Abstract
BACKGROUND:
Recent trials involving pirfenidone suggest a beneficial effect in the treatment of idiopathic pulmonary fibrosis (IPF).
OBJECTIVE:
To report on the efficacy and safety of pirfenidone in the treatment of patients with IPF, at a tertiary care hospital in Saudi Arabia.
METHODS:
The study included 58 patients with IPF who were evaluated from March 2012 to March 2013. During the study period, 33 patients received pirfenidone, and the remaining patients (n = 25) served as a control group. Baseline clinical characteristics, physiological parameters and the results of a 36-Item Short Form Health Survey (SF-36) were compared between the groups. Furthermore, we compared changes in forced vital capacity (FVC), diffusion capacity of the lung for carbon monoxide (DLco), six-minute walk distance (6MWD) and SF-36 for both groups during follow-up. The last follow-up period ended in January 2014.
RESULTS:
There were no significant differences in baseline clinical characteristics between the groups. Furthermore, we found no differences in FVC, DLco and SF-36 during follow-up (median, 12 months). However, patients receiving pirfenidone treatment were less likely to experience reductions in 6MWD compared with the control group (13% vs. 52%, respectively; P = 0.001). Although adverse events were more frequently reported by the pirfenidone group compared with the control group (85 vs. 56%, respectively; P = 0.015), these patients did not require discontinuation of treatment.
CONCLUSION:
Pirfenidone treatment preserves functional capacity, as reflected by the 6MWD. Adverse events associated with pirfenidone treatment were generally well tolerated by the patients.
Keywords: 36-item short form health survey, acetylcysteine, anti-reflux therapy, idiopathic pulmonary fibrosis, pirfenidone, six-minute walk distance
Idiopathic pulmonary fibrosis (IPF) is a subtype of chronic fibrosing interstitial pneumonia that primarily occurs in older adults and is associated with a variable clinical course and poor outcomes.[1,2] Despite extensive research, the pathogenesis of IPF remains poorly understood.
Over the past decade, many studies with a particular focus on agents targeting the ongoing fibro-proliferative process have been performed in an attempt to halt the progressive decline in pulmonary function that is observed in IPF patients. Pirfenidone is a synthetic molecule with unique properties as an antifibrotic, anti-inflammatory and antioxidant agent.[3,4,5] Published studies evaluating the clinical effects of pirfenidone in the treatment of IPF showed that it slows the rate of decline in lung function parameters,[6,7,8,9] reduces the rate of acute exacerbations,[6] leads to improvements in progression-free survival,[9,10] and preserves six-minute walk distance (6MWD).[8,9] Because of these encouraging results, pirfenidone is approved for the treatment of IPF in many countries.
The aim of this study is to describe the efficacy and safety of pirfenidone in the treatment of patients with IPF at a tertiary care hospital in Saudi Arabia. Additionally, we report outcomes such as changes in lung function, walking distance and answers to a 36-Item Short Form Health Survey (SF-36) questionnaire, as well as the number of acute exacerbations, adverse events and deaths.
Methods
Patients
This retrospective study included patients in the database of King Khalid University Hospital and is part of an ongoing large prospective study of current diagnostic assessment and outcomes of interstitial lung diseases (ILDs) in our center. Consecutive patients diagnosed with IPF from March 2012 to March 2013 were included. During this period, pirfenidone was not available in Saudi Arabia, and patients were instructed to purchase pirfenex, the Indian generic form of pirfenidone. As such, IPF patients who started pirfenidone therapy (start date of March 2012) were included in the study, whereas patients who were unable to receive pirfenidone treatment served as a control group. The study was approved by the Institutional Review Board/Ethics Committee of the College of Medicine, King Saud University, Riyadh, Saudi Arabia. Written informed consent was obtained from all study participants seen and evaluated in our ILD center. A standard form was used to collect clinical information, including general symptoms, smoking history, medication use, environmental history, occupational history, family history, and physical exam findings. Surgical lung biopsies were performed in 6 (18%) IPF patients, who received pirfenidone therapy, and in 3 (12%) patients in the control group. The IPF was diagnosed according to established guidelines for the diagnosis and management of IPF.[2] A multidisciplinary approach involving various specialties, including pulmonology, rheumatology, radiology and pathology, was implemented for all IPF cases before a final diagnosis was rendered.
