Abstract
BACKGROUND AND AIM:
We aimed to validate the Turkish version of Berlin Questionnaire (BQ) and developped a BQ-gender (BQ-G) form by adding gender component. We aimed to compare the two forms in defining patients with moderate to severe obstructive sleep apnea (OSA) in sleep clinics.
METHODS:
Four hundred and eighty five consecutive patients, refered to our sleep clinic for snoring, witnessed apnea and/or excessive daytime sleepiness were enrolled to the study. All patients underwent in-laboratory polysomnography (PSG). Patients with sleep efficiency less than 40% and total sleep time less than 4 hours, chronic anxiolitic/sedative drug usage, respiratory tract infection within past two weeks were excluded from the study. All the patients fulfilled BQ. The test and retest for BQ were applied in 15-day interval in 30 patients.
RESULTS:
Totally 433 patients were enrolled to the study (285 male, 148 female). The mean age of the patients was 47,5 ± 10.5 (21-79). 180 patients (41.6%) had apnea-hypopnea index (AHI) ≤ 15, while 253 patients (58,4%) had AHI > 15. The κ value was 48–94 and the the truth value was 69-94% for the test-retest procedure. Sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and area under the curve AUC were 84.2%, 31.7%, 48.7%, 63.4%, and 0.579 in order for BQ and 79.9 %, 51.7%, 63.2% , 69.6%, and 0.652 for BQ-G.
CONCLUSION:
The results showed that BQ-G is relatively better than BQ in determining moderate to severe OSA in sleep clinics where most of the patients are sleep apneic but both of the tests were found to have insufficient validities in defining moderate to severe OSA in sleep clinics.
Keywords: Breathing sleep disorder, Berlin questionnaire, gender, sleep apnea
Obstructive sleep apnea syndrome (OSAS) is a disorder characterized by repetetive upper airway collapse during sleep associated with reversible arterial oxygen desaturation. OSAS leads to repetetive night awakenings and excessive daytime sleepiness[1] The prevalence of obstructive sleep apnea (OSA) is about 2-4% in general population and varies depending on the gender and age in different groups.[2] Overnight polysomnography (PSG) is considered to be gold standard for diagnosis of OSA, however, prohibitive cost of the test and the long waiting lists limit its usage. Questionnaire-based screening tools are used for identifying at risk patients for OSA. Berlin questionnaire (BQ) is the first test used to screen for OSA in the primary care setting,[3] Translation of BQ to Portuguese language[4], Hindi (with modification)[5], Persian[6], Greek[7], Arabic,[8] Korean,[9] and Turkish[10] have been made and validated in the studies. The sensitivities and specificities for defining OSA patients differ in different groups; when applied to a sleep disordered breathing population for different apnea-hypopnea index (AHI) cut-off points 72.1-88.4 % sensitivity but 39.1-50% for specificity values have been reported. The questions did not include gender.[3]
The Turkish version of BQ has been used in a sleep clinic population and BQ was reported to be a poor predictor of OSA in patients admitted to a sleep clinic. We developped a second form of BQ by adding gender (scored as positive if male gender) as the fourth category. We evaluated the effect of adding gender to BQ (BQ-G) in identifying moderate to severe OSA patients in sleep clinics as a screening test.
Methods
Method
A cross sectional study was done in the Department of Respiratory and Sleep Clinic in our hospital between January 2011 and March 2012, for all patients who met the inclusion criteria and provided written informed consent. The study complied with the declaration of Helsinki and was approved by local research ethics committee.
Patients
Four hundred eighty five consecutive patients, refered to our sleep clinic for snoring, witnessed apnea and/or excessive daytime sleepiness were enrolled to the study. All patients underwent in-laboratory PSG. Patients with sleep efficiency less than 40% and total sleep time less than 4 hours, chronic anxiolitic/sedative drug usage, respiratory tract infection within past 2 weeks were excluded from the study. All the patients fulfilled BQ.
Berlin questionnaire (BQ)
The BQ is a widely used screening tool for OSA and has been validated in primary care settings and other specific patient populations.[3,5] BQ contains three categories: category 1 asks about snoring (one introductory question and four follow-up questions about frequency and loudness) and witnessed apneas, category 2 asks about sleepiness and fatigue (three primary questions and one sub-question about drowsy driving), and category 3 asks about having a history of hypertension and also body mass index (BMI) of > 30 kg/m2. Patients are defined to be at high risk for OSA if they have a score of 2 for any of two categories.
In our modified form of BQ-G, we added gender item as the fourth category and it was scored as positive if male gender was present. The whole three positive categories out of four categories were scored as “High risk for OSA” in BQ-G.
