Abstract
OBJECTIVES:
The effects of first-line chemotherapy on overall survival (OS) might be confounded by subsequent therapies in patients with small cell lung cancer (SCLC). We examined whether progression-free survival (PFS), post-progression survival (PPS), and tumor response could be valid surrogate endpoints for OS after first-line chemotherapies for patients with extensive SCLC using individual-level data.
METHODS:
Between September 2002 and November 2012, we analyzed 49 cases of patients with extensive SCLC who were treated with cisplatin and irinotecan as first-line chemotherapy. The relationships of PFS, PPS, and tumor response with OS were analyzed at the individual level.
RESULTS:
Spearman rank correlation analysis and linear regression analysis showed that PPS was strongly correlated with OS (r = 0.97, p < 0.05, R2= 0.94), PFS was moderately correlated with OS (r = 0.58, p < 0.05, R2= 0.24), and tumor shrinkage was weakly correlated with OS (r = 0.37, p < 0.05, R2= 0.13). The best response to second-line treatment, and the number of regimens employed after progression beyond first-line chemotherapy were both significantly associated with PPS ( p ≤ 0.05).
CONCLUSION:
PPS is a potential surrogate for OS in patients with extensive SCLC. Our findings also suggest that subsequent treatment after disease progression following first-line chemotherapy may greatly influence OS.
Keywords: Extensive small cell lung cancer, overall survival, post-progression survival, progression-free survival, tumor response
Lung cancer is one of the leading causes of cancer-related mortality worldwide. In 2007, 1.3 million people were diagnosed with lung cancer, 15-20% of whom were found to have small cell lung cancer (SCLC).[1,2] Overall survival (OS) is considered the most reliable endpoint in cancer studies, and when studies can be conducted to adequately assess survival, it is usually the preferred endpoint.[3] OS is a precise endpoint, is easy to measure, and can be documented by the date of death. Surrogate endpoints such as tumor response and progression-free survival (PFS) are also useful endpoints for phase II oncology clinical trials because they can be measured earlier and more conveniently. Events for these surrogate endpoints occur more frequently than do events for the main endpoints of interest, which are referred to as the true endpoints.
The effects of first-line chemotherapy on OS might be confounded by subsequent therapies. Indeed, PFS improvements do not necessarily result in an improved OS, as shown by recent randomized trials in patients with non-SCLC (NSCLC).[4] In recent years, a growing number of active compounds have become available as second- or third-line chemotherapy for breast, ovarian, and colorectal cancers[5,6,7], as well as advanced NSCLC. However, with respect to the treatment of SCLC, first-line chemotherapy is often beneficial for patients with poor performance status (PS), in contrast with NSCLC cases, albeit at the risk of serious toxic effects. SCLC is a distinct clinical and histological entity within the range of lung cancers. Only a few drugs are available for its treatment, and topotecan is currently the only drug approved for the treatment of relapsed SCLC patients in the United States.[8,9,10] Second-line treatment is an option in only a few patients, owing to rapid disease progression and poor PS.
Although PFS following first-line chemotherapy has not been validated as a surrogate endpoint for OS, post-progression survival (PPS) has been shown to be strongly associated with OS after first-line chemotherapy for advanced NSCLC.[11,12] Furthermore, it has been suggested that OS can be approximated as the sum of PPS and PFS.[3] Very few novel anticancer drugs have become available for extensive SCLC, and the relationship between PPS and OS in extensive SCLC remains unclear.
At the level of the individual patient, it is of interest to assess the effect of therapy administered after disease progression on survival. The validation of surrogate measures for OS after first-line therapy in individual patients with advanced NSCLC has been reported previously.[13] Further, the surrogate endpoint sometimes does not reflect the primary endpoint. The significance of PPS in SCLC also remains unclear at the level of the individual patient. Therefore, it is important to establish whether PFS, PPS, or tumor response could be valid surrogate endpoints for OS after first-line therapy in patients with extensive SCLC using individual-level data.