Exclusion criteria included connective tissue disease, hypersensitivity pneumonitis, idiopathic interstitial pneumonias other than IPF, and a diagnosis of drug-induced or unclassified pulmonary fibrosis.
Physiological measurements
The following pulmonary function tests (PFT Masterscreen; Jaeger, Hoechberg, Germany) were performed using standard methodologies that included spirometry, plethysmography, and measurement of the diffusion capacity of the lung for carbon monoxide (DLco).[11,12,13] Arterial blood gas (ABG) values (Rapid Lab 865; Bayer, Plymouth, UK) were obtained to determine the partial pressure of oxygen (PaO2), the partial pressure of carbon dioxide (PaCO2), and oxygen saturation (SaO2). After the PFTs and ABG sampling, patients were asked to perform a six-minute walk test (6MWT) in accordance with ATS guidelines.[14] Oxygen saturation (SpO2) was recorded at the beginning and end of the six-minute walk. At the end of the test, the total distance walked in meters was documented.
Treatment regimen
Pirfenidone was administered as a 200 mg dose with food three times daily and increased to a full dose (800 mg three times daily) over three weeks. Patients were instructed to avoid direct sunlight exposure and to use sunscreen. Additionally, patients were informed about potential adverse events, including photosensitivity, skin rash, gastrointestinal symptoms, fatigue and liver function abnormalities. Liver function tests were performed every two weeks for eight weeks, followed by tests every month for six months before the testing frequency was decreased to once every three months.
The standard of care in our center is for all IPF patients to receive acetylcysteine at a dose of 600 mg, three times daily, as well as aggressive anti-reflux education and therapy, reflux treatment in the form of pantoprazole at a dose of 40 mg once a day, and the prokinetic agent, domperidone, at 10 mg three times daily.
Outcome measures
Before the initiation of therapy, IPF patients underwent baseline PFT, ABG, and 6MWT measurements and completed a health-related quality of life (HRQL) assessment using a self-administered, validated Arabic version of the SF-36 questionnaire.[15] The SF-36 questionnaire is composed of eight domains and two summary measures, specifically physical and mental health summary scales. The SF-36 questionnaire scoring system ranges from 0-100, with higher scores indicating better health or well-being.
Every three months, patients repeated the PFT, ABG, and 6MWT measurements and the SF-36 questionnaire. Additionally, patients were asked to provide subjective assessments of cough, dyspnea and fatigue at each visit to determine whether these parameters were better, unchanged or worse. Both a dedicated specialist nurse and a clinician were involved in all IPF cases to monitor for adverse events. During the study period, data regarding IPF exacerbations and deaths were also collected. The last follow-up period for our analysis ended in January 2014.
Statistical analysis
Descriptive statistics are presented as the means ± standard deviation, medians with ranges or numbers with percentages. The unpaired Student's t-test, the Mann-Whitney rank sum test, the chi-square test, or Fisher's exact test were used when appropriate to compare variables of interest. Odds ratios and 95% confidence intervals for relative risks were calculated. A two-sided P value < 0.05 was considered statistically significant. Statistical Package for the Social Sciences (SPSS version 18.0, SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
Sixty-five patients diagnosed with IPF comprised the study cohort. A total of 40 patients received pirfenidone from March 2012 to March 2013. Seven patients in the pirfenidone group were excluded from the analysis due to non-compliance (n = 2), inability to tolerate therapy because of abdominal pain (n = 2), rapid disease progression within 12 weeks of initiation of therapy (n = 2) or development of signs and symptoms suggestive of undifferentiated connective tissue disease (n = 1). All patients received acetylcysteine, pantoprazole and domperidone drugs treatment, which is the standard therapy that is prescribed to all IPF patients in our center.
Of the 33 patients in the pirfenidone group, 29 (88%) were native Saudi patients, and 4 (12%) had other origins (2 patients were from Pakistan, and 1 each from Nigeria and Yemen). In the control group (n = 25), 23 (92%) were native Saudi patients, and 2 (8%) had other origins (1 each from Egypt and Yemen).
