the study by Hilbert et al., significantly indicating an advantage of NIV/NISPV versus UMC/conventional therapy alone. The study by Auriant et al. showed somewhat of a border-line significant advantage (p-value = 0.07).
Figure 8.
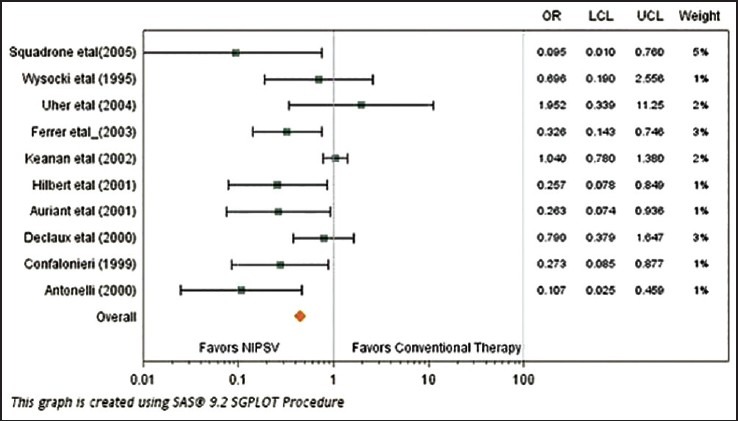
Forest plot contrasting the odds ratio and 95% confidence interval for endotracheal intubation among various studies
Figure 9.
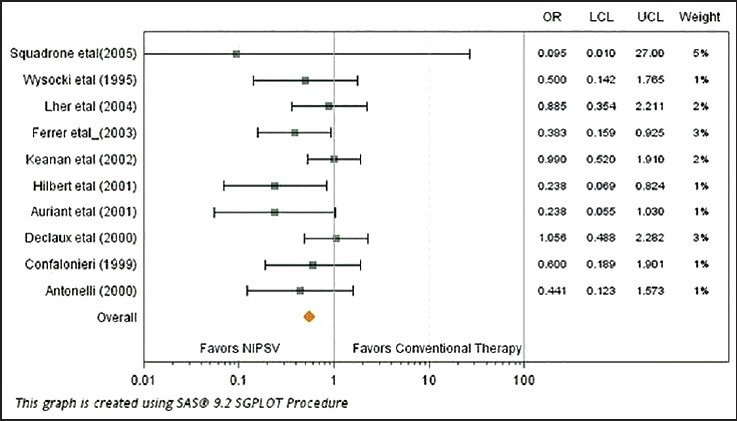
Forest plot contrasting the odds ratio and 95% confidence interval for mortality among various studies
Conclusion
The overall analysis of the 11 RCTs reviewed in this study suggests that the use of NIV as an adjunctive therapy in non-COPD and non-traumatic AHRF patients decreases the need for ETI, ICU length of stay, and mortality rate to significant levels. However, it is still difficult to generalize these findings due to the wide heterogeneity of populations of these RCTs, also due to small sample sizes in some specific trials. Although some RCTs have shown clear benefits of NIV in treating AHRF patients non-related to COPD and trauma as compared to the UMC alone; however, the contradictory results of some other RCTs, like that observed in immunosuppressed patients after lung resection and in patients who underwent abdominal surgery, provide a conflicting opinion. However, this cannot be included as the postoperative study involved only elective procedures. Overall, this study provides strong evidence of significant benefits of NIV in reducing the death rate in AHRF patients.