The first-line treatment of choice in extensive-stage SCLC remains 4 to 6 cycles of platinum combination chemotherapy.[1] Although many patients initially achieve clinical remission or disease control with first-line chemotherapy, most subsequently experience disease progression and eventually die of extensive SCLC. We examined first-line cisplatin and irinotecan combination chemotherapy because it is considered the standard first-line chemotherapy in these cases.[1] Previously, in a phase 3 study of extensive SCLC, first-line chemotherapy with irinotecan plus cisplatin was found to be more effective than etoposide/cisplatin (median survival of 12.8 months versus 9.4 months, p = 0.002).[14] The MST of patients with extensive SCLC was approximately 1 year. For extensive SCLC patients, OS is shorter and options for subsequent chemotherapy are limited.
In the present study, we analyzed the relationships of PFS, PPS, and tumor response with OS in patients with extensive SCLC at the individual level. The patients recruited to this study had only a limited number of options for subsequent-line chemotherapy. We also explored the prognostic value of baseline and tumor characteristics for PPS.
Methods
Patients
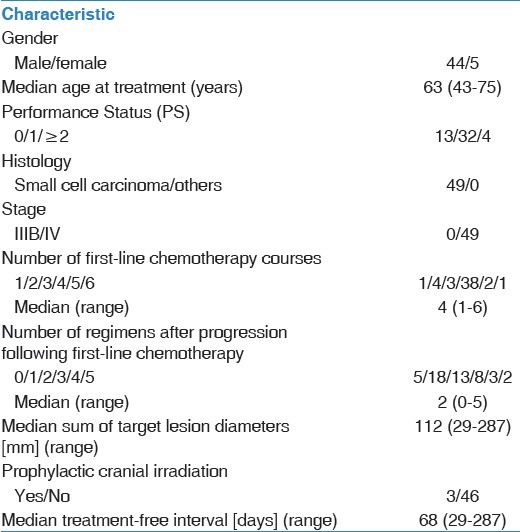
Between September 2002 and November 2012, 60 patients with extensive SCLC were treated with cisplatin and irinotecan as first-line chemotherapy and were enrolled in this study. The tumor response was not evaluated in 10 cases, and PFS data were censored in one case. These 11 patients were excluded from the analyses to maintain uniformity in patient background characteristics. Thus, data from 49 patients were analyzed. The study protocol was approved by the Institutional Review Board of Shizuoka Cancer Center (#.25-J91-25-1-3).
The patients in this study were treated with cisplatin (60 mg·m-2 day-1 for 1 day, followed by a pause of 28 days) and irinotecan (60 mg·m-2 day-1 on days 1, 8, and 15, followed by a pause of 28 days). This cycle was repeated every 28 days for a maximum of six courses.
The best overall response and maximum tumor shrinkage were recorded as tumor responses. Radiographic tumor responses were evaluated according to the Response Evaluation Criteria In Solid Tumors,ver. 1.1[15]: Complete response (CR), disappearance of all target lesions; partial response (PR), at least a 30% decrease in the sum of the target lesion diameters with the summed baseline diameters as a reference; progressive disease (PD), at least a 20% increase in the sum of the target lesion diameters with the smallest sum observed during the study serving as reference; and stable disease (SD), insufficient shrinkage to qualify as PR and insufficient expansion to qualify as PD. PFS was calculated from the start of treatment to the date of PD or death from any cause. OS was recorded from the first day of treatment until death or was censored on the date of the last follow-up consultation. PPS was recorded as the time from tumor progression until death or was censored on the date of the last follow-up consultation. In this study, we defined treatment-free interval (TFI) as the period from the date of completion of first-line treatment to the first relapse. When prophylactic cranial irradiation (PCI) was performed as first-line treatment, the date of completion was defined as the last day of these treatments. We defined sensitive relapse as TFI ≥ 90 days, based on the definition in several previous trials.[16,17]
Statistical analyses
To examine whether PFS, PPS, or tumor shrinkage was correlated with OS, we used Spearman rank correlation analysis and linear regression analysis. In order to identify possible prognostic factors for PPS, the proportional hazards model with a stepwise regression procedure was applied. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated using this model. Because the HR is defined for a 1-unit difference, some factors were converted to an appropriately scaled unit. PPS values were compared using the log-rank test. A P value of ≤0.05 was considered significant for all tests. The two-tailed significance level was also set at 0.05. All statistical analyses were performed using JMP version 9.0 for Windows (SAS Institute, Cary, NC, USA).