A pilot study was conducted on a sample of 30 patients and the test and retest for BQ were applied in 15-day interval.
Sleep study
All participants underwent PSG using Compumedics E series (Compumedics, Melbourne, Victoria, Australia). At least 6 hours of PSG data was recorded. PSG recordings included 6-channel electroencephalography (EEG), 2-channel electrooculography (EOG), 2-channel submental electromyography, oxygen saturation by an oximeter finger probe, respiratory movements via chest and abdominal belts, airflow both via nasal pressure sensor and oro-nasal thermistor, electrocardiography (ECG), and leg movements via both tibial anterolateral electrodes. Sleep stages and respiratory parameters were scored according to the standard criteria of the American Academy of Sleep Medicine (AASM).[11] Based on the guidelines of the AASM, respiratory event was scored as an apnea if there was a drop in the peak signal excursion by ≥ 90% of baseline using an oronasal thermal sensor and the duration of the ≥ 90% drop in sensor signal ≥ 10 seconds and at least 90% of event's duration met amplitude reduction criteria for apnea (recomended criteria). Respiratory event was scored as hypopnea if there was 50% or greater drop in flow of baseline using nasal pressure for 10 seconds or longer associated with ≥ 3% oxygen desaturation with an arousal in which 90% of the event's duration met the amplitude reduction of criteria for hypopnea (alternative criteria).[11] AHI was calculated based on the following formula: total number of obstructive apneas + hypopneas/total sleep time (h). Sleep stage scoring was done by using software (Profusion PSG 3) in 30-seconds epochs by a certified registered PSG technologist according to AASM criteria.[11]
Statistical analysis
Statistical analysis were performed using PASW Statistics 18 (SPSS, Inc, Chicago, Illinois, USA). Continuous variables were summarized as means ± SD or medians (and interquartile ranges), and categorical variables as proportions. Comparisons between groups were done by means of an independent t-test if the data were normally distributed and a Mann–Whitney U-test if not. A P value less than 0.05 was considered statistically significant.
Calculations of sensitivity, specificity, positive predictive value, negative predictive value were done using standard equations.
Results
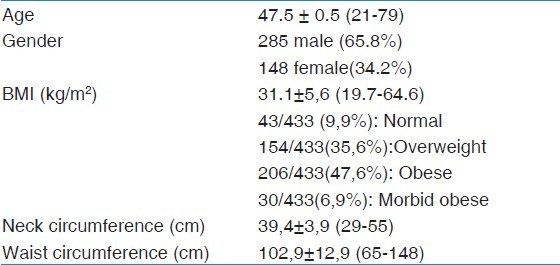
Totally 433 patients were enrolled to the study (285 male, 148 female). The mean age of the patients was 47,5 ± 10.5 (21-79)
The anthropometric measurements were summarized in Table 1. 148 patients (34.1%) had hypertension disease and 121 patients (27.9%) had diabetes mellitus (DM) disease in patient history.
Table 1.
Anthropometric values of the 433 patients
The patients grouped with respect to AHI severity were shown in Table 2.
Table 2.
Patients with respect to AHI severity

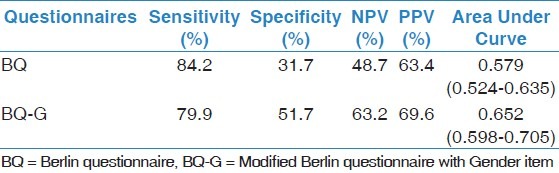
The sensitivity, specificity, Negative Predictive Value (NPV) and Positive Predictive Value (PPV) of the BQ and BQ-G were demonstrated in Table 3. Sensitivity, specificity, NPV and PPV were 84.2%, 31.7%, 48.7%, 63.4% in order for BQ and 79.9 %, 51.7%, 63.2% and 69.6 for BQ-G.
Table 3.
Sensitivity, Specificity, NPV , PPV and AUC for AHI 15 as cut off for BQ and BQ-G
The κ value was 48-94 and the the truth value was 69-94% for the test-retest procedure for BQ.
The sensitivity, specificity and area under the curve (AUC) for BQ and BQ-G were shown in Figure 1. AUC were 0.579 for BQ and 0.652 for BQ-G.
Figure 1.