The increased clinical experience of NIV, patient tolerance, and selection of the most appropriate NIV interface is fundamentally more important.[33] The need of NIV should not be a reason to delay ETI when indicated; rather, the patient's condition should be critically analyzed to decide the safest treatment type. Proper patient monitoring also plays an important role in improving outcomes, therefore, competent personnel, such as respiratory therapists and registered nurses, and highly monitored clinical settings are critical factors for the optimal use of NIV to ensure patient safety. Although many studies demonstrate the effectiveness of NIV, however, considering the variance in results of the studied RCTs, more specific trials that would mainly focus on AHRF patient groups with less heterogeneity in etiology would likely be more reliable to justify this aspect.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Esteban A, Anzueto A, Alia I, Gordo F, Apezteguia C, Palizas F, et al. How is mechanical ventilation employed in the intensive care unit. An international utilization review? Am J Respir Crit Care Med. 2000;161:1450–8. doi: 10.1164/ajrccm.161.5.9902018. [DOI] [PubMed] [Google Scholar]
- 2.McCulloch TM, Bishop MJ. Complications of translaryngeal intubation. Clin Chest Med. 1991;12:507–21. [PubMed] [Google Scholar]
- 3.Beers M, Porter R, Jones T, Kaplan J, Berkwits M. Merck manual of diagnosis and therapy. 18th ed. New Jersey: Merck Research Lab; 2006. Acute hypoxemic respiratory failure (AHRF, ARDS) [Google Scholar]
- 4.Rai SP, Panda BN, Upadhyay KK. Non-invasive positive pressure ventilation in patients with acute respiratory failure. Medical Journal Armed Forces India. 2004;60:224–6. doi: 10.1016/S0377-1237(04)80050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill NS, Brennan J, Garpestad E, Nava S. Non-invasive ventilation in acute respiratory failure. Crit Care Med. 2007;35:2402–7. doi: 10.1097/01.CCM.0000284587.36541.7F. [DOI] [PubMed] [Google Scholar]
- 6.Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease: Positive pressure ventilation through a nose mask. Am Rev Respir Dis. 1987;135:523–4. doi: 10.1164/arrd.1987.135.1.148. [DOI] [PubMed] [Google Scholar]
- 7.Kerby GR, Mayer LS, Pingleton SK. Nocturnal positive pressure ventilation via nasal mask. Am Rev Respir Dis. 1987;135:738–40. doi: 10.1164/arrd.1987.135.3.738. [DOI] [PubMed] [Google Scholar]
- 8.Brochard L, Mancebo J, Elliott MW. Noninvasive ventilation for acute respiratory failure. Eur Respir J. 2002;19:712–21. doi: 10.1183/09031936.02.00295502. [DOI] [PubMed] [Google Scholar]
- 9.Antonelli M, Pennisi MA, Conti G. New advances in the use of noninvasive ventilation for acute hypoxemic respiratory failure. Eur Respir J Suppl. 2003;42:65–71S. doi: 10.1183/09031936.03.00421003. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida Y, Takeda S, Akada S, Hongo T, Tanaka K, Sakamoto A. Factors predicting successful noninvasive ventilation in acute lung injury. J Anesth. 2008;22:201–6. doi: 10.1007/s00540-008-0637-z. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R, Gupta R, Aggarwal AN, Gupta D. Noninvasive positive pressure ventilation in acute respiratory failure due to COPD vs. other causes: Effectiveness and predictors of failure in a respiratory ICU in North India. Int J Chron Obstruct Pulmon Dis. 2008;3:737–43. doi: 10.2147/copd.s3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analysis of randomised controlled trials: The QUOROM statement. Quality of Reporting Meta-analyses. Lancet. 1999;354:1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 13.Antonelli M, Conti G, Bufi M, Costa MG, Lappa A, Rocco M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: A randomized trial. JAMA. 2000;283:235–41. doi: 10.1001/jama.283.2.235. [DOI] [PubMed] [Google Scholar]
- 14.Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, Dupon M, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481–7. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- 15.Hill NS. Noninvasive ventilation for immunocompromised patients. N Engl J Med. 2001;344:522–4. doi: 10.1056/NEJM200102153440711. [DOI] [PubMed] [Google Scholar]