Results
Patient characteristics and treatment efficacy
Of the 49 patients included in the analyses, 43 patients died; the median follow-up time was 14.0 months (range, 0.7-36.8 months). The characteristics of the 49 patients (median age, 63 years; range, 43-75 years) included in the present study are shown in Table 1. Target lesions were not evaluated in one of the cases. One, 38, 5, and 4 patients showed CR, PR, SD, and PD, respectively. The response rate was 79.6% and the disease control rate was 91.8%.
Table 1.
Baseline patient characteristics
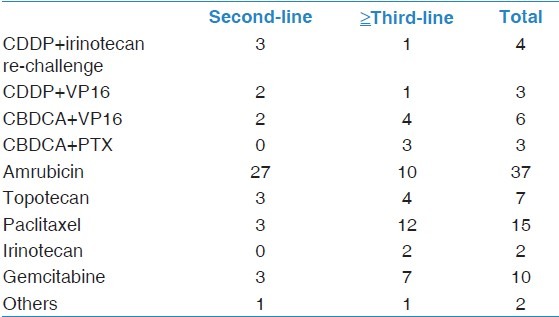
After progressing past first-line chemotherapy, 5 of the 49 patients did not receive further chemotherapy. The other 44 patients received subsequent chemotherapy after completing their first-line chemotherapy. Among the 49 patients, the median number of follow-up therapeutic regimens was 2 (range, 0-5 regimens). The chemotherapy regimens employed, after progressing past the first-line chemotherapy regimen, are shown in Table 2. Amrubicin was the most common second-line chemotherapy agent, and paclitaxel was the most common third-line chemotherapy agent.
Table 2.
Chemotherapy regimens employed after progression following first-line chemotherapy
The median PFS and OS were 5.5 months and 13.9 months, respectively [Figure 1a, b].
Figure 1.
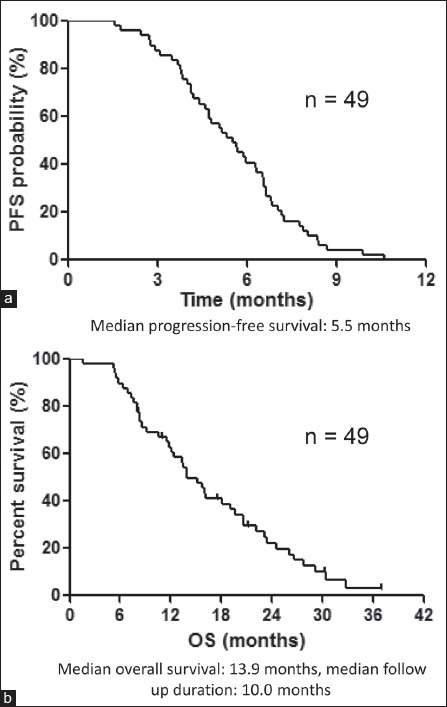
(a) Kaplan-Meier plots showing progression-free survival (PFS) (b) Kaplan-Meier plots showing overall survival (OS)
Relationship between OS and PFS, PPS, and tumor shrinkage
The relationship between OS and PFS, PPS, and tumor shrinkage is shown in Figure 2a, b, and c, respectively. PPS was strongly associated with OS (r = 0.97, p < 0.05, R2= 0.94), based on Spearman's rank correlation coefficient and linear regression, whereas PFS was moderately correlated with OS (r = 0.58, p < 0.05, R2= 0.24). Furthermore, tumor shrinkage was only weakly correlated with OS (r = 0.37, p < 0.05, R2= 0.13).
Figure 2.