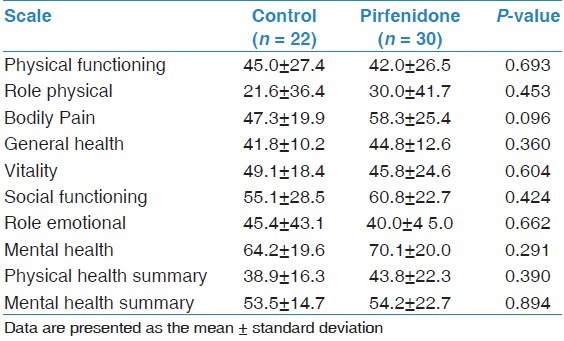
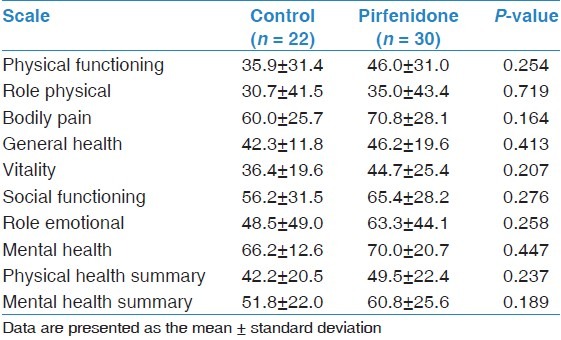
Comparisons of demographic and clinical characteristics, physiological parameters and pulmonary hemodynamics between the two groups at the time of diagnosis are shown in Tables 1 and 2. There were no significant differences between the groups with respect to age, gender, disease duration, smoking status, co-morbidities treatment, PFTs, 6MWT, ABG values or pulmonary hemodynamic parameters. Baseline data for the SF-36 domains showed no significant difference in mean scores between the groups [Table 3].
Table 1.
Baseline demographics and characteristics of study cohort
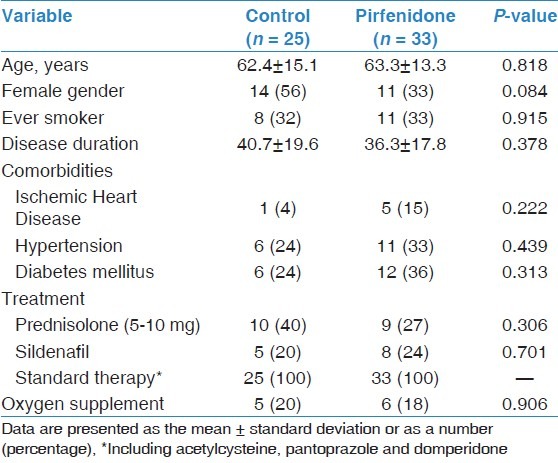
Table 2.
Baseline physiological parameters and pulmonary hemodynamics of study cohort
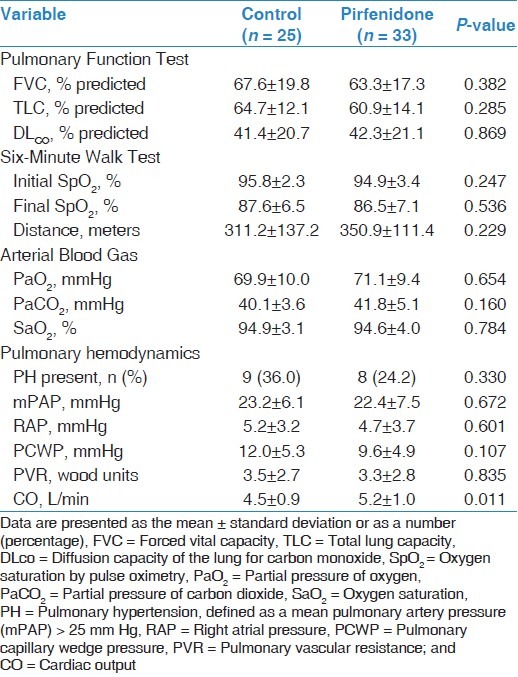
Table 3.