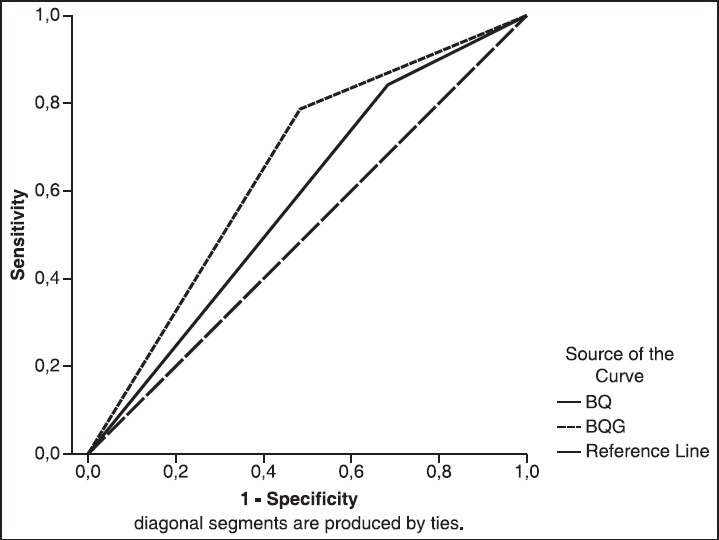
Roc Curves for BQ (Berlin Questionnaire) and BQ-Gender for AHI > 15
Discussion
In the modified form of BQ which was named as BQ-G, gender item was added as the fourth category and was scored as ‘high risk’ in the presence of three positive categories. Gender item helped to raise specificity to 51.7%, NPV to 63.2% and PPV to 69.6% while sensitivity decreased slightly to 79.9% for AHI 15 as cut-off. The results of the BQ-G were found to be relatively better in defining patients with moderate to severe OSA in sleep clinics but the percentages were not found high enough to conclude that neither BQ nor BQ-G were valid enough to be used to define moderate to severe OSA in sleep clinics. BQ was first used by Netzer[3] in primary care sites to define high risk patients for OSA in 744 adults. 100 of the total patients had portable unattended home sleep study in addition to BQ. The sensitivity, specificity, PPV were found as 86%, 77%, 89% in order, for RDI 5 as cut-off. The predictive performance of BQ varied in different populations, the sensitiviy ranging from 54-86% and specificity from 43-77%.[12,13] BQ was reported to have low sensitivity and specificities in sleep clinics in consistent with our results[10,13]; Ulasli et al., reported 73% sensitiviy, 44% specificity for AHI 5 in 1450 patients as cut-off[10] and Ahmadi et al., reported 63 % sensitivity and 43 % specificity for respiratory disturbance index (RDI) 10 as cut-off in 130 patients.[13]
The prevalance of OSA is reported as 2-4%[2] but the percentage varies in different groups. It is reported as 9-24% in middle-aged population.[14] The OSA prevalence is expected to be higher in sleep clinics. 84.8% of 1450 patients were reported to have AHI > 5 in a sleep clinic.[10] In our study, 83.7% of the patient had AHI > 5 and 58.4% had AHI > 15. Nearly all patients had loud snoring and/or witnessed apnea and/or daytime sleepiness which constituted the symptoms of OSA disease. BQ queries these symptoms plus hypertension existence and/or BMI > 30. As most of the patients do have at least two positive answers to the 3 categories, adding differents items such as anthropometric measures or gender can help to increase the validity of BQ. Gender item helped to raise specificity and NPV, but was not found to be helpful as a diagnostic screening method in sleep clinic.
BQ has also been used in bed partners to screen sleep apneic patients[15], in elderly[16], in preoperative identification in elective surgical patients[17], in coronary artery disease[18] with either portable PSG or PSG in sleep laboratories and used to get an idea of the prevalence of OSA in different groups without PSG.[19,20,21]
Prediction formulae for sleep-disordered breathing can be useful for excluding a diagnosis or establishing a priori probability of having a positive test and for prioritizing patient testing. In general prediction models have high sensitivity but low specificity. Clinical prediction rules are necessary so that limited laboratory polysomnographic resources and professional manpower are used optimally.[22] BMI, neck circumference, gender, hypertension, habitual snoring, witnessed apneas, and habitual gasp/choking were found significantly different in patients with OSA in a sleep center.[23] Males have been reported to have higher prevalence of OSA than females.[24] Gender has been used in questionnaires for OSA. STOP-Bang test has four items added to STOP test: BMI, age, neck circumference and gender; in which ‘male’ answer scored positive. Higher STOP-Bang scores were shown to indicate high probability of OSA in sleep clinics.[25] STOP-Bang test was shown to have higher sensitivity and specificity than STOP test and Berlin questionnaire in defining moderate to severe OSA in highway bus drivers.[26] In another study, frequency for various symptoms of sleep apnea and other sleep disorders plus age, BMI and gender were recorded and multiple logistic regression models were used to corporate BMI, age, and gender into a multivariable apnea index (MAP index) and was found potentially useful in defining especially apneic patient[27] similar to STOP-Bang test.