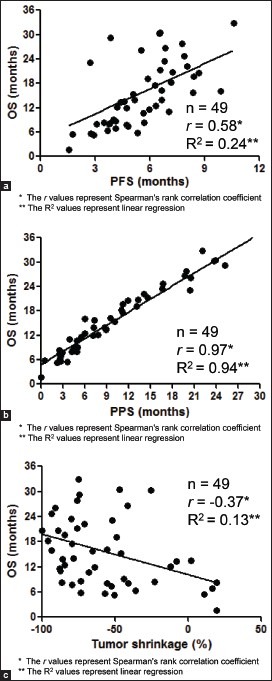
(a) Correlation between overall survival (OS) and progression-free survival (PFS) (b) Correlation between overall survival (OS) and post-progression survival (PPS) (c) Correlation between overall survival (OS) and tumor shrinkage
Factors affecting post-progression survival
PPS was strongly associated with OS. Therefore, the association between PPS and various clinical factors was assessed. In the univariate analysis [Table 3], PS at the end of first-line treatment, at the beginning of second-line treatment, and TFI (≥90/<90 days) as well as the best response at first-line treatment, the best response from the second-line treatment, and the number of regimens employed after progression beyond first-line chemotherapy were found to be associated with PPS (p < 0.05). Next, a multivariate analysis for PPS was conducted [Table 4]. This revealed that the best response after second-line treatment (non-PD/PD), and the number of regimens employed after progression following first-line chemotherapy were significantly associated with PPS (p ≤ 0.05). The log-rank tests confirmed that PPS was significantly associated with the best response at second-line treatment (non-PD/PD), and the number of regimens employed (p < 0.05; Figure 3a and b). Based on the best response at second-line treatment, patients with non-PD had a median PPS of 13.1 months, which was longer than that of their counterparts, who had a median PD of 7.2 months (log-rank, p = 0.05; Figure 3a). According to the number of regimens employed after progression following first-line chemotherapy, the median PPS for those who were not administered additional regimens was 3.5 months; with 1 additional regimen, the median PPS was 5.5 months; and with ≥2 regimens, the median PPS was 14.1 months, (log-rank test, p < 0.01; Figure 3b). These results remained consistent after adjustment using the Cox proportional hazards models [Table 4].
Table 3.
Univariate Cox regression analysis of baseline patient characteristics for post-progression survival
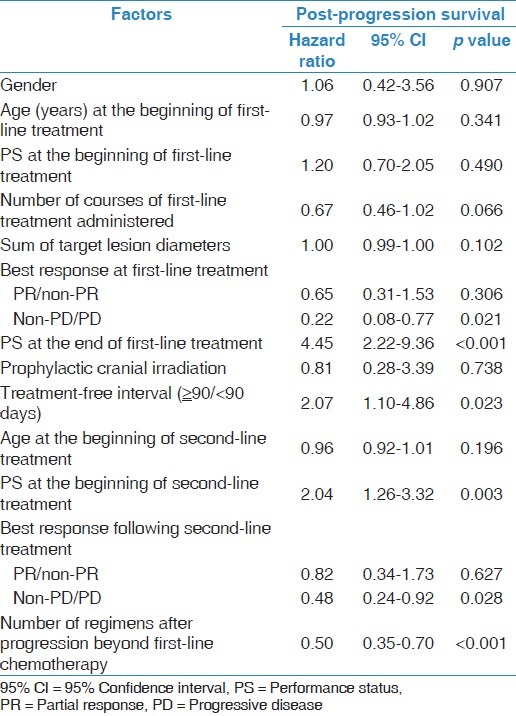
Table 4.
Multivariate Cox regression analysis of performance status (PS) at the end of first-line treatment, PS at the beginning of second-line treatment, best response at first-line treatment, best response at second-line treatment, and number of regimens employed after progression beyond first-line chemotherapy for post-progression survival
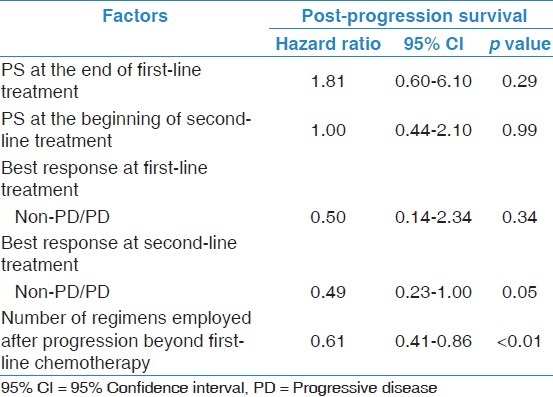
Figure 3.