Baseline data for the Short Form Health Survey (SF-36)
Outcome measures
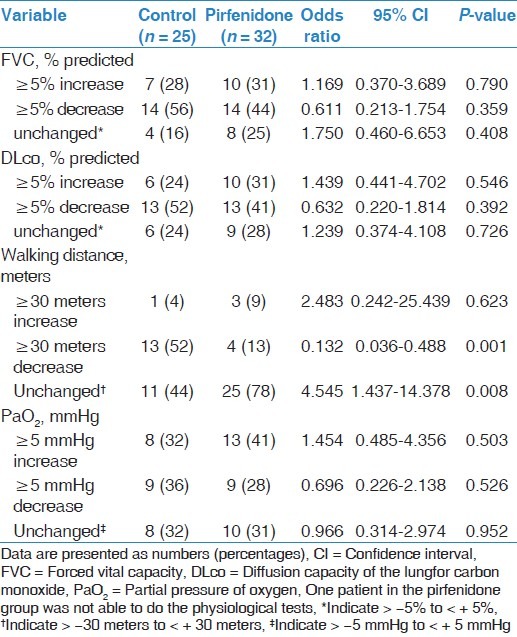
The median duration of treatment with pirfenidone was 12 (range, 8-22) months. In all 17 (52%) patients received pirfenidone therapy for 8 to 12 months, and 16 (48%) patients received treatment for longer than 12 months. During follow-up, changes in PFTs and ABG values were not significantly different between the groups [Table 4]. Moreover, different cutoff values, including ten percentage points or more of the predicted FVC, and 15% points or more of the predicted DLco, were applied and showed no significant difference between the groups (data not shown). Interestingly, patients receiving pirfenidone therapy were less likely to experience a reduction in walking distance th > 30 meters during follow-up compared with those in the control group (13% vs. 52%, respectively; P = 0.001). Furthermore, when follow-up results were compared with baseline values, walking distance did not change in 78% of the pirfenidone treated group compared with 44% of the control group (P = 0.008) [Table 4].
Table 4.
Changes in physiological parameters during follow-up
Subjective assessments of cough, dyspnea, and fatigue during follow-up were not significantly different between the groups [Table 5]. In terms of the SF-36 questionnaire, repeated measurements revealed that the mean scores from each domain were not significantly different between the groups [Table 6]. Moreover, within each group, the mean score changes from each domain were not significantly different compared with baseline values (data not shown).
Table 5.
Subjective symptoms assessments during follow-up
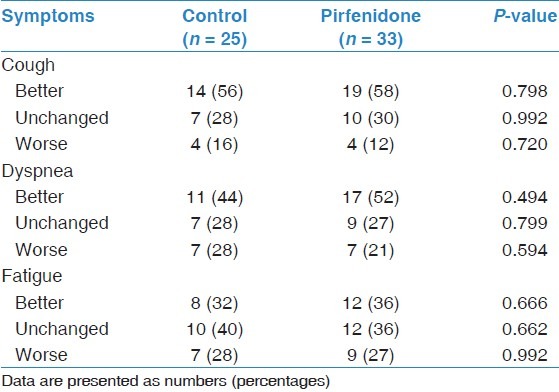
Table 6.
Changes in Short Form Health Survey (SF-36) scores during follow-up
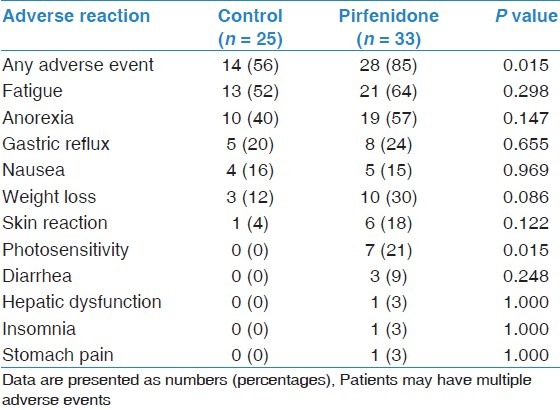
The number of reported adverse events was higher among patients who received pirfenidone treatment compared with those in the control group (85 vs. 56%, respectively; P = 0.015) [Table 7]. The most common adverse event reported by the pirfenidone group was fatigue (64%), followed by anorexia (57%) and weight loss (30%). As expected, photosensitivity was frequently reported by patients who received pirfenidone treatment compared with the control group (21 vs. 0%, respectively; P = 0.015). Serious adverse events requiring discontinuation of therapy were not observed in the pirfenidone group.