Different scoring methods for hypopneas were reported to lead to different AHI values[28,29,30,31]; the values were lowest with AASM-recommended criteria and highest with Chicago criteria.[28] Using ≥ 3% instead of ≥ 4% were reported to increase AHI values.[32] AASM -alternative criteria (2007) was used for scoring hypopneas in our study. Hypopnea was defined as ≥ 30% of pre-event baseline with a duration of the ≥ 30% drop in signal excursion for ≥ 10 second with ≥ 3% oxygen desaturaion or an arousal in the latest AASM manual in 2012[33] Different sensitivities and specificities may be reported with different scoring rules for analysing the value of BQ and BQ-G. we performed only one scoring system. This can be a limitation for our study. Further studies using different scoring rules in the same study can be done to test the validity of both of the tests in sleep clinics in a more confidential design.
The strengths of this study are: 1. The number of patients are high 2. All of the patients had PSG in sleep clinic and scorings were made manually, blind to BQ scores. All the patients had BQ first and then polysomnography.
The specificity of BQ is found low (31.7%) and sensitivity moderately high (84.2%) in defining moderate to severe OSA in our sleep clinic. Adding gender component to BQ (as a modified BQ; BQ-G) helped relatively raise specificity and negative predictive value, but both of the tests were found to have insufficient validities to define moderate to severe OSA in sleep clinics.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Guilleminault C, Tilkian A, Dement WC. The sleep apnoea syndrome. Annu Rev Med. 1976;27:465–84. doi: 10.1146/annurev.me.27.020176.002341. [DOI] [PubMed] [Google Scholar]
- 2.Victor LD. Obstructive sleep apnea. Am Fam Physician. 1999;60:2279–86. [PubMed] [Google Scholar]
- 3.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 4.Vaz AP, Drummond M, Mota PC, Severo M, Almeida J, Winck JC. Translation of Berlin Questionnaire to Portuguese language and its application in OSA identification in a sleep disordered breathing clinic. Rev Port Pneumol. 2011;17:59–65. [PubMed] [Google Scholar]
- 5.Sharma SK, Vasudev C, Sinha S, Banga A, Pandey RM, Handa KK. Validation of the modified Berlin questionnaire to identify patients at risk for the obstructive sleep apnoea syndrome. Indian J Med Res. 2006;124:281–90. [PubMed] [Google Scholar]
- 6.Amra B, Nouranian E, Golshan M, Fietze I, Penzel T. Validation of the Persian version of Berlin sleep questionnaire for diagnosing obstructive sleep apnea. Int J Prev Med. 2013;4:334–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Bouloukaki I, Komninos ID, Mermigkis C, Micheli K, Komninou M, Moniaki V, et al. Translation and validation of Berlin questionnaire in primary health care in Greece. BMC Pulm Med. 2013;13:6. doi: 10.1186/1471-2466-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saleh AB, Ahmad MA, Awadalla NJ. Development of Arabic version of Berlin questionnaire to identify obstructive sleep apnea at risk patients. Ann Thorac Med. 2011;6:212–6. doi: 10.4103/1817-1737.84775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang K, Park KS, Kim JE, Kim SW, Kim YT, Kim JS, et al. Usefulness of the Berlin Questionnaire to identify patients at high risk for obstrutive sleep apnea: A population-based door-to-door study. Sleep Breath. 2013;17:803–10. doi: 10.1007/s11325-012-0767-2. [DOI] [PubMed] [Google Scholar]
- 10.Ulasli SS, Gunay E, Koyuncu T, Akar O, Halici B, Ulu S, et al. Predictive value of Belin Questionnaire and Epworth Sleepiness Scale for obstructive sleep apnea in a sleep clinic population. Clin Respir J. 2013 doi: 10.1111/crj.12070. [DOI] [PubMed] [Google Scholar]
- 11.Conrad I, Ancoli I S, Chesson AL. The AASM Manual for the scoring of Sleep and associated events. Rules, terminology and technical specifications. Westchester: American Academy of Sleep Medicine; 2007. Quan SF for the American Academy of Sleep Medicine. [Google Scholar]
- 12.Strauss RS, Browner WS. Risk for obstructive sleep apnea. Ann Intern Med. 2000;132:758–9. doi: 10.7326/0003-4819-132-9-200005020-00015. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadi N, Chung CA, Gibbs A, Shapiro CM. The Berlin questionnaire for sleep apnea in a sleep clinic population: Relationship to polysomnographic measurements of respiratory disturbances. Sleep Breath. 2008;12:39–45. doi: 10.1007/s11325-007-0125-y. [DOI] [PubMed] [Google Scholar]