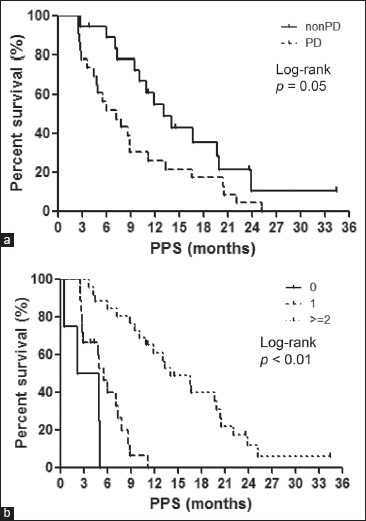
(a)Kaplan-Meier plots showing post-progression survival (PPS), according to the best response following second-line treatment Non-progressive disease (non-PD), median = 13.1 months; progressive disease (PD), median = 7.1 months. (b) Kaplan-Meier plots showing post-progression survival (PPS), according to the number of regimens after progression No further regimen, median = 3.5 months; 1 regimen, median = 5.5 months; 2 regimens, median = 14.1 months
Discussion
We examined the relationships of OS with PFS, PPS, and tumor shrinkage at the individual level in patients with extensive small cell lung cancer. PPS was strongly associated with OS, whereas PFS and tumor shrinkage were moderately and weakly correlated with OS, respectively. In addition, the best response to second-line treatment (non-PD vs. PD), and the number of regimens employed after progression following first-line chemotherapy, independently affected PPS.
The validity of surrogate endpoints has been previously determined through meta-analyses.[18,19] In recent years, biostatisticians have proposed a wide variety of measures for validating surrogate endpoints.[20,21] Although PFS is a potential surrogate endpoint for OS in extensive stage SCLC[22], its validity remains controversial. Broglio et al. recently focused on PPS, which they termed survival post progression (defined as OS minus PFS), in a hypothetical clinical trial setting under the assumption that treatment affected PFS but not PPS.[3] Recently, PPS was found to be strongly associated with OS after first-line chemotherapy for advanced NSCLC in a clinical trial[11,12], and we have previously reported the significance of PPS for advanced NSCLC based on an analysis of individual patients.[13]
In contrast with the findings of a previous study[22], we did not observe that PFS was a surrogate endpoint for OS in extensive stage SCLC, although PPS was not evaluated in the previous study. We analyzed our results pertaining to first-line therapy, which suggested that PFS and tumor response did not adequately reflect OS in such settings. We found that PFS was much shorter than PPS, and thus, PPS was closely related to OS — the relationship was linear. The fact that PPS accounted for the majority of OS suggests that the chemotherapy used was not sufficiently effective for PFS to be a significant component of OS. Thus, in clinical trials with patients expected to have a short PFS after first-line chemotherapy, for example those with extensive SCLC, as was the case in our study, factors that affect PPS need to be considered.
Based on trial-level data for advanced NSCLC, a long PPS is associated with a good PS and the use of first-line monotherapy with a molecular targeted agent.[11] Studies based on individual advanced NSCLC patients revealed that a long PPS was associated with the PS at the beginning of second-line treatment, the best response after second-line treatment (non-PD/PD), and the number of regimens employed after disease progression following first-line chemotherapy.[13] To date, however, no predictive factors for PPS in cases of extensive SCLC have been identified. We studied the prognostic value of baseline factors for PPS in individual patients. We found that the best response after second-line treatment, and the number of regimens employed after progression following first-line chemotherapy were strongly associated with PPS. Moreover, we confirmed the significance of these relationships using log-rank tests. Our findings suggest that patients for whom the disease has been controlled with second-line treatment achieve prolonged PPS after progression following first-line chemotherapy. These patients are also likely to be able to continue chemotherapy and achieve prolonged PPS, which is associated with a longer OS. The number of treatment regimens used after progression following first-line chemotherapy probably reflects the increasing number of available drugs, such as amrubicin, paclitaxel, and topotecan, which are available as second- or third-line chemotherapy for extensive SCLC. In fact, a number of different agents were used to treat our patients, as shown in Table 2.