Table 7.
Reported adverse events in the study cohort
During follow-up, acute exacerbations were noted in 3 (12%) patients in the control group and 4 (12%) patients in the pirfenidone group. Additionally, one death was observed in the control group. Adherence to treatment was 94% in the pirfenidone group and 100% in the control group.
Discussion
The present study is the first to report on the clinical efficacy and safety of pirfenidone for the treatment of patients with IPF in a Saudi Arabian population.
Several studies exploring the effects of pirfenidone in patients with IPF have provided some hope in treating this devastating disease.[6,7,8,9] The most recent study is the ASCEND (Assessment of Pirfenidone to Confirm Efficacy and Safety in Idiopathic Pulmonary Fibrosis) trial, which is a multinational, randomized, double-blinded, placebo-controlled study in which pirfenidone was compared with a placebo.[9] In the cited study, investigators noted that patients in the pirfenidone group experienced a significant reduction in the 1-year rate of decline in FVC and experienced a reduction in the decline in walking distance and an improvement in progression-free survival.[9]
In our study, although pirfenidone was not available in Saudi Arabia during the study period, a relatively high number of our cohort patients were able to receive pirfenex, a generic form of pirfenidone that is manufactured in India. In addition, having a dedicated specialist nurse and constant clinician support resulted in an excellent adherence rate and compliance with pirfenidone therapy despite the frequent dosing regimen and need for repeated liver function tests. Moreover, in spite of the frequency of adverse events reported in the pirfenidone group compared with the control group, the incidence of these events was similar to results reported in other studies.[6,7,8,9] Additionally, these events ranged from mild to moderate in severity and were well tolerated by patients.
Patients who were prescribed pirfenidone were categorized as having mild to moderately severe disease based on their PFT results, which is consistent with previous studies.[6,7,8,9] Surprisingly, we found no significant differences between the groups in terms of changes in FVC, DLco, or PaO2 values during follow-up visits in our clinic, despite similar baseline demographic and clinical characteristics in both groups. A limitation of our study is that the number of patients in each group was relatively small, and our study did not have sufficient power to demonstrate the full range of clinically significant differences in treatment. Nonetheless, important extrapolations can be made from our results. An interesting and important observation noted during the study period was that patients on pirfenidone therapy were less likely to experience a decline in walking distance compared with the control group (13 vs. 52%, respectively; P = 0.001). Additionally, a large proportion of patients in the pirfenidone group were more likely to have stable walking distances (i.e., unchanged) compared with the control group (78 vs. 44%, respectively; P = 0.008). The positive impact of pirfenidone on the 6MWD noted in the present work is consistent with the results of other studies.[8,9] A possible explanation for this finding is that skeletal muscle weakness (i.e., acquired myopathy), which is frequently observed in IPF patients due to hypoxia, stress, malnutrition, corticosteroid therapy and other factors, is associated with increased activity of transforming growth factor beta (TGF-β).[16] Because the anti-fibrotic properties of pirfenidone are mediated by the inhibition of TGF-β expression, as observed in many models of fibrosis, including in the lung, heart, liver and kidney,[17] such actions may also occur in skeletal muscles, resulting in improved functional capacity and exercise tolerance in patients with IPF. However, this hypothesis is only speculative, and future studies are needed to address the precise role of pirfenidone on TGF-β signaling in skeletal muscle.
More than half of the patients who received pirfenidone treatment reported subjective improvements in cough; however, compared with the control group, the difference was not statistically significant (58 vs. 56%, respectively; P = 0.79). Nonetheless, the data partially suggest that aggressive anti-reflux therapy may improve cough symptoms substantially, which may lead to improved quality of life in IPF patients. The HRQL is an assessment tool used by many investigators to determine the effectiveness of a particular intervention. In the present study, we used SF-36 to measure outcomes between patients taking pirfenidone treatment compared with patients receiving standard therapy. Interestingly, we found no significant differences in the SF-36 domains, at baseline or after therapy, in either group. However, it is unclear which study instrument should be used to obtain relevant information after interventions among Saudi patients with IPF, and future studies may need to use different HRQL questionnaires.