- 14.Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, et al. Predictors of sleep-disordered breathing in community dwelling adults: The sleep heart health study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 15.Sapasge P, Leger D, Taillard J, Bayo V, Chaumet G, Philip P. Might the berlin sleep questionnaire applied to bed partners be used to screen sleep apneic patients? Sleep Med. 2010;11:479–83. doi: 10.1016/j.sleep.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Sforza E, Chouchou F, Pichot V, Herrmann F, Barthelemy JC, Roche F. Is the Berlin questionnaire a useful tool to diagnose sleep apnea in the elderly? Sleep Med. 2011;12:142–6. doi: 10.1016/j.sleep.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Chung F, ward B, Ho J, Yuan H, kayumow L, Shapiro C. Preoperative identification of sleep apnea risk in elective surgical atients, using Berlin questionnaire. J Clin Anesth. 2007;19:130–4. doi: 10.1016/j.jclinane.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Martinez D, da Silva RP, Klein C, Fiori CZ, Massierer D, Cassol CM, et al. High risk for sleep apnea in the Berlin questionnaire and coronary artery disease. Sleep Breath. 2011;16:89–94. doi: 10.1007/s11325-010-0460-2. [DOI] [PubMed] [Google Scholar]
- 19.Senthilvel E, Auckley D, Dasarathy J. Evaluation of sleep disoders in the primary care setting: History taking compared to questionnaires. J Clin Sleep Med. 2011;7:41–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symtoms and risk of Sleep Apnea in the US Population: Results from the national sleep foundation sleep in America 2005 poll. Chest. 2006;130:780–6. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 21.Thornton JD, Chandriani K, Thornton JG, Farooq S, Moallem M, Krishnan V, et al. Assessing the prioritization pf primary care referrals for polysomnograms. Sleep. 2010;33:1255–60. doi: 10.1093/sleep/33.9.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harding SM. Prediction formulae for sleep-diordered breathing. Curr Opin Pulm Med. 2001;7:381–5. doi: 10.1097/00063198-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Rowlet JA, Aboussouan LS, Badr MS. The use of clinical prediction formulas in the evaluation of sleep apnea. Sleep. 2000;23:929–38. doi: 10.1093/sleep/23.7.929. [DOI] [PubMed] [Google Scholar]
- 24.Ralls FM. Grigg-Damberger M Roles of gender, age, race/ ethnicity and residential socioeconomics in obstructive sleep apnea syndromes. Curr Opin Pulm Med. 2012;18:568–73. doi: 10.1097/MCP.0b013e328358be05. [DOI] [PubMed] [Google Scholar]
- 25.Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnea. Br J Anaesth. 2012;108:768–75. doi: 10.1093/bja/aes022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fýrat H, Yüceege M, Demir A, Ardýç S. Comparison of four established questionnaires to identify highway bus drivers at risk for obstructive sleep apnea in Turkey. Sleep Biol Rhythms. 2012;10:231–6. [Google Scholar]
- 27.Maislin G, Pack AI, Kribbs NB, Smith PL, Schwartz AR, Kline LR, et al. A survey screen for prediction of apnea. Sleep. 1995;18:158–66. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 28.Guilleminault C, Hagen CC, Huynh NT. Comparison of hypopnea definitions in lean patients with known obstructive sleep apnea hypopnea syndrome (OSAHS) Sleep Breath. 2009;13:341–7. doi: 10.1007/s11325-009-0253-7. [DOI] [PubMed] [Google Scholar]
- 29.Ruehland WR, Rochford PD, O’Donogue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: Impact on the apnea/hypopnea index. Sleep. 2009;32:150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redline S, Kapur VK, Sanders MH, Quan SF, Gottlieb DJ, Rapoport DM, et al. Effects of varying approaches for identifying respiratory disturbances on sleep apnea assessment. Am J Respir Crit Care Med. 2000;161:369–74. doi: 10.1164/ajrccm.161.2.9904031. [DOI] [PubMed] [Google Scholar]
- 31.Manser RL, Rochford P, Pierce RJ, Byrnes GB, Campbell DA. Impact of different criteria for defining hypopneas in the apne-hypopnea index. Chest. 2001;120:909–14. doi: 10.1378/chest.120.3.909. [DOI] [PubMed] [Google Scholar]
- 32.Whitney CW, Golttieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, et al. Reliability of scoring respiartory disturbance indices and sleep staging. Sleep. 1998;21:749–57. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 33.Berry RB, Budhiraja R, Gottieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]