This study has several limitations. First, the sample size was small. However, because relatively few extensive SCLC patients are treated with first-line cisplatin and irinotecan at our institution, this limitation is difficult to overcome, especially as the patients needed to have similar background characteristics. Nevertheless, our institution treats the relatively largest number of such cases, and the practice policy is largely unified simply because this is a single institution. There is of course some bias, but understanding the nature of this bias ensures that the results are still meaningful. In a future study, we will include a larger patient cohort, and more detailed examination is warranted. Second, we could not thoroughly evaluate treatments after progression following second-line chemotherapy, although only a few patients received third-line or subsequent chemotherapy. Third, the date on which a response was recorded was decided by each physician, which might have introduced variance in the PFS and tumor response rate. Fourth, chemotherapy regimens differ between Japan and the USA. In Japan, based on the results of a Japanese phase III trial[14], standard first-line chemotherapy for extensive SCLC currently is cisplatin combined with irinotecan. This combination is also described in the National Comprehensive Cancer Network guidelines as a suitable treatment option. Amrubicin is an effective second-line chemotherapy drug in a number of cancers including SCLC. In a phase III trial, it resulted in a significantly improved response rate compared to topotecan and also improved survival, especially in the subgroup of refractory patients.[23] On the basis of this trial, amrubicin is now the standard second-line chemotherapy agent for extensive SCLC in Japan.
In conclusion, using individual patient data, PFS and tumor response were not found to be ideal surrogates for OS in patients with extensive SCLC who had limited options for subsequent chemotherapy. However, in these patients, PPS, rather than PFS, was strongly associated with OS. In addition, the best response after second-line treatment (non-PD/PD), and the number of regimens employed after disease progression following first-line chemotherapy were prognostic factors for PPS. Thus, the treatment course after progression following first-line chemotherapy greatly influences OS. We believe these findings justify further study to validate PPS as a surrogate marker of OS in patients with extensive SCLC.
Acknowledgements
We wish to thank Ms. Mutsumi Yamazaki, Mr. Taiki Miyauchi, Drs. Takuya Oyakawa, Yasushi Hisamatsu, Ryo Koh, Shota Ohmori, and Kazuhisa Nakashima for their assistance in preparing this manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741–55. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 2.Kalemkerian GP, Akerley W, Bogner P, Borghaei H, Chow LQ, Downey RJ, et al. National Comprehensive Cancer Network. Small cell lung cancer. J Natl Compr Canc Netw. 2013;11:78–98. doi: 10.6004/jnccn.2013.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101:1642–9. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–34. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 5.Saad ED, Katz A, Buyse M. Overall survival and post-progression survival in advanced breast cancer: A review of recent randomized clinical trials. J Clin Oncol. 2010;28:1958–62. doi: 10.1200/JCO.2009.25.5414. [DOI] [PubMed] [Google Scholar]
- 6.Sundar S, Wu J, Hillaby K, Yap J, Lilford R. A systematic review evaluating the relationship between progression free survival and post progression survival in advanced ovarian cancer. Gynecol Oncol. 2012;125:493–9. doi: 10.1016/j.ygyno.2011.12.420. [DOI] [PubMed] [Google Scholar]