Prescribing acetylcysteine to IPF patients is a routine practice in our center. Demedts et al.,[18] noted that IPF patients who received acetylcysteine in addition to prednisone and azathioprine had slower rates of deterioration in vital capacity and DLco after 12 months of therapy. However, a recently published trial comparing acetylcysteine to placebo in patients with IPF suffering from mild to moderate impairment in pulmonary function parameters showed no significant difference in either the study's primary outcome measure (change in FVC over 60 weeks) or in secondary outcome measures, including rate of death, acute exacerbations, DLco, changes in walking distance and responses to a HRQL questionnaire.[19] In the present study, both groups received acetylcysteine; therefore, it is difficult to determine the beneficial effects of acetylcysteine on lung function indices.
The present study had several limitations. The retrospective review of a database from a single tertiary center may have introduced both selection and recall biases. In addition, the number of patients in each group was relatively small, and our study did not have sufficient power to demonstrate clinically significant differences with regards to treatment. Additionally, concomitant administration of other treatments may have affected the bioavailability and distribution of pirfenidone, accounting for the absence of significant improvement in lung function parameters observed in our study.
In conclusion, treatment with pirfenidone was not associated with improved lung function, PaO2, subjective symptoms and responses to a SF-36 questionnaire compared with patients not taking pirfenidone therapy. However, patients treated with pifenidone were more likely to preserve their 6MWD. Moreover, we show that adherence and compliance to treatment was high, and side effects were generally well tolerated by patients.
Acknowledgments
The authors thank the patients who participated in the study, and we are grateful to our research assistant, Ayan Ibrahim, for her excellent patient data monitoring. Thanks to Actelion Pharmaceuticals, Ltd. (Riyadh, Saudi Arabia) for partially funding our research assistants in the Interstitial Lung Disease and Pulmonary Hypertension Center at King Khalid University Hospital, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. , ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;291:367–73. [PubMed] [Google Scholar]
- 4.Oku H, Nakazato H, Horikawa T, Tsuruta Y, Suzuki R. Pirfenidone suppresses tumor necrosis factor-alpha, enhances interleukin-10 and protects mice from endotoxic shock. Eur J Pharmacol. 2002;446:167–76. doi: 10.1016/s0014-2999(02)01757-0. [DOI] [PubMed] [Google Scholar]
- 5.Misra HP, Rabideau C. Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals. Mol Cell Biochem. 2000;204:119–26. doi: 10.1023/a:1007023532508. [DOI] [PubMed] [Google Scholar]
- 6.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone Clinical Study Group in Japan. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 8.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet. 2011;377:1760–9. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 9.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. ASCEND Study Group. A Phase3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 10.Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Walters EH, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010:CD003134. doi: 10.1002/14651858.CD003134.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 12.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 13.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–22. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 14.ATS Committee on Proficiency Standards for Clinical Pulmonary Function. Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 15.Sabbah I, Drouby N, Sabbah S, Retel-Rude N, Mercier M. Quality of life in rural and urban populations in Lebanon using SF-36 health survey. Health Qual Life Outcomes. 2003;1:30. doi: 10.1186/1477-7525-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burks TN, Cohn RD. Role of TGF-beta signaling in inherited and acquired myopathies. Skelet Muscle. 2011;1:19. doi: 10.1186/2044-5040-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20:85–97. doi: 10.1183/09059180.00001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, et al. IFIGENIA Study Group. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–42. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 19.Idiopathic Pulmonary Fibrosis Clinical Research Network. Martinez FJ, de Andrade JA, Anstrom KJ, King TE, Jr, Raghu G. Randomized Trial of Acetylcysteine in Idiopathic Pulmonary Fibrosis. N Engl J Med. 2014;370:2093–101. doi: 10.1056/NEJMoa1401739. [DOI] [PMC free article] [PubMed] [Google Scholar]