- 16.Delclaux C, L’Her E, Alberti C, Mancebo J, Abroug F, Conti G, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: A randomized controlled trial. JAMA. 2000;284:2352–60. doi: 10.1001/jama.284.18.2352. [DOI] [PubMed] [Google Scholar]
- 17.Demoule A, Giro E, Richard JC, Taille S, Brochard L. Increased use of noninvasive ventilation in French intensive care units. Intensive Care Med. 2006;32:1747–55. doi: 10.1007/s00134-006-0229-z. [DOI] [PubMed] [Google Scholar]
- 18.Demoule A, Giro E, Richard JC, Taille S, Brochard L. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med. 2006;32:1756–65. doi: 10.1007/s00134-006-0324-1. [DOI] [PubMed] [Google Scholar]
- 19.Antonelli M, Conti G, Esquinas A, Montini L, Maggiore SM, Bello G, et al. A multi-center survey of the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35:18–25. doi: 10.1097/01.CCM.0000251821.44259.F3. [DOI] [PubMed] [Google Scholar]
- 20.Keenan SP, Powers C, McCormack DG, Block G. Noninvasive positive-pressure ventilation for postextubation respiratory distress: A randomized controlled trial. JAMA. 2002;287:3238–44. doi: 10.1001/jama.287.24.3238. [DOI] [PubMed] [Google Scholar]
- 21.Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apezteguia C, Gonzalez M, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452–60. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]
- 22.Confalonieri M, Potena A, Carbone G, Porta RD, Tolley EA, Umberto Meduri G. Acute respiratory failure in patients with severe community-acquired pneumonia: A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med. 1999;160:1585–91. doi: 10.1164/ajrccm.160.5.9903015. [DOI] [PubMed] [Google Scholar]
- 23.Confalonieri M, Calderini E, Terraciano S, Chidini G, Celeste E, Puccio G, et al. Noninvasive ventilation for treating acute respiratory failure in AIDS patients with Pneumocystis carinii pneumonia. Intensive Care Med. 2002;28:1233–8. doi: 10.1007/s00134-002-1395-2. [DOI] [PubMed] [Google Scholar]
- 24.Ferrer M, Esquinas A, Leon M, Gonzalez M, Alarcon A, Torres A. Noninvasive ventilation in severe hypoxemic respiratory failure: A randomized clinical trial. Am J Respir Crit Care Med. 2003;168:1438–44. doi: 10.1164/rccm.200301-072OC. [DOI] [PubMed] [Google Scholar]
- 25.L’Her E, Duquesne F, Girou E, de Rosiere XD, Le Conte P, Renault S. Noninvasive continuous positive airway pressure in elderly cardiogenic pulmonary edema patients. Intensive Care Med. 2004;30:882–8. doi: 10.1007/s00134-004-2183-y. [DOI] [PubMed] [Google Scholar]
- 26.Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J. 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359:142–51. doi: 10.1056/NEJMoa0707992. [DOI] [PubMed] [Google Scholar]
- 27.Nava S, Carbone G, DiBattista N, Bellone A, Baiardi P, Cosentini R, et al. Noninvasive ventilation in cardiogenic pulmonary edema: A multicenter randomized trial. Am J Respir Crit Care Med. 2003;168:1432–7. doi: 10.1164/rccm.200211-1270OC. [DOI] [PubMed] [Google Scholar]
- 28.Potts JM. Noninvasive positive pressure ventilation: Effect on mortality in acute cardiogenic pulmonary edema: A pragmatic meta-analysis. Pol Arch Med Wewn. 2009;119:349–53. [PubMed] [Google Scholar]
- 29.Auriant I, Jallot A, Herve P, Cerrina J, Ladurie F, Fournier JL, et al. Noninvasive ventilation reduces mortality in acute respiratory failure following lung resection. Am J Respir Crit Care Med. 2001;164:1231–5. doi: 10.1164/ajrccm.164.7.2101089. [DOI] [PubMed] [Google Scholar]
- 30.Squadrone V, Coha M, Cerutti E, Schellino MM, Biolino P, Occella P, et al. Piedmont Intensive Care Units Network (PICUN). Continuous positive airway pressure for treatment of postoperative hypoxemia: A randomized controlled trial. JAMA. 2005;293:589–95. doi: 10.1001/jama.293.5.589. [DOI] [PubMed] [Google Scholar]
- 31.Jaber S, Chanques G, Jung B. Postoperative noninvasive ventilation. Anesthesiology. 2010;112:453–61. doi: 10.1097/ALN.0b013e3181c5e5f2. [DOI] [PubMed] [Google Scholar]
- 32.Wysocki M, Tric L, Wolff MA, Millet H, Herman B. Noninvasive pressure support ventilation in patients with acute respiratory failure: A randomized comparison with conventional therapy. Chest. 1995;107:761–8. doi: 10.1378/chest.107.3.761. [DOI] [PubMed] [Google Scholar]
- 33.Kallet R. Noninvasive ventilation in acute care: Controversies and emerging concepts. Respir Care. 2009;54:259–63. [PubMed] [Google Scholar]