- 7.Petrelli F, Barni S. Correlation of progression-free and post-progression survival with overall survival in advanced colorectal cancer. Ann Oncol. 2013;24:186–92. doi: 10.1093/annonc/mds289. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien ME, Ciuleanu TE, Tsekov H, Shparyk Y, Cucevia B, Juhasz G, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441–7. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 9.Eckardt JR, von Pawel J, Pujol JL, Papai Z, Quoix E, Ardizzoni A, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25:2086–92. doi: 10.1200/JCO.2006.08.3998. [DOI] [PubMed] [Google Scholar]
- 10.von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–67. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 11.Hotta K, Kiura K, Fujiwara Y, Takigawa N, Hisamoto A, Ichihara E, et al. Role of survival post-progression in phase III trials of systemic chemotherapy in advanced non-small-cell lung cancer: A systematic review. PLoS One. 2011;6:e26646. doi: 10.1371/journal.pone.0026646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi H, Okamoto I, Morita S, Taguri M, Nakagawa K. Postprogression survival for first-line chemotherapy of patients with advanced non-small-cell lung cancer. Ann Oncol. 2012;23:1537–41. doi: 10.1093/annonc/mdr487. [DOI] [PubMed] [Google Scholar]
- 13.Imai H, Takahashi T, Mori K, Ono A, Akamatsu H, Shukuya T, et al. Individual-level data on the relationships of progression-free survival, post-progression survival, and tumor response with overall survival in patients with advanced non-squamous non-small cell lung cancer. Neoplasma. 2013;61:233–40. doi: 10.4149/neo_2014_030. [DOI] [PubMed] [Google Scholar]
- 14.Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al. Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Inoue A, Sugawara S, Yamazaki K, Maemondo M, Suzuki T, Gomi K, et al. Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402. J Clin Oncol. 2008;26:5401–6. doi: 10.1200/JCO.2008.18.1974. [DOI] [PubMed] [Google Scholar]
- 17.Jotte R, Conkling P, Reynolds C, Galsky MD, Klein L, Fitzgibbons JF, et al. Randomized phase II trial of single-agent amrubicin or topotecan as second-line treatment in patients with small-cell lung cancer sensitive to first-line platinum-based chemotherapy. J Clin Oncol. 2011;29:287–93. doi: 10.1200/JCO.2010.29.8851. [DOI] [PubMed] [Google Scholar]
- 18.Johnson KR, Ringland C, Stokes BJ, Anthony DM, Freemantle N, Irs A, et al. Response rate or time to progression as predictors of survival in trials of metastatic colorectal cancer or non-small-cell lung cancer: A meta-analysis. Lancet Oncol. 2006;7:741–6. doi: 10.1016/S1470-2045(06)70800-2. [DOI] [PubMed] [Google Scholar]
- 19.Hotta K, Fujiwara Y, Matsuo K, Kiura K, Takigawa N, Tabata M, et al. Time to progression as a surrogate marker for overall survival in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:311–7. doi: 10.1097/JTO.0b013e3181989bd2. [DOI] [PubMed] [Google Scholar]
- 20.Weir CJ, Walley RJ. Statistical evaluation of biomarkers as surrogate endpoints: A literature review. Stat Med. 2006;25:183–203. doi: 10.1002/sim.2319. [DOI] [PubMed] [Google Scholar]
- 21.Fleischer F, Gaschler-Markefski B, Bluhmki E. A statistical model for the dependence between progression-free survival and overall survival. Stat Med. 2009;28:2669–86. doi: 10.1002/sim.3637. [DOI] [PubMed] [Google Scholar]
- 22.Foster NR, Qi Y, Shi Q, Krook JE, Kugler JW, Jett JR, et al. Tumor response and progression-free survival as potential surrogate endpoints for overall survival in extensive stage small-cell lung cancer: Findings on the basis of North Central Cancer Treatment Group trials. Cancer. 2011;117:1262–71. doi: 10.1002/cncr.25526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jotte R, Von Pawel J, Spigel DR, Socinski MA, O’Brien M, Paschold EH, et al. Randomized phase III trial of amrubicin versus topotecan (Topo) as second-line treatment for small cell lung cancer (SCLC) J Clin Oncol. 2011;29(Suppl 15) doi: 10.1200/JCO.2013.54.5392. [DOI] [PubMed] [Google Scholar]


